Significance
B cell depletion via anti-CD20 monoclonal antibodies is a novel, highly efficient therapy for multiple sclerosis (MS). In a murine MS model, we investigated three mechanistic questions that cannot be addressed in humans. First, we established that a fraction of mature B cells in the spleen is resistant to anti-CD20. Second, we determined that, after cessation of treatment, splenic and bone-marrow B cells reconstitute in parallel, substantially preceding B cell reappearance in blood. Third, we observed that, in a model involving activated B cells, the post–anti-CD20 B cell pool contained an elevated frequency of differentiated, myelin-reactive B cells. Together, our findings reveal mechanisms by which pathogenic B cells may persist in anti-CD20 treatment.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, secondary lymphoid organ, anti-CD20, B cell recovery
Abstract
The anti-CD20 antibody ocrelizumab, approved for treatment of multiple sclerosis, leads to rapid elimination of B cells from the blood. The extent of B cell depletion and kinetics of their recovery in different immune compartments is largely unknown. Here, we studied how anti-CD20 treatment influences B cells in bone marrow, blood, lymph nodes, and spleen in models of experimental autoimmune encephalomyelitis (EAE). Anti-CD20 reduced mature B cells in all compartments examined, although a subpopulation of antigen-experienced B cells persisted in splenic follicles. Upon treatment cessation, CD20+ B cells simultaneously repopulated in bone marrow and spleen before their reappearance in blood. In EAE induced by native myelin oligodendrocyte glycoprotein (MOG), a model in which B cells are activated, B cell recovery was characterized by expansion of mature, differentiated cells containing a high frequency of myelin-reactive B cells with restricted B cell receptor gene diversity. Those B cells served as efficient antigen-presenting cells (APCs) for activation of myelin-specific T cells. In MOG peptide-induced EAE, a purely T cell-mediated model that does not require B cells, in contrast, reconstituting B cells exhibited a naive phenotype without efficient APC capacity. Our results demonstrate that distinct subpopulations of B cells differ in their sensitivity to anti-CD20 treatment and suggest that differentiated B cells persisting in secondary lymphoid organs contribute to the recovering B cell pool.
Monoclonal antibodies against CD20 deplete immature and mature B cells and spare plasma cells and hematopoietic stem cells as a result of their lack of CD20 expression. Rituximab was the first anti-CD20 antibody to be tested in clinical multiple sclerosis (MS) trials, which revealed a rapid decline in the development of new CNS lesions in patients with relapsing-remitting (RR) MS (1, 2). In primary progressive (PP) MS, a subgroup of younger patients with ongoing CNS lesion formation experienced a slowing in disease progression (3). All available case series indicate that rituximab is also effective and safe in patients with neuromyelitis optica (NMO) and NMO spectrum disorders (4–6). Testing rituximab’s further humanized successor ocrelizumab confirmed a substantial reduction in the frequency of clinical relapses and CNS lesion formation in RR-MS (7, 8) and a significantly decelerated accumulation of disability in patients with PP-MS (9). Based on these phase III clinical trial findings, ocrelizumab has been approved recently for both MS indications.
Notwithstanding this encouraging development, several mechanistic questions remain to be addressed. To date, it is largely unknown to what extent B cell-rich compartments, such as bone marrow, spleen, and lymph nodes, are depleted by anti-CD20 treatment and how they are repopulated with B cells after cessation of anti-CD20 treatment. Moreover, it is crucial to clarify in which functional status B cells reappear after their transient anti-CD20–mediated extinction and to learn which factors shape the reemerging B cell phenotype. This question is of tremendous clinical relevance, as it determines whether patients need to be continuously depleted of B cells, which may significantly increase the long-term safety risk over time. To approach these mechanistic questions, we investigated de- and repletion of B cells upon transient anti-CD20 treatment in murine model systems with access to all tissues and compartments. To model different functional aspects, we used naïve mice, as well as two models of experimental autoimmune encephalomyelitis (EAE), one in which activated B cells contribute to EAE development and one in which B cells are not activated in an antigen-specific manner.
Results
Anti-CD20 Efficiently Depletes Peripheral Immature and Mature B Cells, Whereas a Subset of CD20+ B Cells Persists in Secondary Lymphoid Organs.
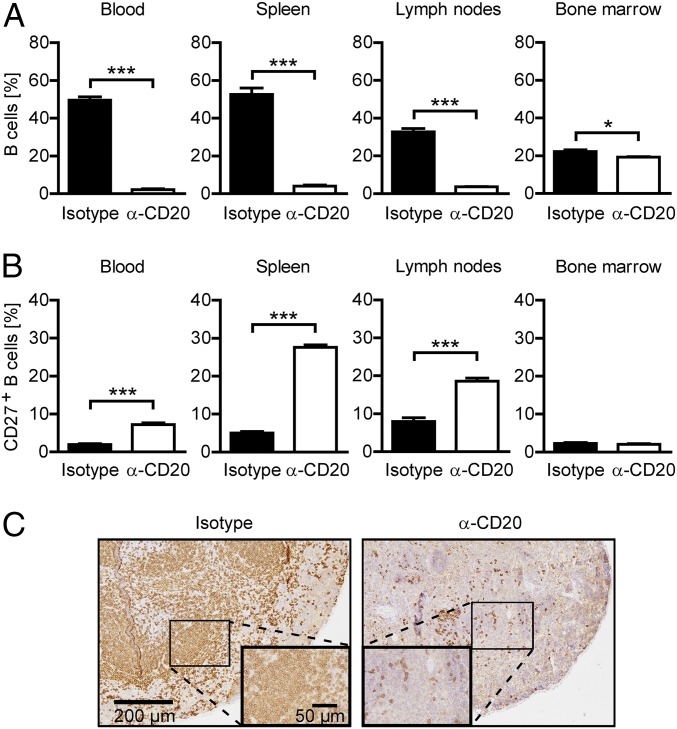
We first investigated the extent of B cell depletion upon systemic application of murine anti-CD20 in the bone marrow, spleen, lymph nodes, and blood of naïve mice. As indicated in Fig. 1A and SI Appendix, Fig. S1A, three weekly injections of 0.2 mg anti-CD20 significantly reduced the frequency of B220+/CD19+ B cells in all compartments. The depicted B cell frequency concomitantly represents the maximum effect of anti-CD20 treatment, as further injections or higher doses of anti-CD20 exerted no additional effect. The highest B cell depletion efficacy was achieved in the blood, where B cells were virtually absent after anti-CD20 treatment. The overall weakest reduction of B220+/CD19+ B cells was observed in the bone marrow. Although CD20+ B cells were efficiently depleted, a large population of CD20− B cell precursors and plasma cells persisted in this compartment (SI Appendix, Fig. S1B, Lower). In contrast, virtually all B cells remaining present in secondary lymphoid organs after anti-CD20 treatment proved to be CD20-positive (SI Appendix, Fig. S1B, Lower), with an enlarged proportion expressing CD27, an activation marker that murine B cells acquire when entering the germinal center reaction (10) (Fig. 1B and SI Appendix, Fig. S1C). In line with this finding, histologic examination revealed a predominant persistence of B cells in areas of follicular structures (Fig. 1C).
Fig. 1.
Characterization of remaining B cells after repetitive anti-CD20 treatment. Mice were treated weekly with anti-CD20 (α-CD20) or isotype antibodies for three consecutive weeks. (A) Frequency of B cells in blood, spleen, inguinal lymph nodes, and bone marrow assessed by flow cytometry using antibodies against CD19 and B220 1 wk after the last injection. Shown is the mean percentage of B cells ± SEM, pregated on intact cells (n = 3 mice per group; *P < 0.05 and ***P < 0.001). (B) Expression of CD27 on B cells in blood, spleen, inguinal lymph nodes, and bone marrow determined by flow cytometry. Shown is the mean percentage of CD27+ cells ± SEM, pregated on CD19+ B220+ cells (n = 3 mice per group; ***P < 0.001). (C) Representative spleen sections stained for B220 by immunohistochemistry. (Scale bars: overview, 200 µm; inlay, 50 µm.)
B Cell Repletion Starts in the Bone Marrow and the Spleen, Whereas B Cells Reappear in Lymph Nodes and Blood Substantially Thereafter.
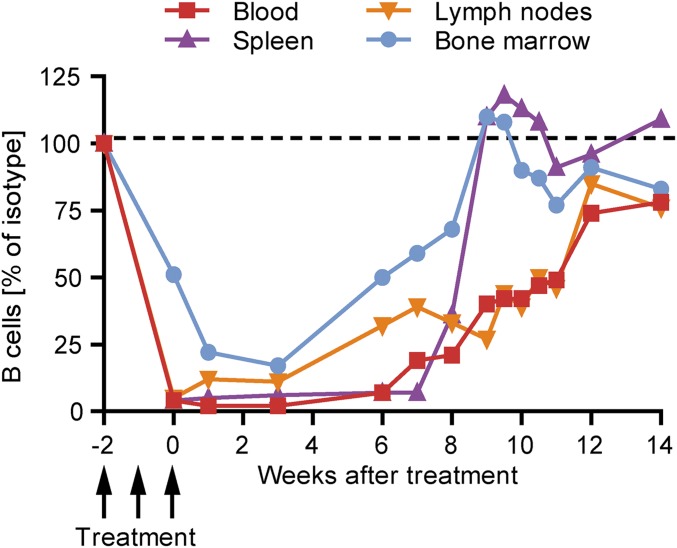
To investigate when and where B cells reappear after systemic B cell depletion, the frequency of B220+CD19+ B cells in the bone marrow, spleen, lymph nodes, and blood was assessed weekly after a full course of three anti-CD20 injections in naïve mice. As shown in Fig. 2, 6 wk after the last anti-CD20 injection, the B cell frequency started to increase in the bone marrow as well as in the spleen. By wk 9, in both of these compartments, B cell numbers were fully restored. In contrast, B cells reappeared slowly and steadily in lymph nodes and blood, with a substantial delay compared with bone marrow and spleen. Accordingly, full B cell repletion was not achieved in blood and lymph nodes before wk 12 after the last anti-CD20 dosing.
Fig. 2.
Kinetic of B cell reappearance in naïve mice after anti-CD20 withdrawal. C57BL/6 mice were treated weekly with anti-CD20 or isotype antibodies for three consecutive weeks. B cell frequencies in blood, spleen, inguinal lymph nodes, and bone marrow were determined by flow cytometry at the indicted time points by using antibodies against CD19 and B220. Shown is the mean percentage of B cells (pregated on all leukocytes) in mice previously treated with anti-CD20 normalized to age- and course-matched isotype-treated controls (n = 1–3 mice per time point).
B Cell Repletion Kinetic Accelerates in the Context of an Active Immunization, Whereas the Clinical Effect of B Cell De- and Repletion Depends on the EAE Model Used.
Next, we assessed B cell depletion and recovery in the context of active EAE. To best reflect distinct aspects of B cell function, we used two EAE models with differential B cell involvement: (i) myelin oligodendrocyte glycoprotein (MOG) protein1–117-induced EAE, a model in which activated, antigen-specific B cells contribute as potent APCs, providers of proinflammatory cytokines and source of antibody-producing plasma cells; and (ii) T cell-mediated MOG peptide35–55-induced EAE, in which B cells are not activated by the immunogen (11) (SI Appendix, Fig. S2).
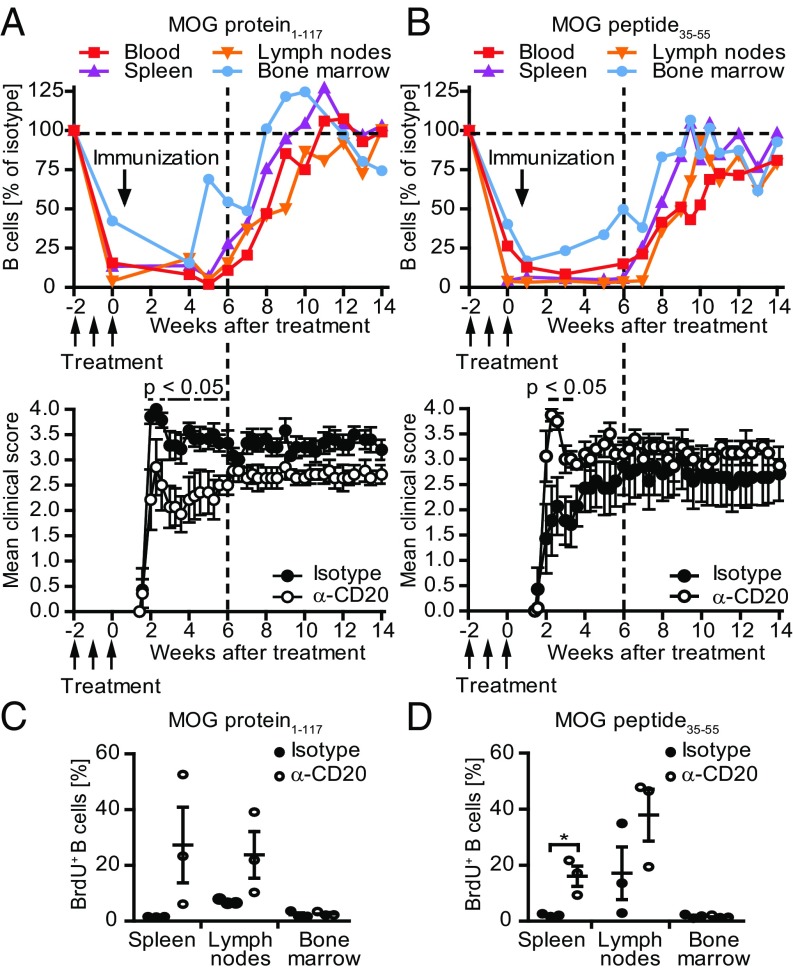
To correct for changes in the relative abundance of B cells caused by the active immunization, we compared the frequency of reappearing B cells to nondepleted, isotype-treated control mice at identical time points after immunization. Compared with naïve mice (Fig. 2), B cell repletion occurred overall faster and more simultaneously throughout the various compartments in the context of an active immunization (Fig. 3 A and B, Upper). The fastest restoration occurred in MOG protein1–117 EAE, and B cell frequencies even exceeded the level of nondepleted controls in most compartments (Fig. 3A, Upper). Analyzing the origin of reoccurring B cells, we observed that, 8 wk after the last anti-CD20 treatment, B cells proliferated in spleen and lymph nodes (Fig. 3 C and D and SI Appendix, Fig. S3 A and B), suggesting that, early in the process of repletion, persisting B cells expanded locally in secondary lymphoid organs.
Fig. 3.
Kinetic of reappearing B cells in transiently B cell-depleted mice immunized with MOG protein1–117 or MOG peptide35–55 and its impact on the EAE course. C57BL/6 mice were treated with anti-CD20 (α-CD20) or isotype antibodies for three consecutive weeks, followed by immunization with (A) MOG protein1–117 or (B) MOG peptide35–55 as indicated. B cell frequencies in blood, spleen, inguinal lymph nodes, and bone marrow were determined by flow cytometry at the indicted time points by using antibodies against CD19 and B220 (Upper). Shown is the mean percentage of B cells in mice previously treated with anti-CD20 normalized to age- and course-matched isotype-treated controls (n = 1–2 mice per time point). Mean clinical score ± SEM of α-CD20 and isotype-treated mice at indicated time points (Lower; n = 5–8 mice per group; *P < 0.05). Eight weeks after the last anti-CD20/isotype treatment, mice immunized with (C) MOG protein1–117 or (D) MOG peptide35–55 received a single i.p. injection of BrdU. Twenty-four hours later, mice were killed and B cells in spleen, inguinal lymph nodes, and bone marrow were analyzed for BrdU incorporation. Shown is the mean percentage ± SEM of BrdU+ cells pregated on CD19+ B cells (n = 3 mice per group; *P < 0.05).
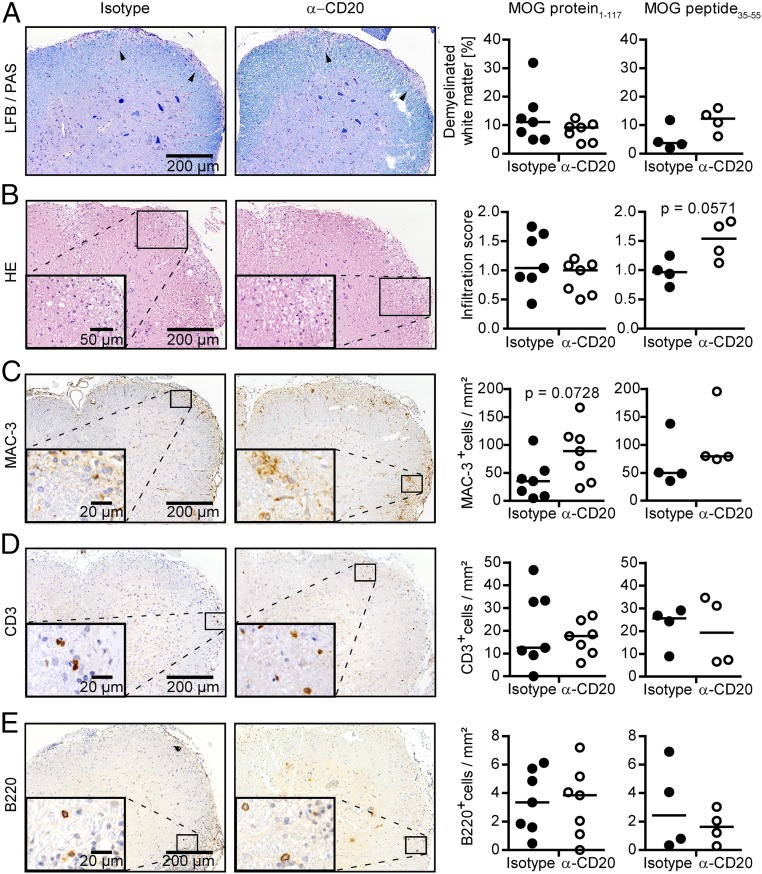
We also evaluated the clinical effect of B cell de- and repletion in both models (Fig. 3 A and B, Lower). Preventive anti-CD20 treatment reduced the severity of MOG protein1–117-induced EAE, as expected by the pathogenic involvement of B cells in this model (12). In contrast, preventive depletion of B cells in MOG peptide35–55-induced disease significantly exacerbated its course (Fig. 3 A and B, Lower). Inversely mirroring the clinical effect of anti-CD20 treatment, reappearance of B cells was associated with gradual clinical worsening in MOG protein EAE, whereas repletion of B cells in purely T cell-mediated EAE dampened the preceding disease exacerbation mediated by anti-CD20 treatment (Fig. 3 A and B, Lower). These findings suggest that the functional outcome of anti-CD20 in treatment of experimental CNS autoimmune disease is determined by the extent of pathogenic B cell involvement and indicate that the absence of regulatory and pathogenic B cell properties upon anti-CD20 treatment is transient. Analyzing CNS histology at the end of the study, the clinically deteriorating effect of transient B cell depletion in mice immunized with MOG peptide35–55 was still reflected by enhanced cellular infiltration and demyelination of the spinal cord (Fig. 4, Right). In contrast, in mice immunized with MOG protein1–117, no difference regarding demyelination and inflammation could be observed anymore (Fig. 4 A and B, Left). This was associated with a fully restored number of B cells, a slight increase in the number of T cells (Fig. 4 D and E, Left), and an accentuated increase in the number of activated MAC-3+ myeloid cells (Fig. 4C, Left).
Fig. 4.
Histologic evaluation of transiently B cell-depleted mice immunized with MOG protein1–117 or MOG peptide35–55. C57BL/6 mice were treated with anti-CD20 (α-CD20) or isotype antibodies for 3 wk, followed by immunization with MOG protein1–117 or MOG peptide35–55 as indicated. Fourteen weeks after the last α-CD20/isotype treatment, mice were killed, and demyelination [Luxol fast blue/periodic acid–Schiff (LFB/PAS)], overall inflammation (H&E), and number of infiltrating immune cells (immunohistochemistry) were determined in spinal cord sections (n = 4–7 mice per group). (A) Representative sections (Left) and group median percentage of demyelinated white matter area (LFB/PAS; Right) and (B) representative sections (Left) and median group score of spinal cord inflammation (HE; Right), determined as follows: 1, slight inflammation; 2, moderate inflammation; 3, strong inflammation. Representative sections (Left) and median numbers (Right) of (C) MAC-3, (D) CD3, and (E) B220 immunopositive cells per square millimeter of spinal cord.
B Cells Returning in an Activating Milieu Reveal a Shift Toward Highly Differentiated Phenotypes Containing an Elevated Frequency of Autoreactive B Cells.
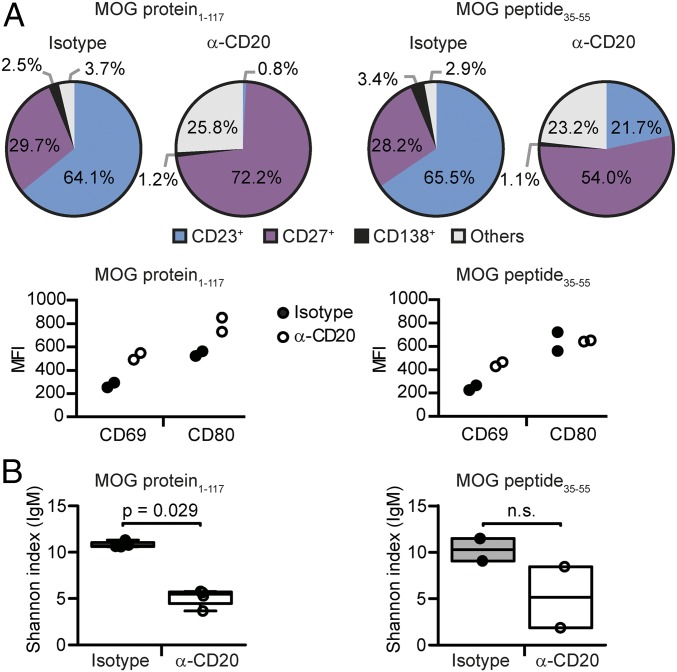
Based on these results, we hypothesized that B cells can generally reappear with predominantly pathogenic or beneficial properties depending on the milieu in which they repopulate. We thus determined the phenotype and composition of reappearing B cells in mice with MOG protein1–117 or MOG peptide35–55 EAE. For simplicity, we divided B cells into four virtually exclusive categories: CD23+ B cells, which represent the vast majority of transitional and follicular B cells; antigen-experienced B cells expressing CD27; and differentiated plasma blasts and plasma cells expressing CD138. Cells expressing none of these markers were defined as “others.” As depicted in Fig. 5A, isotype-treated mice with MOG protein1–117 and MOG peptide35–55 EAE showed a comparable distribution of CD23+, CD27+, and CD138+ cells. Thereafter, we analyzed the distribution of these B cell subgroups in the process of repletion. To our surprise, 8 wk after anti-CD20 treatment, both models revealed a relative expansion of activated CD27+ B cells; in mice immunized with MOG protein1–117, 72.2% of splenic B cells expressed CD27 and CD23+ cells were virtually absent (0.8%), whereas, in MOG peptide35–55 EAE, still 21.7% of all reappearing B cells were found to be CD23+. Although the expression of CD69 was increased on CD19+ cells in both models, CD80 was up-regulated only in MOG protein1–117-immunized mice.
Fig. 5.
Phenotype and BCR diversity of reappearing B cells 8 wk after anti-CD20 withdrawal in mice immunized with MOG protein1–117 or MOG peptide35–55. C57BL/6 mice were treated with anti-CD20 (α-CD20) or isotype antibodies for 3 wk, followed by immunization with MOG protein1–117 or MOG peptide35–55 as indicated. (A) Eight weeks after the last anti-CD20/isotype treatment, differentiation of CD19+ cells was analyzed in spleen by flow cytometry using antibodies against CD23 (naïve B cells), CD27 (activated B cells), and CD138 (plasma blasts and cells); B cells expressing none of these markers are defined as “others.” Shown are the respective B cell subtypes in percentages of all CD19+ cells (Upper). B cell activation was determined by using antibodies against CD69 and CD80. Shown are the respective mean fluorescence intensities (MFIs) of pregated CD19+ cells (Lower; n = 2 mice per group). (B) Eight weeks after the last B cell-depleting treatment, BCR diversity was assessed in lymph node-derived B cells by deep immune repertoire sequencing of the Ig M heavy-chain VDJ region. The clonal diversity of each sample was estimated by using the Shannon diversity index; a higher Shannon index indicates a greater diversity [n = 4 mice per group (Upper, A), n = 2 mice per group (Lower, A)].
To further characterize reappearing B cells, we analyzed their B cell receptor (BCR) repertoire by deep immune repertoire sequencing. B cells reappearing in secondary lymphoid organs 8 wk after cessation of anti-CD20 treatment had a reduced diversity of their B cell repertoire compared with matched control mice (Fig. 5B) per the Shannon diversity index, which, in our setting, represents the diversity of the rearranged Ig variable region heavy-chain mRNA. Specifically, the IgM repertoire in lymph nodes was less diverse in previously B cell-depleted mice (Fig. 5B), and the equivalent alteration could be detected in the spleen in class-switched IgG-positive B cells (SI Appendix, Fig. S4 A and B). Interestingly, a reduced BCR repertoire diversity was observed in both models, EAE induced with MOG protein1–117 and MOG peptide35–55, but reached statistical significance only in MOG protein1–117 EAE. These findings suggest that, after anti-CD20 treatment, B cells reconstitute from a rather limited pool of surviving B cells and/or B cell progenitors.
Importantly, we compared these observations at week 8 to a time point at which the overall frequency of B cells in the spleen was further restored, 12 wk after anti-CD20 treatment. Whereas, in MOG peptide35–55 EAE, the relative shift toward a more experienced B cell phenotype had been completely overwritten, the frequency of antigen-experienced CD27+ B cells remained elevated in MOG protein1–117-immunized mice (SI Appendix, Fig. S4 C and D). These findings indicate that, although enhanced activation of B cells during repletion is transient, B cell removal had lastingly altered their composition toward more mature and differentiated phenotypes in MOG protein1–117-immunized mice.
B Cells Reappear with an Enhanced Antigen-Presenting Function in MOG Protein1–117, but Not in MOG Peptide35–55, EAE.
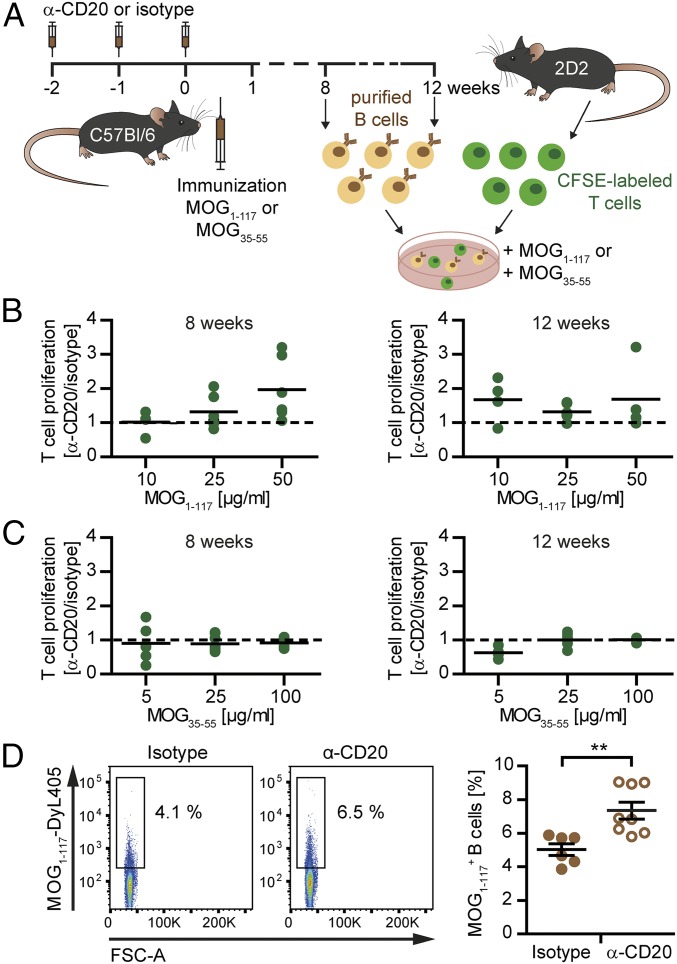
To investigate whether these phenotypical changes translated into enhanced pathogenic properties of reappearing B cells in MOG protein1–117 EAE, we next investigated their capacity to act as APCs (Fig. 6A). In light of the phenotypical changes described here earlier, these assays were performed at wk 8 and 12 after the last anti-CD20 dosing. As indicated in Fig. 6 B and C, at both time points, T cell proliferation was substantially enhanced when B cells reappearing in the context of MOG protein1–117 immunization acted as APCs, whereas this gain of APC function was not observed in MOG peptide35–55 EAE. As the APC capacity of B cells is strongly enhanced when they recognize the antigen to be presented via their BCRs, we next evaluated to what extent B cells isolated from formerly B cell-depleted, MOG protein1–117-immunized mice were capable of binding fluorescence-labeled MOG protein1–117. Compared with matched nondepleted control donors, a significantly higher frequency of B cells reappearing after anti-CD20 treatment recognized and bound MOG protein (Fig. 6D), indicating that this autoreactive population had expanded in the process of repletion.
Fig. 6.
Antigen-presenting capacity of reoccurring B cells 8 and 12 wk after anti-CD20 withdrawal. C57BL/6 mice were treated with anti-CD20 (α-CD20) or isotype antibodies for 3 wk, followed by immunization with MOG protein1–117 (MOG1–117) or MOG peptide35–55 (MOG35–55). At the time points indicated, B cells were isolated from spleen and cocultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve 2D2 T cells for 72 h. According to the preceding immunization, cells were restimulated with MOG1–117 or MOG35–55. (A) Overview of the experimental setup. Shown is the proliferation of 2D2 T cells cocultured with B cells isolated from (B) MOG1–117- and (C) MOG35–55-immunized mice 8 or 12 wk after the last α-CD20/isotype treatment. T cell proliferation was assessed by flow cytometry by using CFSE dilution. Shown is the proliferation of T cells cocultured with reoccurring B cells (previously α-CD20–treated mice) in relation to T cells cocultured with never-depleted B cells (isotype-treated mice). (D) Ten weeks after the last α-CD20/control treatment, B cells were isolated from the spleen of MOG protein1–117-immunized mice and cultured with DyLight-405–labeled MOG protein1–117 (MOG1–117-DyL405) for 2 h. Shown are representative dot plots (Left) and the mean percentage ± SEM of MOG1–117-DyL405+ B cells (Right) in the spleen of α-CD20- and isotype-treated mice (n = 6–8 mice per group; **P < 0.01).
Discussion
In this study, we investigated the clinical and immunological effects of systemic anti-CD20 treatment in experimental CNS autoimmunity with a particular focus on the circumstances of B cell repletion after cessation of therapy. Treating different murine models with anti-CD20, we first confirmed that the depletion of peripherally activated B cells in MOG protein1–117-induced EAE ameliorated its severity, which is mainly attributed to abrogation of potent B cell APC function in this model (12, 13). Of note, transient B cell depletion in this setting was associated with a significant increase in the frequency of activated macrophages and/or microglia within the CNS. In parallel to this preclinical observation, we had reported earlier that, in patients with NMO and MS treated with anti-CD20, peripheral monocytes show signs of an enhanced activation status and proinflammatory differentiation (14), suggesting that B cells physiologically control the activity of myeloid cells and that this desirable B cell property is abolished by anti-CD20 treatment (15). The possible clinical relevance of this regulatory axis between B cells and cells of myeloid origin is highlighted by a recent case report in which a patient with NMO depleted of B and T cells by administration of alemtuzumab died after 20 mo of continuous deterioration, which was associated with a massive CNS infiltration of monocytes (16). In light of the emerging concept that cells of myeloid origin play a central role in maintaining CNS residual inflammation, our observation of an unleashed activity of CNS myeloid cells may furthermore indicate that, despite its outstanding ability to control de novo emerging focal CNS inflammation, anti-CD20–mediated B cell depletion may not positively influence self-sustained CNS-intrinsic inflammation, the projected core process of chronic progression (17).
Analyzing the effect of anti-CD20 treatment on compartments other than blood, we observed that systemic anti-CD20 reduced the frequency of B cells in bone marrow, lymph nodes, and the spleen, whereas a remarkable number of cells remained detectable within follicular structures. These B cells were not only found to be CD20+, but also expressed the maturation marker CD27 (10), suggesting that a fraction of antigen-experienced B cells had escaped from systemic anti-CD20 treatment. A parallel observation was reported in patients with Sjögren’s syndrome, in whom persisting memory B cells could be detected in salivary glands even after 2 y of consecutive rituximab treatment (18). Along the same lines, anti-CD20 treatment of patients with rheumatoid arthritis enriched the relative abundance of memory B cells that coexpressed the proliferation marker Ki-67 (19), confirming that memory B cells can escape systemic anti-CD20–mediated B cell depletion, presumably in organs other than the blood.
Investigating when and in which order B cells repopulated peripheral immune compartments, we found that B cell numbers recovered detectably in the bone marrow starting 6 wk after the last anti-CD20 treatment, which likely reflects the physiological maturation of CD20− precursor cells in this organ. In addition, CD20+ B cells rapidly expanded in the spleen, with a subsequent kinetic indistinguishable from the bone marrow, suggesting a simultaneous recovery of B cells in both compartments. In contrast, blood was repopulated substantially thereafter, presumably because of a secondary distribution of B cells from bone marrow and spleen into this compartment. It is conceivable that our results in mice reflect de- and repletion of B cells in the equivalent compartments in patients with MS treated systemically with rituximab (20) or ocrelizumab (7); in regard to dosing, patients receive ∼8–12 mg/kg of the respective anti-CD20 antibody, and mice were treated with 10 mg/kg of murine anti-CD20 in the present study. Our experimental findings may therefore question whether monitoring circulating blood B cells is truly reflective of functional B cell recovery in patients (21, 22).
One central finding of the present study is that the circumstances under which B cells replete determine the immunological and possibly clinical long-term outcome of noncontinuous B cell depletion. Characterizing and comparing reappearing B cells in different models revealed that antigen-specific stimulation can shape the evolving B cell phenotype in a pathogenic manner as the emerging post–anti-CD20 population showed a substantial increase in the frequency of differentiated, antigen-experienced B cells with a reduced BCR diversity. Functionally, these activation-related changes translated into an enhanced APC function of B cells. Although the BCR repertoire also tended to be less diverse in previously anti-CD20–treated mice immunized with MOG peptide35–55, B cells returned with an approximately unchanged phenotype, and, most importantly, with an unaltered capacity to act as APCs in this model. It may therefore be central to anticipate in which milieu B cells regrow after their therapeutic removal. In this regard, it has been reported that, in patients with type 1 diabetes, the autoimmune B cell response against insulin-producing β-cells in the pancreas reappears immediately after transient B cell depletion with an expansion of existing, as well as newly emerging, B cell clones (23). In rituximab treatment of patients with anti–myelin-associated glycoprotein neuropathy, clinical responders and nonresponders can be distinguished by the persistence and expansion of clonal IgM memory B cells (24). Along the same lines, patients with rheumatoid arthritis exhibited relapse after anti-CD20 treatment depending on the frequency of memory B cells within the reemerging B cell pool (18, 25). These findings further consolidate that the pool of memory B cells can expand in a situation in which the autoantigen is accessible to reemerging B cells and that clonal expansion of antigen-activated B cells is associated with an unfavorable outcome.
Similar to the autoimmune diseases described here earlier, B cells replete with a predominance of CD27+ memory B cells in patients with NMO (26), and the general reoccurrence of B cells is associated with an immediate redevelopment of clinical activity (27, 28). This observation suggests that pathogenic aquaporin-4–recognizing B cells persist and possibly expand, and that patients with NMO accordingly may need to be continuously depleted of B cells (27). In contrast, in treatment of MS, clinical stabilization and silencing of disease activity measured by MRI upon anti-CD20 treatment appears to outlast the absence of B cells (1, 20). In regard to this presumably differential outcome, a recent investigation compared the IgG gene repertoire before and after anti-CD20 treatment in three patients, one with NMO, one with recurrent myelitis, and one with progressive MS (29). Of note, this crucial pilot study detected an expansion of memory B cells within the pool of reemerging B cells in all three patients. Based on this preliminary finding, a relative expansion of peripheral memory B cells may occur independent of the underlying disease entity, which could, however, have differential consequences in NMO vs. MS, as in the latter disease most evidence suggests that B cell pathogenicity develops by a B cell-fostering milieu within the CNS (30).
In conclusion, our findings imply that, in the treatment of CNS demyelinating disorders, systemic anti-CD20 should be used with a sense of proportion. We highlight that, in principle, unselective extinction of B cells concomitantly abolishes preexisting regulatory B cell properties, which may be particularly important for limiting myeloid cell activity and chronic disease progression. In regard to its current use, our experimental study demonstrates how diverse B cells can return after anti-CD20 treatment and highlights the need for biomarkers assessing when, how frequently, and how long an individual patient with MS, NMO, or other disease should receive systemic anti-CD20 (31). Our study furthermore establishes that, in the process of repletion, emerging B cells are susceptible to modulation, giving rise to the concept that a suitable maintenance medication could most efficiently foster regulatory B cell properties and prevent redevelopment of pathogenic B cell function after anti-CD20 induction therapy.
Materials and Methods
Animal experiments were carried out in accordance with the guidelines of the Central Department for Animal Experiments, University Medical Center, Göttingen, and approved by the Office for Consumer Protection and Food Safety of the State of Lower Saxony. Mice were immunized with 100 µg MOG peptide35–55 or 75 µg MOG protein1–117, and EAE severity was assessed daily and scored on a scale from 0 to 5. B cell depletion was achieved by three weekly i.p. injections of 200 µg murine anti-CD20 or isotype control. At the indicated time points, B cell frequencies were assessed by flow cytometry. Detailed descriptions of study materials and methods are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Katja Grondey and Julian Koch for excellent technical support. This work was supported by the Startförderung of the Universitätsmedizin Göttingen (D.H. and S.H.-K.); Deutsche Forschungsgemeinschaft Grants WE 3547/5-1 (to M.S.W.), LE 3079/3-1 (to K.L.-H.), and SFB-TR-128 (to K.L.-H.); Novartis (M.S.W.); TEVA (M.S.W.); Biogen-Idec (M.S.W.); Roche (M.S.W.); Merck (M.S.W.); the ProFutura Programm of Universitätsmedizin Göttingen (M.S.W.); Munich Cluster for Systems Neurology (SyNergy) (K.L.-H.); the Hertie Foundation (MyLab Program) (K.L.-H.); and National Multiple Sclerosis Society Grant G-1508-07064 (K.L.-H.). M.S.W. is serving as an editor for PLoS One.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810470115/-/DCSupplemental.
References
- 1.Hauser SL, et al. HERMES Trial Group B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Or A, et al. Rituximab in relapsing-remitting multiple sclerosis: A 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400, and erratum (2008) 63:803. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 3.Hawker K, et al. OLYMPUS trial group Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 4.Stellmann JP, et al. NEMOS (Neuromyelitis Optica Study Group) Immunotherapies in neuromyelitis optica spectrum disorder: Efficacy and predictors of response. J Neurol Neurosurg Psychiatry. 2017;88:639–647. doi: 10.1136/jnnp-2017-315603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: A systematic review and meta-analysis. JAMA Neurol. 2016;73:1342–1348. doi: 10.1001/jamaneurol.2016.1637. [DOI] [PubMed] [Google Scholar]
- 6.Jacob A, et al. Treatment of neuromyelitis optica with rituximab: Retrospective analysis of 25 patients. Arch Neurol. 2008;65:1443–1448. doi: 10.1001/archneur.65.11.noc80069. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, et al. OPERA I and OPERA II Clinical Investigators Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 8.Kappos L, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 9.Montalban X, et al. ORATORIO Clinical Investigators Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Hendriks J, Langerak P, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172:7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 11.Clements CS, et al. The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis. Proc Natl Acad Sci USA. 2003;100:11059–11064. doi: 10.1073/pnas.1833158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber MS, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monson NL, et al. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e17103. doi: 10.1371/journal.pone.0017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann-Horn K, et al. Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflammation. 2011;8:146. doi: 10.1186/1742-2094-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moulin V, et al. B lymphocytes regulate dendritic cell (DC) function in vivo: Increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelfand JM, Cotter J, Klingman J, Huang EJ, Cree BA. Massive CNS monocytic infiltration at autopsy in an alemtuzumab-treated patient with NMO. Neurol Neuroimmunol Neuroinflamm. 2014;1:e34. doi: 10.1212/NXI.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 18.Pers JO, et al. B-cell depletion and repopulation in autoimmune diseases. Clin Rev Allergy Immunol. 2008;34:50–55. doi: 10.1007/s12016-007-8015-4. [DOI] [PubMed] [Google Scholar]
- 19.Adlowitz DG, et al. Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS One. 2015;10:e0128269. doi: 10.1371/journal.pone.0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzer J, et al. Rituximab in multiple sclerosis: A retrospective observational study on safety and efficacy. Neurology. 2016;87:2074–2081. doi: 10.1212/WNL.0000000000003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen M, et al. Monitoring CD27+ memory B-cells in neuromyelitis optica spectrum disorders patients treated with rituximab: Results from a bicentric study. J Neurol Sci. 2017;373:335–338. doi: 10.1016/j.jns.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg BM, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: Creating strategies for therapeutic success. Mult Scler. 2012;18:1022–1026. doi: 10.1177/1352458511432896. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain N, et al. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest. 2016;126:282–287. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer MA, et al. Rituximab induces sustained reduction of pathogenic B cells in patients with peripheral nervous system autoimmunity. J Clin Invest. 2012;122:1393–1402. doi: 10.1172/JCI58743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 27.Pellkofer HL, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76:1310–1315. doi: 10.1212/WNL.0b013e3182152881. [DOI] [PubMed] [Google Scholar]
- 28.Cassinotto C, Joux J, Chausson N, Smadja D, Cabre P. [Failure of rituximab in relapsing neuromyelitis optica: Case report with two-year prospective follow-up] Rev Neurol (Paris) 2008;164:394–397. doi: 10.1016/j.neurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Maurer MA, et al. Rituximab induces clonal expansion of IgG memory B-cells in patients with inflammatory central nervous system demyelination. J Neuroimmunol. 2016;290:49–53. doi: 10.1016/j.jneuroim.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Corcione A, et al. B-cell differentiation in the CNS of patients with multiple sclerosis. Autoimmun Rev. 2005;4:549–554. doi: 10.1016/j.autrev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Hohlfeld R, Meinl E. Ocrelizumab in multiple sclerosis: Markers and mechanisms. Lancet Neurol. 2017;16:259–261. doi: 10.1016/S1474-4422(17)30048-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.