Significance
A number of plant-associated bacteria possess transcription factors homologous to LuxR that respond to compounds found in plant tissue. These LuxR homologs are known to regulate virulence or symbiosis. Although the first of these proteins were described more than a decade ago, the factors they respond to remain unknown. Here we identify a compound that functions with PipR, a plant-responsive LuxR homolog from a tree root endopytic Pseudomonas isolate. The inducer is an ethanolamine derivative. Ethanolamine is a building block for plant membrane phospholipids and signaling molecules. This discovery connects at least one of the plant-responsive LuxR homolog systems to a growing understanding of ethanolamine chemistry and responses of bacterial cells to ethanolamine and ethanolamine derivatives.
Keywords: ethanolamine, LuxR homolog, plant–microbe interactions, transcription activator, quorum sensing
Abstract
Certain plant-associated Proteobacteria sense their host environment by detecting an unknown plant signal recognized by a member of a LuxR subfamily of transcription factors. This interkingdom communication is important for both mutualistic and pathogenic interactions. The Populus root endophyte Pseudomonas sp. GM79 possesses such a regulator, named PipR. In a previous study we reported that PipR activates an adjacent gene (pipA) coding for a proline iminopeptidase in response to Populus leaf macerates and peptides and that this activation is dependent on a putative ABC-type transporter [Schaefer AL, et al. (2016) mBio 7:e01101-16]. In this study we identify a chemical derived from ethanolamine that induces PipR activity at picomolar concentrations, and we present evidence that this is the active inducer present in plant leaf macerates. First, a screen of more than 750 compounds indicated ethanolamine was a potent inducer for the PipR-sensing system; however, ethanolamine failed to bind to the periplasmic-binding protein (PBP) required for the signal response. This led us to discover that a specific ethanolamine derivative, N-(2-hydroxyethyl)-2-(2-hydroxyethylamino) acetamide (HEHEAA), binds to the PBP and serves as a potent PipR-dependent inducer. We also show that a compound, which coelutes with HEHEAA in HPLC and induces pipA gene expression in a PipR-dependent manner, can be found in Populus leaf macerates. This work sheds light on how plant-associated bacteria can sense their environment and on the nature of inducers for a family of plant-responsive LuxR-like transcription factors found in plant-associated bacteria.
A variety of plant-associated bacteria sense plants by recognizing an unknown signal that binds to one of a family of bacterial transcriptional regulators called plant-associated bacteria LuxR homologs (1–5). These regulators are thought to have evolved from acyl-homoserine lactone (acyl-HSL)-responsive LuxR homologs involved in quorum sensing, which is the population-wide regulation of gene expression in a cell density-dependent manner (6). However, instead of responding to self-produced molecules, plant-associated bacteria LuxR homologs respond to a plant signal (7, 8).
Interkingdom-signaling systems involving plant-derived molecules and bacterial protein receptors have been well studied in the cases of legumes and nitrogen-fixing rhizobial bacteria as well as the Rhizobiaceae genus Agrobacterium and its plant hosts. Examples include the rhizobial transcriptional regulator NodD that senses flavonoids produced by legumes to activate nodulation genes (9, 10) and the two-component system VirA/VirG from Agrobacterium that is involved in sensing plant-derived phenolic compounds to activate virulence genes (11). Whereas these systems are confined to Rhizobiaceae, the putative plant-associated bacteria LuxR homologs are more widespread and can be found in a number of genera belonging to the alpha-, beta- and gammaproteobacteria classes. Of the “Top 10 Plant Pathogenic Bacteria”, five (Pseudomonas syringae pathovars, Xanthomonas oryzae pv. oryzae, Xanthomonas campestris pathovars, Xanthomonas axonopodis pathovars, and Dickeya dadantii and solani) (12) possess putative plant-associated bacteria luxR homologs. These luxR genes are usually in close proximity to genes annotated as coding for a proline iminopeptidase (pip), and the LuxR protein has been shown to bind to specific DNA sequences to activate pip expression (4, 13). This system is important for both plant pathogenesis and mutualism. Pip proteins and the LuxR homologs that control their expression are important for virulence of Xanthomonas spp. and Pseudomonas syringae pv. actinidiae (1, 2, 4, 14). Additionally, plant-associated bacteria LuxR homologs have been shown to be important for biocontrol of wheat by Pseudomonas protegens (3) and for competition for nodulation of Medicago sativa by Sinorhizobium meliloti (15). Although our understanding of plant-associated bacteria LuxR homologs has improved in recent years, progress has been limited because an actual plant signal has not yet been identified.
We recently described a plant-associated bacteria LuxR homolog, called PipR, from the Populus deltoides root endophyte Pseudomonas sp. GM79 (16). Like its homologs in other plant-associated bacteria, PipR activates a downstream pip gene (called pipA in strain GM79) in response to plant leaf macerates. Genes divergently transcribed from pipR are annotated as a peptidase (named aapA) and an ABC-type transporter (Fig. 1A). PipA and AapA were purified and shown to cleave N-terminal amino acids. PipA was most active as a prolyl iminopeptidase, whereas AapA was most active as an alanyl aminopeptidase (16). Strain GM79 ΔpipA and ΔaapA mutants had elevated PipR-dependent responses to leaf macerates, consistent with the hypothesis that they degrade the plant signal as part of a negative feedback loop. In contrast, a mutation in the ABC transporter gene aapB abolished the ability of PipR to activate pipA in the presence of leaf macerates, indicating that the ABC transporter is required for uptake of the plant signal. The involvement of peptidases in the PipR system suggested that the plant signal might be peptide-like, which led to our report that PipR responds to specific small peptides (16). However, the response to peptides was relatively weak and required high peptide concentrations.
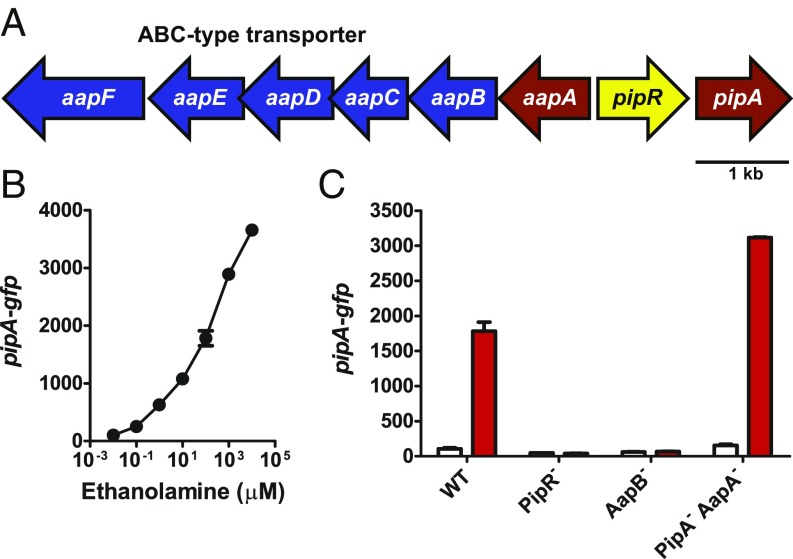
Fig. 1.
The pipR gene region in Pseudomonas GM79 and PipR-dependent ethanolamine activation of PpipA-gfp. (A) The pipR (yellow) genomic region. Genes coding for the predicted ABC-type transporter (blue) and genes coding for the peptidases (red). (B) Activation of gfp expression in strain GM79 containing pPpipA-gfp by ethanolamine. (C) Influence of mutations in ethanolamine (100 µM) activation of gfp expression in strains carrying pPpipA-gfp. AapB−, AapB transporter mutant; PipA− AapA−, PipA, AapA double-peptidase mutant; PipR−, PipR mutant; WT, wild type. Data are GFP fluorescence in cells grown without additions (white bars) or cells grown with 100 µM ethanolamine (red bars). B and C show mean relative fluorescence units (RFU) per optical density (OD600) × 10 of four biological replicates, and the bars represent the SDs.
Here we show that N-(2-hydroxyethyl)-2-(2-hydroxyethylamino) acetamide (HEHEAA), a compound that forms spontaneously from ethanolamine, induces pipA expression at extremely low concentrations (10 pM), and PipR is required for this activation. Our findings suggest that ethanolamine and its derivatives, which serve as important intermediates in plant cell membrane biogenesis and can serve as plant hormones, play a role in plant–bacteria interactions.
Results
PipR Responds to a Compound Derived from Ethanolamine.
In a previous work (16), we screened a small library of compounds (268 dipeptides and 14 tripeptides) for signals capable of serving as PipR coactivators of pipA. In the screen we used Pseudomonas GM79 containing a plasmid with the pipA promoter fused to gfp (pPpipA-gfp). We discovered that the tripeptide SHS induced gfp expression, but only at relatively high concentrations (1 mM). To identify a more active PipR coinducer of pipA expression we performed a screen by using a Pseudomonas GM79 with ΔpipA ΔaapA mutations containing pPpipA-gfp. The response of the double-peptidase mutant to SHS is much more robust than the response in wild-type Pseudomonas GM79 (16). We screened the 760 compounds available in Biolog plates PM1-8. These plates contain compounds that are potential carbon, nitrogen, phosphorous, or sulfur sources and nutrient supplements (17). Consistent with the prior publication (16) the screen showed several peptides served as relatively weak PipR coinducers of GFP. The fluorescence generated by them was on the order of 2- to 17-fold greater than controls without an added compound (SI Appendix, Table S1). By far the greatest response was to ethanolamine (nearly 50 times that of control fluorescence).
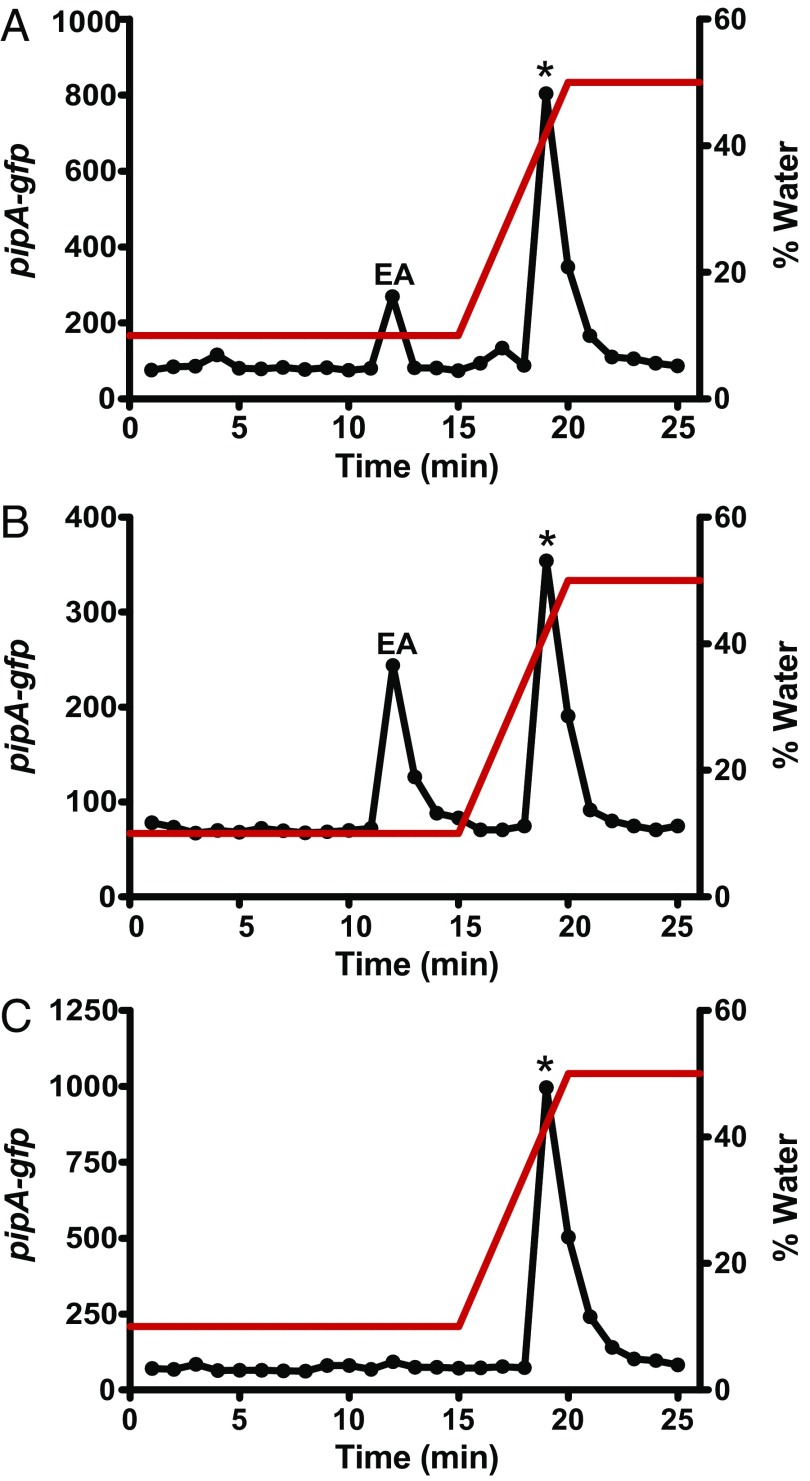
We tested high-purity ethanolamine (99%) to confirm the results of the screen. Ethanolamine at concentrations as low as 100 nM induced GFP fluorescence in the wild-type strain GM79 (Fig. 1B). Moreover, this activity was dependent on PipR and the ABC-type transporter, and ethanolamine induction of GFP was greater in the peptidase double mutant than in the wild type (Fig. 1C). These results are consistent with our previous results with plant macerates and peptides (16). The fact that the mutant analysis showed that the putative ABC-type transporter was necessary for ethanolamine induction of GFP was surprising because strain GM79 has an ethanolamine transporter gene (PMI36_01922) homologous to the eat transporter gene (PA4023) of Pseudomonas aeruginosa, and the GM79 gene is linked to ethanolamine catabolism genes eutBC (PMI36_01923 and PMI36_01924). Ethanolamine can also diffuse into cells, and in Salmonella enterica, its active import is only necessary when its concentration is less than 25 µM (18, 19). That the transporter was required for the response to 100 µM ethanolamine suggested that ethanolamine itself did not serve as a PipR coinducer of pipA transcription, but rather the inducer was a low-level contaminant in the ethanolamine solution. To test this hypothesis we fractionated a solution of ethanolamine by hydrophilic interaction liquid chromatography (HILIC) and found two active peaks (Fig. 2A). One active peak coeluted with ethanolamine, as revealed by high-resolution mass spectrometry (HRMS). Unfortunately, there was not enough material in the other peak for mass spectrometry. That there was activity in the HILIC ethanolamine fraction led us to hypothesize that the active compound was spontaneously formed from ethanolamine. Support for this hypothesis came from the following experiment: we subjected the HILIC-purified ethanolamine fraction to a second round of HPLC, and bioactive material was again found in the ethanolamine fraction and in the second fraction (Fig. 2B). We also subjected the second fraction to another round of HILIC and found that all of the active material eluted at the position of the second activity peak, with no detectable activity where ethanolamine elutes (Fig. 2C).
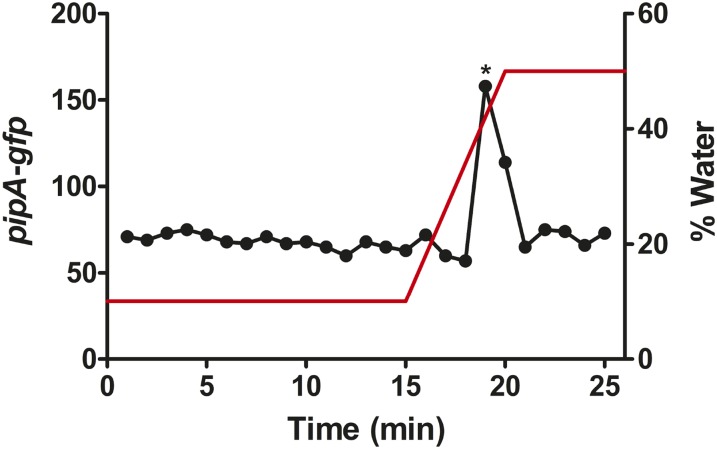
Fig. 2.
A compound formed from ethanolamine activates PpipA-gfp expression. (A) Fractions from HILIC-column chromatography of ethanolamine (12 µg) were tested by using the Pseudomonas GM79 (pPpipA-gfp) bioassay. Ethanolamine (EA) was eluted in fraction 12, as confirmed by MS, and a second peak of activity was eluted in fractions 19 and 20. (B) The material from fraction 12 from A was subjected to a second round of HILIC, and fractions were tested with the bioassay as in A. (C) The material from fraction 19 from A was subjected to a second round of HILIC, and fractions were tested with the bioassay, as in A. The red line shows the gradient of water. The asterisks indicates the fraction where synthetic HEHEAA elutes.
Use of AapF to Trap the Active pipA-gfp Inducer.
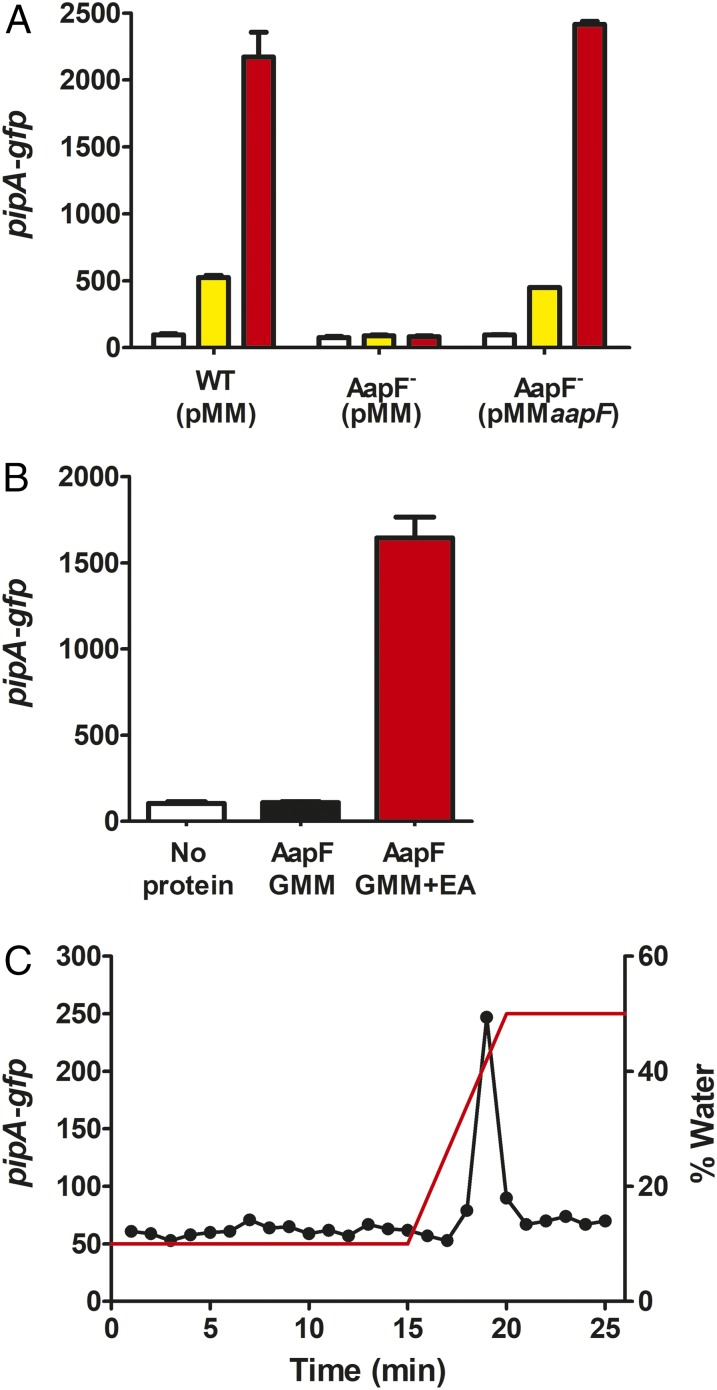
To purify enough of the active spontaneously forming ethanolamine derivative we decided to make use of the periplasmic-binding protein (PBP) of the ABC-type transporter required for the induction of pipA transcription. PBPs, also referred to as substrate-binding proteins, are a common feature of ABC-type transporters and are involved in the initial binding of the substrate and its delivery to the membrane-bound subunits that use ATP hydrolysis to catalyze uptake into cells (20). To confirm that the PBP is important for induction of pipA transcription, we made a deletion of its coding gene, aapF. An aapF mutation abolished the responses to ethanolamine solutions and to leaf macerates (Fig. 3A). Thus, we conclude that the PipR coactivating ligand requires initial binding to the PBP before its transport into the cell.
Fig. 3.
The PBP encoded by aapF is required for activation of the pPpipA-gfp reporter by leaf macerates and ethanolamine, and it can be used to purify the active molecule present in ethanolamine solutions. (A) Strains are wild type (WT) and the AapF mutant (AapF−) carrying pPpipA-gfp and either a pMMB67EH-TetRA vector control (pMM) or the aapF expression vector pMMaapF. Data are the activities of the pPpipA-gfp reporter grown with water control (white bars), 0.5% leaf macerates (yellow bars), or 100 µM ethanolamine (red bars). (B) The aapF gene was expressed in E. coli grown in glucose minimal medium (GMM) ± 10 mM ethanolamine (EA). Purified protein (5 µg) was denatured to release bound molecules. The supernatant fluid containing the released molecules was processed as described in Materials and Methods, and the bioassay was used to measure inducer activity of the released material. A and B show the mean RFU per OD600 × 10 of four biological replicates, and the bars represent SDs. (C) Material released from AapF was separated by HILIC, and fractions were tested with the bioassay. The red line shows the gradient of water.
To collect sufficient amounts of the inducer for HRMS we purified His-tagged AapF from recombinant Escherichia coli grown in glucose minimal medium plus ethanolamine and denatured the purified His-tagged AapF to release bound molecules. As shown in Fig. 3B, active material was released from AapF isolated from E. coli grown in the presence of ethanolamine, but active material was not recovered from protein purified from recombinant E. coli grown without added ethanolamine. Moreover, separation of the active AapF-released material by HILIC showed the presence of only one active peak, with no detectable activity where ethanolamine should elute (Fig. 3C). As expected, AapF binds to the active PipR coactivator and not to ethanolamine.
Purification and High-Resolution Mass Spectrometry of the PipR-Dependent pipA Inducer.
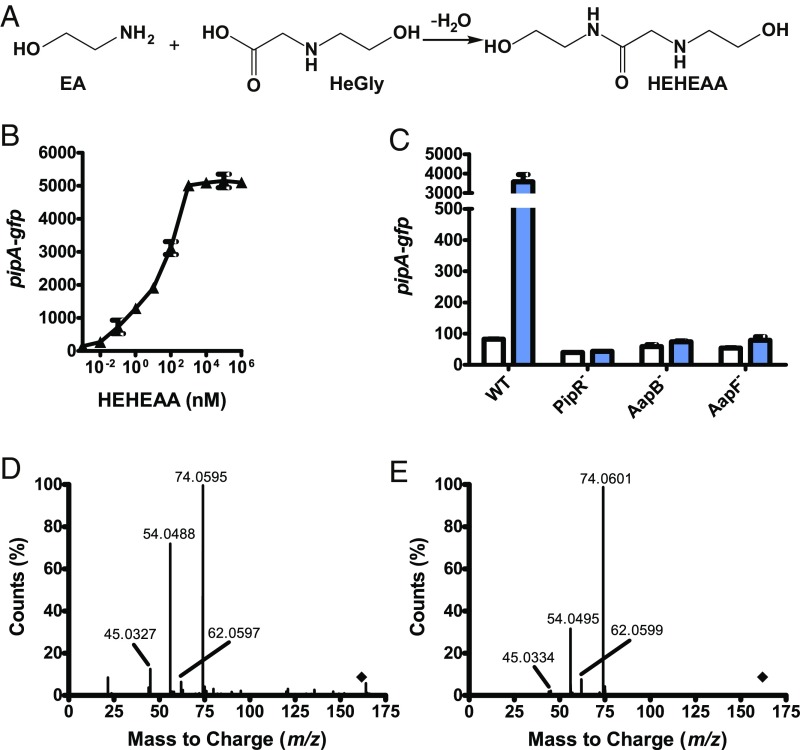
We obtained material trapped in 500 mg of recombinant His-tagged AapF by denaturing the protein purified from E. coli grown in glucose minimal medium plus ethanolamine and, separately, from E. coli grown in glucose minimal medium plus 15N-labeled ethanolamine. The active material released into the buffer by denaturation was further purified by HILIC. The [M+1]+ of the purified material derived from ethanolamine- and 15N-labeled ethanolamine-grown E. coli were 163.1071 and 165.0997, respectively. This result indicates the inducer contains two nitrogen atoms. Based on the molecular mass of the inducer and the indication that it contains two nitrogen atoms we predicted a chemical formula of C6H14N2O3. The chemical engineering literature on compounds formed spontaneously from ethanolamine is extensive (21), and the molecular weight of the purified inducer is consistent with that of N-(2-hydroxyethyl)-2-(2-hydroxyethylamino)acetamide (HEHEAA). HEHEAA is formed by the condensation of ethanolamine and N-(2-hydroxyethyl)glycine (HeGly), another common spontaneously derived product of ethanolamine, through the formation of an amide bond and release of water (Fig. 4A) (21–23).
Fig. 4.
Picomolar levels of HEHEAA, a compound spontaneously formed from ethanolamine, induce gfp expression in Pseudomonas GM79 (pPpipA-gfp). (A) HEHEAA formation from ethanolamine and HeGly (21–23). (B) Activation of gfp expression in the Pseudomonas GM79 (pPpipA-gfp) bioassay by HEHEAA. (C) Influence of mutations in the genes flanking pipR on HEHEAA activation of PpipA-gfp. The strains are AapB−, AapB transporter mutant; AapF, AapF PBP mutant; PipR−, PipR mutant; WT, wild type. Data are GFP fluorescence of cells grown with (blue bars) or without (white bars) 1 µM HEHEAA. B and C show mean RFU per OD600 × 10 of four biological replicates, and the bars represent the SDs. (D) The MS2 fragmentation spectrum of active material purified from ethanolamine, [M+H]+ 163.1071 m/z, at 8.18 min (indicated by the diamond). (E) The MS2 fragmentation spectrum of chemically synthesized HEHEAA, [M+H]+ 163.1101 m/z, at 8.14 min.
HEHEAA Is a PipR-Dependent Inducer of pipA Expression in Pseudomonas GM79.
Our analysis of the material purified by binding to AapF and subsequent HILIC indicated that the pipA inducer is HEHEAA. Thus, we asked whether chemically synthesized HEHEAA purchased commercially served as an inducer. Indeed, fluorescence of Pseudomonas GM79 carrying the pipA promoter-gfp plasmid showed a dose-dependent increase (Fig. 4B). Cells responded to HEHEAA at concentrations of this inducer as low as 10 pM, and saturation of the response was achieved at about 1 μM HEHEAA (Fig. 4B). Thus, synthetic HEHEAA has a potency about 10,000 times that of ethanolamine solutions, suggesting that HEHEAA is present at a level of about 0.01% in solutions of ethanolamine. As expected, the HEHEAA response was dependent on PipR and on the ABC transporter (Fig. 4C). Furthermore, the elution profile (Fig. 2) and the tandem mass spectrum (Fig. 4 D and E) were indistinguishable from those of the material purified from ethanolamine. We note that the commercial preparation of HEHEAA contained a synthetic precursor, HeGly, at about 20% based on 1H NMR. Therefore, we tested HeGly and found it did not serve as a PipR coinducer of pipA over a wide range of concentrations.
Active Inducer from Populus Tissue Coelutes with HEHEAA.
Does the active material isolated from Populus tissue have HEHEAA characteristics? It is possible that the Populus-derived coinducer is HEHEAA or an entirely different small water-soluble compound. Ethanolamine is an important plant metabolite for both the synthesis of phosphatidylethanolamine (PE) and phosphatidylcholine (PC), two major phospholipids in plant membranes (24), and for the production of the plant-signaling lipids N-acylethanolamines that modulate plant physiological processes (25). Plants are able to directly generate ethanolamine by serine decarboxylation, and free ethanolamine levels are estimated to be 50–250 nmol per gram of fresh weight in plant tissues (26, 27). Because HEHEAA is formed spontaneously from ethanolamine, we hypothesized that this molecule would also be present in plants. To test this hypothesis we separated Populus leaf-derived material by HILIC and tested fractions using Pseudomonas GM79 with the pipA-gfp reporter plasmid. We found that the active molecule in leaf macerates coelutes with HEHEAA (Fig. 5). This result is evidence in favor of the hypothesis that the PipR-dependent pipA inducer in plant tissue is HEHEAA.
Fig. 5.
Fractionation of Populus leaf macerates by HILIC suggests that HEHEAA is the active compound in plants. HILIC-fractionated Populus leaf macerates (50 µL of 5% plant macerate wt/vol) were tested by using the bioassay. The red line shows the gradient of water. The asterisk indicates the fraction where synthetic HEHEAA elutes.
Discussion
We identified an ethanolamine-derived small molecule, HEHEAA, as a PipR coinducer of pipA in the plant root endophytic bacterium Pseudomonas GM79. HEHEAA forms spontaneously from ethanolamine and is present in ethanolamine solutions. Our evidence indicates HEHEAA interacts with AapF, a periplasmic-binding protein, which delivers the inducer to an ABC-type transporter. Based on the finding that PipR is required for HEHEAA activity we propose that this LuxR-like protein binds to HEHEAA, and HEHEAA-bound PipR serves to activate pipA expression. Although there is no direct evidence that HEHEAA binds to PipR, our identification of HEHEAA as a potent PipR coactivator of pipA makes it possible to begin to study PipR activity. An important step toward identification of HEHEAA was the use of the PBP, AapF, as “bait” for HEHEAA. This innovation was inspired by previous use of a PBP required for activity of the Vibrio harveyi quorum-sensing signal AI-2. The AI-2 structure was elucidated in the crystal structure of the PBP (28).
HEHEAA induced PipR-dependent transcription of pipA at concentrations as low as 10 pM with a maximal response at about 1 µM HEHEAA. The level of sensitivity is greater than that for most acyl-HSL–responsive LuxR homologs, which function in the range of 10–500 nM with two exceptions of sensitive acyl-HSL systems, BjaR and BraR from Bradyrhizobium spp (29, 30). In the case of the HEHEAA-responsive system in Pseudomonas GM79, active transport of HEHEAA presumably serves to concentrate the signal in cells, and if PipR is in fact the HEHEAA receptor it might have a HEHEAA affinity similar to typical acyl-HSL–responsive LuxR homologs.
An important question is whether HEHEAA is the Pseudomonas GM79 pipA inducer in planta. Ethanolamine is an important metabolite required for synthesis of the membrane phospholipids PE and PC (24), and it can be formed either directly or indirectly from serine (26, 31, 32). Plants are able to directly generate ethanolamine by serine decarboxylation (26, 27). Plants also use ethanolamine in the production of a variety of N-acylethanolamines. These molecules modulate several plant physiological processes such as seed germination, plant–pathogen interactions, chloroplast development, and flowering (25). Our results indicate that HEHEAA is present in Populus tissue (Fig. 5). Based on the activity of pipA-inducing water-soluble material in plant leaves we estimate HEHEAA is at a concentration of about 10 pmol per gram of plant tissue. We know that the material obtained from leaf macerates requires AapF to induce the pipA promoter, coelutes with authentic HEHEAA, and activates the pipA promoter in a PipR-dependent fashion. We have not been able to confirm the molecular mass of the plant material because we have not collected a sufficient amount of inducer for mass spectrometry.
Plant-responsive LuxR homologs, which activate genes coding for putative proline iminopeptidases, occur in a variety of Proteobacteria (7, 33). These genes have been studied in some detail in certain plant pathogens and mutualistic symbionts. In both rice and cabbage pathogenic species of the genus Xanthomonas the PipR homolog responds to plant material by activating the linked proline iminopeptidase gene and other genes, and mutations of the pipR homologs attenuate virulence (1, 2, 4). There have been unsuccessful efforts to identify the inducers for the xanthomonads, but we know that the inducers for these pathogens are water-soluble and low molecular weight (13, 34). Perhaps the activator of some of these related systems is HEHEAA. We believe a sensitive response to HEHEAA would require a HEHEAA transport system like that encoded by genes linked to pipR. In fact the arrangement of pipR-like, pipA-like, and transport genes in a number of bacteria is similar to that in Pseudomonas GM79 (35), but in other bacteria with linked pipR-like, pipA-like genes there is either a set of different putative transporter genes or there are no linked transporter genes. We note that there are dozens of small molecules that form spontaneously from ethanolamine (21). Perhaps some of these compounds will serve as ligands for PipR-like proteins in other bacterial species.
Also of interest is that ethanolamine catabolism by some animal and plant pathogens is important for virulence (36). For instance, ethanolamine utilization promotes survival of Listeria monocytogenes in both intestinal epithelial cells and in bloodstream infection (37, 38), and a mutant of the plant pathogen Erwinia chrysanthemi blocked in ethanolamine metabolism was unable to cause systematic infection in plants (39). Ethanolamine has also been reported to be a signal or cue involved in responses of bacteria to mammalian hosts. For example, this molecule has been reported to activate expression of genes important for virulence of enterohemorrhagic E. coli O157:H7 (EHEC) at concentrations that do not support growth of the bacterium (40). Our findings raise the possibility that the E. coli response might not be to ethanolamine itself but rather to one of the many small molecules formed spontaneously in ethanolamine solutions.
The results presented here substantially advance the understanding of an important group of non–acyl-HSL–responsive LuxR homologs and lend insight into how these transcription factors might be responding to their plant host environments. The work also connects at least one of the plant-responsive systems to a growing understanding of ethanolamine chemistry and responses of bacterial cells to ethanolamine and ethanolamine derivatives.
Methods
Bacterial Strains and Growth Conditions.
Bacterial strains and plasmids are described in SI Appendix, Table S2. Pseudomonas sp. GM79 and its derived strains were grown in LB broth (41) or M9 minimal medium (42) with 10 mM succinate (succinate minimal medium) at 30 °C with shaking, unless otherwise indicated. E. coli strains were grown in LB broth or in M9 minimal medium with 20 mM glucose, 1 µg/mL biotin, and 1 µg/mL thiamine (glucose minimal medium) at 37 °C with shaking. Antibiotics were used as appropriate at the following concentrations: 50 μg/mL (E. coli) or 25 μg/mL (Pseudomonas GM79) kanamycin (Km), 20 μg/mL (E. coli) or 50 μg/mL (Pseudomonas GM79) gentamicin (Gm), and 10 μg/mL tetracycline (Tc). For plating we used media solidified with 1.5% agar.
Chemicals.
Ethanolamine (99% pure) was purchased from Acros Organics, and 15N-ethanolamine (98% pure) was purchased from Sigma-Aldrich. HEHEAA (95% pure) was purchased from Chiron AS; however, our own analysis determined that the compound was only 75% pure, and most of the impurity (20% of the total sample) was identified as HeGly. HeGly (90% pure) was purchased from Santa Cruz Biotechnology, Inc. Partially purified Populus leaf macerates were prepared as described before (16).
Reporter Assays.
Bioassays were performed by growing bacteria containing the plasmid pPpipA-gfp in succinate minimal medium with Km, as described previously (16). Briefly, inocula from 18-h cultures were used to inoculate fresh medium, and the inoculum size was 5%. The inoculated medium was then dispensed into wells of a 384-well microtiter dish (70 μL per well) containing the material to be tested and incubated at room temperature for 8 h. GFP fluorescence (excitation 485 nm, emission 535 nm) and growth (optical density at 600 nm) were assessed by using a Synergy H1 (BioTek) plate reader, and data are reported as relative fluorescence units (RFU) per optical density (OD) × 10.
Screening a Small-Molecule Library in Biolog Plates.
We screened 760 small molecules available in Phenotype Microarray Plates PM1-8 (Biolog, Inc.) by using Pseudomonas GM79 ΔpipA ΔaapA (pPpipA-gfp) as a reporter. Plates were inoculated with 100 µL of cultures prepared as in the previous section and incubated for 18 h, after which GFP fluorescence and culture density were measured. Fluorescence per OD compared with that without added test compounds was used to assess whether a compound could serve as a pipA-gfp activator.
Mutant and Plasmid Construction.
All plasmids and primer sequences are described in SI Appendix, Tables S2 and S3, respectively. To construct the aapF null mutant, we PCR-amplified sequences upstream (642 bp) and downstream (615 bp) of the intended deletion. The upstream fragment was amplified from strain GM79 genomic DNA using the primer pair aapFmut_1F and aapFmut_1R, and the downstream fragment was amplified with the primer pair, aapFmut_2F and aapFmut_2R. Overlap extension PCR was used to combine the fragments with the primers aapFmut_1F and aapFmut_2R. The resulting amplicon was cloned into EcoRI-BamHI–digested pEX19-Gm (43). This suicide vector was then introduced into Pseudomonas GM79 by conjugal mating, and colonies with single crossovers were selected by plating on succinate minimal agar containing Gm. To screen for double-crossover mutants we streaked Gm-resistant isolates on LB agar containing 10% sucrose and screened for loss of Gm resistance. To evaluate PipR activation of the pipA promoter in the AapF mutant we introduced pPpipA-gfp (16) into the mutant by conjugation.
To complement the aapF mutation, a DNA fragment containing aapF was PCR-amplified by using Pseudomonas GM79 genomic DNA as a template with the primer pair aapFcomp_F and aapFcomp_R. The PCR product was cloned into EcoRI-HindIII–digested pMMB67EH-TetRA. The complementing plasmid (or pMMB67EH-TetRA vector control) was introduced into the aapF mutant harboring the pPpipA-gfp reporter by conjugation. All mutant and plasmid constructs were confirmed by DNA sequencing.
Purification of His6-Tagged AapF.
A cytoplasmic version of AapF for overexpression in E. coli was created by cloning the aapF gene with the secretion signal peptide omitted (nucleotides 1–78 of the aapF ORF) into the His6-tag protein expression vector pQE-30 to generate pQEaapF (SI Appendix, Tables S2 and S3). The signal peptide was identified computationally by using SignalIP (44). To obtain purified His6-AapF, E. coli M15 pRep4 containing pQEaapF was grown at 37 °C in glucose minimal medium plus antibiotics to an optical density of 0.6 at 600 nm. We then added 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) and incubated cultures at 18 °C for 16 h, after which cells were pelleted by centrifugation. Cell lysates were prepared by suspending the pelleted cells in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8), lysing them by using a French Pressure Cell, and removing cell debris by centrifugation for 20 min at 14,000 × g. The His6-tagged AapF was purified from the cell lysate by nickel resin column chromatography (Qiagen). To obtain purified His6-AapFcyto bound to the ligand we added 10 mM of either ethanolamine or 15N-labeled ethanolamine to the E. coli M15 pRep4-containing pQEaapF culture together with the IPTG and then proceeded to purify AapF as described above.
HILIC Fractionation.
Materials were fractionated by using a CORTECS HILIC column (4.6 × 100 mm, 2.7 µm particle size; Waters) and the following solvents: A. 10:90 acetonitrile:water 10 mM ammonium formate pH 4 and B. 90:10 acetonitrile:water 10 mM ammonium formate pH 4. Runs were started at 100% solvent B for 15 min, after which a 5-min gradient to 50:50 A:B was executed, and the A:B 50:50 mixture was maintained for an additional 5 min. The flow rate was 1.2 mL per min, and 1-min fractions were collected. Fractions were concentrated under a gentle stream of nitrogen gas at 70 °C and tested for bioactivity as described above.
Purification and Identification of AapF-Released Compounds.
His6-Aap was purified from E. coli as described above, and the buffer was exchanged with 100 mM ammonium acetate pH 7.5 by using an Amicon Ultra-15 filter with a 30,000 nominal molecular weight limit. The protein was then denatured at 70 °C for 20 min, centrifuged at 5,000 × g to separate the protein pellet from the released material, and the supernatant fluid was filtered with an Amicon Ultra-15 filter (3,000 nominal molecular weight limit) to remove any larger-mass, nonactive compounds. The material released from His6-AapF denaturation was dried by a rotary evaporation and suspended in liquid chromatography-mass spectometry (LC/MS)-grade water (Fischer Scientific). The active material was separated by HILIC, and fractions were tested for bioactivity as described above. The bioactive molecule was eluted in fractions 19 and 20 (Fig. 3C). The LC/MS analysis was performed on an Agilent 6530 LC-q-TOF mass spectrometer equipped with an ultrahigh performance liquid chromatography system using a Kinetex 2.6-μm HILIC (100 Å-pore size 100 × 2.1 mm) column under the same gradient described above for purification but with a 0.5 mL/min flow rate. The active metabolite was eluted predominately between 7–8 min.
Supplementary Material
Acknowledgments
We thank Beth Traxler, Howard Shuman, and Chris Neumann for helpful discussions. We thank the Harvard Medical School Institute of Chemistry and Cell Biology-Longwood Screening Facility for use of analytical instruments. This research was sponsored by NIH Grant R01AT009708 (to J.C.) and the Genomic Science Program, US Department of Energy, Office of Science, Biological and Environmental Research, as part of the Plant Microbe Interfaces Scientific Focus Area (pmi.ornl.gov) (E.P.G. and C.S.H.). Oak Ridge National Laboratory is managed by University of Tennesse-Battelle LLC for the US Department of Energy under Contract DE-AC05-00OR227525.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809611115/-/DCSupplemental.
References
- 1.Chatnaparat T, Prathuangwong S, Ionescu M, Lindow SE. XagR, a LuxR homolog, contributes to the virulence of Xanthomonas axonopodis pv. glycines to soybean. Mol Plant Microbe Interact. 2012;25:1104–1117. doi: 10.1094/MPMI-01-12-0008-R. [DOI] [PubMed] [Google Scholar]
- 2.Ferluga S, Bigirimana J, Höfte M, Venturi V. A LuxR homologue of Xanthomonas oryzae pv. oryzae is required for optimal rice virulence. Mol Plant Pathol. 2007;8:529–538. doi: 10.1111/j.1364-3703.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Subramoni S, et al. Bacterial subfamily of LuxR regulators that respond to plant compounds. Appl Environ Microbiol. 2011;77:4579–4588. doi: 10.1128/AEM.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Jia Y, Wang L, Fang R. A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol Microbiol. 2007;65:121–136. doi: 10.1111/j.1365-2958.2007.05775.x. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Zhao Y, Qian G, Liu F. XocR, a LuxR solo required for virulence in Xanthomonas oryzae pv. oryzicola. Front Cell Infect Microbiol. 2015;5:37. doi: 10.3389/fcimb.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 7.González JF, Venturi V. A novel widespread interkingdom signaling circuit. Trends Plant Sci. 2013;18:167–174. doi: 10.1016/j.tplants.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Subramoni S, Venturi V. LuxR-family ‘solos’: Bachelor sensors/regulators of signalling molecules. Microbiology. 2009;155:1377–1385. doi: 10.1099/mic.0.026849-0. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan JT, Long SR. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci USA. 1985;82:6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaink HP, et al. Symbiotic properties of rhizobia containing a flavonoid-independent hybrid nodD product. J Bacteriol. 1989;171:4045–4053. doi: 10.1128/jb.171.7.4045-4053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CH, Winans SC. Functional roles assigned to the periplasmic, linker, and receiver domains of the Agrobacterium tumefaciens VirA protein. J Bacteriol. 1992;174:7033–7039. doi: 10.1128/jb.174.21.7033-7039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansfield J, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferluga S, Venturi V. OryR is a LuxR-family protein involved in interkingdom signaling between pathogenic Xanthomonas oryzae pv. oryzae and rice. J Bacteriol. 2009;191:890–897. doi: 10.1128/JB.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel HK, et al. The kiwifruit emerging pathogen Pseudomonas syringae pv. actinidiae does not produce AHLs but possesses three luxR solos. PLoS One. 2014;9:e87862. doi: 10.1371/journal.pone.0087862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patankar AV, González JE. An orphan LuxR homolog of Sinorhizobium meliloti affects stress adaptation and competition for nodulation. Appl Environ Microbiol. 2009;75:946–955. doi: 10.1128/AEM.01692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer AL, et al. A LuxR homolog in a cottonwood tree endophyte that activates gene expression in response to a plant signal or specific peptides. mBio. 2016;7:e01101-16. doi: 10.1128/mBio.01101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bochner BR, Gadzinski P, Panomitros E. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001;11:1246–1255. doi: 10.1101/gr.186501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren BR, Sarwar Z, Pinto A, Ganley JG, Nomura CT. Ethanolamine catabolism in Pseudomonas aeruginosa PAO1 is regulated by the enhancer-binding protein EatR (PA4021) and the alternative sigma factor RpoN. J Bacteriol. 2016;198:2318–2329. doi: 10.1128/JB.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penrod JT, Mace CC, Roth JR. A pH-sensitive function and phenotype: Evidence that EutH facilitates diffusion of uncharged ethanolamine in Salmonella enterica. J Bacteriol. 2004;186:6885–6890. doi: 10.1128/JB.186.20.6885-6890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouedard C, Picq D, Launay F, Carrette PL. Amine degradation in CO2 capture. I. A review. Int J Greenhouse Gas Control. 2012;10:244–270. [Google Scholar]
- 22.da Silva EF, et al. Understanding 2-ethanolamine degradation in postcombustion CO2 capture. Ind Eng Chem Res. 2012;51:13329–13338. [Google Scholar]
- 23.Vevelstad SJ, Grimstvedt A, Knuutila H, da Silva EF, Svendsen HF. Influence of experimental setup on amine degradation. Int J Greenhouse Gas Control. 2014;28:156–167. [Google Scholar]
- 24.Gibellini F, Smith TK. The Kennedy pathway–De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 25.Blancaflor EB, et al. N-acylethanolamines: Lipid metabolites with functions in plant growth and development. Plant J. 2014;79:568–583. doi: 10.1111/tpj.12427. [DOI] [PubMed] [Google Scholar]
- 26.Rontein D, et al. Plants synthesize ethanolamine by direct decarboxylation of serine using a pyridoxal phosphate enzyme. J Biol Chem. 2001;276:35523–35529. doi: 10.1074/jbc.M106038200. [DOI] [PubMed] [Google Scholar]
- 27.Rontein D, Rhodes D, Hanson AD. Evidence from engineering that decarboxylation of free serine is the major source of ethanolamine moieties in plants. Plant Cell Physiol. 2003;44:1185–1191. doi: 10.1093/pcp/pcg144. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren NA, Harwood CS, Schaefer AL, Giraud E, Greenberg EP. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc Natl Acad Sci USA. 2011;108:7183–7188. doi: 10.1073/pnas.1103821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindemann A, et al. Isovaleryl-homoserine lactone, an unusual branched-chain quorum-sensing signal from the soybean symbiont Bradyrhizobium japonicum. Proc Natl Acad Sci USA. 2011;108:16765–16770. doi: 10.1073/pnas.1114125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent C. Eukaryotic phospholipid biosynthesis. Annu Rev Biochem. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- 32.Voelker DR. Phosphatidylserine decarboxylase. Biochim Biophys Acta. 1997;1348:236–244. doi: 10.1016/s0005-2760(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer AL, et al. LuxR- and luxI-type quorum-sensing circuits are prevalent in members of the Populus deltoides microbiome. Appl Environ Microbiol. 2013;79:5745–5752. doi: 10.1128/AEM.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, et al. XerR, a negative regulator of XccR in Xanthomonas campestris pv. campestris, relieves its repressor function in planta. Cell Res. 2011;21:1131–1142. doi: 10.1038/cr.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramoni S, Florez Salcedo DV, Suarez-Moreno ZR. A bioinformatic survey of distribution, conservation, and probable functions of LuxR solo regulators in bacteria. Front Cell Infect Microbiol. 2015;5:16. doi: 10.3389/fcimb.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaval KG, Garsin DA. Ethanolamine utilization in bacteria. mBio. 2018;9:e00066-18. doi: 10.1128/mBio.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph B, et al. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol. 2006;188:556–568. doi: 10.1128/JB.188.2.556-568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellin JR, et al. Riboswitches. Sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science. 2014;345:940–943. doi: 10.1126/science.1255083. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, et al. Genome-wide identification of plant-upregulated genes of Erwinia chrysanthemi 3937 using a GFP-based IVET leaf array. Mol Plant Microbe Interact. 2004;17:999–1008. doi: 10.1094/MPMI.2004.17.9.999. [DOI] [PubMed] [Google Scholar]
- 40.Kendall MM, Gruber CC, Parker CT, Sperandio V. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. mBio. 2012;3:e00050-12. doi: 10.1128/mBio.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 42.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 43.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 44.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.