Significance
Tissue plasminogen activator (tPA) is employed in stroke patients to lyse blood clots, but its therapeutic potential in brain trauma victims is far from settled, partly due to fears of internal bleeding. The long-term effects of tPA upon the white matter of the brain and neurological outcomes after head trauma are also uncertain. To address these gaps, we performed electrophysiological, anatomical, and behavioral studies in genetically modified or wild-type mice subjected to traumatic brain injury and infused tPA through the nose. We found that natural or synthetic forms of tPA protect or repair white matter tracts without magnifying internal bleeding. Thus, tPA treatment after brain injury promotes communication across neuronal networks through nerve fiber tracts and improves long-term adaptive behavior.
Keywords: white matter, head injury, trauma, contusion, alteplase
Abstract
Recombinant tissue plasminogen activator (tPA) is a Food and Drug Administration-approved thrombolytic treatment for ischemic stroke. tPA is also naturally expressed in glial and neuronal cells of the brain, where it promotes axon outgrowth and synaptic plasticity. However, there are conflicting reports of harmful versus neuroprotective effects of tPA in acute brain injury models. Furthermore, its impact on white matter integrity in preclinical traumatic brain injury (TBI) has not been thoroughly explored, although white matter disruption is a better predictor of long-term clinical outcomes than focal lesion volumes. Here we show that the absence of endogenous tPA in knockout mice impedes long-term recovery of white matter and neurological function after TBI. tPA-knockout mice exhibited greater asymmetries in forepaw use, poorer sensorimotor balance and coordination, and inferior spatial learning and memory up to 35 d after TBI. White matter damage was also more prominent in tPA knockouts, as shown by diffusion tensor imaging, histological criteria, and electrophysiological assessments of axon conduction properties. Replenishment of tPA through intranasal application of the recombinant protein in tPA-knockout mice enhanced neurological function, the structural and functional integrity of white matter, and postinjury compensatory sprouting in corticofugal projections. tPA also promoted neurite outgrowth in vitro, partly through the epidermal growth factor receptor. Both endogenous and exogenous tPA protected against white matter injury after TBI without increasing intracerebral hemorrhage volumes. These results unveil a previously unappreciated role for tPA in the protection and/or repair of white matter and long-term functional recovery after TBI.
Traumatic brain injury (TBI) is a leading cause of death and disability in young adults. Although TBI may damage both gray and white matter in the brain, most preclinical therapies have emphasized protection of neuronal cell bodies in gray matter and have failed to translate into successful clinical trials (1). Aside from direct loss of neuronal somata, brain damage caused by TBI may include hemorrhagic lesions, white matter hyperintensities, and axonal injury in white matter tracts, which elicit retrograde or anterograde degeneration of fiber pathways (2). White matter injury in patients with TBI is particularly visible in the corpus callosum (CC), internal capsule, and corticospinal tracts (CSTs), even when the injury is relatively mild (3). Damage within white matter tracts can disrupt sensorimotor and cognitive function and elicit profound neurological disabilities. Indeed, the extent of white matter disruption is a superior harbinger of clinical outcomes compared with focal tissue lesions (3–5). These observations reflect the dependence of executive function and memory on topographically widespread neuronal circuits that communicate through long white matter tracts (6, 7). The present study was therefore centered on the preservation of white matter, mitigation of axonal injury, and axonal regeneration in corticofugal pathways in an animal model of TBI.
Recombinant tissue plasminogen activator (tPA) is a Food and Drug Administration-approved clot-dissolving treatment for ischemic stroke. Accumulating evidence suggests that tPA has pleiotropic functions extending beyond thrombolytic activity. Aside from being synthesized by the endothelial lining of the vasculature, tPA is endogenously expressed in the brain, including in neurons, astrocytes, microglia, and oligodendrocytes, where it promotes synaptic plasticity and axon outgrowth, among other roles (8, 9). However, the role of endogenous tPA in acute brain injuries is somewhat controversial. For example, tPA is hypothesized to augment blood–brain barrier damage, edema, and hemorrhage in models of acute brain injury (10–16). On the other hand, endogenous tPA also appears to protect neurons and white matter against acute ischemic insults (17, 18). As most studies have not examined extended survival periods after brain injury, the long-term impact of endogenous tPA on white matter integrity and functional outcomes after TBI also remains unclear.
The present study was designed to determine the effects of tPA deletion on long-term outcomes after controlled cortical impact (CCI), an established model of TBI. Measurement outcomes were focused on long-term sensorimotor and cognitive functions and white matter parameters, including axonal injury, demyelination, impulse conduction, and regeneration of nerve fibers in white matter tracts. The results demonstrate that tPA-KO mice exhibit lower white matter integrity and poorer recovery of neurological deficits than WT mice up to 35 d after TBI. Furthermore, intranasal delivery of recombinant tPA after TBI restores the long-term functional recovery in tPA-KO mice to WT levels. tPA evokes these protective and/or restorative effects without increasing TBI-induced intracerebral hemorrhage. Thus, both endogenously expressed and exogenously delivered tPA can play a significant role in promoting white matter integrity and long-term neurological recovery after TBI.
Results
The Absence of Endogenous tPA Expression in tPA-KO Mice Exacerbates Long-Term Neurological Impairments After TBI.
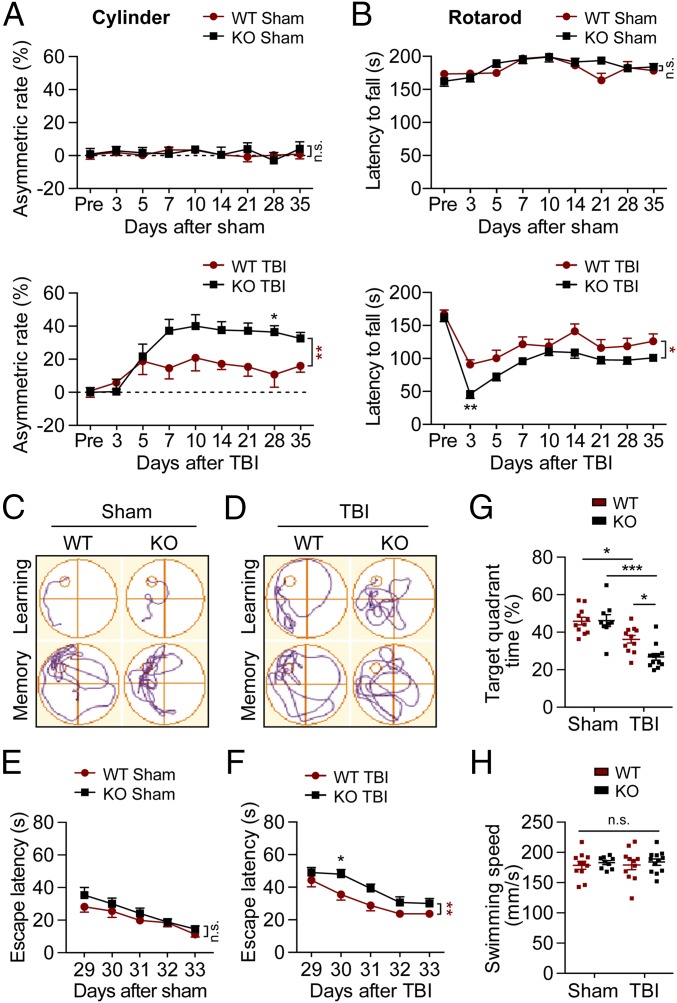
We investigated if deletion of the tPA-encoding gene improves or exacerbates long-term neurological dysfunction up to 35 d after TBI or sham operation. Following sham operation, WT and tPA-KO mice exhibited no forelimb use asymmetry or sensorimotor coordination deficits throughout the entire study, as assessed with the cylinder (Fig. 1A, Upper) and rotarod (Fig. 1B, Upper) tests, respectively. TBI elicited forelimb asymmetry between 5 and 35 d after TBI, and this motor deficit was significantly magnified in tPA-KO mice (Fig. 1A, Lower). Similarly, both WT and tPA-KO mice fell off the accelerating rotating rod sooner than sham-treated control mice between 3 and 35 d after surgery, and this motor deficit was exacerbated in tPA-KO mice (Fig. 1B, Lower).
Fig. 1.
Deletion of tPA exacerbates long-term behavioral impairments after TBI. WT and tPA-KO mice were subjected to sham injury or unilateral CCI (defined here as TBI) in the right hemisphere. (A and B) Sensorimotor functions were evaluated before (3 d of pretraining before CCI) and up to 35 d after unilateral TBI by calculating forelimb asymmetry in the cylinder test (A) and the latency to fall in the rotarod test (B). TBI increased asymmetry and decreased latency to fall; sham operations had no effect. (C–H) Long-term spatial learning (escape latency), spatial memory (target quadrant time), and swim speeds were assessed by the Morris water maze test. (C and D) Representative images illustrate swim paths on days 33 (learning) and 34 (memory) after TBI (C) or sham operation (D). (E–G) Escape latency and target quadrant time were not affected by sham operation but were increased after TBI, and even more in tPA-KO than in WT mice. (H) Swimming speed was unaffected by tPA-KO or TBI. Data are presented as mean ± SEM. n = 9–12 per group. *P ≤ 0.05, **P ≤ 0.01 for KO vs. WT mice by two-way repeated-measures ANOVA followed by Bonferroni post hoc correction in A, B, E, and F (see asterisks next to brackets on the right). *P ≤ 0.05, ***P ≤ 0.001 by two-way ANOVA followed by Tukey post hoc correction in G and H. n.s., not significant. See SI Appendix, Table S1 for statistical details for all figures.
Long-term spatial cognitive functions were assessed by the Morris water maze test from day 29 to day 34 post-TBI (Fig. 1 C–H). All mice exhibited gradual decreases in escape latency over time (Fig. 1 C–F). WT and tPA-KO mice displayed comparable learning and memory capacity after sham surgeries (Fig. 1 E and G). After TBI, tPA-KO mice exhibited greater learning and memory deficits than WT mice, as manifested by significantly longer escape latencies (loss of spatial learning) (Fig. 1F) and decreased time in the target quadrant (loss of spatial memory) (Fig. 1G). Mice from all four groups exhibited similar swim speeds, suggesting that the memory tests were not confounded by major differences in swim abilities (Fig. 1H).
Exacerbation of White Matter Damage in tPA-KO Mice May Impede Long-Term Neurological Recovery After TBI.
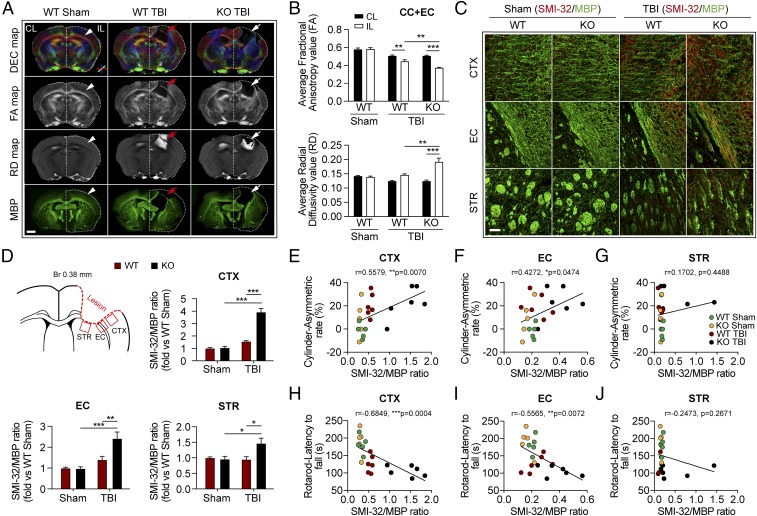
Ex vivo diffusion tensor imaging (DTI) was performed to assess white matter integrity in the CC and external capsule (EC) at 35 d after TBI or sham operation (Fig. 2 A and B and SI Appendix, Fig. S1). Reductions in fractional anisotropy (FA) and increases in radial diffusivity (RD) with DTI indicate the loss of white matter integrity (19). Compared with sham-operated mice, both TBI groups exhibited significant decreases in FA and increases in RD in the CC and EC of the ipsilesional hemisphere (SI Appendix, Fig. S1). However, after TBI the tPA-KO mice exhibited significantly greater changes in both FA and RD values in the ipsilesional hemisphere than WT mice (Fig. 2B), suggesting that tPA KO exacerbates white matter damage induced by TBI.
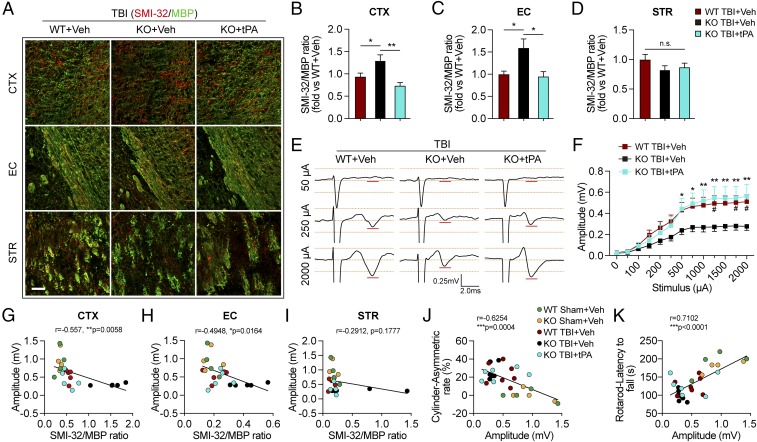
Fig. 2.
Deletion of tPA exacerbates long-term white matter disruption after TBI. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. (A) Representative DTI data of ex vivo brains [directionally encoded color (DEC), FA, and RD maps] and MBP staining 35 d after TBI or sham operation. In DEC axial maps centered on the level of the lesion, the color indicates the direction of water diffusion along white matter fibers (red = mediolateral, blue = dorsoventral, and green = anteroposterior). The contralesional (CL) and ipsilesional (IL) hemispheres are indicated. Arrowheads in the Sham column point to the uninjured CC and EC, and arrows in the TBI columns point to the injured CC and EC. (Scale bar, 1 mm.) (B) Quantification of average FA and RD values in the CC and EC area of the IL and CL hemispheres. (C) Representative images of dephosphorylated neurofilament protein (SMI-32, red) and MBP (green) in CTX, EC, and STR 35 d after TBI. (Scale bar, 50 μm.) (D) Illustration of regions of interest relative to lesion and quantification of the ratios of SMI-32 to MBP fluorescence intensity in the CTX, EC, and STR, illustrated as fold-change compared with the sham WT average value. (E–J) Correlation between the ratio of SMI-32 to MBP intensity in CTX, EC, and STR and forelimb use asymmetry in the cylinder test (E–G) or the latency to fall in the rotarod test (H–J) on day 35 after TBI or sham surgery. Data are presented as mean ± SEM. n = 4 per group in B; n = 8–11 per group in D; n = 5–6 per group in E–J. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 by two-way ANOVA followed by Tukey post hoc correction in B and D; P values stated in E–J were determined by two-tailed linear correlation analyses.
We assessed the integrity of myelin with immunostaining against myelin basic protein (MBP) and the degree of demyelination with immunostaining against nonphosphorylated neurofilament H (Sternberger monoclonal clone 32; SMI-32) in the perilesional cortex (CTX), EC, and striatum (STR) at 35 d after TBI. In both sham-operated groups, the SMI-32 signal in the EC and STR was extremely low. However, the SMI-32 signal was readily detectable in the uninjured CTX, perhaps indicating relatively higher numbers of unmyelinated axons within this region (Fig. 2C). TBI elevated the SMI-32/MBP ratio in the CTX, EC, and STR to significantly higher levels in tPA-KO mice than in WT mice (Fig. 2D). We further observed that the ratios of SMI-32/MBP in the CTX and EC, but not in the STR, were positively correlated with asymmetry in the cylinder test at 35 d after TBI (Fig. 2 E–G) and were inversely correlated with the latency to fall in the rotarod test (Fig. 2 H–J). In addition, the SMI-32/MBP ratios in the CTX and EC were also correlated with spatial learning and memory in the Morris water maze test (SI Appendix, Fig. S2).
The volume of gross tissue loss in the core of the impacted region was measured after immunostaining for the neuronal somatodendritic marker microtubule-associated protein 2 (MAP2) 35 d following TBI (SI Appendix, Fig. S3). WT and tPA-KO mice exhibited similar cortical lesion volumes, suggesting comparable damage to gray matter at the injury core. Thus, tPA KO has little effect on the cortical contusion size in this TBI model.
Collectively, these findings suggest that endogenous tPA mitigates TBI-induced white matter injury at extended survival times and that the extent of white matter injury within the perilesional zones in both the CTX and EC may partly dictate the evolution of long-term sensorimotor deficits after TBI.
TBI Elicits Greater Acute White Matter Damage in tPA-KO Mice than in WT Mice.
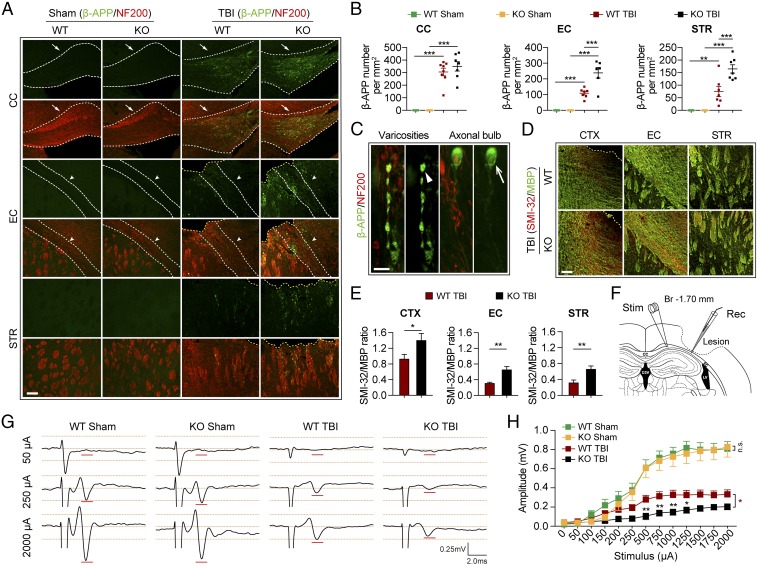
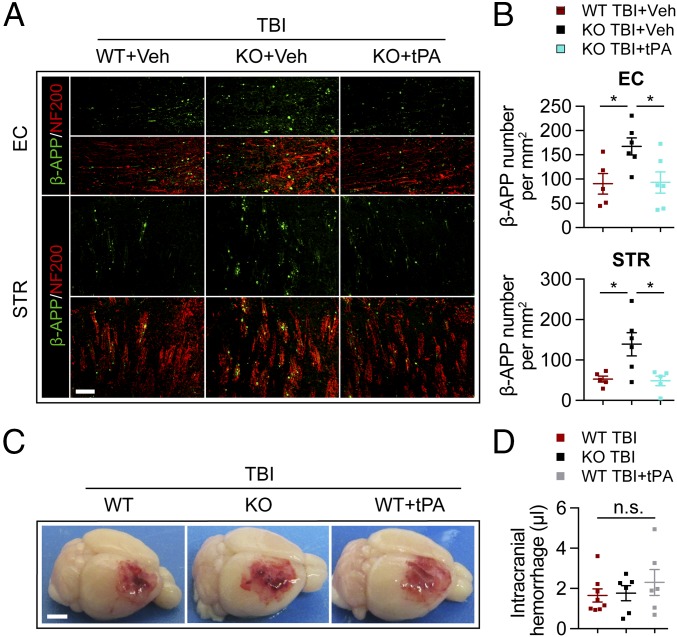
The decline of motor function in tPA-KO mice within 1 wk after TBI (Fig. 1 A and B) suggested that tPA deficiency may also worsen early brain damage. Therefore, we assessed white matter injury at 3 d after TBI with immunofluorescent staining for the accumulation of β-amyloid precursor protein (β-APP), an established indicator of axonal damage (20), and the SMI-32/MBP protein ratio, an established measurement for demyelination (21). The numbers of β-APP+ axonal varicosities were significantly increased along NF200+ neurofilaments in the perilesional CC, EC, and STR at 3 d after TBI in both WT and tPA-KO groups (Fig. 3 A–C). β-APP varicosity densities in the perilesional EC and STR, but not in CC, were significantly higher in tPA-KO mice than in WT mice (Fig. 3 A and B). Moreover, the SMI-32/MBP ratios in perilesional CTX, EC, and STR were significantly increased in tPA-KO mice compared with WT mice (Fig. 3 D and E).
Fig. 3.
tPA KO aggravates acute axonal injury and impairs axonal conduction after TBI. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. (A) Representative images of β-APP (green) and neurofilament heavy polypeptide (NF200) (red) in the perilesional CC, EC, and STR of WT and tPA-KO mice 3 d after TBI or sham operation. Dashed lines define the borders of the lesion and the CC or EC. (Scale bar, 100 μm.) (B) Quantification of β-APP density in striatal fiber bundles as varicosities per square millimeter in 25-μm-thick coronal sections. (C) Representative images showing axonal bulb (arrow) and classic varicosities (arrowhead). (Scale bar, 10 μm.) (D) Representative images of SMI-32 (abnormally dephosphorylated filaments) and MBP staining of WT and tPA-KO mice 3 d after TBI. (Scale bar, 100 μm.) (E) Quantification of SMI-32/MBP intensity ratios. (F–H) CAPs were recorded 3 d after TBI. (F) Diagram showing the location of the stimulating and recording electrodes at the external capsule. The dashed black line defines the lesion border. (G) Representative traces of the evoked CAPs after 50 μA, 250 μA, and 2,000 μA (100 ms) stimulation in sham and TBI groups (0.75 mm from the stimulation point). (H) Quantification of peak-to-peak amplitudes after various stimulation current intensities. Data are presented as mean ± SEM. n = 6–8 per group in A and B; n = 4–6 per group in E; n = 6–10 per group in G and H. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 by two-way ANOVA followed by Tukey post hoc correction in B or Student’s t test in B and E or two-way repeated-measures ANOVA followed by Bonferroni post hoc correction in H. Main effects are indicated next to brackets in H; n.s., not significant.
To determine whether the histological results in white matter were associated with functional changes, we measured evoked compound action potentials (CAPs) in the perilesional CC and EC 3 d after TBI (Fig. 3 F–H). In this assay, the amplitude of the CAPs is a measure of axonal conduction (21, 22). There was no difference in amplitude between sham-injured WT and tPA-KO mice, suggesting that loss of tPA did not affect the baseline conduction properties of myelinated axons (Fig. 3 G and H). The amplitudes were significantly decreased after TBI in the WT group, reflecting the expected axonal injury (Fig. 3 G and H). In accordance with the histological changes in white matter, CAP amplitudes were further significantly decreased in tPA-KO mice compared with WT mice.
Taken together, these results strongly suggest that endogenous tPA mitigates both acute and chronic white matter damage after TBI at the histological and functional levels.
Intranasal Delivery of Recombinant tPA Rescues Neurological Functions After TBI.
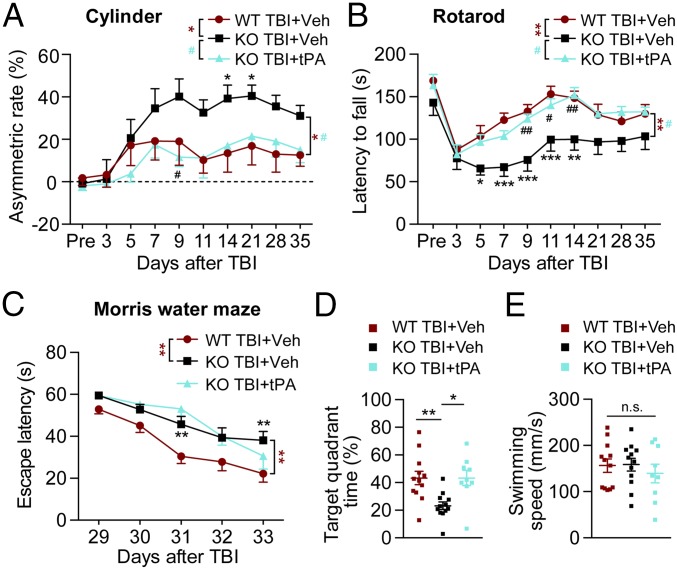
As the absence of endogenous tPA worsens neurological impairments and white matter injury at both acute and delayed time points after TBI, we hypothesized that intracerebral delivery of exogenous tPA to tPA-KO mice would ameliorate neurological deficits after TBI. To test this, we employed the intranasal approach for tPA delivery into the brain (23). In a pilot dose–response study we tested the effects of various intranasal tPA dosages (0, 0.5, 1, and 1.5 mg/kg) on behavioral performance up to 14 d after TBI using the rotarod test. The results showed that 0.5 mg/kg was the lowest dose among the tested doses to improve post-TBI performance in the rotarod test significantly. Higher doses did not exhibit further protection. Therefore, 0.5 mg/kg was selected as the optimal dose for all subsequent studies. tPA-KO and WT mice were subjected to TBI, and equal volumes of PBS or tPA (0.5 mg/kg, freshly dissolved in PBS at 1 μg/μL) were delivered into their nostrils 2 h after the injury and then once every other day for 2 wk (days 0, 2, 4, and so forth.). Sham-operated tPA-KO and WT mice treated with PBS exhibited no sensorimotor deficits in the cylinder or rotarod test and no differences across groups—an important control showing lack of effects on baseline sensorimotor functions (SI Appendix, Fig. S4 A and B). In contrast, after TBI the tPA-KO mice exhibited greater sensorimotor dysfunction than WT mice in both tests (Fig. 4 A and B) as well as greater cognitive dysfunction in the Morris water maze test (Fig. 4 C and D), consistent with the previous behavior data (Fig. 1). However, after post-TBI intranasal tPA administrations, the sensorimotor function of tPA-KO mice was equivalent to that of WT mice (Fig. 4 A and B). Although tPA treatment failed to improve spatial learning significantly in tPA-KO mice (Fig. 4C), tPA rescued long-term spatial memory in tPA-KO mice, raising it to the levels of PBS-treated WT mice (Fig. 4D). Swim speeds were unaffected (Fig. 4E), as in Fig. 1. These results suggest that intranasal administration of recombinant tPA restores most of the neurological functions examined in tPA-KO mice after TBI and improves them to WT levels.
Fig. 4.
Intranasal delivery of exogenous, recombinant tPA facilitates neurological recovery after TBI in tPA-KO mice. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. Recombinant tPA (0.5 mg/kg) or an equivalent volume of vehicle (PBS) was delivered intranasally 2 h after TBI and every other day up to 14 d postinjury (Methods). (A and B) The cylinder test (A) and the rotarod test (B) were used to assess forelimb asymmetry and sensorimotor coordination, respectively, 3 d before and 3–35 d after TBI. Mice were pretrained for 3 d before surgery, and the average latency to fall on the last day before surgery was used as the baseline value. In both tests recovery was improved to nearly WT levels by tPA treatment. (C–E) Cognitive function was assessed by the Morris water maze test. (C) The time required to reach the invisible platform 29–33 d postinjury (spatial learning). Performance measured as escape latency was worse in tPA-KO than in WT mice after TBI and was not significantly improved by tPA treatment. (D) Quantification of time spent in the target quadrant when the platform was removed on day 34 (spatial memory). tPA improved recovery in the tPA-KO mice to WT levels. (E) Average swim speeds were comparable in all three groups. Data are presented as mean ± SEM. n = 9–12 per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 for KO+vehicle vs. WT+vehicle; #P ≤ 0.05, ##P ≤ 0.01 for KO+vehicle vs. KO+tPA by two-way repeated-measures ANOVA followed by Tukey post hoc correction in A–C. *P ≤ 0.05 and **P ≤ 0.01 by one-way ANOVA followed by Tukey post hoc correction in D. n.s., not significant by the Kruskal–Wallis test (E).
Intranasal Delivery of tPA Compensates for the Detrimental Effects of tPA KO on White Matter After TBI.
Macroscopic loss of brain neuronal tissue was assessed by staining for the neuron marker MAP2 at 3 and 35 d after TBI. No significant difference in cortical tissue volume loss was observed between the tPA and PBS treatment groups at either early or extended survival time points (SI Appendix, Figs. S5 and S6), suggesting that tPA administration does not rescue the focal cortical lesion after TBI, consistent with the tPA-KO data (SI Appendix, Fig. S3) and with the severity of the mechanical trauma in the focal injury zone.
We investigated whether intranasal tPA infusions reduce white matter disruption in tPA-KO mice subjected to TBI. SMI-32/MBP staining was performed on brain slices harvested 35 d after TBI. Consistent with our previous findings in untreated mice, PBS-treated tPA-KO mice exhibited a higher SMI-32/MBP ratio in the CTX and EC, but not in the striatum, than PBS-treated WT mice after TBI (Fig. 5 A–D). Furthermore, the increase in SMI-32/MBP ratios in tPA-KO mice was abolished with intranasal tPA infusions in the CTX and EC (Fig. 5 B and C). To assess whether these histological effects were paralleled by functional improvements, we investigated whether tPA treatment increased axonal conduction at 35 d after TBI. Compared with sham controls (Fig. 3H), TBI markedly decreased CAP amplitude in WT mice, and this effect was even more profound in tPA-KO mice (Fig. 5 E and F). tPA treatment significantly enhanced conduction amplitude in tPA-KO mice to levels comparable to those in WT mice after TBI (Fig. 5 E and F). Taken together, these data reveal that intranasal delivery of tPA replaces the endogenous tPA missing from KO mice and prevents long-term demyelination and loss of axonal conduction after TBI. Furthermore, white matter loss was associated with deterioration of axonal conductivity, as shown by a negative correlation between the SMI-32/MBP ratio in the CTX and EC and the amplitude of evoked CAPs (Fig. 5 G–I). Strong correlations were also observed between evoked CAP amplitude and the asymmetry rate in the cylinder test (Fig. 5J) and the latency to fall in the rotarod test (Fig. 5K). These findings indicate that the electrophysiology measurements are an excellent predictor of functional performance after TBI.
Fig. 5.
Intranasal delivery of recombinant tPA mitigates long-term white matter injury and improves axonal conduction after TBI in tPA-KO mice. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. tPA (0.5 mg/kg) or an equivalent volume of vehicle (PBS) was delivered intranasally 2 h after TBI and every other day up to 14 d postinjury. (A) Representative images of SMI-32 (red) and MBP (green) immunostaining 35 d after TBI. (Scale bar, 50 μm.) (B–D) Quantification of SMI-32/MBP intensity ratio in CTX (B), EC (C), and STR (D) after TBI. n = 7–11, normalized to WT+vehicle. (E) Representative traces of evoked CAPs in the ipsilesional CC and EC (stimulus, 50 μA, 250 μA, and 2,000 μA 0.75 mm lateral to the stimulating electrode) at 35 d post-TBI. Recordings were made ventral to the lesion core (Fig. 3F). (F) Action potential conduction along nerve fibers, as measured by the amplitude of the CAPs under different stimulus strengths (0–2,000 μA). (G–I) Correlation analyses of evoked CAPs amplitude (under the stimulus of 2,000 μA) and the ratio of SMI-32/MBP intensity in CTX (G), EC (H), and STR (I). (J and K) The evoked CAP amplitude (under the stimulus of 2,000 μA) was correlated with the forelimb use asymmetry in the cylinder (J) and latency to fall off the rotarod (K). Data are presented as mean ± SEM. n = 7–11 per group in B–D; n = 8–12 per group in E and F; n = 4–7 per group in G–K. *P ≤ 0.05, **P ≤ 0.01 by one-way ANOVA followed by Tukey post hoc correction in B–D; #P ≤ 0.05 WT TBI+vehicle vs. KO TBI+vehicle and *P ≤ 0.05, **P ≤ 0.01 TBI-KO+tPA vs. TBI-KO+vehicle by two-way repeated-measures ANOVA followed by a Tukey post hoc correction in F. P values stated in G–K were determined by two-tailed Pearson product linear correlation analyses. n.s., not significant.
Intranasal Delivery of tPA Reverses Axonal Injury Induced by TBI in tPA-KO Mice Without Magnifying the Intracranial Hemorrhage.
Thus far, we have demonstrated that tPA administration improves long-term white matter integrity in tPA-KO mice and is associated with superior neurological outcomes. As acute axonal damage after TBI was greater in tPA-KO mice (Fig. 3), we examined whether exogenous tPA treatment might alleviate this detrimental effect by measuring β-APP+ varicosities 3 d after TBI. No immunoreactivity for β-APP was detected in either sham injury group. In accordance with our previous results, β-APP accumulated within injured axons in the perilesional EC and STR in PBS-treated WT mice after TBI, and this effect was greater in the tPA-KO mice, as expected (Fig. 6 A and B). However, tPA treatment significantly decreased β-APP staining in tPA-KO mice to levels similar to those in WT mice, suggesting that exogenous tPA administration reduced axonal injury in the acute phase after TBI.
Fig. 6.
Intranasal delivery of recombinant tPA ameliorates axonal injury after TBI in tPA-KO mice without magnifying hemorrhage volume. (A and B) WT and tPA-KO mice were subjected to unilateral TBI in the right hemisphere. tPA (0.5 mg/kg) or an equivalent volume of vehicle (PBS) was delivered intranasally 2 h after TBI and on 2 d post-TBI. (A) Representative images of β-APP (green) and NF200 (red) in perilesional EC and striatum (STR) 3 d after TBI. (Scale bar, 100 μm.) (B) Quantification of β-APP density in the EC and STR. (C and D) WT and tPA-KO mice were subjected to TBI in the right hemisphere, and tPA (0.5 mg/kg) was given intranasally to WT mice 2 and 22 h after TBI, respectively. (C) Representative images of intracranial hemorrhage 24 h after TBI. (Scale bar, 2 mm.) (D) Quantification of intracranial hemorrhage using Drabkin’s assay for hemoglobin concentration. Data are presented as mean ± SEM. n = 5–6 per group in B; n = 6–8 per group in D. *P ≤ 0.05 by one-way ANOVA; n.s., not significant. Quantification of hemorrhage volumes can be viewed in SI Appendix, Fig. S7.
The clinical use of recombinant tPA is viewed as contraindicated in head trauma victims due to the concern that tPA might increase the risk of cerebral hemorrhage. A recent paper reported that TBI induces less brain hemorrhage in tPA-KO mice than in WT mice (15), suggesting that endogenous tPA might augment cerebral bleeding after TBI. Therefore, we tested the safety of intranasal tPA administration and found that intranasal tPA treatment (0.5 mg/kg) failed to increase brain hemorrhage in WT TBI mice (Fig. 6 C and D and SI Appendix, Fig. S7). Furthermore, there was no decrease in the amount of intracranial hemorrhage in tPA-KO mice compared with WT mice 24 h after TBI (Fig. 6 C and D and SI Appendix, Fig. S7B). These results do not support a major contributing role for tPA in TBI-induced brain hemorrhage.
Intranasal Delivery of tPA Prevents the Detrimental Impact of tPA KO on Axonal Sprouting After TBI.
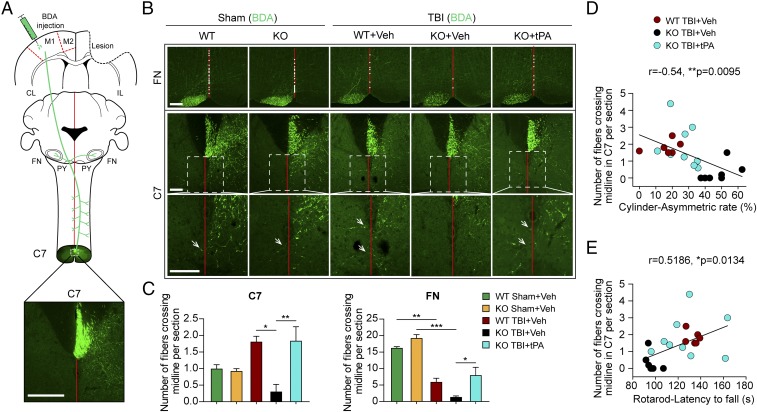
tPA is known to exert neurotrophic growth factor-like effects (24–26). Therefore, we examined whether tPA influences axonal sprouting in the CST and corticobulbar tracts. As illustrated (Fig. 7A), the tract-tracer biotinylated dextran amine (BDA) was injected in the contralesional motor cortex 3 wk after TBI or sham operation. BDA is anterogradely transported along the pyramidal tracts and crosses into the contralateral (ipsilesional) spinal cord before cervical spinal cord segment 7 (C7). The sprouting of BDA+ axons across the midline into the denervated facial nucleus (ipsilesional) and spinal cord (contralesional) is a robust measure of neuroplasticity (27, 28). Thus, brains and spinal cords were harvested 2 wk after BDA infusions, and the numbers of BDA+ fibers in the ipsilesional brainstem at the level of the facial nuclei and the contralesional C7 segment were counted to assess axonal sprouting from motor neurons of the contralesional cortex (Fig. 7 B and C). Sham-operated WT and tPA-KO mice exhibited similar numbers of BDA+ fibers. The absence of endogenous tPA in tPA-KO mice dramatically reduced the number of midline-crossing fibers in C7 after TBI compared with WT mice (Fig. 7C). In the facial nuclei, TBI significantly reduced the number of midline-crossing axons in WT mice, and tPA KO decreased this measure further. Notably, tPA-KO mice infused with recombinant tPA exhibited similar numbers of midline-crossing fibers as WT mice in both areas (Fig. 7C), revealing an essential role of tPA in axonal sprouting during the recovery period after TBI.
Fig. 7.
Intranasal delivery of recombinant tPA promotes axonal sprouting after TBI in tPA-KO mice. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. tPA (0.5 mg/kg) or an equivalent volume of vehicle (PBS) was delivered intranasally 2 h after TBI and every other day up to 14 d postinjury. (A) Schematic diagram of tract-tracing experiments. BDA was injected in the contralesional motor cortex 21 d after TBI in all groups. Animals were killed on day 35 after TBI. The corticobulbar and corticospinal projections from motor cortex (motor areas M1 and M2) into contralesional (CL) and ipsilesional (IL) facial nuclei (FN) and spinal cord (C7) are depicted in green. (Scale bar, 230 μm.) (B) Representative photomicrographs of streptavidin-FITC BDA+ axons. Sprouting axons are indicated by white dots in the midline at the FN level and arrows at the level of C7, respectively. (Scale bars, 100 μm.) The boxed areas in the Middle Row are enlarged in the Bottom Row. (C) Quantification of midline-crossing axons per section from three sections per animal. n = 6–9 per group. (D and E) Linear correlations of sprouting axons with behavioral performance at day 35 postinjury. Correlations between the numbers of midline-crossing fibers and asymmetry of forelimb use in the cylinder test (D) and fall latency in the rotarod test (E). n = 6–9 per group. Data are presented as mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 by one-way ANOVA followed by Tukey post hoc comparison in C. P values stated in D and E were determined by two-tailed Pearson product linear correlation analyses.
To determine the strength of the relationship between increased sprouting and improvements in sensorimotor function by exogenously delivered recombinant tPA, Pearson product correlation analyses were performed. The number of midline-crossing BDA+ fibers at C7 was negatively correlated with the forelimb use asymmetry in the cylinder test and positively correlated with fall latency in the rotarod test on day 35 post-TBI (Fig. 7 D and E). These results are consistent with the view that tPA improves neurological outcomes after TBI partly through an increase in axon sprouting.
Exogenous tPA Application Promotes Neurite Outgrowth in Vitro.
To explore the mechanisms by which tPA promotes axon sprouting under pathological conditions, an in vitro assay was designed to mimic the inhibition of neurite outgrowth after TBI by application of chondroitin sulfate proteoglycan (CSPG) (29). CSPG has been implicated in the inhibition of spontaneous axonal plasticity after TBI (30, 31). The neurotrophic effects of tPA are thought to be partly mediated by the epidermal growth factor receptor (EGFR) (32, 33). As expected, CSPG decreased the length of tau+ axons in neurons in a concentration-dependent manner (SI Appendix, Fig. S8 A and B), and axon length was significantly increased after tPA treatment (SI Appendix, Fig. S8 C and D). The increases in axon length by tPA were diminished by the EGFR inhibitor AG1478. These results suggest that the axon growth-promoting effects of tPA are mediated, at least in part, by EGFR.
Discussion
The present study demonstrates white matter-protective and neurorestorative roles for endogenous tPA after TBI, according to histological, MRI, electrophysiological, tract-tracing, and behavioral criteria. Despite the lack of a tissue-sparing effect at the cortical contusion site, endogenous tPA and intranasally delivered tPA robustly improved the structural and functional integrity of white matter tracts in perilesional brain regions in both acute and chronic injury phases. The salutary effects of tPA on white matter integrity after TBI likely contributed to the improvements in long-term cognitive and sensorimotor functions. These results reveal a previously unappreciated role for tPA in the protection and/or repair of white matter and long-term functional recovery after TBI.
Severe traumatic injury in white matter rapidly sets in motion numerous pathologies, including (i) acute axonal injury, which may lead to degeneration of the entire soma, impaired neuronal network communication, and proinflammatory neuron–glia crosstalk, and (ii) acute oligodendrocyte injury, which may lead to oligodendrocyte loss and demyelination of axons (34). Severe TBI may also evolve into chronic, long-term disruption of white matter unless endogenous repair processes interrupt or decelerate the secondary expansion of inflammatory and degenerative cascades. Although our study was not designed to distinguish between acute protection and long-term repair, the data collected 3 d after TBI strongly suggest that tPA preserves the structural integrity and functional capacity of myelinated axons in acute injury phases. Second, both endogenously expressed and exogenously delivered tPA mitigated the loss of white matter tracts at 35 d post-TBI and promoted axonal sprouting and neurological recovery during late injury phases. Late injury phases after TBI are normally associated with reparatory axon regrowth, the proliferation and maturation of oligodendrocyte progenitor cells, remyelination of white matter, and some degree of spontaneous recovery of neurological function (34). However, longitudinal characterization of the same cohort of injured animals will be needed to further distinguish protective versus reparative effects of tPA.
tPA is naturally expressed in both gray and white matter, including within oligodendrocytes of the CC, but tPA expression in the CC declines with age, perhaps reducing the regeneration potential of white matter (35). After lysolecithin-induced white matter demyelination, endogenous tPA accelerates the migration of oligodendrocyte precursors to the lesion site through its EGF-like domain (36). Furthermore, endogenous tPA protects neurons against ischemic injury, and exogenous tPA mitigates white matter damage 24 h poststroke (17, 18, 35). The neuroprotective properties of tPA after stroke injury may be mediated by tPA-induced production and release of TNF-α from neurons and the subsequent overexpression of the cell-cycle inhibitor gene p21 (18). tPA also prevents apoptosis in both human and mouse oligodendrocytes through its EGF-like functional domain, and these effects are blocked by AG1478 (35). The present study on TBI is distinct from previous work in that we examined both acute and long-term oligotrophic and axonotrophic properties of exogenous and endogenous tPA with (i) a battery of histological markers, (ii) tract-tracing assessment of axons projected from the contralesional motor cortex, (iii) diffusion tensor imaging of white matter bundles, (iv) electrophysiological assessments of white matter function, and (v) repeated measurements of sensorimotor coordination/asymmetry and spatial learning/memory. We also confirmed the internal consistency between functional and histological datasets with correlation analyses.
In the present study, both endogenous and exogenous tPA promoted postinjury axonal sprouting in corticofugal efferents from the contralesional motor cortex during the chronic recovery phase after TBI. Previous work in stroke models supports prosprouting or remodeling effects of tPA (27, 37, 38). tPA also promotes axonal outgrowth of cultured peripheral neurons on purified myelin (39). As a potential mechanism underlying axonal sprouting, EGFR activation mediated tPA-induced axonal extension in cultured primary cortical neurons after exposure to CSPG. Endogenous tPA is known to be released in an activity-dependent manner from neurons after exposure to stimuli associated with synaptic plasticity and is critical for long-term potentiation (10, 11, 40, 41). These collective observations suggest that superior neuroplasticity under conditions of tPA repletion may facilitate improvements in long-term outcomes.
Although tPA treatment has shown neuroprotective effects and promotes neurovascular remodeling or regeneration (17, 18, 27, 36–38), the use of tPA is not currently indicated in head trauma due to somewhat contradictory perceptions of an increased risk of brain hemorrhage and edema (42). Intravascular delivery of the free form, but not of erythrocyte-bound tPA, was reported to increase cortical tissue injury and hemorrhage after TBI (43). Exogenous tPA is believed to cause brain hemorrhage via protease-dependent activation of matrix metalloproteinases and breakdown of the blood–brain barrier, because mutant forms of tPA devoid of the protease activities, such as tPA-S481A, do not induce brain hemorrhage (12, 13, 16). The discrepancies in the literature on the beneficial versus toxic properties of tPA are likely attributed to variable doses and/or the timing of tPA treatment (10, 44). The dose of tPA for thrombolytic therapy in humans is 0.9 mg/kg, which is associated with an increased risk of intracerebral hemorrhage only if the delivery of tPA is delayed for >4.5 h after the onset of stroke (45). In rodent models of TBI and stroke, intracerebral hemorrhage is typically induced by i.v. delivery of tPA of 5 and 10 mg/kg, respectively (15, 46, 47). In a rat model of physiological thrombus induced by crushing and stenosis of the common carotid artery, the i.v. “human dose” of 0.9 mg/kg was ineffective for rodent clot lysis, and the authors concluded that “rat doses” between 1.8 and 4.5 mg/kg were more likely to achieve the upper limits of the clinical recanalization rates of 10–30% (48). In a rat stroke model, intranasal delivery of 2.2–2.4 mg/kg tPA failed to increase intracerebral hemorrhage (49). Nevertheless, the neuroprotective tPA dose tested in the current study (0.5 mg/kg) is considerably lower than the reported doses that cause intracerebral hemorrhage in rodents (15, 46, 47). Furthermore, TBI-induced brain hemorrhage was not reduced in tPA-KO mice. These collective results do not support a major contributing role for endogenous or low-dose exogenous tPA in TBI-induced intracerebral hemorrhage.
The intranasal route is thought to be a superior method for quickly accessing the brain, as it minimizes systemic exposure and bypasses the blood–brain barrier by employing alternative routes, such as the olfactory and trigeminal nerves, interstitial space, and lymphatics (50–52). I.v. tPA has a short half-life of 5–10 min in human plasma (53, 54), which contrasts with recent findings that intranasal delivery of 1.86 mg/kg tPA in rats leads to dramatically higher tPA protein and activity levels in the brain as late as 24 h postinfusion (55). However, brain bioavailability will depend upon formulation and other chemical properties of the intranasal infusate, as the intranasal routes were found to be similar to the i.v. route for some drugs (56, 57). Furthermore, we caution that generalizations of effective drug dosages from rodents to human patients are fraught with caveats, including the absence of underlying vascular pathologies in otherwise healthy rodents. Further studies are warranted to identify the pharmacokinetics and pharmacodynamics properties of a range of low doses of intranasal tPA and to determine the relationship between tPA concentrations (in brain and blood after intranasal delivery) and the risk of cerebral hemorrhage or other adverse events in a variety of injury models.
In conclusion, our findings suggest that the roles of the pleiotropic tPA-encoding gene extend to protection of neuronal network communication and improvement of functional outcomes in acute and chronic phases of TBI. If similar mechanisms operate in humans, they might partly contribute to the normal life expectancy of TBI survivors and the absence of progressive neurodegeneration, unless the TBI is severe or repetitive in nature (58). Therefore, low-dose, recombinant forms of tPA should continue to be tested for their potential to preserve or rescue white matter tracts and improve long-term functional recovery after acute brain injuries.
Materials and Methods
Adult male C57BL/6J WT mice and global tPA-KO mice were purchased from The Jackson Laboratory at 8–12 wk of age. TBI was elicited in randomly assigned mice (within genotype constraints) by unilateral CCI, as described (22). A right parietal craniotomy (diameter 3.5 mm) was centered 0.5 mm anterior and 2.0 mm lateral to bregma in anesthetized mice. CCI was performed with a pneumatically driven CCI device (Precision Systems and Instrumentation) utilizing a 3-mm flat-tipped impactor to compress the exposed brain tissue to a depth of 1.5 mm for 150 ms at a velocity of 3.5 m/s. All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (59). Every attempt was made to reduce animal suffering and numbers used.
Animals were randomly assigned to receive either PBS or tPA 2 h after TBI (on day zero) and subsequently every other day for 14 d postsurgery. Human recombinant tPA (Actilyse; Boehringer Ingelheim GmbH) was freshly dissolved in PBS and delivered intranasally at a dose of 0.5 mg/kg. Thus, a 30-g, 8- to 12-wk-old mouse would receive 15 µg tPA in 15 µL PBS over the course of ∼25 min (five 3-µL injections of 1 µg/µL tPA, spaced ∼5 min apart). All infusions were made in mice lying on their backs and fully anesthetized with isoflurane delivered through a nose cone. All outcomes were assessed by blinded investigators and analyzed by two-tailed parametric or nonparametric tests depending on whether assumptions such as Gaussian distribution were met. The statistical tests employed, F and two-tailed P values, the t-statistic, and the Pearson correlation coefficient r, are listed in SI Appendix, Table S1. For further methodological details, please consult SI Appendix.
Supplementary Material
Acknowledgments
We thank Carol Culver for editorial assistance and Patricia Strickler for administrative support. This project was supported by NIH Grants NS095029 and NS108695 (to M.V.L.B. and J.C.), and US Department of Veterans Affairs (VA) Merit Review BX003377 (to J.C.). J.C. is the Richard King Mellon Professor of Neurology and a recipient of a VA Senior Research Career Scientist Award. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810693115/-/DCSupplemental.
References
- 1.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong KA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 3.Kinnunen KM, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigler ED. Distinguished Neuropsychologist Award Lecture 1999. The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Arch Clin Neuropsychol. 2001;16:95–131. [PubMed] [Google Scholar]
- 5.Sidaros A, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 7.Nusbaum F, et al. Hemispheric differences in white matter microstructure between two profiles of children with high intelligence quotient vs. controls: A tract-based spatial statistics study. Front Neurosci. 2017;11:173. doi: 10.3389/fnins.2017.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsirka SE. Tissue plasminogen activator as a modulator of neuronal survival and function. Biochem Soc Trans. 2002;30:222–225. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 9.Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- 10.Lee TW, Tsang VW, Birch NP. Physiological and pathological roles of tissue plasminogen activator and its inhibitor neuroserpin in the nervous system. Front Cell Neurosci. 2015;9:396. doi: 10.3389/fncel.2015.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yepes M, Lawrence DA. New functions for an old enzyme: Nonhemostatic roles for tissue-type plasminogen activator in the central nervous system. Exp Biol Med (Maywood) 2004;229:1097–1104. doi: 10.1177/153537020422901103. [DOI] [PubMed] [Google Scholar]
- 12.Armstead WM, et al. tPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012;29:1794–1802. doi: 10.1089/neu.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstead WM, et al. tPA-S(481)A prevents impairment of cerebrovascular autoregulation by endogenous tPA after traumatic brain injury by upregulating p38 MAPK and inhibiting ET-1. J Neurotrauma. 2013;30:1898–1907. doi: 10.1089/neu.2013.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstead WM, Riley J, Yarovoi S, Higazi AA, Cines DB. Tissue-type plasminogen activator-A296-299 prevents impairment of cerebral autoregulation after stroke through lipoprotein-related receptor-dependent increase in cAMP and p38. Stroke. 2016;47:2096–2102. doi: 10.1161/STROKEAHA.116.012678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hijazi N, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125:2558–2567. doi: 10.1182/blood-2014-08-588442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstead WM, Hekierski H, Yarovoi S, Higazi AA, Cines DB. tPA variant tPA-A296-299 prevents impairment of cerebral autoregulation and necrosis of hippocampal neurons after stroke by inhibiting upregulation of ET-1. J Neurosci Res. 2018;96:128–137. doi: 10.1002/jnr.24112. [DOI] [PubMed] [Google Scholar]
- 17.Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest. 2010;120:2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haile WB, et al. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α. J Cereb Blood Flow Metab. 2012;32:57–69. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 21.Pu H, et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1474–1484. doi: 10.1038/jcbfm.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Chevilley A, et al. Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front Cell Neurosci. 2015;9:415. doi: 10.3389/fncel.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–650. doi: 10.1126/science.284.5414.647. [DOI] [PubMed] [Google Scholar]
- 26.Liot G, et al. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J Neurochem. 2006;98:1458–1464. doi: 10.1111/j.1471-4159.2006.03982.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, et al. Subacute intranasal administration of tissue plasminogen activator increases functional recovery and axonal remodeling after stroke in rats. Neurobiol Dis. 2012;45:804–809. doi: 10.1016/j.nbd.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos CM, et al. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 29.Hynds DL, Snow DM. Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: Stalling/stopping exceeds turning in human neuroblastoma growth cones. Exp Neurol. 1999;160:244–255. doi: 10.1006/exnr.1999.7212. [DOI] [PubMed] [Google Scholar]
- 30.Yi JH, et al. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J Comp Neurol. 2012;520:3295–3313. doi: 10.1002/cne.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris NG, Carmichael ST, Hovda DA, Sutton RL. Traumatic brain injury results in disparate regions of chondroitin sulfate proteoglycan expression that are temporally limited. J Neurosci Res. 2009;87:2937–2950. doi: 10.1002/jnr.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand T, et al. Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR. Cell Death Dis. 2015;6:e1924. doi: 10.1038/cddis.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemarchand E, et al. Stressed neurons protect themselves by a tissue-type plasminogen activator-mediated EGFR-dependent mechanism. Cell Death Differ. 2016;23:123–131. doi: 10.1038/cdd.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology. 2016;110:654–659. doi: 10.1016/j.neuropharm.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Correa F, et al. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonetti C, et al. Tissue-type plasminogen activator exerts EGF-like chemokinetic effects on oligodendrocytes in white matter (re)myelination. Mol Neurodegener. 2017;12:20. doi: 10.1186/s13024-017-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou T, et al. Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J Neuropathol Exp Neurol. 2006;65:78–86. doi: 10.1097/01.jnen.0000195942.25163.f5. [DOI] [PubMed] [Google Scholar]
- 38.Shen LH, et al. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke. 2011;42:459–464. doi: 10.1161/STROKEAHA.110.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minor K, Phillips J, Seeds NW. Tissue plasminogen activator promotes axonal outgrowth on CNS myelin after conditioned injury. J Neurochem. 2009;109:706–715. doi: 10.1111/j.1471-4159.2009.05977.x. [DOI] [PubMed] [Google Scholar]
- 40.Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YY, et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 43.Stein SC, et al. Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 2009;26:1585–1592, and erratum (2010) 27:299. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: Therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hacke W, et al. ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 46.Crumrine RC, et al. Intra-arterial administration of recombinant tissue-type plasminogen activator (rt-PA) causes more intracranial bleeding than does intravenous rt-PA in a transient rat middle cerebral artery occlusion model. Exp Transl Stroke Med. 2011;3:10. doi: 10.1186/2040-7378-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao L, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain. 2017;140:1914–1931. doi: 10.1093/brain/awx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomkins AJ, Hood RJ, Levi CR, Spratt NJ. Tissue plasminogen activator for preclinical stroke research: Neither “rat” nor “human” dose mimics clinical recanalization in a carotid occlusion model. Sci Rep. 2015;5:16026. doi: 10.1038/srep16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Velumian AA, Samoilova M, Fehlings MG. A novel approach for studying the physiology and pathophysiology of myelinated and non-myelinated axons in the CNS white matter. PLoS One. 2016;11:e0165637. doi: 10.1371/journal.pone.0165637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 51.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Gravanis I, Tsirka SE. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin Ther Targets. 2008;12:159–170. doi: 10.1517/14728222.12.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verstraete M, Bounameaux H, de Cock F, Van de Werf F, Collen D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans. J Pharmacol Exp Ther. 1985;235:506–512. [PubMed] [Google Scholar]
- 55.Meng Y, et al. Subacute intranasal administration of tissue plasminogen activator promotes neuroplasticity and improves functional recovery following traumatic brain injury in rats. PLoS One. 2014;9:e106238. doi: 10.1371/journal.pone.0106238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christrup LL, Foster D, Popper LD, Troen T, Upton R. Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: A randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther. 2008;30:469–481. doi: 10.1016/j.clinthera.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Babhair SA, Tariq M, Abdullah ME. Comparison of intravenous and nasal bioavailability of clonidine in rodents. Res Commun Chem Pathol Pharmacol. 1990;67:241–248. [PubMed] [Google Scholar]
- 58.Brown AW, et al. A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. J Neurotrauma. 2011;28:167–176. doi: 10.1089/neu.2010.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. National Research Council (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.