Significance
Each neuron forms a single axon and multiple dendrites, and this configuration is important for wiring the brain. How only a single axon extends from a neuron, however, remains unknown. This study demonstrates that CAMSAP3, a protein that binds the minus-end of microtubules, preferentially localizes along axons in hippocampal neurons. Remarkably, mutations of CAMSAP3 lead to production of multiple axons in these neurons. In attempts to uncover mechanisms underlying this abnormal axon extension, the authors found that CAMSAP3-anchored microtubules escape from acetylation, a process mediated by α-tubulin acetyltransferase-1, and depletion of this enzyme abolishes abnormal axon formation in CAMSAP3 mutants. These findings reveal that CAMSAP3 controls microtubule dynamics, preventing tubulin acetylation; this mechanism is required for single-axon formation.
Keywords: axon, neuronal polarity, CAMSAP, microtubule, αTAT1
Abstract
The molecular mechanisms that guide each neuron to become polarized, forming a single axon and multiple dendrites, remain unknown. Here we show that CAMSAP3 (calmodulin-regulated spectrin-associated protein 3), a protein that regulates the minus-end dynamics of microtubules, plays a key role in maintaining neuronal polarity. In mouse hippocampal neurons, CAMSAP3 was enriched in axons. Although axonal microtubules were generally acetylated, CAMSAP3 was preferentially localized along a less-acetylated fraction of the microtubules. CAMSAP3-mutated neurons often exhibited supernumerary axons, along with an increased number of neurites having nocodazole-resistant/acetylated microtubules compared with wild-type neurons. Analysis using cell lines showed that CAMSAP3 depletion promoted tubulin acetylation, and conversely, mild overexpression of CAMSAP3 inhibited it, suggesting that CAMSAP3 works to retain nonacetylated microtubules. In contrast, CAMSAP2, a protein related to CAMSAP3, was detected along all neurites, and its loss did not affect neuronal polarity, nor did it cause increased tubulin acetylation. Depletion of α-tubulin acetyltransferase-1 (αTAT1), the key enzyme for tubulin acetylation, abolished CAMSAP3 loss-dependent multiple-axon formation. These observations suggest that CAMSAP3 sustains a nonacetylated pool of microtubules in axons, interfering with the action of αTAT1, and this process is important to maintain neuronal polarity.
Neurons typically generate a single axon and multiple dendrites, creating a cellular basis for their network formation. The polarity of neurons is established during early processes of neuronal differentiation, which have been well studied using hippocampal neurons in culture. The cell initially extends multiple neurites (stage 1–2), and then one of them is selected to differentiate into the axon (stage 3), whereas others give rise to dendrites, followed by maturation of the respective neurites at subsequent stages (stage 4–5) (1). Formation of such polarized axon formation is regulated by numerous factors and mechanisms, as has been extensively reviewed (2–4). However, a holistic picture of the mechanisms by which neuronal polarity is established remains obscure.
Microtubules are a major cytoskeletal component of neurites and are known to regulate axon formation and functions (5). It has been shown that local stabilization of the cytoskeleton is sufficient to initiate the extension of axons (6). Microtubule dynamics are regulated by posttranscriptional modification, including tubulin acetylation (7). Acetylation occurs in long-lived microtubules (8, 9), and α-tubulin acetyltransferase 1 (αTAT1/MEC-17) is responsible for the acetylation of α-tubulins at lysine 40 located at the luminal side of microtubules (10–12). αTAT1/MEC17-mediated acetylation modifies structural or mechanical properties of microtubules, such as protofilament organization (13, 14) and molecular flexibility (15, 16). In cortical neurons, αTAT1/MEC17 serves to reduce microtubule dynamics, suppressing axon overbranching (17). How microtubule stability or posttranscriptional modification controls axon formation is, however, not fully understood.
Microtubules are anchored to the centrosome in many, but not all, cell types. In neurons, the centrosome is actually not required for axon extension (18), suggesting that microtubules involved in this process are derived from noncentrosomal sites. Recent studies have revealed that a group of proteins, termed CAMSAP1–CAMSAP3 (in vertebrates), Patronin (in Drosophila), and PTN-1 (in Caenorhabditis elegans), bind and stabilize the minus-ends of microtubules (19–22), and they act as a seed for tubulin polymerization at noncentrosomal sites (22, 23). These proteins regulate microtubule assembly in various cell types, such as epithelial cells (23–25) and oocytes (26). In neurons, CAMSAP2 takes part in neurite morphogenesis (27), and PTN-1 is required for axon morphogenesis and regeneration (28–30). In the present study, we investigated the potential role of CAMSAP3 in axon development, in comparison with CAMSAP2, using mouse hippocampal neurons. Remarkably, CAMSAP3 mutations promoted multiple axon formation, whereas CAMSAP2 loss had no such effect, indicating that CAMSAP3 is specifically required for neuronal polarity. Further analysis suggests that CAMSAP3 maintains a dynamic pool of microtubules by counteracting αTAT1-mediated tubulin acetylation to retain single axons.
Results
Loss of CAMSAP3 Leads to Supernumerary Axon Formation.
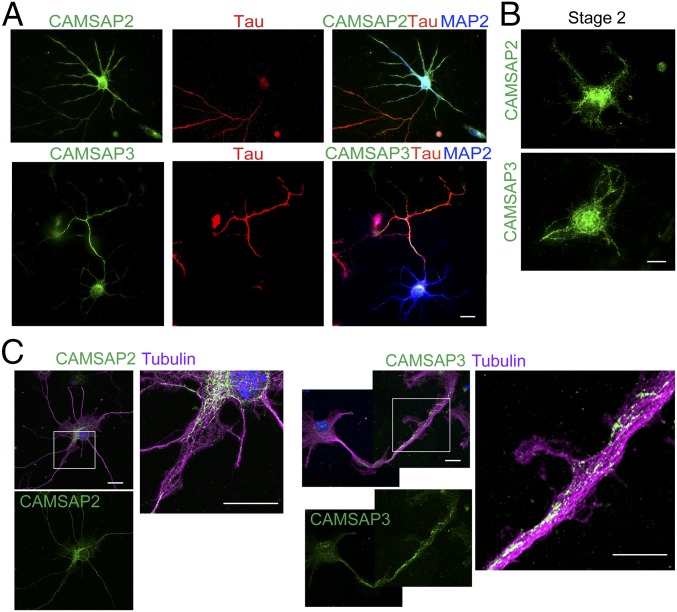
Developing brains expressed CAMSAP2 and CAMSAP3, although CAMSAP3 expression in hippocampus was delayed (SI Appendix, Fig. S1A). We collected hippocampal neurons from E16.5 embryos and cultured them for 6 d in vitro (DIV6), followed by double-immunostaining for CAMSAPs and a dendrite or axon marker [MAP2 for dendrite; Tau, ankyrin-G or axonal neurofilaments (SMI312 antigen) for axon]. The results showed that CAMSAP2 was distributed in both axons and dendrites, whereas CAMSAP3 was consistently enriched in axons (Fig. 1A). In earlier stages of culture (stage 2), however, both CAMSAP2 and CAMSAP3 were detected in all neurites, as well as in the cell body (Fig. 1B), and the restriction of CAMSAP3 to axons took place at stage 3 (SI Appendix, Fig. S1B), indicating that the axon-enriched localization of CAMSAP3 coincides with axon differentiation (31). Double-immunostaining for CAMSAPs and microtubules showed that CAMSAPs were detected as punctate signals that overlap with microtubules (Fig. 1C), as observed in other cell types (23). High-magnification views showed that CAMSAPs were localized only along a subset of microtubule bundles (Fig. 1C, enlarged panels).
Fig. 1.
Distribution of CAMSAP2 and CAMSAP3 in hippocampal neurons in culture. (A) Neurons were triple-immunostained for CAMSAP2 or CAMSAP3 (green), Tau (red), and MAP2 (blue) at DIV6. (B) Neurons (stage 2) were immunostained for CAMSAP2 or CAMSAP3 at DIV2. (C) Neurons (stage 3) were triple-stained for CAMSAP2 or CAMSAP3, α-tubulin (magenta) and DNA (blue) at DIV4. Boxed areas are enlarged. (Scale bars, 20 μm in A and 10 μm in B and C.)
To investigate the role of these CAMSAPs in neuron development, we generated a line of Camsap2 mutant mice by a gene knockout method (SI Appendix, Fig. S2 A–C). This mouse line is conventionally called Camsap2−/−, as CAMSAP2 proteins are not detectable. These mice were born without gross developmental defects, although they showed growth retardation (SI Appendix, Fig. S2D). For the analysis of Camsap3 mutants, we used Camsap3dc/dc mice that express a C terminus-truncated CAMSAP3 protein that is unable to bind microtubule minus-ends (24). In addition, we obtained another gene-targeted Camsap3 mutant line, designated Camsap3tm1a(EUCOMM)Wtsi, the homozygous mice of which (conventionally called Camsap3−/−) did not express CAMSAP3 proteins (SI Appendix, Fig. S2E). In the following studies, we used Camsap3dc/dc mutants principally and CAMSAP3−/− mice for confirmatory purposes, as the former were more thoroughly characterized. Brains of Camsap2−/− and Camsap3dc/dc mice were smaller than control brains, but their gross structures, including those of hippocampus, did not appear defective (SI Appendix, Fig. S1F).
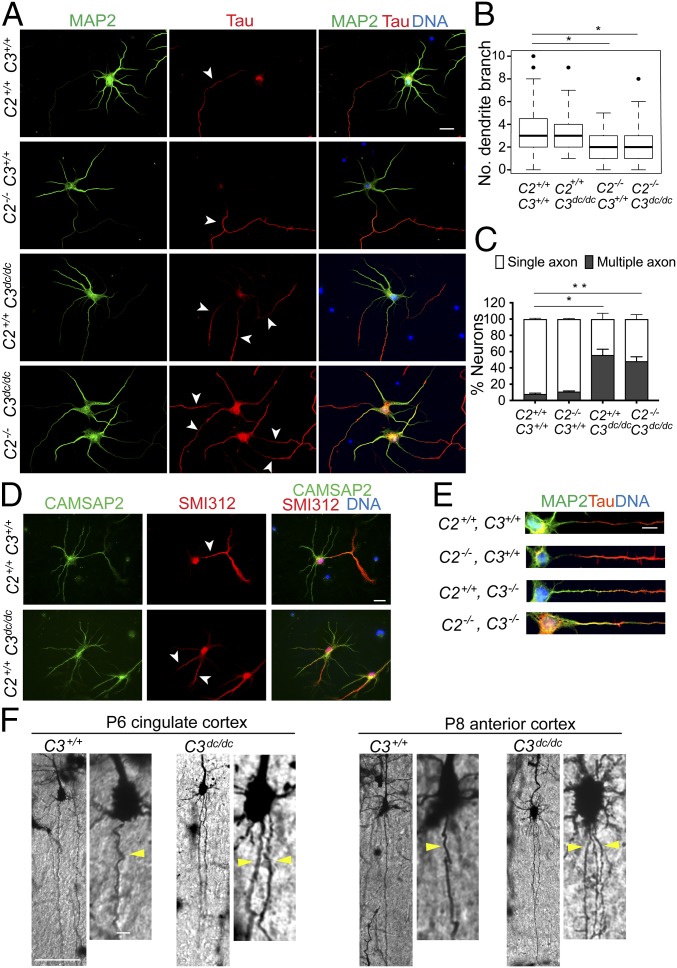
We collected hippocampal neurons from the mutant mice and cultured them in vitro. CAMSAP2−/− neurons developed a single axon and multiple dendrites, although the branching of their dendrites tended to be diminished (Fig. 2 A–C), consistent with a previous report (27). In contrast, CAMSAP3dc/dc neurons showed polarity defects: Around half of them generated multiple axons, ranging from two to four, as assessed by immunostaining for axon markers (Fig. 2 A and C). Neurons derived from Camsap3−/− mice also showed enhanced multiaxon formation (SI Appendix, Fig. S3A). We also depleted (knocked down, KD) CAMSAP3 in wild-type neurons, using specific siRNAs (SI Appendix, Fig. S3B), finding similar defects in neurite differentiation (SI Appendix, Fig. S3C). Thus, various types of CAMSAP3 deficiency equally induced multiple axon formation. Immunostaining of CAMSAP3dc/dc neurons for the mutated CAMSAP3 protein detected diffuse signals along neurites, which were never enriched in axons (SI Appendix, Fig. S3D), indicating that CAMSAP3 binding to microtubule minus-ends is required for it to concentrate in axons.
Fig. 2.
Effects of Camsap2 (C2) or Camsap3 (C3) mutations on neurite extension and patterning. (A) Neurons derived from wild-type (C2+/+, C3+/+), C2−/− (C2−/−, C3+/+), C3dc/dc (C2+/+, C3dc/dc), and double-mutated (C2−/−, C3dc/dc) hippocampi were triple-stained for MAP2 (green), Tau (red), and DNA (blue) at DIV6. Arrowheads point to examples of Tau-positive neurites. (B and C) Number of secondary branching in the dendrites (B), and the ratio of neurons with single to multiple axons (C). Approximately 500 neurons at DIV6 were analyzed in each measurement. *P < 0.05 and **P < 0.005 versus the wild-type group. (D) Detection of CAMSAP2 in wild-type and C3dc/dc neurons at DIV6. Neurons were triple-stained for CAMSAP2 (green), SMI312 (red) and DNA (blue). Arrows point to examples of SMI312-positive neurites. (E) Comparisons of MAP2 (green) and Tau (red) distribution at the proximal region of axons. Representative samples are shown. (F) Cortical neurons in wild-type and C3dc/dc mice at P6 or P8, stained by the Golgi method. Singly labeled neurons located in the two indicated regions of the cortex are shown. A portion of each image was enlarged. Yellow arrowheads indicate the axons extending from the soma. The ventricular side of the cortex is oriented toward the bottom. Representative examples of images obtained from five independent experiments are shown. [Scale bars, 20 μm in A and D; 5 μm in E; 100 μm in F (10 μm in the enlarged images).]
Because CAMSAP2 is expressed in Camsap3 mutants, we tested whether CAMSAP2 plays any active role in multiple axon formation. To do this, we obtained double mutants of Camsap2 and Camsap3 by crossing Camsap2−/− and Camsap3dc/dc mice. Neurons derived from double mutants again showed multiple axon phenotypes (Fig. 2 A and C). In addition, the pattern of CAMSAP2 distribution did not particularly differ between wild-type and Camsap3dc/dc neurons (Fig. 2D). These observations suggest that CAMSAP2 was not required for multiple axon formation. Comparisons of axon and dendrite markers (Tau and MAP2, respectively) between control and Camsap3-mutated neurons indicated that MAP2 tended to spread into Tau-positive neurites more deeply in the mutants than in control patients (Fig. 2E). This suggests that the excessively formed axons might have acquired a hybrid axon/dendrite nature, unlike authentic axons. When Camsap3dc/dc neurons were cultured up to maturation, synaptic proteins came to accumulate along neurites, suggesting that axon and dendrite differentiation took place normally in these neurons, despite the excess number of putative axons (SI Appendix, Fig. S3E).
We also observed neuron morphology in vivo, using sections of postnatal brains stained by the Golgi method, which sporadically labels neurons and their processes. This method allowed us to correctly trace the axons extending from the soma only if labeled neurons were sparsely distributed without overlapping with others. Although such neurons were rare in our specimens, we observed some isolated pyramidal neurons at the cerebral cortex. Pyramidal neurons are known to extend a single axon straight toward the ventricular side. In Camsap3dc/dc brains, we found that at least a few percent of pyramidal neurons produced multiple axon-like processes, and that such neurons were undetectable in wild-type cortices (Fig. 2F). We did not examine neurons in the hippocampus, as they were too densely labeled by the Golgi method.
CAMSAP3 Is Involved in Control of Tubulin Acetylation.
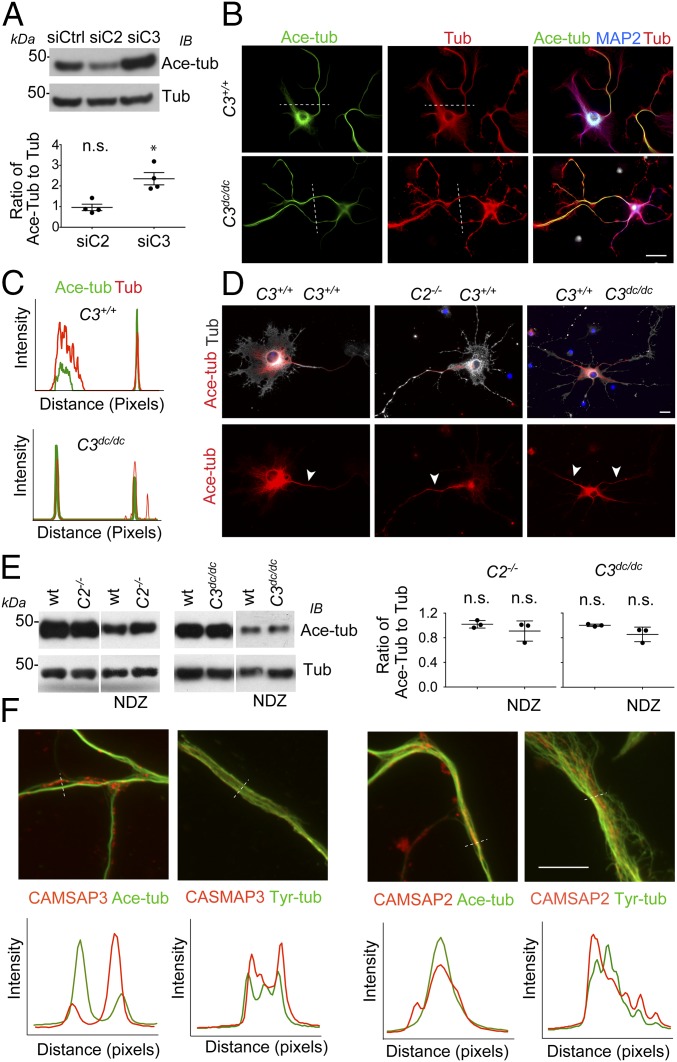
We then investigated how CAMSAP3 maintains single axons and why its mutations lead to multiple axon formation. Previous studies showed that stabilization of microtubules is sufficient for driving a neurite to differentiate into an axon (6), and tubulin acetylation is a hallmark of stable microtubules (7). Therefore, we examined whether CAMSAP loss affected microtubule dynamics by observing tubulin acetylation. In the initial experiments, we used the Neuro-2a (N2a) neuroblastoma line (32) as a neuronal model, which allowed us to conduct efficient preliminary analysis. N2a cells expressed both CAMSAP2 and CAMSAP3, exhibiting their scattered distribution in the cytoplasm with the highest concentration at perinuclear zones (SI Appendix, Fig. S4A). When these CAMSAPs were knocked down using siRNAs (SI Appendix, Fig. S3B), acetylated tubulin was increased after CAMSAP3 KD, but it did not change or was slightly decreased in CAMSAP2 KD cells (Fig. 3A). Morphologically, N2a cells exhibited short filopodium-like processes at the peripheries, and this shape of cells was not affected by CAMSAP2 KD. In contrast, ∼30% of CAMSAP3 KD cells produced long neurite-like processes, the number of which varied from cell to cell (SI Appendix, Fig. S4B), as observed in N2a cells whose differentiation was induced by treatment with pharmacological reagents such as retinoic acid (33). Acetylated tubulin was detected from the cytoplasm in control or CAMSAP2 KD cells, but its distribution extended to the elongated processes in CAMSAP3 KD cells (SI Appendix, Fig. S4B). To check whether acetylated microtubules are stable, we treated control and CAMSAP3 KD cells with nocodazole, a reagent to promote microtubule depolymerization. In the treated cells, although α-tubulin became to diffuse losing its meshwork architecture, acetylated microtubules remained in the cytoplasm, as well as in the neurite-like processes of CAMSAP3 KD cells (SI Appendix, Fig. S4C). Taken together, these observations suggest that CAMSAP3 loss led to an increase of acetylated, stable microtubules, simultaneously promoting neurite-like process formation in N2a cells.
Fig. 3.
Tubulin acetylation in Camsaps-mutated cells. (A) Western blot analysis of acetylated tubulin in N2a cells treated with siRNA specific for CAMSAP3 (siC3) or CAMSAP2 (siC2), or with control siRNA (siCtrl). The ratio of acetylated tubulin (Ace-tub) to total α-tubulin (Tub) was measured by Fiji software, using four independent samples, and the values were normalized by setting the ratio in siCtrl samples to “one.” *P < 0.05 versus siCtrl. n.s., not significant. (B and C) Immunostaining for acetylated tubulin (green), MAP2 (blue), and α-tubulin (red) in neurons at DIV5, derived from wild-type and C3dc/dc hippocampi. Immunostaining signals were scanned along the dotted lines in C. (D) Immunostaining for acetylated tubulin (red), α-tubulin (white), and DNA (blue) in neurons at DIV6, derived from wild-type, C2−/−, and C3dc/dc hippocampi. Cells were treated with 10 μM nocodazole for 1 h at 37 °C before fixation. Arrowheads indicate examples of elongated processes. A representative image from more than 50 neurons obtained from three independent experiments is shown. (E) Western blot analysis of acetylated tubulin in neurons at DIV6, derived from wild-type (wt) and C2−/− or C3dc/dc hippocampi. Some cultures were treated with 10 μM nocodazole (NDZ) for 1 h at 37 °C before sample preparation. The ratio of acetylated tubulin (Ace-tub) to total α-tubulin (Tub) was measured by Fiji software, using three independent samples, and the values were normalized by setting the ratio in wt samples to “one.” *P < 0.05 versus wt. n.s., not significant. (F) Localization of CAMSAP2 or CAMSAP3 (red), in relation to acetylated (Ace-tub) or tyrosinated (Tyr-tub) tubulins (green) in wild-type neurons at DIV4. Immunostaining signals were scanned along the dotted lines. (Scale bars, 20 μm in B and D; 10 μm in E.)
Next, we observed tubulin acetylation in hippocampal neurons. Immunostaining showed that, in wild-type neurons, acetylation was broadly detected in various regions of the neuron. Nevertheless, the ratio of acetylated tubulin to the total tubulin tended to be higher in axons compared with dendrites (Fig. 3B, Upper and C), consistent with previous reports (6, 34). In Camsap3dc/dc neurons, this relative increase of acetylated tubulins came to be observed in multiple neurites (Fig. 3B, Lower and C). When these neurons were treated with nocodazole, nocodazole-resistant, acetylated microtubules were detectable in various regions of a neuron, regardless of whether CAMSAPs were present or not. However, concerning the thin and long processes extending from the cell body, which are putative axons, only single processes held nocodazole-resistant microtubules in most of CAMSAP2 knockout or wild-type neurons, whereas multiple processes had them in CAMSAP3-mutated neurons (Fig. 3D). These observations support the idea that CAMSAP3 dysfunction leads to an increase of neurites with stable microtubules also in neurons.
Unlike in N2a cells, however, in hippocampal neurons, the total level of acetylated tubulin did not particularly change in Camsap2 or Camsap3 mutants, as assessed by Western blotting (Fig. 3E). This was also the case for cells treated with nocodazole. Immunostaining for acetylated tubulin also did not suggest apparent differences in its overall level between these samples (Fig. 3 B and D). To investigate whether CAMSAP3 mutations could affect tubulin dynamics in hippocampal neurons, we closely observed the distribution of CAMSAPs in relation to that of acetylated and tyrosinated microtubules, which represent stable and dynamic microtubules, respectively, in axons. Microtubules are densely packed in axon shafts, preventing us from resolving individual microtubules; therefore, we purposely observed specific portions of axons, where microtubules happened to be untied. Our observations showed a tendency for CAMSAP3 puncta to localize along a subpopulation of microtubules that are less acetylated, although this protein well coincided with tyrosinated microtubules (Fig. 3F, Left). In contrast, the distribution of CAMSAP2 in relation to microtubule posttranscriptional modification was not so clear-cut as that of CAMSAP3. Unlike CAMSAP3, CAMSAP2 did not avoid acetylated microtubules (Fig. 3F, Right). Given that CAMSAP3 is localized to restricted zones in neurons (Fig. 1C), these observations suggest that, in neurons, CAMSAP3 may affect tubulin acetylation only for a small fraction of microtubules and at levels not detectable using whole-cell lysates.
CAMSAP3 Actively Suppresses Tubulin Acetylation.
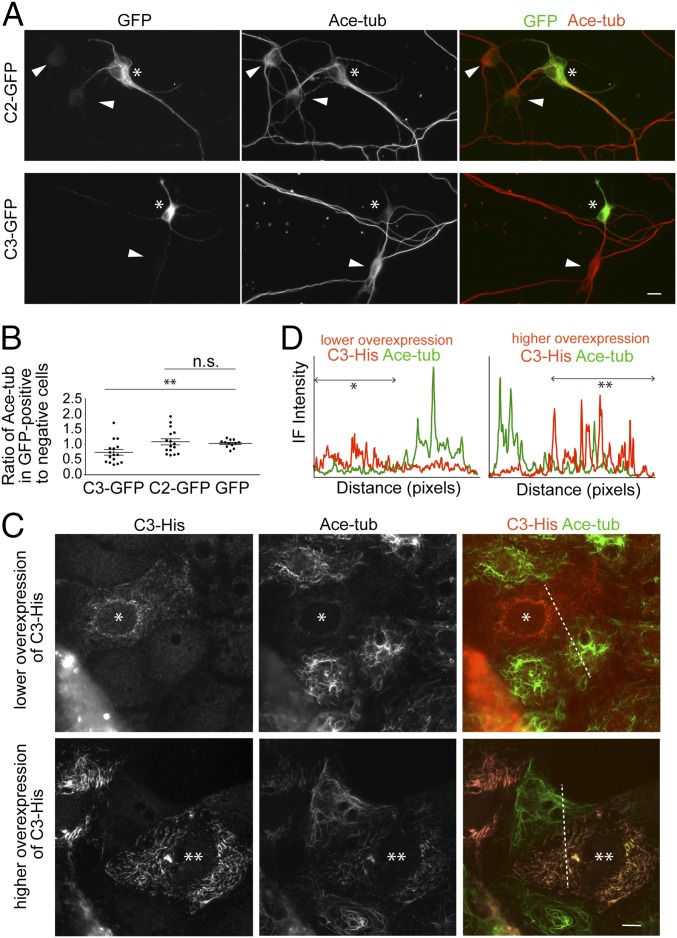
To confirm whether CAMSAP3 is capable of affecting tubulin acetylation in hippocampal neurons, we transiently overexpressed GFP-tagged CAMSAP2 (C2-GFP) or CAMSAP3 (C3-GFP) in them, and observed tubulin acetylation at their cell bodies, as acetylation levels considerably fluctuated between neurites even in untransfected neurons. Our repeated observations showed that tubulin acetylation levels tended to be lower in neurons expressing C3-GFP, whereas this tendency was not observed in those expressing C2-GFP (Fig. 4 A and B). These findings support the idea that CAMSAP3 works to suppress tubulin acetylation in axons as well.
Fig. 4.
Effects of CAMSAPs overexpression on tubulin acetylation. (A and B) Neurons were transfected with GFP-tagged CAMSAP2 (C2-GFP), CAMSAP3 (C2-GFP), or GFP alone at DIV1, and fixed at DIV5, followed by double-immunostaining for GFP and acetylated tubulin (A). Asterisk indicates a neuron expressing C2-GFP or C3-GFP. For comparison, nontransfected cells are also indicated (arrowheads). The graph in B shows the ratio of immunofluorescence (IF) signals for acetylated tubulin in cells expressing C3-GFP, C2-GFP, or GFP alone to those in cells not expressing the constructs, which are present in the same cultures. For this quantification, average IF intensity for acetylated tubulin was measured at the cytoplasmic region of neuronal cell body, using 16 (for C3-GFP and C2-GFP) and 12 (for GFP) cultures obtained from three independent experiments. **P < 0.005 versus GFP alone. n.s. is not significant. (C and D) Caco2 cells were transfected with C3-His and then double-immunostained for His tag and acetylated tubulin (C). Single asterisk indicates a cell expressing a lower level of His-C3, whereas double asterisks indicate a cell expressing a higher level of His-C3. IF signals were scanned along the dotted lines, as shown in D. Lower His-C3 expression eliminated acetylated microtubules, whereas higher His-C3 expression rearranged their assembly. A representative image from more than 60 transfected cells obtained from three independent experiments is shown. (Scale bars, 10 μm.)
For more detailed analysis of CAMSAP3-dependent control of tubulin acetylation, we used Caco2 cells in monolayer cultures, which allowed us to observe individual CAMSAP3 puncta and microtubules at high resolution (23). Double-immunostaining for CAMSAP3 and acetylated tubulins showed that the majority of CAMSAP3 puncta were free from acetylated microtubules, and acetylated microtubules were generally not associated with CAMSAP3 puncta, although a small population of CAMSAP3 puncta attached to an end of these microtubules (SI Appendix, Fig. S4D). We then overexpressed His-tagged CAMSAP3 (C3-His) in Caco2 cells. The results showed that a moderate level of C3-His expression completely eliminated acetylated microtubules (Fig. 4 C and D). In contrast, when ectopic C3-His expression was further increased, this protein began to show streak-like distributions along microtubules, probably because of their excess binding to the microtubule lattice; and these microtubules were acetylated again. These observations confirmed that unless CAMSAP3 is overly expressed, CAMSAP3-bound microtubules are prevented from acetylation.
α-Tubulin Acetyltransferase-1 Is Required for Supernumerary Axon Formation.
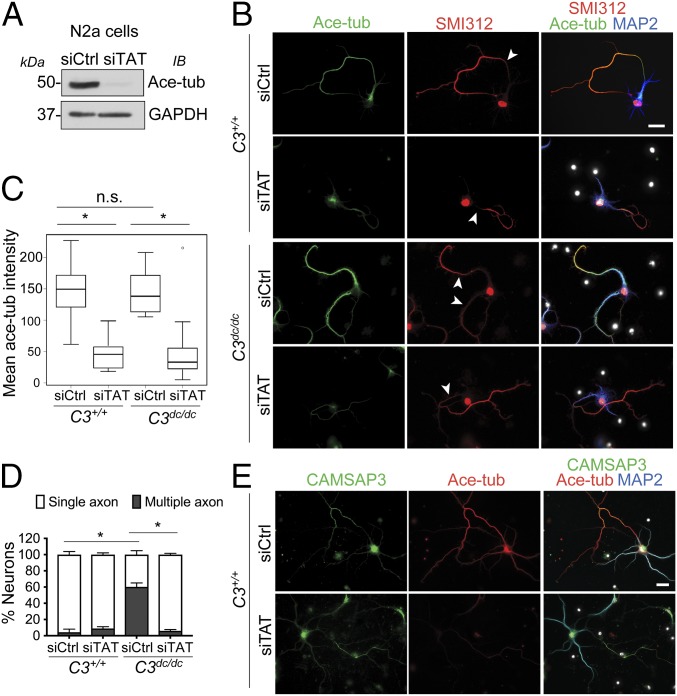
Next, we examined whether tubulin acetylation itself had any influence on axon behavior. Acetylation of tubulins is mediated with α-tubulin acetyltransferase 1 (αTAT1/MEC-17) (10–12). We confirmed that tubulin acetylation was strongly inhibited by treating N2a cells with αTAT1-specific siRNAs (Fig. 5A), and conversely it was dramatically increased by overexpressing this enzyme in these cells (SI Appendix, Fig. S5A). CAMSAP3 loss-dependent up-regulation of tubulin acetylation was also abolished by depleting αTAT1 in these cells (SI Appendix, Fig. S5B). To confirm whether this αTAT1 siRNA-dependent elimination of tubulin acetylation depended on the catalytic activity of the enzyme, we tested whether the catalytic domain-mutated αTAT1 (D157N) (11) could rescue this elimination. The results showed that only wild-type αTAT1 restored tubulin acetylation in CAMSAP3/αTAT1 codepleted cells (SI Appendix, Fig. S5B).
Fig. 5.
Requirement of α-tubulin acetyltransferase-1 for supernumerary axon formation. (A) Knockdown efficiency of αTAT1-specific siRNA (siTAT), as assessed by suppression of tubulin acetylation. (B–D) Neurons derived from wild-type and C3dc/dc brains were transfected with siTAT or siCtrl at DIV1, and then immunostained for acetylated tubulin (green), SMI312 (red), and DNA (blue) at DIV6 (B). Intensity of the stains for acetylated tubulin per neuron (C), and the ratio of neurons with single to multiple axons (D) was measured. *P < 0.005 versus the control group. At least 100 neurons were analyzed for each quantification. (E) Expression of CAMSAP3 in siTAT-treated neurons. Wild-type and C3dc/dc neurons were transfected with siTAT or siCtrl at DIV1, and then immunostained for CAMSAP3 (green), acetylated tubulin (red), and MAP2 (blue) at DIV6. (Scale bars, 20 μm.)
αTAT1 depletion also reduced tubulin acetylation in neurons, although axons still elongated, as assessed by immunostaining for axon markers (Fig. 5 B and C), confirming that axonal tubulin acetylation is αTAT1-dependent. Camsap3dc/dc neurons similarly responded to αTAT1 depletion, and importantly, in these αTAT1-depleted neurons, supernumerary axon formation was abolished (Fig. 5 B and D). These results suggest that αTAT1 is required to maintain the multiple axons generated by CAMSAP3 mutation. Next, we tested whether excess tubulin acetylation could induce multiple axon formation by overexpressing GFP-tagged αTAT1 or its D157N mutant in wild-type neurons, and found that the expression of either molecule did not particularly increase axon number (SI Appendix, Fig. S5C). Overexpression of αTAT1 in N2a cells also did not affect their process formation, even though tubulin acetylation level was greatly elevated (SI Appendix, Fig. S5D). These results suggest that increase of tubulin acetyltransferase alone is not sufficient for disrupting neuronal polarity or for promoting neurite extension. We also found that CAMSAP3 remained in axons when αTAT1 was knocked down (Fig. 5E), indicating that axonal accumulation of CAMSAP3 occurs independent of tubulin acetylation.
Discussion
Our findings demonstrate that CAMSAP3 is required to maintain single axons in hippocampal neurons in culture. We found CAMSAP3 to be enriched in axons, localizing along a subpopulation of microtubules that are less acetylated. Loss of CAMSAP3 resulted in up-regulation of acetylated tubulins in N2a cells, as found with other cells (23). Conversely, moderate overexpression of CAMSAP3 actively reduced tubulin acetylation in both epithelial and neuronal cells. Given that tubulin acetylation is known to occur in long-lived microtubules, our observations suggest that CAMSAP3-anchored microtubules are not stable when CAMSAP3 density is properly high, thereby escaping from αTAT1-mediated acetylation. CAMSAP3 thus seems to serve the purpose of retaining a pool of dynamic microtubules in axons, although their microtubules are generally acetylated. We suspect that CAMSAP3 removal abolished this system, causing a reduction in dynamic microtubules, and the resultant local increase of stable microtubules induced supernumerary axon formation, as previous studies demonstrated that taxol-induced stabilization of microtubules resulted in multiple axon generation (6) or extra neurite formation (35). We also found that CAMSAP3 depletion in N2a cells induced neurite-like process formation. Although the identity of these processes remains unknown, this observation suggests that CAMSAP3 may have a general ability to control neurite formation, loss of which leads to unregulated growth of neurites, and this might have occurred in axons in the case of hippocampal neurons.
Our results also demonstrate that αTAT1 is required to maintain multiple axons in CAMSAP3-deficient neurons. This finding suggests that although enhanced tubulin acetylation might have primarily occurred as a result of microtubule stabilization induced by CAMSAP3 removal, tubulin acetylation per se has a role in extra axon formation. It has been shown that tubulin acetylation alters various mechanical properties of microtubules (13–16), and it keeps microtubules consolidated in axons (17). Such effects of acetylation on microtubule properties could contribute to neurite regulation. In contrast, overexpression of αTAT1 did not induce supernumerary axons or neurite elongation, suggesting that simple enhancement of αTAT1 activity is not sufficient to bring about these events. Stabilization of microtubules might be a prerequisite for αTAT1 to contribute to the disturbance of neurite genesis.
How, then, does CAMSAP3-dependent regulation of microtubule dynamics retain single axons in normal neurons? CAMSAP3 might merely serve to protect microtubules against overstabilization that would lead to supernumerary axon formation. However, it is equally possible that CAMSAP3 could have other active roles. It has been hypothesized that single axons are maintained by feedback loops between signals that are responsible for axon and dendrite differentiation (3, 36, 37). A recent study showed that a Ca2+ wave derived from the axon terminal suppresses the growth of other neurites (minor neurites) through activation of the CaMKI/GEF-HI/Rho kinase signaling pathway at the cell body region (38). CAMSAP3-bound dynamic microtubules might play a role in the transfer of such axonal signals to the cell body, loss of which leads to uncontrolled growth or differentiation of minor neurites.
Elucidating how CAMSAP3, as a minus-end stabilizer, regulates microtubule dynamics remains an important future subject. It is noteworthy that CAMSAP2 loss did not enhance microtubule acetylation, which suggests that CAMSAP2 and CAMSAP3 differently regulate microtubule dynamics. In fact, some differences in their ability to stabilize the microtubule minus-ends have been suggested by previous in vitro analysis (22). Furthermore, unlike in the case of CAMSAP3 mutants, polarity defects were not observed in Camsap2-knockout neurons, supporting the notion that CAMSAP2 and CAMSAP3 differ in biological functions. A previous study, in contrast, reported polarity defects in CAMSAP2-deficient neurons (27). In this study, RNAi was used to suppress CAMSAP2 expression, and such methodological differences between the studies might have resulted in nonidentical conclusions. Despite this discrepancy, both studies showed reduced branching of dendrites in CAMSAP2-deficient neurons, suggesting that this CAMSAP subtype plays a role in neurite growth. In C. elegans neurons, PTRN-1 works to stabilize microtubules (30), and its mutations impair axon regeneration (28), suggesting that PTRN-1 might be similar to CAMSAP2 rather than CAMSAP3 in function. In mammalian epithelial cells, CAMSAP2 and CAMSAP3 show overlapping distributions and functions, and their removal similarly up-regulated tubulin acetylation (23). These observations suggest that CAMSAP subtypes change their roles in a cell type-dependent manner. To explore the molecular basis of how differently CAMSAP2 and CAMSAP3 regulate microtubule dynamics is an important future subject. It also remains to be investigated how the CAMSAP subtypes localize in axons and dendrites in distinct patterns.
The supernumerary axon formation phenotypes were observed in vivo, but at lower frequencies than in vitro, suggesting that the phenotypes might have been enhanced by in vitro environments. As αTAT1 activity has been shown to be promoted under stress conditions (39), this enzyme might have responded to in vitro environments more sensitively than in vivo, resulting in more profound phenotypes of CAMSAP3 mutants in vitro. Given that developmental phenotypes were less prominent, it is possible that the CAMSAP3/αTAT-dependent mechanisms uncovered by the present study are more important for homeostasis of neuronal polarity rather than for development, as suggested in the case of PTRN-1 (28). Exploring the potential involvement of CAMSAP3 mutation in brain diseases is therefore another intriguing subject for future research.
Materials and Methods
Generation of CAMSAP knockout mice are described in SI Appendix, Materials and Methods. Cell cultures, RNA interference, and cDNA transfection are described in SI Appendix, Materials and Methods. Immunoblotting, immunocytochemistry, and Golgi staining are described in SI Appendix, Materials and Methods. Image analysis and statistical analysis are also described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Shuichi Hayashi, Nobutoshi Tanaka, and Mika Toya for experimental advice, discussion, and preliminary data; Miwa Kawasaki for technical support; and the RIKEN Kobe light microscopy facility for confocal microscope operation, where imaging experiments were performed. We also thank the Wellcome Trust Sanger Institute Mouse Genetics Project and its funders for providing the mutant mouse line (Allele: Camsap3tm1a(EUCOMM)Wtsi). Funding and associated primary phenotypic information may be found at https://www.sanger.ac.uk/science/collaboration/mouse-resource-portal. This work was supported by the program Grant-in-Aid for Scientific Research (S) (Grant 25221104) from the Japan Society for Promotion of Science (to M.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803875115/-/DCSupplemental.
References
- 1.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schelski M, Bradke F. Neuronal polarization: From spatiotemporal signaling to cytoskeletal dynamics. Mol Cell Neurosci. 2017;84:11–28. doi: 10.1016/j.mcn.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Arimura N, Kaibuchi K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 4.Yogev S, Shen K. Establishing neuronal polarity with environmental and intrinsic mechanisms. Neuron. 2017;96:638–650. doi: 10.1016/j.neuron.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Kapitein LC, Hoogenraad CC. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506. doi: 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 8.Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003;421:230. doi: 10.1038/421230a. [DOI] [PubMed] [Google Scholar]
- 9.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalebic N, et al. αTAT1 is the major α-tubulin acetyltransferase in mice. Nat Commun. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
- 11.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akella JS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cueva JG, Hsin J, Huang KC, Goodman MB. Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules. Curr Biol. 2012;22:1066–1074. doi: 10.1016/j.cub.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalidou I, et al. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol. 2012;22:1057–1065. doi: 10.1016/j.cub.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portran D, Schaedel L, Xu Z, Théry M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol. 2017;19:391–398. doi: 10.1038/ncb3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science. 2017;356:328–332. doi: 10.1126/science.aai8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan Wei, et al. α-Tubulin acetylation restricts axon overbranching by dampening microtubule plus-end dynamics in neurons. Cereb Cortex. 2017:1–15. doi: 10.1093/cercor/bhx225. [DOI] [PubMed] [Google Scholar]
- 18.Stiess M, et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 19.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin SS, Vale RD. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell. 2010;143:263–274. doi: 10.1016/j.cell.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendershott MC, Vale RD. Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proc Natl Acad Sci USA. 2014;111:5860–5865. doi: 10.1073/pnas.1404133111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang K, et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014;28:295–309. doi: 10.1016/j.devcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka N, Meng W, Nagae S, Takeichi M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc Natl Acad Sci USA. 2012;109:20029–20034. doi: 10.1073/pnas.1218017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toya M, et al. CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc Natl Acad Sci USA. 2016;113:332–337. doi: 10.1073/pnas.1520638113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanal I, Elbediwy A, Diaz de la Loza MdelC, Fletcher GC, Thompson BJ. Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia. J Cell Sci. 2016;129:2651–2659. doi: 10.1242/jcs.189076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nashchekin D, Fernandes AR, St Johnston D. Patronin/shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev Cell. 2016;38:61–72. doi: 10.1016/j.devcel.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau KW, et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Chuang M, et al. The microtubule minus-end-binding protein patronin/PTRN-1 is required for axon regeneration in C. elegans. Cell Rep. 2014;9:874–883. doi: 10.1016/j.celrep.2014.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcette JD, Chen JJ, Nonet ML. The Caenorhabditis elegans microtubule minus-end binding homolog PTRN-1 stabilizes synapses and neurites. eLife. 2014;3:e01637. doi: 10.7554/eLife.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson CE, et al. PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. eLife. 2014;3:e01498. doi: 10.7554/eLife.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher TL, Banker GA. The establishment of polarity by hippocampal neurons: The relationship between the stage of a cell’s development in situ and its subsequent development in culture. Dev Biol. 1989;136:446–454. doi: 10.1016/0012-1606(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 32.Graham DI, Gonatas NK, Charalampous FC. The undifferentiated and extended forms of C1300 murine neuroblastoma. An ultrastructural study and detection of concanavalin A binding sites on the plasma membrane. Am J Pathol. 1974;76:285–312. [PMC free article] [PubMed] [Google Scholar]
- 33.Wu G, Fang Y, Lu ZH, Ledeen RW. Induction of axon-like and dendrite-like processes in neuroblastoma cells. J Neurocytol. 1998;27:1–14. doi: 10.1023/a:1006910001869. [DOI] [PubMed] [Google Scholar]
- 34.Cambray-Deakin MA, Burgoyne RD. Posttranslational modifications of alpha-tubulin: Acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol. 1987;104:1569–1574. doi: 10.1083/jcb.104.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirszenblat L, Neumann B, Coakley S, Hilliard MA. A dominant mutation in mec-7/β-tubulin affects axon development and regeneration in Caenorhabditis elegans neurons. Mol Biol Cell. 2013;24:285–296. doi: 10.1091/mbc.E12-06-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inagaki N, Toriyama M, Sakumura Y. Systems biology of symmetry breaking during neuronal polarity formation. Dev Neurobiol. 2011;71:584–593. doi: 10.1002/dneu.20837. [DOI] [PubMed] [Google Scholar]
- 37.Namba T, et al. Extracellular and intracellular signaling for neuronal polarity. Physiol Rev. 2015;95:995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 38.Takano T, et al. Discovery of long-range inhibitory signaling to ensure single axon formation. Nat Commun. 2017;8:33. doi: 10.1038/s41467-017-00044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackeh R, et al. Reactive oxygen species, AMP-activated protein kinase, and the transcription cofactor p300 regulate α-tubulin acetyltransferase-1 (αTAT-1/MEC-17)-dependent microtubule hyperacetylation during cell stress. J Biol Chem. 2014;289:11816–11828. doi: 10.1074/jbc.M113.507400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.