Significance
While clonally propagated individuals should share identical genomes, there is often substantial phenotypic variation among them. Both genetic and epigenetic modifications induced during regeneration have been associated with this phenomenon. Here we investigated the fate of the epigenome after asexual propagation by generating clonal individuals from differentiated somatic cells through the manipulation of a zygotic transcription factor. We found that phenotypic novelty in clonal progeny was linked to epigenetic imprints that reflect the organ used for regeneration. Some of these organ-specific imprints can be maintained during the cloning process and subsequent rounds of meiosis. Our findings are fundamental for understanding the significance of epigenetic variability arising from asexual reproduction and have significant implications for future biotechnological applications.
Keywords: epigenetics, Arabidopsis thaliana, DNA methylation, transgenerational inheritance, asexual reproduction
Abstract
Plants differ from animals in their capability to easily regenerate fertile adult individuals from terminally differentiated cells. This unique developmental plasticity is commonly observed in nature, where many species can reproduce asexually through the ectopic initiation of organogenic or embryogenic developmental programs. While organ-specific epigenetic marks are not passed on during sexual reproduction, the fate of epigenetic marks during asexual reproduction and the implications for clonal progeny remain unclear. Here we report that organ-specific epigenetic imprints in Arabidopsis thaliana can be partially maintained during asexual propagation from somatic cells in which a zygotic program is artificially induced. The altered marks are inherited even over multiple rounds of sexual reproduction, becoming fixed in hybrids and resulting in heritable molecular and physiological phenotypes that depend on the identity of the founder tissue. Consequently, clonal plants display distinct interactions with beneficial and pathogenic microorganisms. Our results demonstrate how novel phenotypic variation in plants can be unlocked through altered inheritance of epigenetic marks upon asexual propagation.
Compared with animals, in plants somatic cells can be much more easily coaxed into regenerating entire individuals (1). A potential reason why differentiated plant cells can rapidly acquire “stemness” is that the epigenome of plants is much more flexible than that of animals. Thus, asexual reproduction is much more common in plants than in animals (2, 3), and this has been traditionally exploited by humans for the clonal propagation and genetic manipulation of many economically important plant species, including grapevines, nearly all tuber and root crops, and fruit and forest trees (4).
Although clonal propagation provides ecological and evolutionary benefits, the resulting restricted genetic variation could be detrimental to fitness. Notably, clonally propagated plants are not always phenotypically identical to their parents, a phenomenon termed somaclonal variation. While somaclonal variation is often attributed to the accumulation of random genetic mutations in form of single-base changes or transposon activation (5, 6), increasing evidence suggests that genetic changes are not solely responsible. For example, genome-wide DNA methylation patterns can be perturbed during clonal propagation through hormone-dependent tissue culture, and these epimutations can be associated with altered expression of protein-coding genes (7–10). In some cases, such tissue-culture induced epimutations can be stably inherited across multiple sexual generations (7, 8, 10) and can be responsible for phenotypes that distinguish clonal descendants from their parents (11). Increased genetic and epigenetic diversity may be deleterious, but also potentially result in advantageous traits. For all these reasons, we would like to better understand the precise origin and mechanistic basis of the molecular and phenotypic changes created during plant regeneration.
Depending on the species, different types of organs, such as roots, stems, and leaves, as well as entire embryos, can be used for clonal propagation. The starting material thus includes tissue with different patterns of gene expression and epigenetic profiles. For example, 1.6% of methylated regions in the Arabidopsis thaliana genome differ in methylation status between shoots and roots, and more than 2,000 genes differ in expression levels (12).
An attractive hypothesis is that complete or partial maintenance of variant epigenetic landscapes present in the starting material will contribute to phenotypic variation among regenerants. We have tested this hypothesis by regenerating A. thaliana plants from different postembryonic organs. Frequent limitations of plant regeneration are the lengthy periods of tissue culture and the hormone mixtures that need to be optimized for each tissue, which confounds the interpretation of phenotypic differences in regenerated plants. To circumvent this problem, we have exploited the fact that somatic embryogenesis can be induced in different tissues of A. thaliana by ectopic expression of certain zygotic factors (2). Here we used transgenic lines carrying an inducible version of the RWP-RK DOMAIN-CONTAINING 4 (RKD4) transcription factor gene (13). We provide evidence that certain organ-specific epigenetic differences are partially maintained during regeneration of A. thaliana. Whole plants derived from roots inherit many aspects of root-specific methylation and gene expression patterns not just in roots, but also in leaves. These epigenetic profiles and the resulting macroscopic and molecular phenotypes are stably transmitted during meiosis for at least four self-crossing generations. Our findings demonstrate that plants with novel methylation and gene expression patterns, as well as physiological phenotypes, can be created using specific regeneration strategies.
Results
Tissue Origin of Regenerants Affects Activity of Defense-Related Genes.
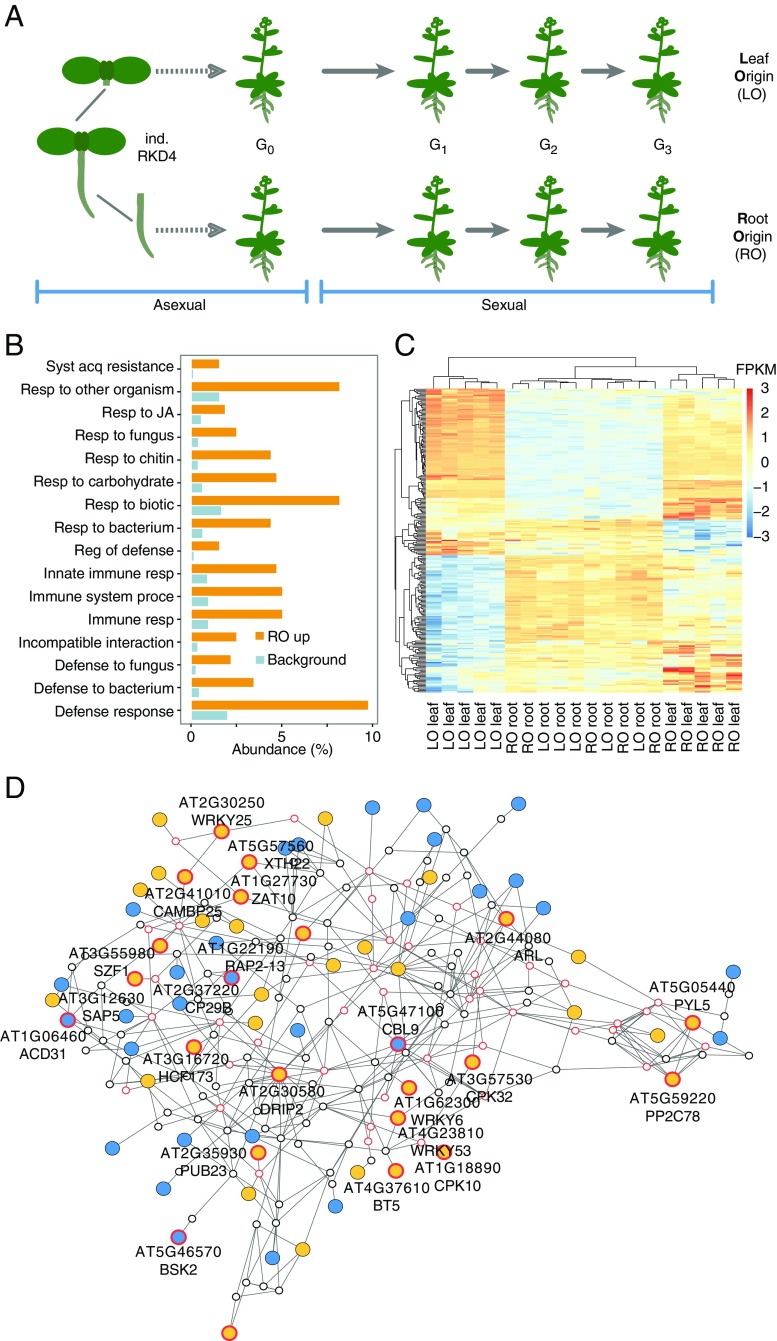
Plants can reproduce asexually from both belowground and aboveground organs, which are known to be epigenetically distinct (12, 14). We took advantage of this situation to determine the extent to which the organ-specific origin of the epigenome could influence phenotypes of clonal progeny. To mimic naturally occurring events associated with asexual propagation (2, 15), we did not resort to hormone-induced regeneration in tissue culture, but instead produced somatic embryos from distinct root (root origin; RO) and leaf (leaf origin; LO) tissues of A. thaliana (Col-0 strain) by controlled expression of the RKD4 zygotic factor (13) (SI Appendix, Fig. S1). We collected seeds from independently regenerated G0 individuals after self-pollination and further propagated each line by selfing for over three consecutive generations (G1–G3) (Fig. 1A). Visual examination revealed no obvious morphological differences between RO and LO plants.
Fig. 1.
Differential expression of defense-related genes in RO and LO plants. (A) Experimental design. RO and LO regenerated plants (n = 5 independent lines each) were propagated through self-fertilization over three generations. (B) GO analysis of DEGs (FDR <0.01; absolute log-twofold change >1.5) between leaves of RO and LO G2 plants revealing enrichment for defense-related functions. (C) Heatmap of scaled, normalized log-transformed read counts for genes underlying the significant enrichment of GO terms in B. FPKM, fragments per kilobase per million. (D) Interaction gene network of DEGs. To facilitate network analysis, we lowered the FDR threshold to <0.05. Of the resulting 4,585 DEGs, 2,752 were represented in the ANAP protein interaction database. Nodes represent genes; triangles highlight defense-related genes from B; edges indicate evidence for gene interaction. Blue filled circles, high expression in LO plants; orange filled circles, high expression in RO plants; red outlined circles, genes with stress-related GO terms.
To determine any potential differences at the molecular level, we performed whole-genome transcriptome analyses in five randomly selected G2 lines. Using stringent thresholds [false discovery rate (FDR) <0.01; absolute log-twofold change >1.5], only 13 differentially expressed genes (DEGs) differentiated roots of RO plants from roots of LO plants, but almost 20-fold more DEGs (n = 239) were identified between leaves of RO and LO plants (SI Appendix, Fig. S2 and Dataset S1). Gene ontology (GO) analysis revealed that these DEGs were enriched for stress- and defense-related genes (FDR <0.05) (Fig. 1B). The 51 genes in these two categories were mostly up-regulated in leaves of RO plants and primarily involved in cellular signaling (27%) and transcriptional regulation (22%). Clustering of all samples based on DEG expression levels suggested that leaves of RO plants had partial root characteristics, sharing apparent similarity with roots from both RO and LO plants (Fig. 1C); in contrast, leaves of LO plants formed a single distinct group (Fig. 1C). To gain further insight into this transcriptional variation, we performed a network analysis of DEGs that distinguished leaves of RO and LO plants, based on a less stringent threshold (FDR <0.05) (16). This network contained the 239 stringent DEGs (SI Appendix, Fig. S2 and Dataset S1), of which 213 had known interactions with other DEGs and 69 were part of a single functional network (Fig. 1D). This network included five genes (WRKY6, SZF1, PYL5, PUB23, and DRIP2) that had been previously implicated in the negative regulation of abiotic and biotic stress responses (17–21).
Tissue of Origin of Regenerants Affects Interaction with Microbes.
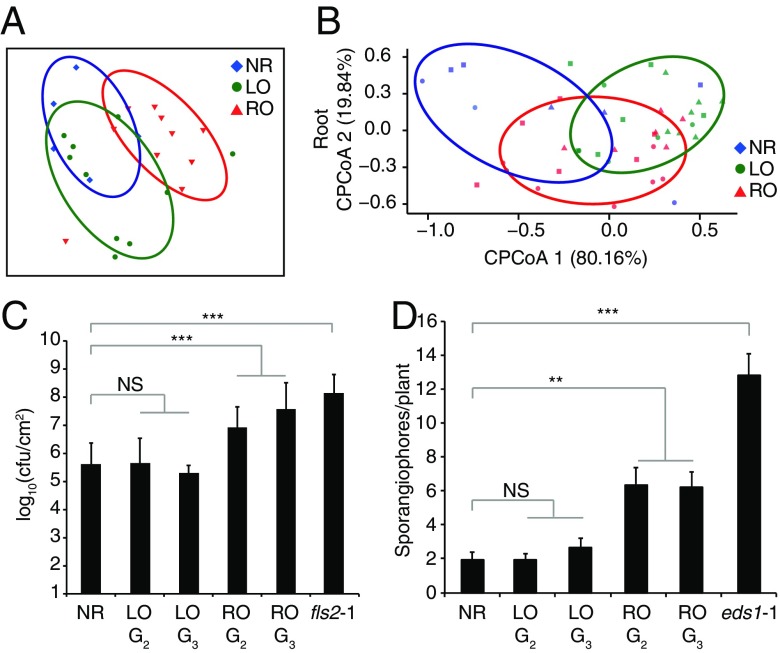
We next sought to define whether the gene expression differences between RO and LO plants were functionally meaningful and affected whole-plant or tissue-specific functions. Plant microbiota are known to be affected by developmental factors as well as immune system activity (22, 23), and both the altered expression of tissue-specific genes in RO plants as well as the enriched GO categories suggested that it would be relevant to test for a change in the interaction with the biotic environment. We grew plants in natural soils and after 4 weeks assessed the bacterial communities that became associated with their roots. Bacterial communities from roots of RO plants differed from those of both nonregenerated plants (ANOSIM, r = 0.485, P = 0.007) and LO plants (ANOSIM, r = 0.216, P = 0.056) (Fig. 2A and SI Appendix, Fig. S3). In addition, in vitro root colonization by Bacillus amyloliquefaciens FZB42, a soil-borne plant growth-promoting bacterium (24), differed significantly between RO and nonregenerated plants (Wilcoxon rank-sum test, W = 3862.5, P = 5.9e-05) (SI Appendix, Fig. S4). Finally, we inoculated roots of regenerated and nonregenerated plants with synthetic communities (SynComs) consisting of abundant soil- and root-derived bacterial isolates (25). Again, the root bacterial communities differed between regenerated and nonregenerated individuals (7.3% variance explained by genotype; permutation-based ANOVA, P = 0.07) (SI Appendix, Fig. S5); this was especially obvious for the Alcaligenaceae family (Betaproteobacteria) (Fig. 2B and SI Appendix, Fig. S5). Similarly, when analyzing leaves of SynCom-inoculated plants (9.6% variance explained by genotype; P = 0.023), the most notable differences were observed for Xanthomonadaceae (Gammaproteobacteria) (ANOVA, P < 0.05) (SI Appendix, Figs. S5 and S6), which include several phytopathogenic strains.
Fig. 2.
Plants regenerated from roots or leaves interact differently with beneficial and pathogenic microbes. (A) PCA of Bray–Curtis distances of bacterial communities present in roots of nonregenerated and regenerated plants grown in natural soils (n = 10). (B) Canonical analysis of principal coordinates (based on Bray–Curtis distances) showing different root-associated communities of SynComs colonized on nonregenerated and regenerated plants (n = 12). (C) Susceptibility of nonregenerated and regenerated plants to P. syringae pv. tomato strain DC3000 infection. Bacterial growth was determined at 3 d after inoculation (100 cfu mL−1). Data are mean ± SD values from three independent experiments. Statistical significance according to Fisher’s exact test: **P < 0.01, ***P < 0.001; NS, not significant. (D) Susceptibility of nonregenerated and regenerated plants to H. arabidopsidis (Hpa) Noks1 infection, as indicated by the number of conidiospores on leaves at 3 d after inoculation with a suspension of 300,000 spores/mL. Data are mean ± SD values from two independent experiments. P values were determined using Student’s t test.
These differences in microbiota led us to further assess the response to known pathogens. For this, we inoculated leaves with the bacterium Pseudomonas syringae pv. tomato strain DC3000 and the oomycete Hyaloperonospora arabidopsidis isolate Noks1, as the A. thaliana Col-0 strain lacks gene-for-gene resistance to both of these pathogens (26, 27). We found that RO plants were more sensitive than LO plants to infection by either pathogen, and that these differences were stably inherited for at least three generations (Fig. 2 C and D). This result was in line with our previous observation of the up-regulation of negative regulators of biotic stress in RO plants (Fig. 1D).
Heritable Differences in Genome-Wide DNA Methylation in Regenerants.
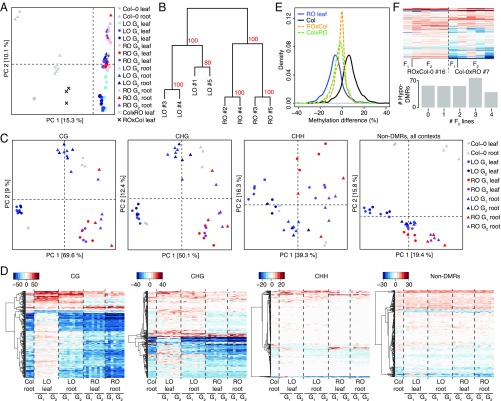
Given the unexpected widespread and heritable differences between transcriptional profiles and microbe responses in RO and LO plants, which should have very few, if any, group-wise genetic differences, we investigated the potential epigenomic basis for phenotypic differentiation between RO and LO plants. DNA methylation is an important epigenetic mark, for which excellent statistical methods for genome-wide comparisons are available (28). Moreover, dynamic changes in DNA methylation during sexual reproduction have been well documented (29). We used whole-genome bisulfite sequencing of leaves and roots from RO and LO individuals to monitor methylome changes over three consecutive generations (Dataset S2). Principal component analysis (PCA) of 736,413 differentially methylated positions (DMPs) discovered in pairwise contrasts revealed clear differences between root and leaf samples from regenerated and nonregenerated plants (PC1; Fig. 3A). Regenerated samples clustered according to their tissue of origin before regeneration and not, as might be expected, by tissue identity at the time of DNA extraction (PC2; Fig. 3A). When repeating PCA with the methylation level of positions not classified as DMPs, we found little residual variance, indicating a low number of false negatives (SI Appendix, Fig. S7).
Fig. 3.
DNA methylation variation in regenerated plants is stably inherited after selfing and back-crossing. (A) PCA of DNA methylation levels at DMPs identified from pairwise sample comparisons. Numbers in brackets indicate the fraction of overall variance explained by the respective PC. (B) Clustering of LO and RO leaf samples in generation G2, based on 765 DMRs identified in all-against-all pairwise comparisons. (C) PCA of methylation at 255 DMRs identified in G2 RO vs. LO leaf comparison, divided by cytosine sequence context. (Right) PCA on methylation in all contexts within randomly chosen non-DMRs. Numbers in brackets indicate the amount of variance explained by the respective PC. (D) Gains and losses of DNA methylation in DMRs identified between RO and LO leaves in the G2 generation. Color keys indicate methylation rate differences in relation to leaves of nonregenerated plants. (Right) Differences in a random subset of non-DMRs. (E) Methylation frequencies at DMRs in leaves of nonregenerated Col-0, RO, and reciprocal crosses (F1) between nonregenerated Col-0 and RO plants. (F) Methylation analysis of progeny from F1 reciprocal hybrids. The heatmap shows DMR methylation levels in individual F1 hybrid plants (#7 and #16) and each of four independent descendants. The bar plot shows the frequency of hypomethylation in F2 plants of DMRs that were hypomethylated in the F1 hybrid.
Because the functional relevance of individual DMPs in plants is unclear, we also analyzed differentially methylated regions (DMRs). Cluster analysis based on 765 DMRs identified in pairwise comparisons between all G2 leaf samples validated the use of the independent RO and LO lines as replicate groups (Fig. 3B). We found 255 consistent RO vs. LO DMRs in G2 leaves (Dataset S3). Compared with methylated regions in general, RO vs. LO DMRs were overrepresented in exons of gene coding sequences and in 2-kb regions flanking genes (SI Appendix, Fig. S8). Similar distributions of DMRs across genomic features have been reported in the context of biotic and abiotic stresses (30–32), suggesting that gene-proximal DNA methylation changes might be a common feature of induced epimutations.
Methylation patterns are known to differ between shoots and roots in A. thaliana and closely related species (12, 14). Methylation in LO leaves was similar to that in nonregenerated leaves (PC 1 in Fig. 3C); likewise, roots from RO samples showed patterns similar to those in nonregenerated roots. However, leaves from RO populations had methylation patterns closer to those of roots, especially in a symmetric cytosine context (Fig. 3C). In contrast, methylation levels at these DMRs in roots of LO plants were similar to those seen in nonregenerated root samples (Fig. 3C). As expected, methylation levels at non-DMRs grouped samples primarily by their tissue identity, regardless of regenerant origin (Fig. 3C).
Our DMR analysis also revealed that leaves from RO plants had less overall CG and CHG (but not CHH) methylation in the identified DMRs compared with leaves of nonregenerated plants or LO plants (Fig. 3D). To account for sampling bias and stochastic effects, we repeated the analyses using randomly selected non-DMRs, which did not produce any evidence of reduced methylation in roots of RO plants (Fig. 3D). The reduced DNA methylation in RO leaves was stably inherited over at least three generations (Fig. 3 C and D), indicating that root-specific DNA methylation patterns are not lost during subsequent sexual reproduction.
To understand the importance of the methylome status at the time of regeneration, we compared the leaf methylome of RO plants with the methylation profiles of roots from nonregenerated seedlings and mature plants (SI Appendix, Fig. S9). The RO leaf methylome was closer to that of roots from nonregenerated seedlings, implying that regeneration had been induced at the seedling stage and that the cell-specific methylation pattern was maintained throughout the regeneration process and in subsequent sexually reproduced progeny.
Because there is evidence of methylation information being transferable between chromosomes (33, 34), we tested whether such information transfer could occur in trans in our system. To this end, we performed reciprocal crosses between RO and nonregenerated plants. DNA methylation in both F1 hybrids was at midparent values, indicating that the DMRs on chromosomes inherited from the RO parent retained their hypomethylated status (Fig. 3E and SI Appendix, Fig. S10). We assessed the heritability of the observed differential methylation at these loci by sequencing individual F2 plant progenies. More than two-thirds (80%) of DMRs with midparental methylation in the F1 hybrid retained their hypomethylated state in at least one F2 descendant, indicating that allele-specific methylation was stably inherited through both mitotic and meiotic divisions (Fig. 3F).
The establishment and maintenance of DNA methylation in plants rely on a series of partially interconnected pathways, depending on the genomic features that are methylated (35). Consequently, when genome-wide demethylation is induced by various mutations, some regions can be remethylated upon restoration of the methylation machinery, while others cannot, and this is a function of the underlying methylation pathways (36–38). To gain insight into the mechanisms that supported the partial maintenance of root-specific methylation patterns in RO plants, we investigated whether methylated regions that became hypomethylated in RO plants were under the control of a specific epigenetic pathway, by comparing the methylation changes in different mutant contexts (39). We found that CG methylation in such regions was affected in the chromatin remodeling mutant ddm1 and in the DNA methylation maintenance mutants met1 and vim1 vim2 vim3, while CHG methylation was altered in the de novo methyltransferase cmt3 mutant and in mutants with a compromised H3K9 methylation machinery (SI Appendix, Fig. S11).
Contrary to our expectations, the regions that were hypo-methylated in RO plants were not affected in the triple ros1 dml2 dml3 (rdd) mutant, which lacks three DNA demethylases, suggesting that DNA hypomethylation in RO plants is due to differences in the establishment and/or maintenance of DNA methylation in root initials during embryogenesis, rather than to subsequent active demethylation. Induced methylation changes are often associated with changes in activity of transposable elements (TEs), which in turn is reflected in altered expression patterns of siRNAs, which are a central component of TE silencing pathways that involve RNA-directed DNA methylation (RdDM) (40–42). LO vs. RO DMRs tend to not directly overlap with TEs and 24-nt siRNA loci (43), but are often closer to them than would be expected by chance (SI Appendix, Fig. S12).
Differential Methylation Affects Expression of RSM1.
To establish a connection between the molecular and phenotypic variation generated by plant regeneration, we searched for correlations between changes in DNA methylation (765 DMRs) and gene expression in leaves of regenerated plants, using a combined set of 1,537 DEGs from G1 and G2 generations (FDR <0.01). We found 29, mostly hypomethylated, DMRs that were within 2 kb upstream or downstream of DEGs (SI Appendix, Fig. S13 and Dataset S4). To confirm that such DMRs can indeed affect gene expression, we selected a DMR approximately 1 kb downstream of RADIALIS-LIKE SANT/MYB1 (RSM1)/MATERNAL EFFECT EMBRYO ARREST 3 (MEE3), a regulator of growth and flowering time (44, 45). This DMR was hypomethylated in RO leaves compared with LO leaves, in both G1 and G2 generations (SI Appendix, Fig. S14). To manipulate DNA methylation experimentally, we introduced an inverted repeat (IR) hairpin to force DNA hypermethylation at the RSM1-DMR (RSM1-IR) by RdDM. Bisulfite sequencing confirmed that the targeted genome region became specifically hypermethylated in IR transgenic lines (Fig. 4A), and this was accompanied by reduced RSM1 expression (Fig. 4B). RSM1_IR lines suffered from pleiotropic developmental defects, including accelerated senescence and earlier flowering (Fig. 4C). When we transformed RSM1_IR plants with a synthetic RSM1 construct (synRSM1) resistant to RSM1_IR targeting, the RSM1-IR developmental phenotypes were suppressed (Fig. 4D), indicating that RSM1 is the causative gene responsible for the underlying developmental defects observed in IR lines. In aggregate, these observations are consistent with the DMR containing a regulatory region, the activity of which is influenced by DNA methylation.
Fig. 4.
Activity of an RSM1 regulatory element is affected by DNA methylation. (A) Snapshot of DNA methylation in different sequence contexts at the RSM1 locus in wild-type (WT) Col-0 plants and plants carrying an inverted repeat transgene (RSM1-IR). The black horizontal bar indicates the region targeted by RSM1-IR–induced RdDM for DNA hypermethylation. Green ticks, CG methylation; blue, CHG methylation; orange, CHH methylation. (B) RSM1 expression in leaves and roots of WT plants, RSM1-IR plants, and RSM1-IR plants complemented with synRSM1. White bar, leaf; black bar, root. (C) Induced DNA hypermethylation of RSM1-DMR affects morphology. (D) Genetic complementation of RSM1-IR with a synthetic RSM1 transgene (synRSM1) resistant to RSM1-IR–induced hypermethylation.
Discussion
We have set out to determine whether epigenetic phenomena contribute to phenotypic variation arising from asexual propagation in plants. Our data show that the epigenetic profile of certain somatic cells can be at least partially maintained when clonal regenerants are produced by inducing an embryogenic developmental program in these somatic cells. Inappropriate maintenance of somatic epigenetic profiles has also been observed when mammalian clones are produced by nuclear transfer, where it can result in embryonic lethality and postnatal growth defects (46, 47).
Because somatic embryogenesis closely mimics many aspects of normal embryogenesis (48), the partial retention of tissue-specific epigenetic signatures in the primary regenerants is not so surprising, as these marks have not passed through gametogenesis and early stages of embryogenesis. A less expected finding is that progeny of regenerated plants, which have undergone multiple cycles of sexual reproduction, can continue to retain some organ-specific epigenetic marks, as well as transcriptional and phenotypic signatures typical of the founder tissue used for the initial propagation. The stability of these epigenetic marks and the reduced methylation levels of the affected loci resemble epialleles induced by the inactivation of positive regulators of DNA methylation. Such epialleles have been shown to be heritable over multiple generations in epigenetic recombinant inbred lines (36, 37, 49), where they are also associated with stable phenotypic variation (36, 50).
Plant regeneration induced by phytohormones can lead to heritable genome-wide DNA hypomethylation that is correlated with altered gene expression. In both A. thaliana and rice, such epimutations seem to occur in a stochastic manner (7, 9), but they appear to be more consistent with and to be targeted to specific genomic regions in maize (10). While we also observe some random epigenetic changes after somatic embryogenesis induced by zygotic factors, most methylation changes following tissue-specific regeneration are reproducible and reflect the epigenetic state of the tissue of origin.
In A. thaliana, asymmetric CHH methylation is actively reprogrammed during male gametogenesis and proper methylation is rapidly restored in the embryo after fertilization (51–53). On the other hand, symmetric CHG and CG methylation is thought to remain stable throughout both male and female gametogenesis and in embryos (51–55). Unfortunately, detailed methylation maps of embryonic lineages and stem cells are not available, but the leaf methylation pattern can be envisioned to resemble a ground state more closely than the root methylation pattern, which is distinguished from the leaf pattern primarily by hypomethylation. With roots being further away from the ground state than leaves, one might then expect RO plants to be epigenetically more distinct from nonregenerated plants compared with LO plants.
Targeted hypomethylation likely takes place already during embryogenesis, when root cell initials are first specified, because hypomethylation is a characteristic of many cell types in the postembryonic root (51). The mechanisms underlying epigenetic reprogramming in roots are unknown, but methylation differences between roots and leaves have been found in other plant species as well (12, 14, 56, 57). One possibility is that such marks are involved in regulating distinct tissue-specific transcriptional responses to environmental factors, such as exposure and interactions with microorganisms. In support of this argument, A. thaliana leaves challenged with bacterial pathogens rapidly remodel their DNA methylation profiles (31), and root resistance to fungal pathogens requires active DNA demethylation (58). However, whether DNA methylation is the primary epigenetic mark that changes upon regeneration and whether it is the most important cause for the expression and phenotypic differences observed remains to be clarified.
In summary, we propose that organ-specific epigenetic marks captured during somatic embryogenesis are also likely to contribute to the phenotypic somaclonal variation observed in plants propagated in vitro (5, 11, 59, 60), as well as in natural asexually reproducing plant populations (61). The partial maintenance of organ-specific epigenetic marks during cloning might not be a unique feature of plants, and may also occur in clonal animals. Our findings thus not only suggest new and exciting possibilities for enhancing or, perhaps more importantly, limiting phenotypic variation in clonally propagated elite lines (11), but also raise pertinent questions regarding the adaptive significance of developmental epigenomic changes captured during asexual reproduction in plants.
Materials and Methods
A. thaliana Col-0 was grown in a controlled environment (16-h light/8-h dark, 22 °C). For the direct plant regeneration of plants from differentiated organs, we used transgenic lines carrying a dexamethasone-inducible transgene to overexpress the GRANDE (GRD)/RWPRK motif-containing (RKD4) transcription factor (13). Seeds from a transgenic indRKD4 line were germinated on Murashige and Skoog (MS) plates. After 4–6 d, plants were transferred to MS plates with 20 µM dexamethasone, on which they were incubated for 7 d. Plants were transferred to dexamethasone-free MS plates to allow the formation of somatic embryos, which were dissected manually by micromanipulation using tungsten needles. Isolated somatic embryos were transferred to MS plates to aid whole plant regeneration from leaves (LO) or roots (RO). We generated 10 independent regenerants from each organ (G0 generation), which were grown in soil to produce seeds. We grew 24 plants from each line (G1 generation) and selected 10 individuals at random for each regenerated line to produce seed. We continued the sexual propagation of each regenerant population for two additional generations (G2 and G3) following the same scheme.
Additional information is provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank C. Lanz and J. Hildebrandt for help with Illumina sequencing, J. Engelhorn for assistance with RNA sampling, Rainer Borriss for providing the B. amyloliquefaciens FZB42 strain, and Liliana M. Costa for discussions and comments on the manuscript. This work was supported by a European Research Council (ERC) Marie Sklodowska-Curie Fellowship (751204-H2020-MSCA-IF-2016, to A.W.); the ERC AdG IMMUNEMESIS Project, the Deutsche Forschungsgemeinschaft SPP1529 Program, and the Max Planck Society (D.W.); the ERC AdG ROOTMICROBIOTA Project, the Cluster of Excellence on Plant Sciences, and the Max Planck Society (P.S.-L.); and the Biotechnology and Biological Sciences Research Council (Grants BB/L025892/1, to G.D.B., and BB/L003023/1, BB/N005279/1, BB/N00194X/1, and BB/P02601X/1, to J.G.-M.).
Footnotes
A.W., C.B., D.W., and J.G.-M. are inventors on patent application “Stable epigenetic plant variants” (PCT/EP2016/055377 and WO2016146552A1) filed by University of Warwick and Max Planck Society.
Data deposition: Sequencing reads have been deposited at the European Nucleotide Archive (accession nos. PRJEB26932 and PRJEB14117). DNA methylation data have been uploaded to the EPIC-CoGe epigenome browser and can be accessed at https://genomevolution.org/CoGe/NotebookView.pl?lid=835.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805371115/-/DCSupplemental.
References
- 1.Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21:212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K. Plant regeneration: Cellular origins and molecular mechanisms. Development. 2016;143:1442–1451. doi: 10.1242/dev.134668. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto K, Jiao Y, Meyerowitz EM. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell. 2010;18:463–471. doi: 10.1016/j.devcel.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 4.McKey D, Elias M, Pujol B, Duputié A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010;186:318–332. doi: 10.1111/j.1469-8137.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang C, et al. Regenerant Arabidopsis lineages display a distinct genome-wide spectrum of mutations conferring variant phenotypes. Curr Biol. 2011;21:1385–1390. doi: 10.1016/j.cub.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyao A, et al. Molecular spectrum of somaclonal variation in regenerated rice revealed by whole-genome sequencing. Plant Cell Physiol. 2012;53:256–264. doi: 10.1093/pcp/pcr172. [DOI] [PubMed] [Google Scholar]
- 7.Stroud H, et al. Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife. 2013;2:e00354. doi: 10.7554/eLife.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stelpflug SC, Eichten SR, Hermanson PJ, Springer NM, Kaeppler SM. Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics. 2014;198:209–218. doi: 10.1534/genetics.114.165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanurdzic M, et al. Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol. 2008;6:2880–2895. doi: 10.1371/journal.pbio.0060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Z, et al. Heritable epigenomic changes to the maize methylome resulting from tissue culture. Genetics. 2018;209:983–995. doi: 10.1534/genetics.118.300987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong-Abdullah M, et al. Loss of Karma transposon methylation underlies the mantled somaclonal variant of oil palm. Nature. 2015;525:533–537. doi: 10.1038/nature15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widman N, Feng S, Jacobsen SE, Pellegrini M. Epigenetic differences between shoots and roots in Arabidopsis reveals tissue-specific regulation. Epigenetics. 2014;9:236–242. doi: 10.4161/epi.26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waki T, Hiki T, Watanabe R, Hashimoto T, Nakajima K. The Arabidopsis RWP-RK protein RKD4 triggers gene expression and pattern formation in early embryogenesis. Curr Biol. 2011;21:1277–1281. doi: 10.1016/j.cub.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Seymour DK, Koenig D, Hagmann J, Becker C, Weigel D. Evolution of DNA methylation patterns in the Brassicaceae is driven by differences in genome organization. PLoS Genet. 2014;10:e1004785. doi: 10.1371/journal.pgen.1004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcês HM, Koenig D, Townsley BT, Kim M, Sinha NR. Truncation of LEAFY COTYLEDON1 protein is required for asexual reproduction in Kalanchoë daigremontiana. Plant Physiol. 2014;165:196–206. doi: 10.1104/pp.114.237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warde-Farley D, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lackman P, et al. Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc Natl Acad Sci USA. 2011;108:5891–5896. doi: 10.1073/pnas.1103010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin F, et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell. 2008;20:1693–1707. doi: 10.1105/tpc.107.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robatzek S, Somssich IE. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002;16:1139–1149. doi: 10.1101/gad.222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, et al. The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis. Plant Cell Physiol. 2007;48:1148–1158. doi: 10.1093/pcp/pcm088. [DOI] [PubMed] [Google Scholar]
- 21.Trujillo M, Ichimura K, Casais C, Shirasu K. Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr Biol. 2008;18:1396–1401. doi: 10.1016/j.cub.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 22.Lebeis SL, et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- 23.Chaparro JM, Badri DV, Vivanco JM. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014;8:790–803. doi: 10.1038/ismej.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury SP, et al. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One. 2013;8:e68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y, et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature. 2015;528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- 26.Tomé DF, Steinbrenner J, Beynon JL. A growth quantification assay for Hyaloperonospora arabidopsidis isolates in Arabidopsis thaliana. Methods Mol Biol. 2014;1127:145–158. doi: 10.1007/978-1-62703-986-4_12. [DOI] [PubMed] [Google Scholar]
- 27.Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagmann J, et al. Century-scale methylome stability in a recently diverged Arabidopsis thaliana lineage. PLoS Genet. 2015;11:e1004920. doi: 10.1371/journal.pgen.1004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadrana L, Colot V. Plant transgenerational epigenetics. Annu Rev Genet. 2016;50:467–491. doi: 10.1146/annurev-genet-120215-035254. [DOI] [PubMed] [Google Scholar]
- 30.Wibowo A, et al. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife. 2016;5:e13546. doi: 10.7554/eLife.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowen RH, et al. Widespread dynamic DNA methylation in response to biotic stress. Proc Natl Acad Sci USA. 2012;109:E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popova OV, Dinh HQ, Aufsatz W, Jonak C. The RdDM pathway is required for basal heat tolerance in Arabidopsis. Mol Plant. 2013;6:396–410. doi: 10.1093/mp/sst023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaves IK, Groszmann M, Wang A, Peacock WJ, Dennis ES. Inheritance of trans chromosomal methylation patterns from Arabidopsis F1 hybrids. Proc Natl Acad Sci USA. 2014;111:2017–2022. doi: 10.1073/pnas.1323656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves IK, et al. Trans chromosomal methylation in Arabidopsis hybrids. Proc Natl Acad Sci USA. 2012;109:3570–3575. doi: 10.1073/pnas.1201043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matzke MA, Mosher RA. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat Rev Genet. 2014;15:394–408. doi: 10.1038/nrg3683. [DOI] [PubMed] [Google Scholar]
- 36.Cortijo S, et al. Mapping the epigenetic basis of complex traits. Science. 2014;343:1145–1148. doi: 10.1126/science.1248127. [DOI] [PubMed] [Google Scholar]
- 37.Reinders J, Paszkowski J. Unlocking the Arabidopsis epigenome. Epigenetics. 2009;4:557–563. doi: 10.4161/epi.4.8.10347. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira FK, et al. A role for RNAi in the selective correction of DNA methylation defects. Science. 2009;323:1600–1604. doi: 10.1126/science.1165313. [DOI] [PubMed] [Google Scholar]
- 39.Stroud H, Greenberg MV, Feng S, Bernatavichute YV, Jacobsen SE. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152:352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L, Cao X. The effect of transposable elements on phenotypic variation: Insights from plants to humans. Sci China Life Sci. 2016;59:24–37. doi: 10.1007/s11427-015-4993-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhai J, et al. Small RNA-directed epigenetic natural variation in Arabidopsis thaliana. PLoS Genet. 2008;4:e1000056. doi: 10.1371/journal.pgen.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borges F, Martienssen RA. The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol. 2015;16:727–741. doi: 10.1038/nrm4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajagopalan R, Vaucheret H, Trejo J, Bartel DP. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006;20:3407–3425. doi: 10.1101/gad.1476406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamaguchi A, et al. A small subfamily of Arabidopsis RADIALIS-LIKE SANT/MYB genes: A link to HOOKLESS1-mediated signal transduction during early morphogenesis. Biosci Biotechnol Biochem. 2008;72:2687–2696. doi: 10.1271/bbb.80348. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Zhou Y, Fan L-M. A novel repressor of floral transition, MEE3, an abiotic stress regulated protein, functions as an activator of FLC by binding to its promoter in Arabidopsis. Environ Exp Bot. 2015;113:1–10. [Google Scholar]
- 46.Wong AHC, Gottesman II, Petronis A. Phenotypic differences in genetically identical organisms: The epigenetic perspective. Hum Mol Genet. 2005;14:R11–R18. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 47.Rideout WM, 3rd, Eggan K, Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001;293:1093–1098. doi: 10.1126/science.1063206. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman JL. Somatic embryogenesis: A model for early development in higher plants. Plant Cell. 1993;5:1411–1423. doi: 10.1105/tpc.5.10.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johannes F, et al. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 2009;5:e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kooke R, et al. Epigenetic basis of morphological variation and phenotypic plasticity in Arabidopsis thaliana. Plant Cell. 2015;27:337–348. doi: 10.1105/tpc.114.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawakatsu T, Nery JR, Castanon R, Ecker JR. Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 2017;18:171. doi: 10.1186/s13059-017-1251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouyer D, et al. DNA methylation dynamics during early plant life. Genome Biol. 2017;18:179. doi: 10.1186/s13059-017-1313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker J, et al. Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat Genet. 2018;50:130–137. doi: 10.1038/s41588-017-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calarco JP, et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151:194–205. doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh P-H, et al. Arabidopsis male sexual lineage exhibits more robust maintenance of CG methylation than somatic tissues. Proc Natl Acad Sci USA. 2016;113:15132–15137. doi: 10.1073/pnas.1619074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chwialkowska K, Nowakowska U, Mroziewicz A, Szarejko I, Kwasniewski M. Water-deficiency conditions differently modulate the methylome of roots and leaves in barley (Hordeum vulgare L.) J Exp Bot. 2016;67:1109–1121. doi: 10.1093/jxb/erv552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira LJ, Azevedo V, Maroco J, Oliveira MM, Santos AP. Salt-tolerant and -sensitive rice varieties display differential methylome flexibility under salt stress. PLoS One. 2015;10:e0124060. doi: 10.1371/journal.pone.0124060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le TN, et al. DNA demethylases target promoter transposable elements to positively regulate stress responsive genes in Arabidopsis. Genome Biol. 2014;15:458. doi: 10.1186/s13059-014-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miguel C, Marum L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J Exp Bot. 2011;62:3713–3725. doi: 10.1093/jxb/err155. [DOI] [PubMed] [Google Scholar]
- 60.Zhang D, et al. Tissue culture-induced heritable genomic variation in rice, and their phenotypic implications. PLoS One. 2014;9:e96879. doi: 10.1371/journal.pone.0096879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piquot Y, et al. Variation in sexual and asexual reproduction among young and old populations of the perennial macrophyte Sparganium erectum. Oikos. 1998;82:139–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.