Abstract
Background
Global climate change is likely to increase the geographic range and seasonality of malaria transmission. Areas suitable for distribution of malaria vectors are predicted to increase with climate change but evidence is limited on future distribution of malaria with climate in China.
Objective
Our aim was to assess a potential effect of climate change on Plasmodium vivax (P. vivax) and Plasmodium falciparum (P. falciparum) malaria under climate change scenarios.
Methods
National malaria surveillance data during 2005–2014 were integrated with corresponding climate data to model current weather-malaria relationship. We used the Generalized Additive Model (GAM) with a spatial component, assuming a quasi-Poisson distribution and including an offset for the population while accounting for potential non-linearity and long-term trend. The association was applied to future climate to project county-level malaria distribution using ensembles of Global Climate Models under two climate scenarios - Representative Concentration Pathways (RCP4.5 and RCP8.5).
Results
Climate change could substantially increase P. vivax and P. falciparum malaria, under both climate scenarios, but by larger amount under RCP8.5, compared to the baseline. P. falciparum is projected to increase more than P. vivax. The distributions of P. vivax and P. falciparum malaria are expected to increase in most regions regardless of the climate scenarios. A high percentage (>50%) increases are projected in some counties of the northwest, north, northeast, including northern tip of the northeast China, with a clearer spatial change for P. vivax than P. falciparum under both scenarios, highlighting potential changes in the latitudinal extent of the malaria.
Conclusion
Our findings suggest that spatial and temporal distribution of P. vivax and P. falciparum malaria in China will change due to future climate change, if there is no policy to mitigate it. These findings are important to guide the malaria elimination goal for China.
Keywords: P. vivax, P. falciparum, Climate, Malaria, RCP, Scenario
1. Introduction
Malaria is a lethal vector-borne parasitic disease mainly affecting people in tropical and subtropical countries (World Health Organization (WHO), 2015). The burden of malaria is decreasing over the recent years because of intensive control interventions but the disease is still a significant public health problem (Murray et al., 2012) with 214 million cases and 438, 000 deaths reported globally in 2015, and about 3.2 billion people in the world are at risk of the disease (World Health Organization (WHO), 2015). In China, >30 million malaria cases were recorded annually in the 1940s. Following the establishment of the malaria control program and several decades of control interventions, the malaria burden greatly declined. In 2010, the Chinese government endorsed the National Action Plan for Elimination of Malaria, with the aim of disease elimination by 2020 (Yin et al., 2013). However, there is possibility of malaria resurgence following reduction of transmission because the risk factors in endemic areas still exist and the environment may be more conducive for transmission owing to the ongoing climate change (Cohen et al., 2012). Change in vectorial capacity, population movement, response to reintroduced cases, and public awareness in non or low endemic areas are another determinants of dealing with malaria resurgence (Qian et al., 2014). In addition to the historical challenges in maintaining malaria control in the country (Yin et al., 2014; Zhou et al., 2005), the most recent study in some provinces of the country estimated a potential increase of malaria by 19–29% in 2020s (Song et al., 2016). Hence, improving the understanding of the potential impact of climate change on the spatial and temporal dynamism of malaria transmission is of great importance.
Malaria is caused by four species of the genus Plasmodium - P. vivax, P. falciparum, P. malaria, and P. ovale. In China, P. vivax and P. falciparum are the two most important Plasmodium species (Lu et al., 2014). P. vivax was the most common Plasmodium parasite for a long time, accounting for 76.9% of all reported malaria cases during 2004–2012, with a peak in 2006 (Zhou et al., 2007; Zhang et al., 2014). P. vivax has a wider geographical coverage with stable and unstable transmission spanning the south, central, southeast and some province in the north of the country. P. falciparum, a causative agent of severe malaria, has a lower incidence in China. The disease is transmitted by four malaria vectors under genus Anopheles: A. dirus, A. lesteri, A. minimus and A. sinensis. Malaria is acknowledged as one of the most climate-sensitive infectious diseases (Githeko et al., 2000; Bai et al., 2013) because the growth and development of Anopheles mosquitoes, and the Plasmodium development in the mosquito called sporogonic cycle or extrinsic incubation period are affected by changes in the climatic factors (Shapiro et al., 2017). Although the sporogonic stages specific effect of climate factors is not well defined, available evidence indicated that the rate of ookinate maturation (Noden et al., 1995) and ex-flagellation and sporozoite formation in the oocyct (Rastogi et al., 1987) are more responsive and are regarded as bottleneck stage in the lifecycle of malaria (Eling et al., 2001). Climate-malaria relationship has been widely investigated in China and other neighbouring countries, with the most frequently reported significant climate predictors: temperature, precipitation, and relative humidity (Zhang et al., 2010; Huang et al., 2011; Bi et al., 2003; Yang et al., 2010; Liang et al., 2008; Bi et al., 2013; Bouma et al., 1996).
There is a general consensus that global warming is mainly due to atmospheric concentrations of greenhouse gasses (IPCC, 2013). Evidences have indicated that with rising global climate, many areas of the world will become more favorable for the survival of climate-sensitive vectors such as mosquitoes. Likewise, an assessment of the potential impact of climate change on malaria showed an increasing risk of malaria in previously malaria-free areas (Martens et al., 1995; Caminade et al., 2014; Dhimal et al., 2015). The spatial limits of malaria distribution are predicted to follow the change of climatic factors, including rainfall and temperature (Astrom et al., 2012; Zell, 2004; Pascual et al., 2006; Siraj et al., 2014; Zhou et al., 2004). Using global climate models, studies have predicted a latitudinal and longitudinal increase in distribution of malaria and suitable areas in some regions (Pakdad et al., 2017; Khormi and Kumar, 2016; Ryan et al., 2015), while others have predicted a reductions in the geographic range of the disease distribution in some regions (Khormi and Kumar, 2016). In China, a warmer climate is predicted by 2081–2100, with an annual average temperature increase of 1.3 °C to 5.2 °C, and an increase in average annual precipitation of 5%–12%, compared to 1986–2005. The temperature and precipitation pattern increases from south to north, and the northern regions are expected to experience hotter and wetter climate (Tian et al., 2015; Chong-Hai and Ying, 2012). This means that the previously cooler areas could then be more suitable for malaria transmission. Using ecological niche modelling, one study predicts a potential increase of areas suitable for distribution of the four common malaria vectors in China under climate change scenarios (Ren et al., 2016). For example, A. sinensis, the broadly distributed mosquito was predicted to consistently increase and expand northward along the margin of endemicity. Projecting the magnitude and location of future weather-related changes in malaria are of significant public health importance, and will inform developing sustainable strategies for mitigation of climate change effects (Tonnang et al., 2010). However, evidence on the effect of future climate on potential malaria distribution in China is limited. One study conducted recently in China using a remotely sensed environmental predictors in a Genetic Program model estimated a potential increase of malaria (by 19–29% in 2020), and expansion of high-risk areas (Song et al., 2016). However, this study focused on only a few provinces of Northern China. The study linked future malaria risk to climate variables with more emphasis on temperature and precipitation without considering the potential effect of other important climate predictor such as relative humidity (Bouma et al., 1996; Tonnang et al., 2010; Li et al., 2013) previously used to estimate an extent of malaria transmission in China (Yang et al., 2010). Furthermore, there are limitations to the usefulness of modelling changes in future distributions of malaria due to climate change when other drivers of transmission, such as land use change (Tatem et al., 2008; Lindblade et al., 2000) are not considered in scenarios. This study aims to predict future weather-related malaria in China under the recent climate scenarios-RCP4.5 and RCP8.5, while considering a potential effect of population increase.
2. Methodology
2.1. Study site
This study was conducted in China, a country with a population of approximately 1.34 billion according to the 2010 census (Haub, 2011). The climate is extremely diverse across the country, with the southern regions exhibiting tropical climate while the northern region is subarctic (Climate features in China, 2017). Substantial temperature and precipitation changes are ongoing in the country owing to climate change over the last decades (Chong-Hai and Ying, 2012), establishing an ideal environment for malaria transmission.
2.2. Malaria data
Detailed malaria data used for this study were previously described (Hundessa et al., 2016). Briefly, the national malaria case data at a county-level was obtained from the China Information System for Disease Control and Prevention (CISDCP) for the period from 2005 to 2014. In 2004, the government of China enhanced infectious disease surveillance, establishing the online Nationwide Notifiable Infectious Diseases Reporting Information System (NIDRIS) which includes malaria as a notifiable disease (Wang et al., 2013). Laboratory-confirmed (microscopy and/or Rapid Diagnostic Test) and suspected cases are reported to the county-level CDC within 24 h (Qian et al., 2014). Case investigation and identification are conducted within three days of receiving the case report following standard criteria (Cao et al., 2014). The malaria dataset included laboratory diagnosed malaria species, demographic factors, residential location (county code) and associated geo-coordinate. The county-level population data available from China Bureau of Statistics were used to generate the data for each consecutive year through linear interpolation.
2.3. Observed weather-malaria relationship
In China, meteorological information is regularly recorded at several weather stations on a daily basis. For this study, we used county-level weather variables, including annual temperature, precipitation, and relative humidity obtained from the China Meteorological data Sharing Centre for the period 2005–2014, giving nationwide coverage of climatic factors. An average annual value of weather variables from all stations was calculated and linked to the county-level annual malaria data to establish a database for estimating the baseline weather-malaria relationship.
2.4. Future weather data
Global Climate Model (GCM) projected climate data from the IPCC Data Distribution Centre (IPCC, 2007) was downscaled to each region by using NWAI-WG, a weather generator based statically downscaling model developed at NSW DPI's Wagga Wagga Agricultural Institute (Liu and Zuo, 2012). This method includes a bias correction of the monthly raw GCMs data, where the observed and raw GCM projected monthly values of the historical period 1961–2000 are used to establish the relationship using a qq-plot technique for adjusting GCMs distribution to match with the observed distribution. The same relationship is applied to adjust the GCM projected future data (Liu and Zuo, 2012). The daily values of the climate variables for the baseline and future time periods were disaggregated by a modified WGEN (Richardson and Wright, 1984) to simulate a series of county-specific future climate change scenarios RCP 4.5 and RCP 8.5. We used all GCMs available under RCP4.5 and RCP8.5 scenarios, resulting 26 GCMs for each counties. RCP is the scenario used in climate research to give possible description of change in future climate with respect to anthropogenic greenhouse gasses emission, air-pollutants, land use change and climate policies. We selected two scenarios, RCP4.5 and RCP8.5 which represent medium stabilization, and high emission scenario, respectively (Van Vuuren et al., 2011). Average annual temperature, precipitation, and relative humidity were considered as weather predictors in this study based on their relative importance in previous studies (Song et al., 2016; Ryan et al., 2015; Tonnang et al., 2010; Shen, 2007). To incorporate the effect of population change on future malaria distribution, a 10% increase in population was assumed extending the previous estimation that China population will increase by 10% until 2050 (Wei and Jinju, 2009).
2.5. Statistical modelling
Nationwide historical weather variables were used with the national malaria surveillance data to examine the current weather-malaria relationship. A GAM model with a spatial component, assuming a quasi-Poisson distribution and including an offset for the population. This type of GAMs allows modelling potential non-linear associations while adjusting for long-term trends and spatial correlation (Hastie and Tibshirani, 1990). This method has been found to perform better than other modelling approaches (Walsh and Kleiber, 2001; Moisen and Frescino, 2002) and has been widely used for projection purposes (Drexler and Ainsworth, 2013; Guisan et al., 2002). Specific details of the GAM models have been previously described (Hastie and Tibshirani, 1990; Dominici et al., 2002). We used smooth terms for the effect of climatic variables and potential confounders as relationships are usually non-linear (Cao et al., 2014). The spatial dependency between the neighbouring counties was modelled through a tensor product spline function of latitude and longitude at each county's centroid. To evaluate the performance of the model, cross-validation was performed with 90% randomly selected for the training set and 10% for the test set. Five hundred replicates were used to ensure reliability of the model measured by R square. The entire data set was used for the final projection of weather-related P. vivax and P. falciparum malaria.
Weather-related malaria was projected for the time period 1985–2100. The malaria projections were based on an average of 26 global climate models (GCMs), each run under two Representative Concentration Pathway scenarios RCP 4.5 and RCP 8.5. The malaria projections for the period of 1985–2014 was used to compare the historical malaria observations for examining possible bias for the baseline malaria occurrences so it can ensure confidence of the future projections. The average of all available GCMs (Table S1) were used to capture a plausible ranges of responses and performances in the GCM models because the projection results from multiple models could minimize uncertainty in future climate (McSweeney and Jones, 2016; Semenov, 2009).
Then, future malaria cases for P. vivax and P. falciparum were separately reported as a percentage (%) change across time periods and regions, compared to baseline. The spatial extent of projected change in malaria was reported following the regional classification previously described by Xie et al. (2011). All statistical analysis were performed using the “mgcv” package in R software (R Core Team, 2015) and projections were mapped using ArcGIS software (ESRI, 2001). The protocol for this study was approved by the Ethics Committee of The University of Queensland, Australia.
3. Results
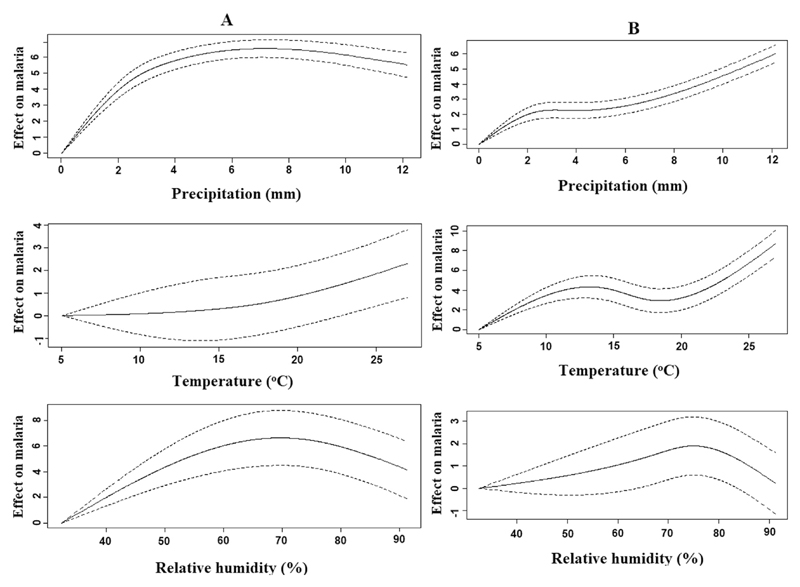
Cross-validation of our model showed that the model effectively captured the current distribution of P. vivax (R2 = 0.94) and P. falciparum (R2 = 0.88) malaria. Fig. 1 presents baseline (2005–2014) weather-malaria relationships for P. vivax and P. falciparum malaria. Temperature, rainfall and relative humidity were associated with the malaria incidence.
Fig. 1.
The relationships between weather variables and malaria in China during 2005–2014. A. P. vivax; B. P. falciparum.
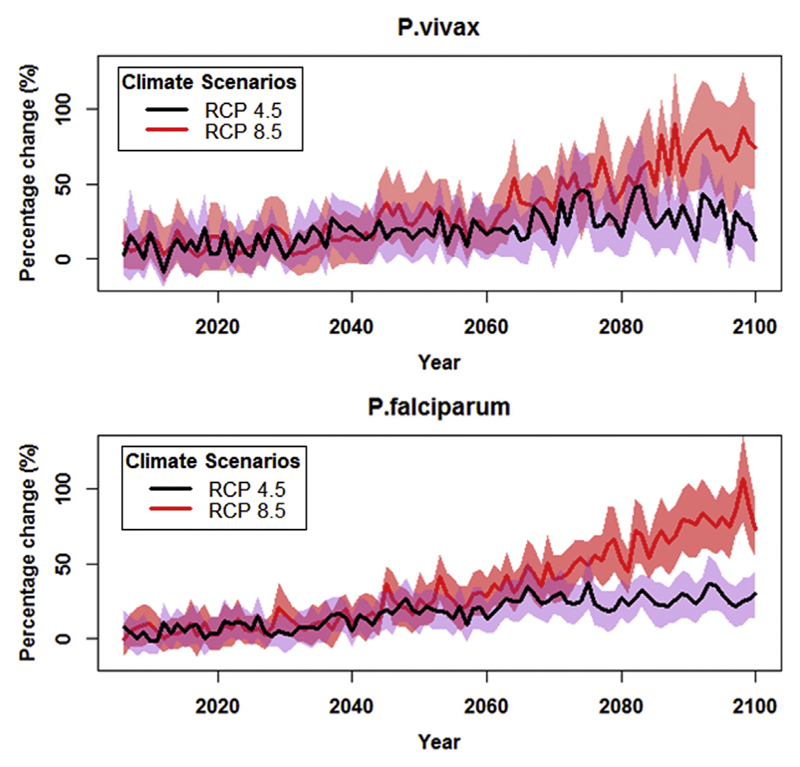
As shown in Fig. 2, future climate could potentially increase cases of P. vivax and P. falciparum malaria up to 2100, under both scenarios. Both P. vivax and P. falciparum were projected to increase more substantially under RCP8.5 than RCP4.5. Nevertheless, different GCMs provided slightly different projections, especially under the RCP4.5 climate scenario (Fig. S1).
Fig. 2.
Time series plot of percentage change in the weather-related P. vivax and P. falciparum malaria cases in China, compared to baseline (1985–2014). The black line indicates change in weather-related P. vivax under RCP 4.5 scenario. The red line indicates change in weather-related P. vivax under RCP 8.5 scenario. These projections used an average of 26 GCMs (Table S2). Polygon area indicates 95%CI. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1. Change in P. vivax and P. falciparum malaria
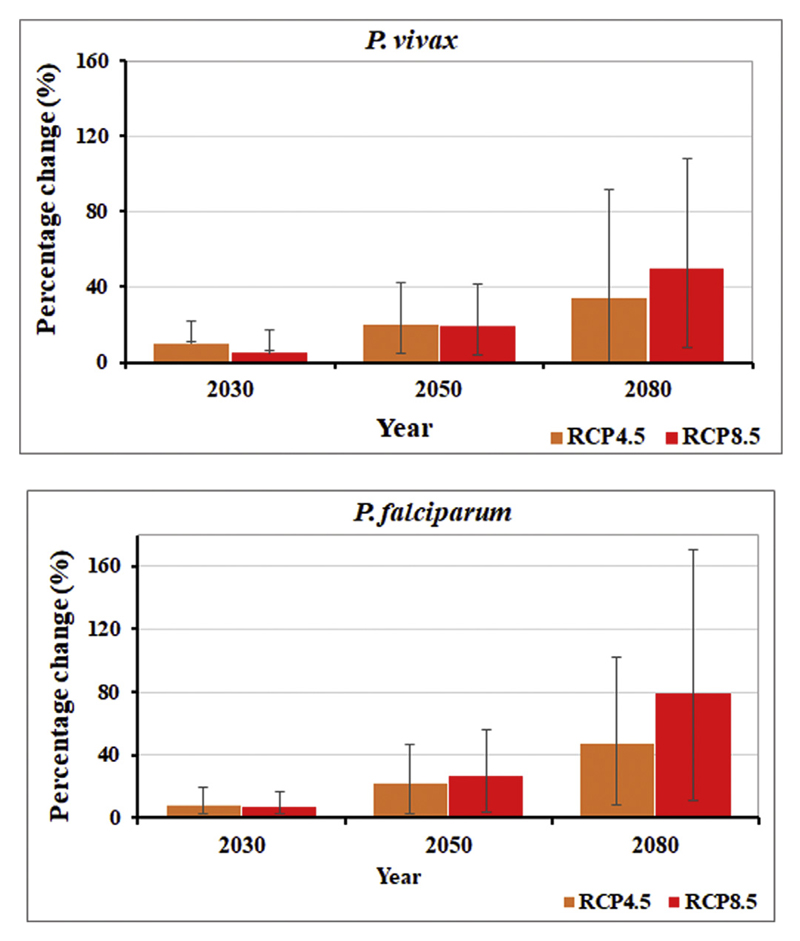
The percentage change in weather-related P. vivax in the 2030s, 2050s, and 2080s time periods using 26 GCMs under two scenarios (RCP4.5 and RCP8.5), compared to baseline (1985–2014) are depicted in Table 1 and Fig. 3. The projections show consistent increase of P. vivax and P. falciparum malaria throughout the study period under both scenarios. Compared to baseline period (1985–2014), P. vivax is predicted to increase by an average of 9.8%, 19.5%, and 34.3% in the 2030s, 2050s, and 2080s, respectively, under RCP8.5, and by an average of 5.5%, 18.7%, and 49.8%, respectively, under RCP4.5. Similarly, P. falciparum malaria is predicted to increase under RCP8.5 scenario by an average of 6.9%, 26.2%, and 79.6% in the 2030s, 2050s, and 2080s, respectively, and under RCP4.5, by 8.4%, 22.0%, and 47.1%, respectively. Generally, both P. vivax and P. falciparum would increase consistently up to the end of this century regardless of the scenarios (Table 1 and Fig. 3).
Table 1. Percentage (%) change in malaria with climate change scenarios-RCP8.5 and RCP4.5.
| Malaria | Scenarios | Periods |
||
|---|---|---|---|---|
| 2030s | 2050s | 2080s | ||
| P. vivax | RCP4.5 | 9.8% | 19.5% | 34.3% |
| RCP8.5 | 5.5% | 18.7% | 49.8% | |
| P. falciparum | RCP4.5 | 8.4% | 22.0% | 47.1% |
| RCP8.5 | 6.9% | 26.2% | 79.6% | |
Fig. 3.
Percentage (%) change in the P. vivax and P. falciparum malaria in China under RCP 4.5 and RCP 8.5 in 2030s, 2050s, and 2080s compared to the baseline period (1985–2014).
3.2. Spatial change in malaria
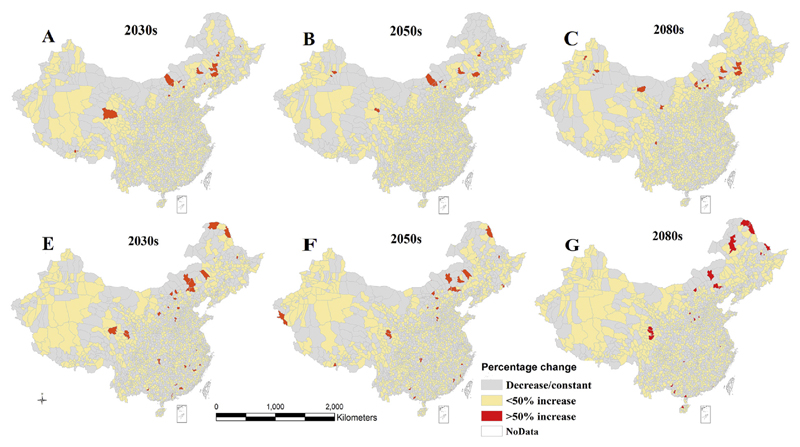
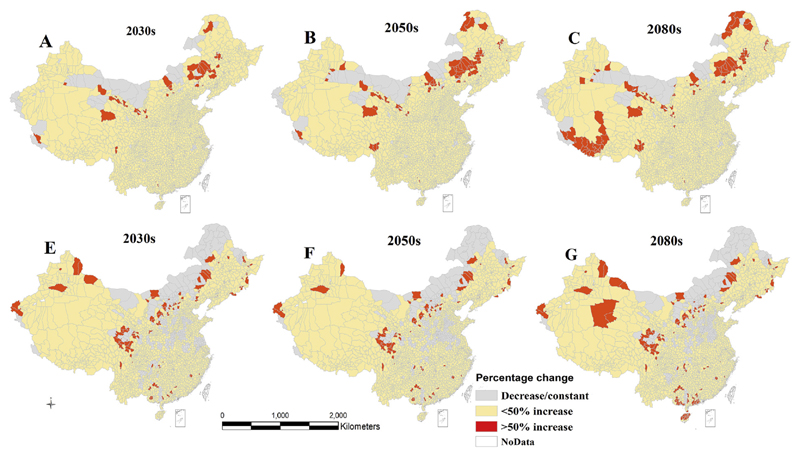
There would be considerable changes in the spatial distribution of P. vivax malaria across different regions of China in the 2030s, 2050s, and 2080s under RCP4.5 and 8.5 (Figs. 4–5). These projections are based on averages of 26 GCMs, and show a consistent increase of P. vivax in most counties of southern, southeastern, southwestern, central, and some parts of northeast and northwest of China under both scenarios in the 2030s, 2050s, and 2080s, compared to the baseline distribution (1985–2014). A large percentage increase (>50%) of P. vivax is projected in some counties of the north, northeast, and northwest, under both climate scenarios, with a greater increase under RCP4.5. The projection under RCP4.5 scenario indicated that a large percentage increase of P. vivax will involve some counties in the southwest, and northern tip of the northeast of the country in the 2050s and 2080s (Fig. 4A–C).
Fig. 4.
Percentage change (%) in P. vivax and P. falciparum malaria at the county level in China under RCP 8.5, compared to baseline period (1985–2014). A–C indicates the distribution of P. vivax, while E–G indicates the distribution of P. falciparum malaria. Projections are based on an average of 26 GCMs.
Fig. 5.
Percentage change (%) in the spatial distribution of projected P. vivax and P. falciparum malaria in China under the RCP 4.5 scenario, compared with the baseline period (1985–2014). A–C indicates the distribution of P. vivax, while E–G indicates the distribution of P. falciparum malaria. Projections are based on an average of 26 GCMs.
Climate change will also increase the potential distribution of P. falciparum malaria under both climate scenarios (Figs. 4–5). Compared to the baseline period, P. falciparum malaria will increase in most counties of the south, southeast, southwest, central, northwest and some counties of the north and northeast regions in 2030s, 2050s and 2080s. Similar to P. vivax, a higher percentage (>50%) increase of P. falciparum is predicted in some counties of the northwest, north, northeast, including northern tip of the northeast China under RCP8.5, but some areas of the south and central southwestern regions are also expected to have high percentage increase during the same period (Fig. 4E–G). However, the spatial pattern of this increase is not clear under RCP4.5 (Fig. 5E–G).
4. Discussion
This is the first national study to project the long-range possible future distribution of P. vivax and P. falciparum malaria in China using ten years of malaria surveillance data and 26 global climate models under two emission pathways (RCP8.5 and RCP4.5). Cross-validation of the projection model showed good agreement between predicted and observed malaria cases, and was therefore used to project the malaria distribution. The findings indicate that P. vivax and P. falciparum malaria will increase in China, but by a larger amount under RCP8.5 scenario. Both P. vivax and P. falciparum are projected to increase in most parts of the regions throughout the century under both emission scenarios. A high percentage (>50%) increase of malaria is predicted in some counties of the northwest, north, and northeast, including northern tip of the northeast China, with a clearer spatial change for P. vivax than P. falciparum. These findings are crucial for providing evidence-based information to achieve the goal of malaria elimination in China.
The findings indicated that climate change could potentially increase both P. vivax and P. falciparum malaria. Studies have predicted increase in average surface temperature, and percentage change in precipitation (in northwest region of China) by 2081–2100 (Tian et al., 2015). There have also been increased occurrence and intensity of extreme weather events such as floods, floods, landslides and droughts. These changes in climate will possibly enhance the transmission of malaria and other climate-sensitive vector-borne diseases. In addition to climate change with high impact on the transmission of malaria, the projected increase of malaria in the present study could be explained by some other factors. For example, urbanization has been accelerating in China, with increasing urban population over the last few decades (Yeh et al., 2011). This and increased population migration (Gong et al., 2012) can lead independently and synergistically to malaria transmission.
There was a difference in the magnitude of malaria increase across the two emission pathways, which is likely to be related to differences in their underlying assumptions. The RCP4.5 emission pathway leads to low greenhouse gas concentration levels (4.5 W/km2) through resilient climate policy intervention (Thomson et al., 2011). In contrast, RCP8.5, the highest emission scenario, assumes no climate policy, and that greenhouse gas concentration will consistently rise associated with high population growth, followed by over-use of land and high energy demands (Wayne, 2013). Thus, the malaria transmission may be more enhanced under the latter scenario.
The magnitude of change was slightly higher for P. falciparum than P. vivax regardless of the scenario, which may indicate that the two malaria types respond differently to climatic factors (Casman and Dowlatabadi, 2002; An, 2011; Gething et al., 2011). Climate factors plays a vital role in malaria transmission because the survival and development of Anopheles mosquitoes and rate of malaria parasites developmental within the mosquitoes also called sporogonic cycle or extrinsic incubation period (Teklehaimanot et al., 2004) is sensitive to change in environmental conditions. The species-specific effect of climate factors on malaria parasite is not well defined. Some evidences suggest that P. falciparum is more sensitive to climate factors than P. vivax (Bi et al., 2013; Casman and Dowlatabadi, 2002) which concur with our findings. More recently P. vivax is reported to have shorter extrinsic incubation period (reviewed in Howes et al. (2016)), but no reported evidence comprising specific duration and temperature ranges of different malaria parasites. Some other literatures reported a likely shift towards a predominance of P. vivax malaria attributed to difference some biological features of the malaria parasites. They pointed out that i) the gametocyte of P. vivax appear earlier in victim's erythrocytes enabling transmission before P. falciparum. ii) P. vivax is characterized by hyponozoites (dormant life stage in human liver) which cause relapse long time after original infection, meaning that transmission of the latter poses greater challenge on the malaria elimination goal (Howes et al., 2016). However, these biological processes and malaria transmission can happen when and where environment is suitable.
The distribution of P. vivax and P. falciparum malaria will change in future decades, but the magnitude of change and patterns vary by the scenario. Both P. vivax and P. falciparum might increase in most parts of the south, southeast, central, southwest and some parts of the north, northwest, and northeast regions across all decades for the rest of the century under both emission scenarios, if there is no policy to mitigate climate change damage. Historically, P. vivax was mostly distributed in the provinces of the southwest, central, south and southeast regions of China (Sheng et al., 2003a). During 1999–2004, around 910–1336 counties reported malaria most of which were from southern and central provinces. More than 50% of the national malaria cases during 2002–2004 were reported from Yunnan and Hainan provinces (Sheng et al., 2003a; Zhou et al., 2005, 2006). Although malaria cases and number of affected counties slightly decreased after several years of interventions, malaria is still public health problem in some of the southern and central provinces with a consistent malaria transmission and focal outbreaks, especially in the A. sinensis transmission areas (Zhou et al., 2005, 2006; Sheng et al., 2003b). Human behavioural factors, such as population movement has also the potential to contribute to the geographic distribution of malaria, especially P. falciparum in China. Using only local malaria cases, potential increases are predicted in this study along the margins of existing transmission (Yang et al., 2010), as well as malaria/vector-free areas, indicating the potential of future climate in sustaining malaria transmission in current endemic areas, and geographic expansion of the disease in the future.
The highest percentage increase (>50%) of both P. vivax and P. falciparum malaria is predicted in some of the presently cooler regions of China (i.e., the north, northeast, northwest regions) under the high emission scenario. Furthermore, P. falciparum is expected to be expanded to the northern tip of the northeastern region under RCP8.5 scenario, while P. vivax is predicted in the areas under both scenarios. This may be explained by the expectation that future climate change will have a significant effect on expanding suitable habitat, therefore transmission of malaria (Park, 2011) (Hales et al., 2002) or a potential northward shift of malaria. Coinciding with this, a latitudinal change of malarious areas was reported by a study in Africa (Hales et al., 2002). Similarly, some regions of India, including the northern and northeast were predicted to have malaria in 2050s (Bhattacharya et al., 2006). In China, the northern and northwest regions (currently cooler regions) were predicted to be wetter and warmer (Tian et al., 2015; Shen, 2007), resulting in greater future malaria transmission associated with creation of an environment suitable for malaria vectors (Ryan et al., 2015; Hales et al., 2002). Previous studies have indicated that the average surface temperature have risen in China, and projected to increase by 2.6 °C (under RCP4.5) during 2080–2100 (Tian et al., 2015). Compared to the south, the north of China, the northwest and northeast are estimated to have a high average temperature by 2080s (Tian et al., 2015; Xu et al., 2006). The percentage change in precipitation was also projected to increase by a larger amount in the north and northwest regions of the country (Wang and Chen, 2014; Zhou et al., 2014), which was expected to create a suitable environment for malaria vectors in the regions (Ren et al., 2016). However, the geographic distribution of the principal (and efficient) malaria vectors such as A. lesteri and A. dirus has been shrinking following several years of control interventions, and shifting to predominance of A. sinensis - the exophilic and zoophilic mosquito (Zhang et al., 2017). This vector has developed resistance to insecticide, and projected to increase with climate change (Ren et al., 2016; Zhang et al., 2017) but China would have the economic resources to contain the spread of malaria through vector control intervention, improved housing or medical treatment.
The projection showed a clearer spatial change of malaria for P. vivax than P. falciparum, with a high percentage increase of P. vivax projected only in the north, northeast, and parts of the northwest of China under both scenarios. Although a high percentage increase is projected in these regions, some parts of south, southwest and central China are also expected to have P. falciparum under both scenarios. This may indicates differential impact of change in the climatic factors on malaria parasites, hence their spatiotemporal distribution. For example, a study indicated the minimum temperature for development of malaria parasite is lower for P. vivax (15 °C) than for P. falciparum (18 °C) (Shapiro et al., 2017; Patz and Olson, 2006). Thus, small increase in this climatic factor may enhanced vivax transmission in cooler area while limiting the spread of malaria in the previously hotter areas of the south, southwest and central China where temperature is expected to exceed the malaria transmission threshold (Mordecai et al., 2013).
4.1. Limitations of the study
There are several limitations that need caution when interpreting the results. First, the future projections of malaria under climate change need to consider the observed national decline in the disease over the last few decades, mainly owing to the control interventions. Second, although climate change is of major concern and present the framework within which malaria transmission is possible, other non-climate factors such as socioeconomic growth may contribute to future outcomes (Wang et al., 2014; Qi et al., 2015). Second, the underlying spatial distribution of malaria modelled with the bi-dimensional spline was assumed fixed in time during projection for the future periods. Third, several technologies are available for climate change mitigation and adaptation, and others are under research and development to minimize carbon emission (Leung et al., 2014; Lin et al., 2016). Therefore, in future, human being may control the public health effect of climate change through effective use of technology together with socioeconomic improvement may promote local capacity of the diseases control, better environmental management and land-use patterns, and implementation of health warning systems. However, the possible effects of socioeconomic and technological information were not considered, as these data were not available. These might have overestimated the impact of climate change. Fourth, we used annual data based on its importance in previous studies (Caminade et al., 2014) and clearer trends to help long-term planning. However, we concede that using county-level data, rather than individual data might have introduced uncertainty in the malaria-weather relationship. Finally, even though we used well-established model (GAM), every model has limitations, and we haven't reported uncertainty associated with our projections, which would have arose from the climate data, model fit or distribution of malaria data.
5. Conclusion
Our findings suggest that spatial and temporal distribution of P. vivax and P. falciparum malaria in China will increase due to future climate change, if there is no policy to mitigate climate change. These findings are important to guide China's malaria elimination goal and will provide targeted, evidence-based information to plan malaria control intervention.
Based on these findings, it is important that possible risk management strategies should be developed, and surveillance-response system enhanced, including in the currently malaria-free areas projected to have malaria in future. Although this study presents the results from an average of 26 GCMs projections, future study should evaluate an accuracy of every GCM in each region for the most plausible projections. Future research will be benefit by combing the RCPs with the Shared Socioeconomic Pathway (SSP) that consider the key scenario drivers such as socioeconomic growth, urbanization and population for the estimation of future malaria.
Highlights.
Potential effect of climate change on malaria was assessed under climate scenarios.
We used the GAM with spatial component and project malaria under climate scenarios.
We found a substantial increase of future malaria by a larger amount under RCP8.5.
We found a potential changes in the latitudinal extent of the malaria with climate.
Acknowledgments
YG was supported by the Career Development Fellowship of Australian National Health and Medical Research Council (APP1107107). We are grateful to the China Information System for Disease Control and Prevention (CISDCP) provided malaria data and China Meteorological data Sharing Centre for providing weather data.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2018.01.300.
References
- An Grace. Influence of climate on malaria in China. Penn McNair Res J. 2011;3 [Google Scholar]
- Astrom C, Rocklov J, Hales S, Beguin A, Louis V, Sauerborn R. Potential distribution of dengue fever under scenarios of climate change and economic development. EcoHealth. 2012;9:448–454. doi: 10.1007/s10393-012-0808-0. [DOI] [PubMed] [Google Scholar]
- Bai L, Morton LC, Liu Q. Climate change and mosquito-borne diseases in China: a review. Glob Health. 2013;9:10. doi: 10.1186/1744-8603-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Sharma C, Dhiman R, Mitra A. Climate change and malaria in India. Curr Sci. 2006;90:369. [Google Scholar]
- Bi P, Tong S, Donald K, Parton KA, Ni J. Climatic variables and transmission of malaria: a 12-year data analysis in Shuchen County, China. Public Health Rep. 2003;118:65–71. doi: 10.1093/phr/118.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Yu W, Hu W, Lin H, Guo Y, Zhou XN, Tong S. Impact of climate variability on Plasmodium vivax and Plasmodium falciparum malaria in Yunnan Province, China. Parasit Vectors. 2013;6:357. doi: 10.1186/1756-3305-6-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma MJ, Dye C, van der Kaay HJ. Falciparum malaria and climate change in the northwest frontier province of Pakistan. Am J Trop Med Hyg. 1996;55:131–137. doi: 10.4269/ajtmh.1996.55.131. [DOI] [PubMed] [Google Scholar]
- Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colon-Gonzalez FJ, Stenlund H, Martens P, Lloyd SJ. Impact of climate change on global malaria distribution. Proc Natl Acad Sci U S A. 2014;111:3286–3291. doi: 10.1073/pnas.1302089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, Tang L, Gosling RD, Feachem RG, Gao Q. Communicating and monitoring surveillance and response activities for malaria elimination: China's “1-3-7” strategy. PLoS Med. 2014;11:e1001642. doi: 10.1371/journal.pmed.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casman EA, Dowlatabadi H. The Contextual Determinants of Malaria. Resources for the Future. 2002 [Google Scholar]
- Chong-Hai X, Ying X. The projection of temperature and precipitation over China under RCP scenarios using a CMIP5 multi-model ensemble. Atmos Ocean Sci Lett. 2012;5:527–533. [Google Scholar]
- Climate features in China. 2017 http://www.china.org.cn/english/environment/222902.htm.
- Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhimal M, Ahrens B, Kuch U. Climate change and spatiotemporal distributions of vector-borne diseases in Nepal–a systematic synthesis of literature. PLoS One. 2015;10:e0129869. doi: 10.1371/journal.pone.0129869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Zeger SL, Samet JM. On the use of generalized additive models in time-series studies of air pollution and health. Am J Epidemiol. 2002;156:193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- Drexler M, Ainsworth CH. Generalized additive models used to predict species abundance in the Gulf of Mexico: an ecosystem modeling tool. PLoS One. 2013;8:e64458. doi: 10.1371/journal.pone.0064458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling W, Hooghof J, van de Vegte-Bolmer M, Sauerwein R, Van Gemert G. Tropical temperatures can inhibit development of the human malaria parasite Plasmodium falciparum in the mosquito. Proceedings of the Section Experimental and Applied Entomology-Netherlands Entomological Society. 2001:151–156. [Google Scholar]
- ESRI. Components M: Environmental Systems Research Institute. California, USA: 2001. [Google Scholar]
- Gething PW, Van Boeckel TP, Smith DL, Guerra CA, Patil AP, Snow RW, Hay SI. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasit Vectors. 2011;4:92. doi: 10.1186/1756-3305-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Gong P, Liang S, Carlton EJ, Jiang Q, Wu J, Wang L, Remais JV. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Edwards TC, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model. 2002;157:89–100. [Google Scholar]
- Hales S, De Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. CRC Press; 1990. [Google Scholar]
- Haub C. Population Reference Bureau. China Releases First 2010 Census Results. 2011 http://www.prb.org/Publications/Articles/2011/china-census-results.aspx.
- Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, Hay SI. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95:15–34. doi: 10.4269/ajtmh.16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Zhou S, Zhang S, Zhang H, Li W. Meteorological factors-based spatio-temporal mapping and predicting malaria in central China. Am J Trop Med Hyg. 2011;85:560–567. doi: 10.4269/ajtmh.2011.11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundessa S, Williams G, Zhang W, Li S, Chen L, Guo Y. Spatial change in the risks of Plasmodium vivax and Plasmodium falciparum malaria in China, 2005–2014. Infect Dis Health. 2016;21:89–96. [Google Scholar]
- IPCC. IPoCC: Data Distribution Center. AR4. SRES Scenarios; 2007. (pp. SRES Scenarios) [Google Scholar]
- IPCC. Climate change 2013: the physical science basis summary for policymakers. IPCC WGI Fifth Assessment Report. Vol. 5 IPCC; 2013. [Google Scholar]
- Khormi HM, Kumar L. Future malaria spatial pattern based on the potential global warming impact in South and Southeast Asia. Geospat Health. 2016;11:416. doi: 10.4081/gh.2016.416. [DOI] [PubMed] [Google Scholar]
- Leung DY, Caramanna G, Maroto-Valer MM. An overview of current status of carbon dioxide capture and storage technologies. Renew Sust Energ Rev. 2014;39:426–443. [Google Scholar]
- Li Liu D, Zuo H. Statistical downscaling of daily climate variables for climate change impact assessment over New South Wales, Australia. Clim Chang. 2012;115:629–666. [Google Scholar]
- Li T, Yang Z, Wang M. Temperature, relative humidity and sunshine may be the effective predictors for occurrence of malaria in Guangzhou, southern China, 2006–2012. Parasit Vectors. 2013;6:155. doi: 10.1186/1756-3305-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Wen, Run-He SHI, De-Zhoni Xu, Zhe Y, Yi Cheng, Yong W. Correlations between land surface temperature, land use type and malaria epidemics of Hainan Province, China. Fourth Mil Med Univ. 2008;29 [Google Scholar]
- Lin E, Jiang K, Hu X, Zuo J, Li M, Ju H. Climate Change Mitigation and Adaptation: Technology and Policy Options. Climate and Environmental Change in China: 1951–2012. Springer; 2016. pp. 107–127. [Google Scholar]
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Med Int Health. 2000;5:263–274. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- Lu G, Zhou S, Horstick O, Wang X, Liu Y, Muller O. Malaria outbreaks in China (1990–2013): a systematic review. Malar J. 2014;13:269. doi: 10.1186/1475-2875-13-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens WJ, Niessen LW, Rotmans J, Jetten TH, McMichael AJ. Potential impact of global climate change on malaria risk. Environ Health Perspect. 1995;103:458–464. doi: 10.1289/ehp.95103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney CF, Jones RG. How representative is the spread of climate projections from the 5 CMIP5 GCMs used in ISI-MIP? Clim Serv. 2016;1:24–29. [Google Scholar]
- Moisen GG, Frescino TS. Comparing five modelling techniques for predicting forest characteristics. Ecol Model. 2002;157:209–225. [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111(Pt 5):539–545. doi: 10.1017/s0031182000077003. [DOI] [PubMed] [Google Scholar]
- Pakdad K, Hanafi-Bojd AA, Vatandoost H, Sedaghat MM, Raeisi A, Moghaddam AS, Foroushani AR. Predicting the potential distribution of main malaria vectorsGlob Anopheles stephensi, An. culicifacies s.l. and An. fluviatilis s.l. in Iran based on maximum entropy model. Acta Trop. 2017;169:93–99. doi: 10.1016/j.actatropica.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Park JW. Changing transmission pattern of Plasmodium vivax malaria in the Republic of Korea: relationship with climate change. Environ Health Toxicol. 2011;26:e2011001. doi: 10.5620/eht.2011.26.e2011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci U S A. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Olson SH. Malaria risk and temperature: influences from global climate change and local land use practices. Proc Natl Acad Sci U S A. 2006;103:5635–5636. doi: 10.1073/pnas.0601493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Wang Y, Li Y, Meng Y, Chen Q, Ma J, Gao GF. The effects of socioeconomic and environmental factors on the incidence of dengue fever in the Pearl River Delta, China, 2013. PLoS Negl Trop Dis. 2015;9:e0004159. doi: 10.1371/journal.pntd.0004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y-J, Zhang L, Xia Z-G, Vong S, Yang W-Z, Wang D-Q, Xiao N. Preparation for malaria resurgence in China: approach in risk assessment and rapid. Malaria Control and Elimination Program in the People's Republic of China. 2014;86:267. doi: 10.1016/B978-0-12-800869-0.00010-X. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- Rastogi M, Pal NL, Sen AB. Effect of variation in temperature on development of Plasmodium berghei (NK 65 strain) in Anopheles stephensi. Folia Parasitol. 1987;34:289–297. [PubMed] [Google Scholar]
- Ren Z, Wang D, Ma A, Hwang J, Bennett A, Sturrock HJ, Fan J, Zhang W, Yang D, Feng X, et al. Predicting malaria vector distribution under climate change scenarios in China: challenges for malaria elimination. Sci Rep. 2016;6 doi: 10.1038/srep20604. 20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CW, Wright DA. WGEN: A Model for Generating Daily Weather Variables. 1984.
- Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K, Lafferty KD. Mapping physiological suitability limits for malaria in Africa under climate change. Vector Borne Zoon Dis. 2015;15:718–725. doi: 10.1089/vbz.2015.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov M. The use of multi-model ensembles from global climate models for impact assessment of climate change. EGU General Assembly Conference Abstracts; 2009. p. 12732. [Google Scholar]
- Shapiro LLM, Whitehead SA, Thomas MB. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017;15:e2003489. doi: 10.1371/journal.pbio.2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. Temperature and precipitation changes in China during the Holocene. Adv Atmos Sci. 2007;24:1024–1036. [Google Scholar]
- Sheng HF, Zhou SS, Gu ZC, Zheng X. Malaria situation in the People's Republic of China in 2002. Chin J Parasitol Parasit Dis. 2003a;21:193–196. [PubMed] [Google Scholar]
- Sheng HF, Zhou SS, Gu ZC, Zheng X. Malaria situation in the People's Republic of China in 2002. Zhongguo J Sheng Chong Xue Yu J Sheng Chong Bing Za Zhi. 2003b;21:193–196. [PubMed] [Google Scholar]
- Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science. 2014;343:1154–1158. doi: 10.1126/science.1244325. [DOI] [PubMed] [Google Scholar]
- Song Y, Ge Y, Wang J, Ren Z, Liao Y, Peng J. Spatial distribution estimation of malaria in northern China and its scenarios in 2020, 2030, 2040 and 2050. Malar J. 2016;15:345. doi: 10.1186/s12936-016-1395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Guerra CA, Kabaria CW, Noor AM, Hay SI. Human population, urban settlement patterns and their impact on Plasmodium falciparum malaria endemicity. Malar J. 2008;7(1) doi: 10.1186/1475-2875-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teklehaimanot HD, Lipsitch M, Teklehaimanot A, Schwartz J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar J. 2004;3:41. doi: 10.1186/1475-2875-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Calvin KV, Smith SJ, Kyle GP, Volke A, Patel P, Delgado-Arias S, Bond-Lamberty B, Wise MA, Clarke LE. RCP4. 5: a pathway for stabilization of radiative forcing by 2100. Clim Chang. 2011;109:77–94. [Google Scholar]
- Tian D, Guo Y, Dong W. Future changes and uncertainties in temperature and precipitation over China based on CMIP5 models. Adv Atmos Sci. 2015;32:487–496. [Google Scholar]
- Tonnang HE, Kangalawe RY, Yanda PZ. Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malar J. 2010;9:111. doi: 10.1186/1475-2875-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F. The representative concentration pathways: an overview. Clim Chang. 2011;109:5. [Google Scholar]
- Walsh WA, Kleiber P. Generalized additive model and regression tree analyses of blue shark (Prionace glauca) catch rates by the Hawaii-based commercial longline fishery. Fish Res. 2001;53:115–131. [Google Scholar]
- Wang L, Chen W. A CMIP5 multimodel projection of future temperature, precipitation, and climatological drought in China. Int J Climatol. 2014;34:2059–2078. [Google Scholar]
- Wang L, Wang Y, Yang G, Ma J, Wang L, Qi X-P. China Information System for Disease Control and Prevention (CISDCP) 2013 [Google Scholar]
- Wang SQ, Li YC, Zhang ZM, Wang GZ, Hu XM, Qualls WA, Xue RD. Prevention measures and socio-economic development result in a decrease in malaria in Hainan, China. Malar J. 2014;13:362. doi: 10.1186/1475-2875-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne GP. The beginner’s guide to representative concentration pathways. Skept Sci. 2013 https://skepticalscience.com/docs/RCP_Guide.pdf, Version 1.0. [Google Scholar]
- Wei C, Jinju L. Future Population Trends in China: 2005–2050. Monash University, Centre of Policy Studies and the Impact Project; 2009. [Google Scholar]
- World Health Organization (WHO) World Malaria Report-2014. WHO; Geneva: 2015. [Google Scholar]
- Xie B, Zhang Q, Ying Y. Trends in precipitable water and relative humidity in China: 1979–2005. J Appl Meteorol Climatol. 2011;50:1985–1994. [Google Scholar]
- Xu Y, Zhang Y, Lin E, Lin W, Dong W, Jones R, Hassell D, Wilson S. Analyses on the climate change responses over China under SRES B2 scenario using PRECIS. Chin Sci Bull. 2006;51:2260–2267. [Google Scholar]
- Yang G-J, Gao Q, Zhou S-S, Malone JB, McCarroll JC, Tanner M, Vounatsou P, Bergquist R, Utzinger J, Zhou X-N. Mapping and predicting malaria transmission in the People's Republic of China, using integrated biology-driven and statistical models. Geospat Health. 2010;5:11–22. doi: 10.4081/gh.2010.183. [DOI] [PubMed] [Google Scholar]
- Yeh AG, Xu J, Liu K. China's Post-reform Urbanization: Retrospect, Policies and Trends. IIED; 2011. [Google Scholar]
- Yin JH, Yang MN, Zhou SS, Wang Y, Feng J, Xia ZG. Changing malaria transmission and implications in China towards National Malaria Elimination Programme between 2010 and 2012. PLoS One. 2013;8:e74228. doi: 10.1371/journal.pone.0074228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JH, Zhou SS, Xia ZG, Wang RB, Qian YJ, Yang WZ, Zhou XN. Historical patterns of malaria transmission in China. Adv Parasitol. 2014;86:1–19. doi: 10.1016/B978-0-12-800869-0.00001-9. [DOI] [PubMed] [Google Scholar]
- Zell R. Global climate change and the emergence/re-emergence of infectious diseases. Int J Med Microbiol. 2004;293(Suppl. 37):16–26. doi: 10.1016/s1433-1128(04)80005-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bi P, Hiller JE. Meteorological variables and malaria in a Chinese temperate city: a twenty-year time-series data analysis. Environ Int. 2010;36:439–445. doi: 10.1016/j.envint.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lai S, Zheng C, Zhang H, Zhou S, Hu W, Clements AC, Zhou XN, Yang W, Hay SI, et al. The epidemiology of plasmodium vivax and plasmodium falciparum malaria in China, 2004–2012: from intensified control to elimination. Malar J. 2014;13:419. doi: 10.1186/1475-2875-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Guo S, Feng X, Afelt A, Frutos R, Zhou S, Manguin S. Anopheles vectors in mainland China while approaching malaria elimination. Trends Parasitol. 2017;33:889–900. doi: 10.1016/j.pt.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci U S A. 2004;101:2375–2380. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SS, Tang LH, Sheng HF. Malaria situation in the People's Republic of China in 2003 (in Chinese) Zhongguo J Sheng Chong Xue Yu J Sheng Chong Bing Za Zhi. 2005;23:385–387. [PubMed] [Google Scholar]
- Zhou SS, Tang LH, Sheng HF, Wang Y. Malaria situation in the People's Republic of China in 2004. Zhongguo J Sheng Chong Xue Yu J Sheng Chong Bing Za Zhi. 2006;24:1–3. [PubMed] [Google Scholar]
- Zhou SS, Wang Y, Tang LH. Malaria situation in the People's Republic of China in 2006 (in Chinese) Chin J Parasit Parasit Dis. 2007;25:439–441. [PubMed] [Google Scholar]
- Zhou B, Wen QH, Xu Y, Song L, Zhang X. Projected changes in temperature and precipitation extremes in China by the CMIP5 multimodel ensembles. J Clim. 2014;27:6591–6611. [Google Scholar]