Summary
Background:
In a phase 2 short-term (6 months) study of patients with congenital adrenal hyperplasia (CAH), continuous subcutaneous hydrocortisone infusion (CSHI) was found to be a safe, effective, and well-tolerated method of replacing cortisol with improved disease and patient related outcomes.
Objective:
Evaluate the safety and efficacy of long-term CSHI.
Design:
Single centre, open label, phase 2 extension study.
Patients:
Five adults with classic CAH.
Measurements:
Biomarkers of disease control, metabolic indices and health-related quality-of-life (HRQoL) estimates.
Results:
Six of eight patients chose to continue on long-term CSHI therapy. Compared to baseline, eighteen months of CSHI resulted in decreased (P = 0.043) 0700–hour ACTH, 17-hydroxyprogesterone, androstenedione, and progesterone; increased whole-body lean mass (P = 0.024); and improved HRQoL, especially symptoms of adrenal insufficiency (P = 0.003). Findings at six and eighteen months did not differ and improvements achieved in androgen control, lean body mass and HRQoL after six months of CSHI were maintained at eighteen months. The hydrocortisone dose appeared to decrease with time [6 vs. 18 months: 38.3 ± 8.8 vs. 33.6 ± 12.2 mg/day (P = 0.062)], especially in women receiving oral contraceptives. Reduction of testicular adrenal rest and adrenal size observed at 6 months remained stable. In one patient, an adrenal adenoma continually decreased over time. Subjective improvement in hirsutism was reported.
Conclusions:
Long-term use of CSHI is a safe and well-tolerated treatment option in a select set of adults with classic CAH. Improvements observed short-term in disease control and subjective health status continued long-term.
Keywords: Congenital adrenal hyperplasia, continuous subcutaneous hydrocortisone infusion, circadian
1 |. INTRODUCTION
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21-OHD) is characterized by impairment in cortisol and aldosterone production along with androgen excess.1 Management goals in CAH include replacement of deficient hormones, control of excess androgen and its consequences, and avoidance of glucocorticoid over-replacement and its consequences. Conventional cortisol replacement with oral glucocorticoid is suboptimal and many patients suffer from adverse outcomes due to hyperandrogenism, hypercortisolism, or a combination of these two states.2 Current conventional glucocorticoid replacement therapies also fail to mimic the physiological cortisol rhythm, which has a distinct circadian pattern.3 Novel glucocorticoid replacement approaches aiming to replace cortisol in a physiological manner are under study, including the use of a modified-release formulation of hydrocortisone (Chronocort®) and continuous subcutaneous hydrocortisone infusion (CSHI).4, 5 We previously reported improvements in adrenal androgen control and health-related quality-of-life (HRQoL) estimates with the use of CSHI therapy in adults with difficult-to-treat CAH, defined as having androgen and glucocorticoid related co-morbidities on conventional oral glucocorticoid therapy.6 Overall, short-term (6 months) use of CSHI was found to be safe, effective and a well-tolerated method of replacing cortisol in adults with classic CAH with improved outcomes; positive effects on HRQoL were the most significant.6 The aim of this study was to evaluate the safety and efficacy of long-term use of CSHI.
2 |. PATIENTS AND METHODS
2.1 |. Subjects
Eight patients with difficult-to-treat classic CAH due to 21-hydroxylase deficiency were enrolled in a six month phase II clinical trial (NCT01859312) using CSHI. 6 Following completion of the short-term segment of the trial, six patients (four females) opted to continue on CSHI therapy for an additional year. Two patients switched back to oral glucocorticoid therapy as they found CSHI therapy did not suit their lifestyle. Details of the study participants and baseline clinical and hormonal characteristics have been previously described.6 One patient underwent bariatric surgery for morbid obesity and was excluded from this study; however, she currently is maintained on CSHI therapy.7 The long-term study was approved by the Eunice Kennedy Shriver National Institute of Child Health & Human Development Institutional Review Board, and all patients provided written informed consent.
2.2 |. Study Design
The objective of this study was to assess the long-term safety and efficacy of CSHI therapy. Specifically, our goal was to observe if the improvements achieved in androgen control and HRQoL estimates after six months of CSHI therapy were maintained at eighteen months.6 All patients remained on their daily fludrocortisone replacement and dose adjustments were made if necessary based on plasma renin activity levels and clinical symptomatology. All patients returned to the National Institutes of Health (NIH) Clinical Center (Bethesda, Maryland) for two to three visits over the additional one-year period completing a total duration of eighteen months of CSHI therapy. At each visit, all patients had a history and examination, anthropometric measurements (weight, waist and hip circumference). Hirsutism was assessed using Ferriman–Gallwey score and was defined as total score ≥ 8.8 Laboratory evaluation included 24-hour serial hormone sampling and fasting laboratory evaluation for bone turnover markers (cross-linked telopeptide [CTX] and osteocalcin), insulin, glucose, and liver enzymes [(aspartate aminotransferase (AST), alanine aminotransferase (ALT)]. Homeostasis model assessment–insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR = [fasting insulin (U/mL) * fasting glucose (mmol/L)]/22.5.
2.3 |. CSHI Therapy
Details of the CSHI therapy have been previously described.6 Briefly, hydrocortisone sodium succinate (100 mg/2 mL Solu-Cortef ACT-O-VIAL, Pfizer Inc) was reconstituted to a 50 mg/mL solution, resulting in 0.5 mg of hydrocortisone per pump-administered unit. Medtronic MiniMed Paradigm REAL-Time (MMT-722) insulin pump, MMT-332A reservoir and Mio 9 mm/32” infusion sets were used. Hydrocortisone dosing adjustments were made based on adrenal steroid profiles and clinical symptomatology, while maintaining a near physiological diurnal pattern of cortisol replacement.3, 6 Clinical symptomatology was prioritized in the presence of conflicting data. Compliance with CSHI was assessed by medication/supply accountability and review of total basal dose logs for a month prior to the visit.
2.4 |. Hormonal Assays
Assays for ACTH, cortisol, 17-hydroxyprogsterone (17-OHP), androstenedione, progesterone, and testosterone were performed at the NIH Clinical Center, Bethesda, MD, USA. Plasma ACTH was analyzed by chemiluminescent immunoassay on Siemens Immulite 2000 XPi analyzer with an analytical sensitivity of 1.1 pmol/L (5 pg/mL), intra-assay coefficient of variation (CV) 2.5% and interassay CV 3.6%. Serum cortisol, 17-OHP, androstenedione, progesterone, and testosterone were analyzed by liquid chromatography-tandem mass spectrometry. Cortisol assay had a sensitivity of 16.6 nmol/L (0.6 µg/dL), inter-assay CV ranged from 3.1 – 3.2% and intra-assay CV from 5.0 –7.7%. For 17-OHP, androstenedione, progesterone, and for testosterone the intra-assay CV ranged from 2.5 – 9.5% and inter-assay CV from 2.9 – 11.1%. 6
2.5 |. Imaging studies
Radiological studies performed at baseline, six- and eighteen-months (end of the study) included dual-energy x-ray absorptiometry (DXA) to evaluate body composition and bone mineral density (BMD), pelvic ultrasound and magnetic resonance imaging (MRI) in females to assess for ovarian morphology and adrenal rest tumours 9, testicular ultrasound in males to assess adrenal rest tumours. Abdominal MRI with liver proton magnetic resonance spectroscopy (MRS) was performed to assess adrenal morphology, liver fat, visceral fat and subcutaneous fat. Liver fat was classified as mild (5–20%), moderate (20–45%) or severe (> 35%). DXA data was corrected according to the National Health and Nutrition Examination Survey.10
2.6 |. Quality-of-life Assessment
At each visit, patients completed questionnaires evaluating fatigue [multi-dimensional assessment of fatigue (MAF)]11, HRQoL [36-item short-form health survey (SF-36 v2)12, adrenal insufficiency specific questionnaire (AddiQoL)]13, and signs and symptoms of adrenal insufficiency or glucocorticoid overtreatment. MAF questionnaire data was used to compute the Global Fatigue Index (GFI) score. GFI scores range from 1 (no fatigue) to 50 (severe fatigue).11 OptumInsight software was used to compute SF-36 score. The software provides a norm-based score, which employs a linear T-score transformation (mean 50, standard deviation 10).12 AddiQoL scores range from 30 (worst possible) to 120 (best possible) as previously described.13
2.7 |. Statistical Analysis
Data are expressed as mean ± SEM, unless otherwise stated. IBM SPSS Statistics 21 was used for data analysis. Depending on the data distribution, parametric (paired t-test) and non-parametric (Wilcoxon signed-rank) tests were used to compare changes in area-under-the-curves (AUC), metabolic indices, hormone biomarkers, and HRQoL estimates from baseline (conventional glucocorticoid therapy) to six- and eighteen-months of CSHI therapy. Categorical data between these intervals were compared using McNemar’s test. Despite the small sample size, all tests were two-tailed and P-values < 0.05 were considered statistically significant.
3 |. RESULTS
3.1 |. Patient data
Five adults (three females; age 25 – 41 years) with classic CAH (four salt-wasting, one simple-virilizing) participated in the long-term study (Table 1). Three females (two with history of secondary amenorrhoea for > 2 years) were initiated on combined OCP. One male patient refused abdominal MRI with liver proton MRS study at the eighteen-month visit. Medication accountability showed all patients were compliant with CSHI therapy.
Table 1:
Baseline characteristics of five patients with classic congenital adrenal hyperplasia on long-term continuous subcutaneous hydrocortisone infusion therapy
| Pt | Age/ Sex |
Phenotype | Glucocorticoid Dose as hydrocortisone a equivalent (mg/day) |
BMI (kg/m2) |
Insulin Resistance b |
Fatty Liver c |
Low BMD d |
GI Intolerance to GC |
TART /PCOS e |
Adrenal mass/ hypertrophy |
Hirsutism i |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25/M | SW | 40 | 34.8 | + | + | − | + | + | + f | NA |
| 2 | 38/F | SW | 50 | 39.8 | + | + | − | − | + | + f, g | + |
| 3 | 32/F | SW | 32.5 | 54.1 | + | + | + | + | + | − | + |
| 4 | 25/F | SV | 25 | 40.3 | + | − | + | + | + | − | + |
| 5 | 41/M | SW | 25 | 27.7 | − | − | + | − | + | + f | NA |
Abbreviations: Pt, patient; BMI, body mass index; BMD, bone mineral density; GI, gastrointestinal; GC, glucocorticoid; TART, testicular adrenal rest tissue; PCOS, polycystic ovary syndrome; M, Male; F, Female; SV, simple virilizing; SW, salt-wasting; +, present; −, not present; NA, not applicable.
Glucocorticoid equivalent dose (mg/day): hydrocortisone X 1, prednisone and prednisolone X 5, and dexamethasone X 80 (2)
HOMA-IR > 2.6
AST/ALT <1 or steatosis by ultrasound
DEXA T-score <−1
12 or more follicles 2–9 mm in diameter and/or an increased ovarian volume > 10 mL by ultrasound (9)
Bilateral adrenal hyperplasia
Adrenal adenoma
Hirsutism was defined as a Ferriman-Gallwey total score ≥ 8 (8)
3.2 |. Cortisol profiles and biomarkers of disease control
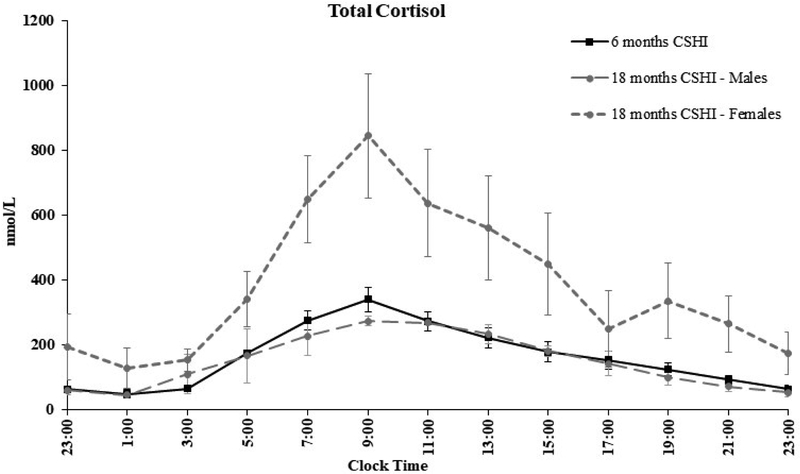
The cortisol profile continued to approximate a near physiological diurnal pattern of cortisol replacement at eighteen months (Figure 1). An increase (~ 45%) in serum total cortisol levels was observed in all three women following initiation of OCP therapy. Measurement of free cortisol was not performed.
Figure 1:
Serum total cortisol levels over 24-hours on continuous subcutaneous hydrocortisone infusion (CSHI) therapy at 6 months and 18 months by sex. Females were receiving OCP therapy at 18 months. An increase of approximately 45% in serum total cortisol levels was observed following initiation of OCP therapy. Measurement of free cortisol was not performed.
Following 18 months of CSHI therapy, all patients had lower 0700–hour ACTH (P = 0.043), 17-OHP (P = 0.043), androstenedione (P = 0.043), and progesterone (P = 0.043) compared to baseline (on oral glucocorticoid therapy). Identical p-values were obtained as non-parametric testing was used. Early morning (0700-hour) and 24-hour biomarker profiles on CSHI therapy at the end of the short–term study phase (six months) and at the end of the long-term study phase (eighteen months) were similar (P > 0.10) (Figure 2).
Figure 2:
Comparison of 24-hour serial hormone levels on conventional glucocorticoid therapy (baseline) vs. continuous subcutaneous hydrocortisone infusion (CSHI) therapy for 6 and 18 months.
Sub-group analysis by sex did not show differences in biomarkers of disease control; but hydrocortisone dose requirements decreased by 13 – 31% in women following introduction of OCPs (n=3). In general, the hydrocortisone dose decreased with time, although this decrease did not reach statistical significance [6 vs. 18months: 38.3 ± 8.8 vs. 33.6 ± 12.2 mg/day, (P = 0.062)]. Two female patients had improvements in testosterone levels (baseline: 5.7, 2.8; 6 months: 1.6, 2.6; 18 months: undetectable, 0.7 nmol/L, respectively). Testosterone remained stable in the normal range for male participants.
3.3 |. Disease-related metabolic parameters and co-morbidities
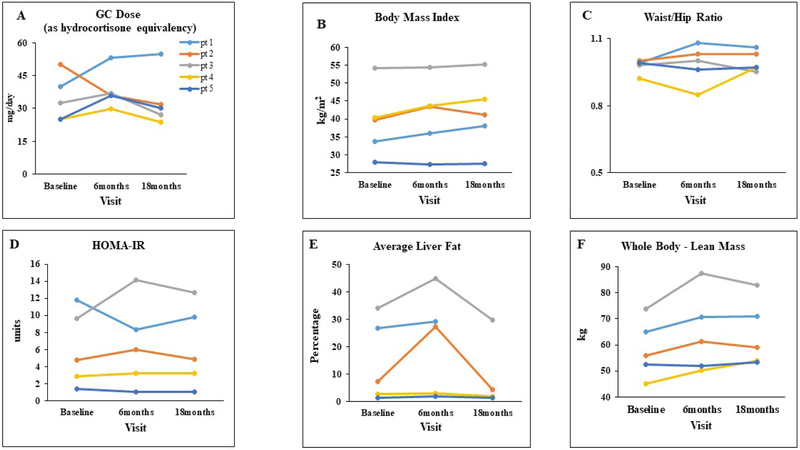
Compared to baseline and following 18 months of CSHI therapy, there were no significant changes in weight, body mass index (Figure 3B), waist/hip circumference, AST and ALT, and HOMA-IR (Figure 3D). At baseline, three of five patients were noted to have mild - severe liver steatosis on MRS. Overall, MRS imaging at the end of the study, showed minimal change in hepatic steatosis with average liver fat (including liver dome, right and left lobes) decreasing from 14.4 ± 6.7% at baseline to 9.3 ± 6.9% at 18 months (P = 0.144). These changes were not statistically significant; however the two patients with the greatest increase in liver fat following 6 months of CSHI therapy had dramatic improvements in liver fat measurements at 18 months, with percent liver fat less than baseline measurements (Figure 3E). No significant changes were observed in visceral fat and subcutaneous fat measurements.
Figure 3:
Glucocorticoid dose (Panel A) and metabolic characteristics (Panel B-F) on conventional glucocorticoid therapy at baseline, and following continuous subcutaneous hydrocortisone infusion therapy for 6 and 18 months.
Abbreviations: GC, glucocorticoid; HOMA-IR, Homeostasis model assessment–insulin resistance
Glucocorticoid equivalent dose (mg/day): hydrocortisone X 1, prednisone and prednisolone X 5, and dexamethasone X 80 (2)
No significant changes were noted over time in the serum osteocalcin or CTX. Whole-body BMD and total fat mass by DXA remained stable over the course of the study. However, an increase was noted in whole-body lean mass (58.48 ± 5 vs. 64.1 ± 5.7 kg, P = 0.024; Figure 3F).
At baseline, both males in the study had testicular adrenal rest tissue (TART). A reduction in TART size was noted in one male at the end of 6 months which remained stable at the end of the study. The largest dimension of the TART lesion in this patient was 1.3 cm, 0.9 cm, and 1.0 cm at baseline, 6, and 18 months respectively. TART size remained stable in the other male patient. Pelvic sonography and MRI demonstrated polycystic ovaries in two females and findings remained stable over the course of the study. Of note, there was no restoration of menses post-OCP initiation in the two females with a history of secondary amenorrhoea. At baseline, all three females had hirsutism (Ferriman–Gallwey scale 10 – 15). Subjective improvement in hirsutism was reported by all three females with reports of decreased frequency of use of depilatories.
Adrenal imaging (MRI) at baseline demonstrated a left sided adrenal adenoma (4.2 × 3.2 cm) along with bilateral adrenal hyperplasia in one female patient. The size of the adenoma decreased over the course of the study (3.5 × 2.5 cm at 6 months and 3 × 2.3 cm at 18 months). At baseline, two male patients had bilateral adrenal hypertrophy. One male patient had mild improvements in the adrenal sizes at the end of 6 months (maximum adrenal width 1.1 cm and 0.6 cm at baseline and 6 months respectively). This patient refused abdominal MRI at the 18 months visit. No other changes were observed in adrenal morphology.
3.4 |. Quality-of-life estimates
Compared to baseline, HRQoL estimates remained improved following 18 months of CSHI therapy. Based on the SF-36 questionnaire, improvements from baseline were observed in the Vitality (P = 0.035), Physical Functioning (P = 0.043), General Health (P = 0.018), and Mental Health (P = 0.049) domains. The most significant improvement maintained long-term was in symptoms of adrenal insufficiency based on the AddiQoL, an adrenal insufficiency specific questionnaire (P = 0.003). There were no significant changes observed in HRQoL measures between the 6 to 18 month time periods (Figure 4).
Figure 4:
Health-Related Quality-of-Life Scores (HRQoL) on Conventional Glucocorticoid Therapy at Baseline and following 6 and 18 months of continuous subcutaneous hydrocortisone infusion therapy.
Abbreviations: SF-36, 36-item short-form health survey; PF, Physical Functioning; GH, General Health; VT, Vitality; MH, Mental Health, AddiQoL, Adrenal insufficiency-specific quality-of -life questionnaire.
3.5 |. Safety and Tolerability
CSHI therapy was well tolerated long-term with no major mechanical issues with the pump. No serious adverse events occurred over the course of the study. The most common adverse event was local skin reaction and pruritus at the catheter site (Table 2). Three short-lived adverse events were associated with the pump: 1 report of dizziness and 1 report of fatigue and weakness were associated with a kink in the catheter; 1 episode of dizziness was associated with the patient forgetting to turn the pump on. Following completion of the long-term study phase, all patients opted to continue on CSHI therapy for disease management. Currently, four patients continue on CSHI therapy and one patient switched back to oral glucocorticoid therapy due to financial constraints.
Table 2.
Adverse events during long-term phase (6 to 18 months) of continuous subcutaneous hydrocortisone infusion therapy.
| Adverse Event | No. of patients |
Outcome |
|---|---|---|
| Skin erythema with pruritus at the infusion site |
4 | Self-limited in all patients, in one patient related to delay in infusion set change |
| Local skin infection at the infusion set site |
1 | Resolved after oral antibiotics |
| Dizziness | 2 | Intermittent (n=2, one episode associated with kink in pump catheter, one episode associated with patient forgetting to turn on pump after reconnecting) |
| Lightheadedness | 2 | Intermittent (n=1, noted when moving from sitting to standing position, resolved with fludrocortisone dose increase) and episodes related to exposure to heat and prolonged standing (n=1), both self-resolved. |
| Fatigue | 3 | Intermittent (n=3), associated with kink in pump catheter (n=1), related to sleep problems (n=1) |
| Weakness | 1 | One episode associated with kink in pump catheter |
| Nausea | 2 | Intermittent and self-limited (n=1), one episode associate with viral illness |
| Headache | 2 | Intermittent (n=2), one patient with history of chronic headaches reported worsening headaches, neurology consult obtained and was diagnosed with stress related headaches |
| Decreased appetite | 1 | Intermittent |
| Difficulty falling asleep or Frequent wakening |
1 | Intermittent (history of sleep problems), some improvement with dose adjustment |
| Early awakening | 2 | Some improvement with dose adjustments |
| Small joint stiffness | 1 | Diagnosed with arthritis |
| Elbow pain | 1 | Patient diagnosed with ulnar entrapment syndrome, resolved with change in sleep position |
| Chest pain | 1 | Self-limited, related to stress at work |
4 |. DISCUSSION
Our study is the first to systematically evaluate the long-term safety and efficacy of CSHI in adults with classic CAH. We demonstrate that CSHI therapy is safe and well tolerated long-term. We observed that improvements achieved in adrenal hormonal control (17-OHP and androstenedione), HRQoL, and tumour size following 6 months of CSHI therapy were maintained long-term. Over time, the hydrocortisone dose needed to maintain optimal clinical status decreased in women receiving estrogen containing OCPs, and stable disease control was achieved for 18 months on glucocorticoid doses similar to or lower than treatment doses on conventional oral therapy. In general, the co-morbidities observed at baseline while on oral glucocorticoid therapy improved or remained stable while receiving CSHI therapy.
Similar to our observations in the short-term study phase, despite achieving near-physiological cortisol profiles, ACTH remained mildly elevated overnight (23:00 – 07:00 hours), early morning (07:00 hour) and daytime (07:00 – 15:00 hours) resulting in elevated adrenal androgens during these times respectively. We were limited in our attempts to address the elevated ACTH levels by increasing cortisol infusion rates as patients reported sleep disturbances with higher cortisol rates overnight. Sleep is a complex physiological process which involves the interplay of several neurotransmitters and hormonal pathways including the hypothalamic-pituitary-adrenal (HPA) axis.14 As patients with classic CAH have inherent cortisol deficiency, we speculate that they may be easily susceptible to stimulation of ACTH and CRH when exposed to varying glucocorticoid levels. Glucocorticoids likely influence sleep mostly through indirect effects on CRH.15 Formal assessments of sleep (questionnaires, sleep study) or electrical activity of the brain might be useful to evaluate the interplay between the HPA axis and sleep in CAH patients, but were not performed as part of our study.
Overall, weight and other anthropometric indices of metabolic syndrome remained stable over the course of the study. Although insulin resistance (assessed by HOMA-IR) and fat-mass remained stable, a significant increase in whole-body lean mass was observed. We also observed an increase in whole-body lean mass following 6 months of CSHI therapy6 and in the study of Chronocort®, an oral modified-release form of hydrocortisone that aims to replicate the diurnal pattern of cortisol secretion.4 The aetiology of this finding is unclear. However, it is well established that the hypothalamic-pituitary–adrenal axis has a profound impact on body composition16. Most notably, excess cortisol results in increased fat mass, decreased bone mass and reduced lean mass, and disruption of normal circadian rhythms has been linked to obesity/metabolic syndrome and has an effect on adipose tissue.16, 17 We speculate that with the use of CSHI therapy, both the achievement of diurnal cortisol replacement and improvements in HRQoL may have played a role in increasing lean body mass. Improvement in HRQoL, resulting in increased physical functioning, and resolution of adrenal insufficiency symptomatology may have contributed indirectly by increasing physical activity with an overall increase in functional capacity.
Non-alcoholic fatty liver disease (NAFLD) is commonly associated with obesity. Prolonged and high dose glucocorticoid treatment has been implicated in the pathogenesis of NAFLD.18 All of our patients were receiving longer-acting glucocorticoid preparations at supraphysiological doses with poor disease control at baseline. All (except one male) patients were obese and had insulin resistance at study entry. Three of four patients had varying degree of hepatic steatosis at baseline. There were no significant changes seen in weight/BMI or HOMA-IR over the course of the study. . However, measures of liver fat content showed some improvement at the end of 18 months, especially in two patients. Another variable that may have influenced liver fat was the introduction of OCP. Estrogen containing OCP use has been reported to be associated with significantly reduced odds for developing of NAFLD.19 We speculate that several factors may have contributed to the improvements noted in hepatic steatosis including decrease in glucocorticoid dose, use of OCP and possible alterations in the enzymes involved in glucocorticoid metabolism/action (11β-hydroxysteroid dehydrogenase and A-ring reductases) with the use of diurnal cortisol replacement via CSHI therapy.20, 21
The use of OCPs in three females may have influenced our ability to decrease hydrocortisone dose over time. Physiological cortisol delivery likely played a role in our ability to reduce hydrocortisone, especially initially. But in our long-term study, we were able to reduce hydrocortisone further after initiation of OCPs. The anti-androgenic effects of OCP likely played a role in our ability to further reduce hydrocortisone dose. Estrogen-containing pills address hyperandrogenism in CAH via several mechanisms: an increase in hepatic production of sex hormone binding globulin increases androgen binding in serum thus reducing free androgen; suppression of the hypothalamic-pituitary-ovarian axis lowers ovarian androgen production; mild blockage of androgens to their receptor occurs.22 Estrogen containing pills have been shown to reduce ACTH secretion and thus may reduce adrenal androgen production.22, 23 In addition, mean plasma ACTH concentrations have been shown to be lower in women receiving OCPs compared to women not receiving OCPs, thus an effect on pituitary ACTH may also play a role in reducing adrenal androgens.23 In our study, significant improvements were noted in androgen levels allowing for reduction in hydrocortisone dose by 13 – 31%.
The effect of estrogen on cortisol metabolism is complex and the impact of this interaction on the management of our CAH patients is unknown. Estrogen containing OCPs affect cortisol metabolism by increasing protein-bound cortisol and total cortisol, and potentially free cortisol. The effects of increased cortisol at the tissue level are not seen as protein-bound cortisol contributes to the rise in plasma cortisol. 24, 25 Women on OCP therapy show greater increase in cortisol levels following exogenous cortisol administration when compared to women not on OCP suggesting a role of OCP in cortisol metabolism.26 As expected, we did note increases in total cortisol levels post-OCP initiation (approximately 45%). Unfortunately, free cortisol and salivary cortisol were not measured as part of this study. .
Our study population consisted of challenging patients with multiple co-morbidities at baseline. Poor disease control has been implicated in tumour growth in patients with CAH.27 Adrenal masses, enlarged adrenals and TART are commonly reported in CAH, especially in patients with suboptimal therapy.28, 29 In our study, adrenal size, TART size and ovarian morphology were mostly unchanged following CSHI therapy, but one patient with a unilateral benign adrenal adenoma had a progressive decrease in the size of the lesion. In addition, at 6 months, one patient had a decrease in TART size and one patient with adrenal hypertrophy had a decrease in adrenal size; these decreased sizes remained stable over time. Despite significant improvements in androgen levels and OCP therapy, two females with secondary amenorrhoea did not have resumption of menses. However, subjective improvement in hirsutism was reported by all three females.
In our previous report on the short-term use of CSHI, we described significant improvements in HRQoL and fatigue, and this was the primary reason why patients wished to continue CSHI therapy. After 6 months of CSHI, the most significant HRQoL improvements were observed in the SF-36 Vitality Score, a domain previously reported to be impaired in patients with CAH 30, 31, AddiQoL, an adrenal insufficiency specific questionnaire,13 and fatigue.6 After 18 months of CSHI, improved HRQoL was maintained, especially as measured by the AddiQoL questionnaire, an instrument developed to evaluate within-individual well-being in patients with adrenal insufficiency in clinical trials.32 After 18 months, patients continued to report improved HRQoL as the main reason for wishing to continue with CSHI therapy. Overall, the significant improvements in HRQoL associated with CSHI therapy seen short-term were maintained long-term.
Previous studies evaluating CSHI therapy in CAH and Addison’s disease have been restricted to case reports or to clinical trials of short duration. 32–36 Merza et al, investigated the effect of CSHI therapy on disease biomarkers for 24-hours and demonstrated improvements in 17-OHP levels in two patients with CAH and ACTH (morning) levels in two Addison’s disease patients.34 Two well-designed short-term (1 – 3 months) clinical trials in patients with Addison’s disease by Gagliardi et al and Øksnes et al. demonstrated that it was possible to achieve cortisol exposures close to the normal cortisol circadian rhythm on CSHI therapy. 32, 36 Although our study has a number of limitations including a small group of patients, open-labelled design with no randomization or blinding, and confounding results from OCP use, this is the first long-term study to systematically evaluate the safety and efficacy of CSHI therapy in patients with CAH.
From our experience with long-term use of CSHI therapy in patients with CAH, we conclude that this novel way of replacing cortisol is safe and well-tolerated. Although we are reassured that there were no serious adverse events related to mechanical failure, CSHI therapy is a more time-intensive therapy compared to oral glucocorticoid replacement regimens and 25% of our patients switched to oral therapy due to the inconvenience of managing a pump. Pruritus around the catheter site was a common complaint. In addition to the cost of the pump and pump supplies, CSHI therapy requires patient commitment, attention to pump alerts, and personal hygiene. Thus, CSHI therapy may be an option for a select group of patients with CAH.
Diurnal cortisol replacement via CSHI may improve HRQoL in patients with CAH and multiple co-morbidities, and some patients with tumour formation (adrenal and gonadal) may benefit. Long-standing metabolic risk factors are mostly unchanged with long-term CSHI, indicating that preventative strategies are needed. The role that glucocorticoids play in the establishment of adverse outcomes in CAH is mostly unknown, but institution of diurnal cortisol replacement early in life might prevent many of the co-morbidities commonly observed during adulthood. Paediatric studies are needed.
Acknowledgements:
We thank our patients for their participation in this study and the 5SWN Metabolic Unit nursing staff for their support in implementing the study. We thank Medtronic Diabetes Company for providing pump devices and supplies, training and technical support.
Funding information: This research was supported by the Intramural Research Program of the National Institutes of Health.
Footnotes
Conflict of Interest/Disclosures: Deborah P. Merke, received unrelated research funds from Diurnal Limited and Millendo Therapeutics, through NIH Cooperative Research and Development Agreements. All other authors have no conflicts of interest to declare.
Clinical Trial Registration Number: NCT01859312
REFERENCES
- 1.El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94(5):1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(3):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97(2):473–481. [DOI] [PubMed] [Google Scholar]
- 6.Nella AA, Mallappa A, Perritt AF, et al. A Phase 2 Study of Continuous Subcutaneous Hydrocortisone Infusion in Adults With Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallappa A, Nella AA, Kumar P, et al. Alterations in Hydrocortisone Pharmacokinetics in a Patient With Congenital Adrenal Hyperplasia Following Bariatric Surgery. J Endocr Soc. 2017;1(7):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005;353(24):2578–2588. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81(5):1018–1025. [DOI] [PubMed] [Google Scholar]
- 11.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22(4):639–643. [PubMed] [Google Scholar]
- 12.Ware JE Jr., Gandek B Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–912. [DOI] [PubMed] [Google Scholar]
- 13.Oksnes M, Bensing S, Hulting AL, et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab. 2012;97(2):568–576. [DOI] [PubMed] [Google Scholar]
- 14.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90(5):3106–3114. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014;1318:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Rodriguez E, Stewart PM, Cooper MS. The pituitary-adrenal axis and body composition. Pituitary. 2009;12(2):105–115. [DOI] [PubMed] [Google Scholar]
- 17.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31(1):1–24. [DOI] [PubMed] [Google Scholar]
- 18.Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103. [DOI] [PubMed] [Google Scholar]
- 19.Liu SH, Lazo M, Koteish A, et al. Oral contraceptive pill use is associated with reduced odds of nonalcoholic fatty liver disease in menstruating women: results from NHANES III. J Gastroenterol. 2013;48(10):1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83(5):1806–1809. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed A, Rabbitt E, Brady T, et al. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS One. 2012;7(2):e29531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(4):1233–1257. [DOI] [PubMed] [Google Scholar]
- 23.Carr BR, Parker CR Jr., Madden JD, MacDonald PC, Porter JC. Plasma levels of adrenocorticotropin and cortisol in women receiving oral contraceptive steroid treatment. J Clin Endocrinol Metab. 1979;49(3):346–349. [DOI] [PubMed] [Google Scholar]
- 24.Burke CW. The effect of oral contraceptives on cortisol metabolism: J Clin Pathol Suppl (Assoc Clin Pathol). 1969;3:11–8. [Google Scholar]
- 25.Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clin Chim Acta. 1987;165(2–3):379–385. [DOI] [PubMed] [Google Scholar]
- 26.Gaffey AE, Wirth MM, Hoks RM, Jahn AL, Abercrombie HC. Circulating cortisol levels after exogenous cortisol administration are higher in women using hormonal contraceptives: data from two preliminary studies. Stress. 2014;17(4):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(7):2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nermoen I, Rorvik J, Holmedal SH, et al. High frequency of adrenal myelolipomas and testicular adrenal rest tumours in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2011;75(6):753–759. [DOI] [PubMed] [Google Scholar]
- 29.Stikkelbroeck NM, Otten BJ, Pasic A, et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(12):5721–5728. [DOI] [PubMed] [Google Scholar]
- 30.Han TS, Krone N, Willis DS, et al. Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur J Endocrinol. 2013;168(6):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nermoen I, Husebye ES, Svartberg J, Lovas K. Subjective health status in men and women with congenital adrenal hyperplasia: a population-based survey in Norway. Eur J Endocrinol. 2010;163(3):453–459. [DOI] [PubMed] [Google Scholar]
- 32.Oksnes M, Bjornsdottir S, Isaksson M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of addison’s disease: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99(5):1665–1674. [DOI] [PubMed] [Google Scholar]
- 33.Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94(9):3477–3480. [DOI] [PubMed] [Google Scholar]
- 34.Merza Z, Rostami-Hodjegan A, Memmott A, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2006;65(1):45–50. [DOI] [PubMed] [Google Scholar]
- 35.Sonnet E, Roudaut N, Kerlan V. Results of the prolonged use of subcutaneous continuous infusion of hydrocortisone in a man with congenital adrenal hyperplasia. ISRN Endocrinol. 2011;2011:219494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagliardi L, Nenke MA, Thynne TR, et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison’s disease: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99(11):4149–4157. [DOI] [PubMed] [Google Scholar]