Abstract
Preserving energy homeostasis in the presence of stressors such as proinflammatory cytokines and nutrient overload is crucial to maintaining normal cellular function. Six-transmembrane epithelial antigen of the prostate 4 (STEAP4), a metalloreductase involved in iron and copper homeostasis, is thought to play a potentially important role in the cellular response to inflammatory stress. Genome-wide association studies have linked various mutations in STEAP4 with the development of metabolic disorders such as obesity, metabolic syndrome, and type 2 diabetes. Several studies have shown that expression of Steap4 is modulated by inflammatory cytokines, hormones, and other indicators of cellular stress, and that STEAP4 may protect cells from damage, helping to maintain normal metabolic function. STEAP4 appears to be particularly relevant in metabolically oriented cells, such as adipocytes, hepatocytes, and pancreatic islet cells. These cells struggle to maintain their function in iron or copper overloaded states, presumably due to increased oxidative stress, suggesting STEAP4’s role in metal homeostasisis critical to the maintenance of cellular homeostasis in general, and in preventing the onset of metabolic disease. In this review, we explore genetic associations of STEAP4 with metabolic disorders, and we examine STEAP4 tissue expression, subcellular localization, regulation, structure, and function as it relates to metabolic diseases. We then examine how STEAP4’s role as a regulator of cellular iron and copper may relate to type 2 diabetes.
Keywords: T2D, STEAP4, islets, beta cells, cytokines, low-grade inflammation, iron homeostasis, iron overload, copper
Introduction
Six-Transmembrane Epithelial Antigen of the Prostate 4 (STEAP4) is anintegral membrane protein that functions as a metalloreductase involved in the transport of copper and iron (Ohgami et al. 2006; Grunewald et al. 2012). The expression of Steap4, also known as TNFα- induced adipose-related proteins (TIARP) (Moldes et al. 2001) or six-transmembrane protein of prostate 2 (STAMP2) (Korkmaz et al. 2005), is modulated in response to inflammation, and metabolism of fatty acids and glucose. Several studies have identified genetic variants in STEAP4 that are associated with numerous metabolic disorders. In line with the hypothesis that defects in STEAP4 are implicated in metabolic disorders, expression of Steap4 is associated with protection against inflammatory-mediated cellular damage. How the metalloreductase actions of STEAP4 may or may not be associated with STEAP4’s putative protective effects are discussed below.
STEAP4 Structure and Function
STEAP4 is a metalloreductase
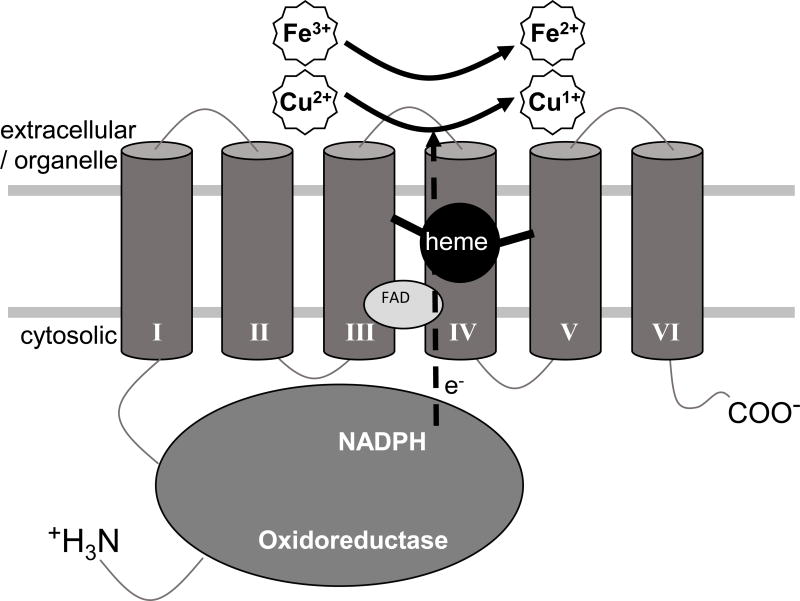
Reduction of extracellular Fe3+ to Fe2+ and Cu2+ to Cu1+ are prerequisites for the transport of each of these metals across the membrane, into the cell. Steap4 and the related Steap2 and Steap3 family members are integral membrane metalloreductases that move electrons from cytosolic NADPH to extracellular iron or copper. STEAP4 (Figure 1) is composed of two domains, an N-terminal oxidoreductase domain present on the cytoplasmic face and, as its name implies (STEAP4: Six Transmembrane Epithelial Antigen of the Prostrate 4), a C-terminal transmembrane domain composed of six membrane spanning α-helices that envelop a single heme binding site (Ohgamiet al. 2005; Kleven et al. 2015). Early hypotheses based upon homology to other ferric- and oxidoreductases suggested the oxidoreductase domain would catalyze electron flow from NADPH to an unidentified flavin, that would then donate electrons to the transmembrane domain where they would move through the heme group to an extracellular metal binding site, reducing iron and copper (Ohgami et al. 2005, 2006).
Figure 1.
Structure and function of STEAP4. Steap4 is composed of two domains, an N-terminal oxidoreductase domain, and a C-terminal transmembrane domain composed of six alpha-helices that coordinate a single heme. The N-terminal oxidoreductase domain draws two electrons from cytoplasmic NADPH and passes these to FAD at the cytosolic face of the transmembrane domain, reducing it to FADH2. FADH2, in turn, passes electrons, one at a time, through the heme in the transmembrane domain, to the cell surface metal binding site where Fe3+ is reduced to Fe2+, or Cu2+ to Cu1+, a prerequisite for transport into the cell or across organelle membranes.
Mutational, kinetic and crystallographic analysis now definitively show that the N-terminal domain does indeed bind and oxidize NADPH (Gauss et al. 2013; Kleven et al. 2015). While the N-terminal domain does reduce flavin, contrary to earlier predictions, it does not harbor a high affinity flavin binding site (Gauss et al. 2013). Instead, the major constituents of the high affinity FAD binding site are found on the cytosolic face of the transmembrane domain of STEAP3 (Kleven et al. 2015). Because the residues involved in FAD binding in the STEAP3 transmembrane domain are strictly conserved among all Steap family members (Steap1–4), it was concluded that Steap family members in general, including STEAP4, bind flavin primarily through the cytosolic face of the transmembrane domain. This work also strongly suggests that FAD is the preferred flavin for all Steap family members (Kleven et al. 2015), a conclusion that was recently verified for STEAP1 (Kim et al, 2016).
In initial studies, the specific activity of Steap4 for reduction of copper suggested copper might also be a physiologically relevant substrate (Ohgami et al. 2006). Importantly, Gauss et al subsequently determined the affinity of rat Steap4 for iron and copper, and found similar Km values for each of these substrates. Further, the affinity of rat Steap4 for both iron and copper is equal to or greater than those of other characterized mammalian ferric and cupric reductases (Gauss et al. 2013). Kleven et al also identified a conserved Fe3+ binding site on the extracellular face of STEAP3. Again, because these sequence motifs are present in STEAP4 and all other Steap family members, this strongly suggests the presence of a conserved metal binding site on the extracellular face (or endosome/organelle membrane) of STEAP4 (Kleven et al. 2015). Further, biochemical and structural studies also suggest that STEAP3 and STEAP4 each function as homodimers (Sendamarai et al. 2008; Gauss et al. 2013; Kleven et al. 2015).
Mechanistically, then, the current view is that the Steap4 oxidoreductase domain draws two electrons from cytoplasmic NADPH (oxidizing it to NADP+), passing these to FAD at the cytosolic face of the transmembrane domain, reducing it to FADH2. FADH2, in turn, passes electrons, one at a time, through the transmembrane heme, to the cell surface metal binding site where Fe3+ is reduced to Fe2+, or Cu2+ to Cu1+ (Kleven, et al. 2015). Fe2+ and Cu1+ are then ready for transport across the membrane by their respective transporters.
Steap4 and cellular uptake of iron and copper
In healthy individuals there is little extracellular free iron. Most free iron is bound by the 80 kDa protein, transferrin. Transferrin serves to solubilize Fe3+, which would otherwise complex with OH− and precipitate (rust).
To meet their iron needs, erythroid cells in particular are dependent upon the transferrin cycle. In this cycle, iron-loaded transferrin (Tf) binds to the cell surface transferrin receptor (TfR) (Lawrence et al. 1999). The Tf:TfR complex then enters the endosome via receptor mediated endocytosis. Within the low-pH endosome, iron is released from Tf and reduced from Fe+3 to Fe+2 by Steap3, permitting transport across the endosomal membrane by divalent metal iron transporter 1 (DMT1), which is selective for Fe2+. The apo-Tf:TfR complex is then recycled to the cell surface, where, at neutral pH, the apo-Tf is released to participate in the cycle once again (Andrews et al. 2015). Other cells with reduced iron needs can, however, take advantage of non-transferrin bound iron. For non-transferrin bound iron, other metal transporters such as Zip8 or Zip14 might also play a role (Zhao et al. 2010; Wang et al. 2012a; Wang & Knutson 2013; Kleven et al. 2015).
Copper transporter 1 (CTR1) is the major copper transporter involved in cellular copper uptake in mammals (Kaplan & Lutsenko 2009; Wang et al. 2011; Kidane et al. 2012). Thus, while the specific identity of the iron and copper transporters is not known, overexpression of mouse Steap4 in HEK-293 cells stimulates cellular uptake of these metals. In fact, in this assay, Steap4 shows the highest copper and iron uptake values of any member of the Steap family (Ohgamiet al. 2006). This strongly suggests that Steap4 plays a role in the cellular uptake of iron and copper, and that STEAP4 may be critical to both iron and copper homeostasis at the cellular level, and within the body in general.
Iron and the innate immune system
Iron withholding is an important strategy of the innate immune system. Transferrin, for example, serves to sequester Fe3+ from pathogenic invaders, for which iron is often the rate limiting nutrient. Thus, several proteins involved in iron transport and homeostasis, including hepcidin, ferritin and transferrin are up or down regulated in individuals suffering from chronic infection.
The increased expression of STEAP4 in response to inflammatory cytokines (see below) suggests Steap4 is also linked to inflammation and the innate immune response. Increased expression of cell surface Steap4 might therefore be expected to increase iron and/or copper import into the cell. While this strategy might reduce the concentration of circulating Fe3+ (and Cu2+) available to pathogens, it might also be expected to result in increased intracellular Fe2+, which if mishandled could lead to increased oxidative stress. The upregulation of Steap4 and potentially iron import by proinflammatory cytokines suggests, at least at the systemic level, that STEAP4-mediated iron transport into the cell is beneficial, perhaps because it reduces the concentration of circulating iron.
STEAP4 Tissue and Cellular Expression
One approach to understanding STEAP4’s role in metabolic dysfunction begins with determining the tissues and organs in which it is expressed. Reviews of tissue expression patterns showed that STEAP4 is found to varying levels in most organs with the exception of the central nervous system (Gomes et al. 2012; Grunewald et al. 2012). More specifically, analysis of metabolic tissues has revealed that STEAP4 is found in adipose tissue, hepatocytes, and pancreatic islets/beta-cells. These tissues are discussed in more detail below.
STEAP4 in Adipocytes
Relative to other cell types, the effects of STEAP4 expression and misexpression are most studied in adipocytes. STEAP4 expression is higher in mature adipocytes rather than young undifferentiated preadipocytes (Moldeset al. 2001; Chen et al. 2009; Moreno-Navarrete et al. 2011; Narvaez et al. 2013; Sikkeland & Saatcioglu 2013). Emerging evidence has demonstrated a role for STEAP4 in the cellular response to nutritional and inflammatory signals (Wellen et al. 2007). When overexpressed, STEAP4 has been shown to reduce inflammation and better regulate glucose metabolism in a mouse model of streptozotocin-induced diabetes (Chuang et al. 2015). In another study, STEAP4 overexpression shifted macrophage polarization to enhance protection of adipose tissue, leading to reduced insulin resistance in diabetic ApoE−/−/LDLR−/− mice (Han et al. 2013).
In contrast, reducing or eliminating STEAP4 negatively impacts adipose tissue. STEAP4 expression has also been implicated in translocation of glucose transporter 4 (GLUT4) to the cell surface, correlating reduced or abolished STEAP4 with increased insulin resistance and the pathophysiology of type 2 diabetes (T2D) (Cheng et al. 2011; Qin et al. 2011). At the whole animal level, Steap4 KO mice are prone to developing obesity, insulin resistance, glucose intolerance (Wellen et al. 2007), and hyperglycemia, hallmarks of metabolic syndrome and T2D. Thus, STEAP4 appears to play a protective role against metabolic and proinflammatory stress in adipocytes and adipose tissue.
STEAP4 Expression in Hepatocytes
In hepatocytes, the lack of STEAP4 has been suggested to correlate with dysfunctional responses to fat and nutrient influx, and the onset of fatty liver disease (Wellen et al. 2007). On the other hand, expression of STEAP4 in hepatocytes has been suggested to cause a suppression of lipogenesis and gluconeogenesis (Wang et al. 2012b). Overexpression of STEAP4 has been shown to ameliorate steatosis and insulin resistance caused by high fat diet (Kim et al. 2015). Hepatic STEAP4 overexpression also decreases hepatitis B virus X-protein signaling and subsequent metabolic dysfunction (Kim et al. 2012a). In mice with STEAP4 deficiency, liver size is elevated, hepatic insulin receptor signaling is impaired, and rates of fatty liver disease are increased (Wellen et al. 2007). As with adipose tissue, STEAP4 thus appears to play a protective role against metabolic and inflammatory stresses.
STEAP4 in pancreatic islets
Steap4 is expressed in pancreatic beta-cell lines (Berner et al. 2015) and primary mouse islets (Sharma et al. 2015) and is upregulated by exposure to cytokines or by free fatty acids (Sharma et al. 2015). In human islets, STEAP4 expression is reduced in obesity and hyperglycemia (HbA1c), but elevated in donors with high white blood cell count (Gordon et al. 2017). IsletSTEAP4 expression also appears to be slightly higher in women compared to men (Gordon et al. 2017). To date, little is known about the function or localization of STEAP4 in islets and warrants further study.
Cellular localization of STEAP4
STEAP4 seems to localize specifically to the Golgi apparatus network and the plasma membrane of cells (Korkmaz et al. 2005; Yoo et al. 2014). One study suggests that STEAP4 co-localizes with caveolin-1, which is known to play a role in insulin signaling in adipose tissues (Chambaut-Guerin & Pairault 2005). Studies of osteoclasts also suggest that STEAP4 can be found in endosomes important in osteoclast development and function (Zhou et al. 2013). Further, with regard to the subcellular distribution of STEAP4, rat STEAP4 shows ferric and cupric reductase activity at acidic pH suggesting it also functions within intracellular organelles, particularly endosomes and granules, to reduce these metals (Gauss et al. 2013). As STEAP4 is examined for localization in more cell types, it would be intriguing if the STEAP4 subcellular distribution differed with cell types, i.e., was found in endosomes and/or the plasma membrane of some cell types, but in the Golgi, ER, nuclear membranes or other organelles in other cell types. While purely speculative as this time, this would suggest STEAP4 has tissue-specific actions that may differ by cell type.
Regulatory Influences on STEAP4 Expression
With potential roles in metabolism, inflammation, cell growth, and cancer, STEAP4 expression is regulated by a number of different factors. Known regulatory factors are described below and summarized in Figure 2.
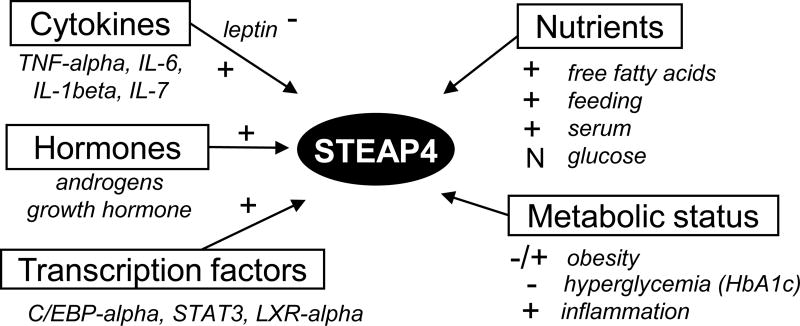
Figure 2.
Factors known to regulate STEAP4. “+” indicates a factor that upregulates STEAP4 expression; “−”indicates a factor that downregulates STEAP4 expression. “−/+” indicates both increased STEAP4 or decreased STEAP4 have been observed in obesity. N = no significant effect detected in adipose tissue (Wellen et al. 2007) or pancreatic islets (Sharma et al. 2015); note that high glucose increases STEAP4 expression in mesangial cells (Chuang et al. 2015).
Cytokines
Studies have suggested STEAP4 plays a fundamental role in cellular homeostasis during inflammatory stress, and a key regulator of STEAP4 expression is cytokine exposure. Several reports show that Steap4 mRNA increases in a dose-dependent manner with TNFα exposure, suggesting TNFα accelerates STEAP4 synthesis (Moldes et al. 2001; Chen et al. 2009; Tanaka et al. 2012a, b). Similar to the effect of TNFα, IL-6 exposure also results in an increase in Steap4 mRNA (Fasshauer et al. 2004) and STEAP4 protein levels in human adipocytes (Chen et al. 2010). Moreover, simultaneous exposure to multiple cytokines including IL-1β, TNFα, and IL-6 stimulates STEAP4 expression in a synergistic manner in adipocytes (Kralisch et al. 2009). Steap4 expression is also increased in hepatocytes when cells are exposed to IL-17 or TNFα, and together, these cytokines produce a synergistic effect (Sparna et al. 2010; Wu et al. 2015). The only cytokine/chemokine found to inhibit STEAP4 expression thus far is leptin (Chen et al. 2010).
Nutrients
Exposure to nutrients also plays a fundamental role in the regulation of STEAP4. Adipocyte exposure to high serum and fatty acids markedly increases Steap4 expression, whereas glucose and insulin treatment show no effect on expression (Waki & Tontonoz 2007; Wellen et al. 2007). Supporting these findings, islet expression of STEAP4 also increased in response to 48-hour exposure to free fatty acids but not to high glucose (Sharma et al. 2015). Moreover, Steap4 expression was increased following meals in normal lean mice, but this effect was lost in obese mice (Wellen et al. 2007).
It is not clear, however, whether STEAP4 is elevated or depressed in conditions of obesity resulting from chronic nutrient excess. Initial studies found that STEAP4 levels in adipocytes and white adipose tissue were increased in obesity (Arner et al. 2008; Catalan et al. 2012). Other studies, however, demonstrated that STEAP4 protein and/or gene expression is downregulated in obese patients (Zhang et al. 2008; Moreno-Navarrete et al. 2011; Kim et al. 2015; Ozmen et al. 2016; Xu et al. 2016). Additionally, STEAP4 expression in both fat and muscle was reduced among the most insulin resistant individuals independent of BMI (Elbein et al. 2011). Further, in human pancreatic islets, reduced STEAP4 gene expression correlates with increased BMI (Gordon et al. 2017) among non-diabetic donors, but not among donors with T2D. Donors with T2Dshowed reduced STEAP4 gene expression with increased HbA1c, a key indicator of chronic hyperglycemia (Gordon et al. 2017). On balance, these findings suggest that reduced STEAP4 expression is associated with obesity.
Hormones
In addition to cytokine and nutrient exposure, STEAP4 expression is regulated by hormones such as growth hormone (GH) and testosterone. Maneschi et al. established that an increase in testosterone increases STEAP4 expression in visceral adipose tissue (Maneschi et al. 2012). Other research has shown that STEAP4 expression is markedly increased in prostate cancer tissues in which testosterone production is high (Korkmaz et al. 2005). Interestingly, testosterone is important in normal glucose metabolism, insulin signaling, and the prevention of metabolic syndrome, and it has been suggested that STEAP4 acts synergistically with testosterone to achieve this effect (Maneschi et al. 2012; Vignozzi et al. 2012). Not surprisingly, an increase in GH, which impacts glucose homeostasis among other effects, has also been shown to increase the expression of STEAP4 in a dose dependent manner (Fasshauer et al. 2003). Although the exact connection between GH and insulin resistance is unclear, an excess of GH hinders the action of insulin in tissues, which may act as a stressor to promote increased STEAP4 expression to counter the effect of testosterone.
Transcription Factors
At the transcriptional level, STEAP4 expression is driven by several transcription factors and signaling cascades. Recent studies have shown that two factors involved in adipocyte differentiation, CCAAT/enhancer-binding protein α (C/EBPα) and liver-X-receptor-alpha activate the STEAP4 promoter, whereas PPARγ does not (Wellenet al. 2007). Other studies have demonstrated that STEAP4 expression is regulated by C/EBPα and STAT3 in the liver (Ramadoss et al. 2010). These transcription factors are activated in response to inflammatory and nutritional signals, suggesting that STEAP4 might play a protective role at the cellular or systemic level (Ramadoss et al. 2010). Similarly, in mesangial cells where high glucose induces Steap4 expression, the increased expression was found to be dependent upon S100B, JNK, PI3K, JAK2 and STAT3 (Chuang et al. 2015).
Regulatory and Protective Influences of STEAP4
Although STEAP4 functions as a metalloreductase at the molecular level, overexpression of STEAP4 has been shown to modulate the expression of several genes. For example, in the mesangial cells discussed immediately above, STEAP4 overexpression attenuates high glucose induced expression of collagen IV, fibronectin and COX2, as well as the expression of TGF-β, ERK1/2, Akt, Smad2/3 and STAT3 (Chuang et al. 2015). The observation that high glucose induces STAT3 dependent expression of STEAP4 (above), and that overexpression of STEAP4 in turn attenuates expression of STAT3, as well as other important signaling molecules like TGF-β, ERK1/2, Akt and Smad2/3, suggests STEAP4 may participate in a feedback loop that modulates the effects of high glucose on mesangial cells.
Steap4 is also reported to show protective effects at the systemic level. STEAP4 overexpression reduced rates of atherosclerosis and plaque formation in diabetic mice (Wang et al. 2014), while its deficiency promoted atherosclerosis (Freyhaus et al. 2012). Similarly, STEAP4 overexpression reduced migration of neutrophil-like HL60 (Tanaka et al. 2012a) and reduced IL-6 and IL-8 cytokine expression (Tanaka et al. 2012b), whereas siRNA knockdown of STEAP4 increased cytokine signaling in patients with rheumatoid arthritis (Tanaka et al. 2012b), again consistent with a role for Steap4 in a negative feedback loop. These protective effects have also been observed in adipocytes and hepatocytes as discussed above.
STEAP4 in Metabolic Disorders
Genome-wide Associations between STEAP4 and Metabolic Disorders
A number of genome-wide association studies have identified genetic variants of STEAP4 associated with obesity and obesity-related disorders. For example, metabolic syndrome is known to impair glucose tolerance, insulin sensitivity, and other conditions linked with obesity. It was found that individuals with metabolic syndrome showed associations with several Single Nucleotide Polymorphisms (SNPs) within or near the STEAP4 gene (Nanfang et al. 2010; Guo et al. 2011a; Chen et al. 2014), particularly in the Uyghur people of western China who are genetically a mix of east Asian and western European genetic lineages (Yao et al. 2004). STEAP4 variants were also associated with metabolic syndrome among the Han Chinese (Qi et al. 2015). Among western Europeans, an epidemiological study examined constitutive parameters of metabolic syndrome in French Caucasians and found that STEAP4 variants were associated with higher triglyceride levels, fasting glucose, and fat intake, however, no relationship between STEAP4 and the prevalence of metabolic syndrome was found (Miot et al. 2010).
Genetic variations in STEAP4 have also been found to impact obesity and insulin secretion. A pair of studies associated a common variant in the STEAP4 gene (rs1981529 Gly75Asp, 224A/G) with obesity in the Uyghur population (Guo et al. 2011b; Han et al. 2012). However, no relationship was found between STEAP4 and severe obesity in a Norwegian cohort (Wangensteen et al. 2011). Finally, an islet-targeted genome-wide association study of Hispanic Americans implicated a STEAP4 polymorphism with measures of acute insulin response to glucose (Sharma et al. 2015), suggesting a role for STEAP4 in insulin-secreting beta-cells. In each case, it is not known whether loss-of-function or gain-of-function mutations in STEAP4 are related to disease status in these studies. Collectively, however, these associations suggest a potentially important role for STEAP4 in obesity and related metabolic disorders, and further mechanistic work is clearly needed.
STEAP4, Iron and T2D
Several excellent reviews detail cellular iron regulation and the relationship between iron and diabetes (Swaminathan et al. 2007; Simcox & McClain 2013; Backe et al. 2016). Further, iron regulation is governed by multiple processes involving heme vs. non-heme dietary iron intake, iron absorption, inflammation, and cellular iron regulation that are more complex than we can address within the scope of this review. For this reason, our discussion of iron will be largely limited to STEAP4. Critical to this discussion, however, is the understanding that while iron is essential for a variety of reasons (DNA synthesis, electron transport chain and ATP production, etc), excess iron results in the production of hydroxyl radicals (via Fenton Chemistry) and reactive oxygen species (ROS) in general. In turn, these ROS react indiscriminately with lipids, proteins and DNA, leading to oxidative damage and cellular stress. In short, iron is a double-edged sword that cuts both ways.
Not surprisingly then, long-standing evidence links T2D with numerous conditions of iron overload including hereditary hemochromatosis (Dymock et al. 1972; McClain et al. 2006) and blood transfusions associated with blood disorders such as beta-thalassemia major (Merkel et al. 1988; Dmochowski et al. 1993; Cario et al. 2003). Markers for elevated iron levels are also strongly associated with increased risk of T2D in the general population (Ford & Cogswell 1999; Fernandez-Real et al. 2002a) and in gestational diabetes (Lao & Tam 1997; Rawal et al. 2016). There is evidence that simply reducing iron levels by phlebotomy can reduce insulin resistance, improve insulin secretion, and lower blood glucose in patients with T2D (Facchini 1998; Fernandez-Real et al. 2002b).
Studies have shown that iron overload can cause insulin resistance in adipocytes (Dongiovanni et al. 2013; Wlazlo et al. 2013; Gao et al. 2015), impair hepatocytes (Ramm & Ruddell 2005; Fargion et al. 2011), and cause beta-cell dysfunction (Cooksey et al. 2004). In contrast, when DMT1 was knocked out in beta-cells, the result was iron depletion and subsequent reductions in ROS, mitochondrial activity, and insulin secretion (Hansen et al. 2012). Consistent with this, under conditions of increased inflammatory stress, DMT1 deficiency was found to protect beta-cells from dysfunction and apoptosis (Hansen et al. 2012). These studies suggest that excessive iron uptake and the resulting oxidative stress inhibit normal beta-cell function, whereas inhibition of iron uptake is protective under conditions of inflammatory stress.
To date, a single report links STEAP4 with changes in iron regulation associated with metabolic disorders. STEAP4 and lipocalin-2 (aka NGAL, which sequesters bacterial siderophores) were both negatively correlated with serum iron levels and positively correlated with markers of inflammation in obese individuals (Catalan et al. 2012). These findings suggest that increased STEAP4 expression from low-grade inflammation in obesity could drive down circulating free iron levels and presumably drive up cellular iron accumulation. Considering the detrimental effect of iron overload and STEAP4’s role in iron transport, it could be argued that knocking out STEAP4 might be beneficial under conditions of stress by reducing iron accumulation and reducing ROS production as occurs for DMT1. However, the opposite appears to be true; STEAP4 deficiency leads to dysfunction in several tissues including liver hepatocytes and adipocytes and exacerbates inflammatory environments. How changes in STEAP4 expression or activity would impact cellular function in these metabolic tissues, particularly in conditions of iron overload, acute vs. chronic inflammation, or nutrient-associated stress, is yet another open question warranting future study.
STEAP4, Copper and Type-2 Diabetes
Copper is also important for many biological functions. Copper is necessary for the activity of superoxide dismutase, an important antioxidant enzyme that scavenges the free radical superoxide (McCord & Fridovich 1969). Beta-cells are thought to be especially susceptible to oxidative stress because of very low anti-oxidant enzyme activity (Karunakaran & Park 2013). Thus, copper import by Steap4 may facilitate increased activity of cytosolic superoxide dismutase, protecting the cell against oxidative stress.
Copper is also a cofactor for cytochrome c oxidase, and is thus critical for mitochondrial electron transport and ATP synthesis (Kim et al. 2012b, Xu et al, 2013). Cytochrome c oxidase activity is thus reduced in copper deficient cells, which could lead to mitochondrial dysfunction. Supporting this idea, increasing dietary copper in Cohen diabetic rats restored insulin secretion and improved glycemia by increasing respiratory-chain enzyme cytochrome c oxidase (Weksler-Zangen et al. 2013).
However, a classic study of biological metals in humans links elevated levels of circulating copper with T2D (Walter et al. 1991), and a recent systematic review also concludes increased copper is associated with both type 1 and type 2 diabetes (Qiu et al. 2016). In addition, when diabetic (db/db) mice with elevated copper levels were given a copper chelator, the mice showed reduced copper and ROS levels and improved glucose tolerance (Tanaka et al. 2009). Thus, the evidence appears to favor a link between increased circulating copper and diabetes, but the mechanism for this link is not well understood.
To date, there has been little study of STEAP4 with respect to copper homeostasis. Evidence of a role for STEAP4 in copper transport comes fromi) the demonstration of Steap4 reduction of Cu2+ to Cu+ with a Cu+-sensitive chelating dye (Ohgami et al. 2006), ii) measurements of cellular copper uptake utilizing transiently transfected HEK-293T cells with STEAP4 expression plasmids (Ohgami, et al. 2006), and iii) the subsequent determination of a high affinity (physiologically relevant, low Km) for copper (Gauss, et al. 2013). In this regard, we point out that increased intracellular copper levels and reduced circulating levels are not mutually exclusive, as STEAP4 mediated transport of circulating copper into the cell would explain both observations. This might also contribute to a functional electron transport chain, or the reduction in ROS in cells expressing STEAP4. Future studies will hopefully precisely pinpoint the links between STEAP4, copper homeostasisand metabolic disorders.
Interacting Partners for STEAP4
While the ferric reductase activity of Steap4 can explain a number of observations related to over, under, or mis-expression of STEAP4, it is difficult to rationalize the reported protective effects of STEAP4 with potentially increased iron import and production of ROS. This suggests Steap4 might possess metal-independent actions, and that understanding these actions will be important in reconciling these apparently contradictory observations. In this light, the literature suggests that STEAP proteins in general, and Steap4 in particular, may be involved in protein-protein interactions that modulate STEAP activity, or the activities of the interacting partners. For example, STEAP3 has been reported to interact with NIX and MYT1 kinase, localizing these proteins at the plasma membrane, and suggesting a role in apoptosis or cell cycle control. STEAP3 is also reported to interact with translationally controlled tumor protein (Amzallag et al. 2004), and there is strong genetic evidence for an interaction between STEAP3 and transferrin receptor (Jabara et al. 2016). Importantly, Steap3 can also form a heterodimer with STEAP4(Kleven 2015). Increased STEAP4 expression could then conceivably modulate the activity of STEAP3 and the activities or subcellular locations of proteins interacting with STEAP3 (NIX, MYT1), thus potentially impacting metal homeostasis, apoptosis and cell cycle control.
Similarly, Steap4 has also been reported to interact with BNIP3L (Passer et al. 2003), focal adhesion kinase-1(Tamura & Chiba 2009) and S100B (Chuang et al. 2015), again suggesting possible links to apoptosis, differentiation and cell cycle progression. In addition, STEAP4 is a reported target of the rhomboid protease RHBDL4/RHBDD1 (Wan et al. 2012). Finally, large scale interactome studies and their related data bases (BioGrid, IntAct, STRING, etc.) provide an expanded list of potential interactions that might help explain the protective effects. Importantly, most of these potential interactions have not been characterized with respect to metalloreductase activity, metal import and the protective effects of STEAP4, and much additional work is clearly needed. However, one possible explanation for the protective activity of Steap4 is that it localizes one or more of these interacting partners to the membrane, thus modulating the activity of the respective signaling pathway, completing a regulatory feedback loop.
STEAP4 in Cancer
In addition to its role in cellular or systemic homeostasis in the presence of inflammatory stress, STEAP4 is also associated with tumorigenesis. STEAP4 overexpression has been suggested to increase ROS, which may contribute to increased mutational rates and further prostate cancer progression (Jin et al. 2015). At the same time, elevated levels of STEAP4 in prostate cancer cells might also help meet the increased need for iron in rapidly multiply cells, signifying its role in cell growth and maintenance (Korkmaz et al. 2005). Thus, while STEAP4 may have a beneficial role in protection from inflammatory stress in a host of chronic metabolic and inflammatory diseases (Grunewald et al. 2012; Gomes et al. 2012; Wu et al. 2015; Jin et al. 2015; Tamura & Chiba 2009; Lindstad et al. 2010, 2016), its mis-expression may also promote cancer cell proliferation and cancer progression, adding to the complexity in understanding the role of STEAP4 in health and disease.
Conclusion
STEAP4 has emerged as a key player in inflammatory responses in metabolic tissues and in cellular iron and copper homeostasis. It is well established that iron and copper, among other trace metals, are essential to maintaining many cellular processes. Numerous studies have pointed to iron overload (Swaminathan et al. 2007; Simcox & McClain 2013; Backe et al. 2016) and copper overload (Walter et al. 1991; Tanaka et al. 2009; Qiu et al. 2016) as contributing factors to insulin resistance and beta-cell dysfunction. With such obvious links, it is somewhat surprising that STEAP4’s function as a metalloreductase has not been examined in the context of metabolic diseases. Several other iron-regulating genes have been recognized as important to both inflammation and metabolic disorders including lipocalin-2 (Wang et al. 2007), hepcidin and ferritin (Andrews et al. 2015). It has yet to be determined whether STEAP4’s protective effects against metabolic and inflammatory damageare mediated by iron regulation, copper regulation, immunomodulation, by some combination of these, or by some completely novel mechanism. Given the clear links between STEAP4 and numerous metabolic disorders, defining these putative functions of STEAP4 is a goal worthy of future discovery and discussion.
Plain Language Summary.
Problems with the cellular regulation of iron and copper have long been associated with diseases of metabolism, including type 2 diabetes. In this review, we examine a protein involved with iron and copper handling in cells called STEAP4 and its connections to inflammation and diseases of obesity.
Acknowledgments
The authors thank the Research and Scholarly Advancement Fellowship (RSAF) program at Ohio University for providing R.T.S. the opportunity to participate in the project.
Funding: This work was supported by the National Institutes of Health R01 DK089182 to CSN, the Osteopathic Heritage Foundation, and the Heritage College of Osteopathic Medicine at Ohio University.
Footnotes
Disclosure Statement: The authors declare that there are no conflicts of interest regarding the publication of this paper.
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Author contribution statement: R.T.S., C.M.L., H.M.G and C.S.N. all contributed to research and writing this manuscript.
References
- Amzallag N, Passer BJ, Allanic D, Segura E, Thery C, Goud B, Amson R, Telerman A. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. The Journal of Biological Chemistry. 2004;279:46104–46112. doi: 10.1074/jbc.M404850200. [DOI] [PubMed] [Google Scholar]
- Andrews M, Soto N, Arredondo-Olguin M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition (Burbank, Los Angeles County, Calif.) 2015;31:51–57. doi: 10.1016/j.nut.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Arner P, Stenson BM, Dungner E, Naslund E, Hoffstedt J, Ryden M, Dahlman I. Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. The Journal of Clinical Endocrinology and Metabolism. 2008;93:2249–2254. doi: 10.1210/jc.2008-0206. [DOI] [PubMed] [Google Scholar]
- Backe MB, Moen IW, Ellervik C, Hansen JB, Mandrup-Poulsen T. Iron Regulation of Pancreatic Beta-Cell Functions and Oxidative Stress. Annual Review of Nutrition. 2016;36:241–273. doi: 10.1146/annurev-nutr-071715-050939. [DOI] [PubMed] [Google Scholar]
- Berner A, Bachmann M, Bender C, Pfeilschifter J, Christen U, Muhl H. Though Active on RINm5F Insulinoma Cells and Cultured Pancreatic Islets, Recombinant IL-22 Fails to Modulate Cytotoxicity and Disease in a Protocol of Streptozotocin-Induced Experimental Diabetes. Frontiers in Pharmacology. 2015;6:317. doi: 10.3389/fphar.2015.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario H, Holl RW, Debatin K-MM, Kohne E. Insulin sensitivity and beta-cell secretion in thalassaemia major with secondary haemochromatosis: assessment by oral glucose tolerance test. European Journal of Pediatrics. 2003;162:139–146. doi: 10.1007/s00431-002-1121-7. [DOI] [PubMed] [Google Scholar]
- Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Six-transmembrane epithelial antigen of prostate 4 and neutrophil gelatinase-associated lipocalin expression in visceral adipose tissue is related to iron status and inflammation in human obesity. European Journal of Nutrition. 2012 doi: 10.1007/s00394-012-0464-8. [DOI] [PubMed] [Google Scholar]
- Chambaut-Guerin AM, Pairault J. Tumour necrosis factor alpha-induced adipose-related protein (TIARP): co-localization with caveolin-1. Biology of the Cell / under the Auspices of the European Cell Biology Organization. 2005;97:339–347. doi: 10.1042/BC20040062. [DOI] [PubMed] [Google Scholar]
- Chen XH, Zhao YP, Zhu C, Ji CB, Zhang CM, Zhu JG, Gao CL, Guo XR. Regulative role of TNFalpha on STEAP4 gene in matured human adipocytes. Zhongguo dang dai er ke za zhi = Chinese journal of contemporary pediatrics. 2009;11:1008–1011. [PubMed] [Google Scholar]
- Chen X, Zhu C, Ji C, Zhao Y, Zhang C, Chen F, Gao C, Zhu J, Qian L, Guo X. STEAP4, a gene associated with insulin sensitivity, is regulated by several adipokines in human adipocytes. International Journal of Molecular Medicine. 2010;25:361–367. doi: 10.3892/ijmm_00000353. [DOI] [PubMed] [Google Scholar]
- Chen X, Huang Z, Zhou B, Wang H, Jia G, Liu G, Zhao H. STEAP4 and insulin resistance. Endocrine. 2014;47:372–379. doi: 10.1007/s12020-014-0230-1. [DOI] [PubMed] [Google Scholar]
- Cheng R, Qiu J, Zhou XY, Chen XH, Zhu C, Qin DN, Wang JW, Ni YH, Ji CB, Guo XR. Knockdown of STEAP4 inhibits insulin-stimulated glucose transport and GLUT4 translocation via attenuated phosphorylation of Akt, independent of the effects of EEA1. Molecular Medicine Reports. 2011;4:519–523. doi: 10.3892/mmr.2011.443. [DOI] [PubMed] [Google Scholar]
- Chuang C-T, Guh J-Y, Lu C-Y, Wang Y-T, Chen H-C, Chuang L-Y. Steap4 attenuates high glucose and S100B-induced effects in mesangial cells. Journal of Cellular and Molecular Medicine. 2015;19:1234–1244. doi: 10.1111/jcmm.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factors determining glucose tolerance in patients with thalassemia major. The Journal of Clinical Endocrinology and Metabolism. 1993;77:478–483. doi: 10.1210/jcem.77.2.8345055. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, Girelli D, Cairo G, Magni P, Fargion S, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. The American Journal of Pathology. 2013;182:2254–2263. doi: 10.1016/j.ajpath.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Dymock IW, Cassar J, Pyke DA, Oakley WG, Williams R. Observations on the pathogenesis, complications and treatment of diabetes in 115 cases of haemochromatosis. The American Journal of Medicine. 1972;52:203–210. doi: 10.1016/0002-9343(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes. 2011;60:1019–1029. doi: 10.2337/db10-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini FS. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care. 1998;21:2190. [PubMed] [Google Scholar]
- Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43:89–95. doi: 10.1016/j.dld.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Krahlisch S, Lossner U, Klier M, Bluher M, Paschke R. GH is a positive regulator of tumor necrosis factor alpha-induced adipose related protein in 3T3-L1 adipocytes. The Journal of Endocrinology. 2003;178:523–531. doi: 10.1677/joe.0.1780523. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Chambaut-Guerin AM, Klein J, Paschke R. Interleukin-6 is a positive regulator of tumor necrosis factor alpha-induced adipose-related protein in 3T3-L1 adipocytes. FEBS Letters. 2004;560:153–157. doi: 10.1016/S0014-5793(04)00096-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002a;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002b;51:1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- Freyhaus HT, Calay ES, Yalcin A, Vallerie SN, Yang L, Calay ZZ, Saatcioglu F, Hotamisligil GS. Stamp2 Controls Macrophage Inflammation through Nicotinamide Adenine Dinucleotide Phosphate Homeostasis and Protects against Atherosclerosis. Cell Metabolism. 2012;16:81–89. doi: 10.1016/j.cmet.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li Z, Gabrielsen JS, Simcox JA, Lee S, Jones D, Cooksey B, Stoddard G, Cefalu WT, McClain DA. Adipocyte iron regulates leptin and food intake. The Journal of Clinical Investigation. 2015;125:3681–3691. doi: 10.1172/JCI81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss GH, Kleven MD, Sendamarai AK, Fleming MD, Lawrence CM. The crystal structure of six-transmembrane epithelial antigen of the prostate 4 (Steap4), a ferri/cuprireductase, suggests a novel interdomain flavin-binding site. The Journal of Biological Chemistry. 2013;288:20668–20682. doi: 10.1074/jbc.M113.479154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes IM, Maia CJ, Santos CR. STEAP proteins: from structure to applications in cancer therapy. Molecular Cancer Research: MCR. 2012;10:573–587. doi: 10.1158/1541-7786.MCR-11-0281. [DOI] [PubMed] [Google Scholar]
- Gordon HM, Majithia N, MacDonald PE, Fox JEM, Sharma PR, Byrne FL, Hoehn KL, Evans-Molina C, Langman L, Brayman KL, et al. STEAP4 expression in human islets is associated with differences in body mass index, sex, HbA1c, and inflammation. Endocrine. 2017;56:528–537. doi: 10.1007/s12020-017-1297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald TG, Bach H, Cossarizza A, Matsumoto I. The STEAP Protein Family: Versatile Oxidoreductases and Targets for Cancer Immunotherapy with Overlapping and Distinct Cellular Functions. Biology of the Cell / under the Auspices of the European Cell Biology Organization. 2012 doi: 10.1111/boc.201200027. [DOI] [PubMed] [Google Scholar]
- Guo YY, Li NF, Wang CM, Yan ZT, Zhang JH, Wang HM, Zhou L, Luo WL. Genetic variation and association of STEAP4 gene with metabolic syndrome in Chinese Uygur patients. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2011a;28:78–82. doi: 10.3760/cma.j.issn.1003-9406.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Guo YY, Li NF, Zhou L, Yao XG, Wang HM, Zhang JH, Luo WL. A common variation within the STEAP4 gene exons is associated with obesity in Uygur general population. Chinese Medical Journal. 2011b;124:2096–2100. [PubMed] [Google Scholar]
- Han RM, Li NF, Yan ZT, Guo YY, Zhang JH, Wang HM, Hong J, Zhou L. Genetic polymorphism of six transmembrance protein of prostate 2 associated with diabetes mellitus in Xinjiang Uygur population. Zhongguo Yi Xue Ke Xue Yuan Xue bao.Acta Academiae Medicinae Sinicae. 2012;34:509–514. doi: 10.3881/j.issn.1000-503X.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Han L, Tang MX, Ti Y, Wang ZH, Wang J, Ding WY, Wang H, Zhang Y, Zhang W, Zhong M. Overexpressing STAMP2 Improves Insulin Resistance in Diabetic ApoE (−/−)/LDLR(−/−) Mice via Macrophage Polarization Shift in Adipose Tissues. PloS One. 2013;8:e78903. doi: 10.1371/journal.pone.0078903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, Friberg J, Grunnet LG, Heller RS, Nielsen AO, Storling J, Baeyens L, et al. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic beta cell fate in response to cytokines. Cell Metabolism. 2012;16:449–461. doi: 10.1016/j.cmet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, Bainter W, Fraulino D, Rahimov F, Sieff C, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nature Genetics. 2016;48:74–78. doi: 10.1038/ng.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Wang L, Qu S, Sheng X, Kristian A, Maelandsmo GM, Pallmann N, Yuca E, Tekedereli I, Gorgulu K, et al. STAMP2 increases oxidative stress and is critical for prostate cancer. EMBO Molecular Medicine. 2015;7:315–331. doi: 10.15252/emmm.201404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. The Journal of Biological Chemistry. 2009;284:25461–25465. doi: 10.1074/jbc.R109.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran U, Park KG. A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes & Metabolism Journal. 2013;37:106–112. doi: 10.4093/dmj.2013.37.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane TZ, Farhad R, Lee KJ, Santos A, Russo E, Linder MC. Uptake of copper from plasma proteins in cells where expression of CTR1 has been modulated. Biometals: An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine. 2012;25:697–709. doi: 10.1007/s10534-012-9528-8. [DOI] [PubMed] [Google Scholar]
- Kim HY, Cho HK, Yoo SK, Cheong JH. Hepatic STAMP2 decreases hepatitis B virus X protein-associated metabolic deregulation. Experimental & Molecular Medicine. 2012a;44:622–632. doi: 10.3858/emm.2012.44.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Khalimonchuk O, Smith PM, Winge DR. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochimica et Biophysica Acta. 2012b;1823:1604–1616. doi: 10.1016/j.bbamcr.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Park SY, Lee MH, Rho JH, Oh YJ, Jung HU, Yoo SH, Jeong NY, Lee HJ, Suh S, et al. Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and insulin resistance. Journal of Hepatology. 2015;63:477–485. doi: 10.1016/j.jhep.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Kleven MD. Ph.D. Dissertation (3708774) Montana State University; Bozeman, Montana, USA: 2015. Biochemical characterization of the six-transmembrane epithelial antigen of the prostate family of metalloreductases. [Google Scholar]
- Kleven MD, Dlakic M, Lawrence CM. Characterization of a single b-type heme, FAD, and metal binding sites in the transmembrane domain of six-transmembrane epithelial antigen of the prostate (STEAP) family proteins. The Journal of Biological Chemistry. 2015;290:22558–22569. doi: 10.1074/jbc.M115.664565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz CG, Korkmaz KS, Kurys P, Elbi C, Wang L, Klokk TI, Hammarstrom C, Troen G, Svindland A, Hager GL, et al. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene. 2005;24:4934–4945. doi: 10.1038/sj.onc.1208677. [DOI] [PubMed] [Google Scholar]
- Kralisch S, Sommer G, Weise S, Lipfert J, Lossner U, Kamprad M, Schrock K, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta is a positive regulator of TIARP/STAMP2 gene and protein expression in adipocytes in vitro. FEBS Letters. 2009;583:1196–1200. doi: 10.1016/j.febslet.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Lao TT, Tam KF. Maternal serum ferritin and gestational impaired glucose tolerance. Diabetes Care. 1997;20:1368–1369. doi: 10.2337/diacare.20.9.1368. [DOI] [PubMed] [Google Scholar]
- Lawrence CM, Ray S, Babyonyshev M, Galluser R, Borhani DW, Harrison SC. Crystal structure of the ectodomain of human transferrin receptor. Science (New York, N.Y.) 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- Lindstad T, Jin Y, Wang L, Qu S, Saatcioglu F. STAMPs at the crossroads of cancer and nutrition. Nutrition and Cancer. 2010;62:891–895. doi: 10.1080/01635581.2010.509836. [DOI] [PubMed] [Google Scholar]
- Lindstad T, Qu S, Sikkeland J, Jin Y, Kristian A, Maelandsmo GM, Collas P, Saatcioglu F. STAMP2 is required for human adipose-derived stem cell differentiation and adipocyte-facilitated prostate cancer growth in vivo. Oncotarget. 2016 doi: 10.18632/oncotarget.11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneschi E, Morelli A, Filippi S, Cellai I, Comeglio P, Mazzanti B, Mello T, Calcagno A, Sarchielli E, Vignozzi L, et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. The Journal of Endocrinology. 2012;215:347–362. doi: 10.1530/JOE-12-0333. [DOI] [PubMed] [Google Scholar]
- McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49:1661–1669. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) The Journal of Biological Chemistry. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Merkel PA, Simonson DC, Amiel SA, Plewe G, Sherwin RS, Pearson HA, Tamborlane WV. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. The New England Journal of Medicine. 1988;318:809–814. doi: 10.1056/NEJM198803313181303. [DOI] [PubMed] [Google Scholar]
- Miot A, Maimaitiming S, Emery N, Bellili N, Roussel R, Tichet J, Velho G, Balkau B, Marre M, Fumeron F, et al. Genetic variability at the six transmembrane protein of prostate 2 locus and the metabolic syndrome: the data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. The Journal of Clinical Endocrinology and Metabolism. 2010;95:2942–2947. doi: 10.1210/jc.2010-0026. [DOI] [PubMed] [Google Scholar]
- Moldes M, Lasnier F, Gauthereau X, Klein C, Pairault J, Feve B, Chambaut-Guerin AM. Tumor necrosis factor-alpha-induced adipose-related protein (TIARP), a cell-surface protein that is highly induced by tumor necrosis factor-alpha and adipose conversion. The Journal of Biological Chemistry. 2001;276:33938–33946. doi: 10.1074/jbc.M105726200. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Ortega F, Serrano M, Perez-Perez R, Sabater M, Ricart W, Tinahones F, Peral B, Fernandez-Real JM. Decreased STAMP2 expression in association with visceral adipose tissue dysfunction. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E1816–E1825. doi: 10.1210/jc.2011-0310. [DOI] [PubMed] [Google Scholar]
- Nanfang L, Yanying G, Hongmei W, Zhitao Y, Juhong Z, Ling Z, Wenli L. Variations of six transmembrane epithelial antigen of prostate 4 (STEAP4) gene are associated with metabolic syndrome in a female Uygur general population. Archives of Medical Research. 2010;41:449–456. doi: 10.1016/j.arcmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. Journal of Cellular Physiology. 2013;228:2024–2036. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nature Genetics. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmen F, Ozmen MM, Gelecek S, Bilgic I, Moran M, Sahin TT. STEAP4 and HIF-1alpha gene expressions in visceral and subcutaneous adipose tissue of the morbidly obese patients. Molecular Immunology. 2016;73:53–59. doi: 10.1016/j.molimm.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Passer BJ, Nancy-Portebois V, Amzallag N, Prieur S, Cans C, Roborel de Climens A, Fiucci G, Bouvard V, Tuynder M, Susini L, et al. The p53-inducible TSAP6 gene product regulates apoptosis and the cell cycle and interacts with Nix and the Myt1 kinase. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2284–2289. doi: 10.1073/pnas.0530298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Yu Y, Wu Y, Wang S, Yu Q, Shi J, Xu Z, Zhang Q, Fu Y, Fu Y, et al. Genetic Variants in Six-Transmembrane Epithelial Antigen of Prostate 4 Increase Risk of Developing Metabolic Syndrome in a Han Chinese Population. Genetic Testing and Molecular Biomarkers. 2015;19:666–672. doi: 10.1089/gtmb.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin DN, Zhu JG, Ji CB, Chunmei-Shi, Kou CZ, Zhu GZ, Zhang CM, Wang YP, Ni YH, Guo XR. Monoclonal antibody to six transmembrane epithelial antigen of prostate-4 influences insulin sensitivity by attenuating phosphorylation of P13K (P85) and Akt: possible mitochondrial mechanism. Journal of Bioenergetics and Biomembranes. 2011;43:247–255. doi: 10.1007/s10863-011-9360-9. [DOI] [PubMed] [Google Scholar]
- Qiu Q, Zhang F, Zhu W, Wu J, Liang M. Copper in Diabetes Mellitus: a Meta-Analysis and Systematic Review of Plasma and Serum Studies. Biological Trace Element Research. 2016 doi: 10.1007/s12011-016-0877-y. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Chiappini F, Bilban M, Hollenberg AN. Regulation of hepatic six transmembrane epithelial antigen of prostate 4 (STEAP4) expression by STAT3 and CCAAT/enhancer-binding protein alpha. The Journal of Biological Chemistry. 2010;285:16453–16466. doi: 10.1074/jbc.M109.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. Seminars in Liver Disease. 2005;25:433–449. doi: 10.1055/s-2005-923315. [DOI] [PubMed] [Google Scholar]
- Rawal S, Hinkle SN, Bao W, Zhu Y, Grewal J, Albert PS, Weir NL, Tsai MY, Zhang C. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia. 2016 doi: 10.1007/s00125-016-4149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PR, Mackey AJ, Dejene EA, Ramadan JW, Langefeld CD, Palmer ND, Taylor KD, Wagenknecht LE, Watanabe RM, Rich SS, et al. An Islet-Targeted Genome-Wide Association Scan Identifies Novel Genes Implicated in Cytokine-Mediated Islet Stress in Type 2 Diabetes. Endocrinology. 2015;156:3147–3156. doi: 10.1210/en.2015-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkeland J, Saatcioglu F. Differential expression and function of stamp family proteins in adipocyte differentiation. PloS One. 2013;8:e68249. doi: 10.1371/journal.pone.0068249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox JA, McClain DA. Iron and diabetes risk. Cell Metabolism. 2013;17:329–341. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparna T, Retey J, Schmich K, Albrecht U, Naumann K, Gretz N, Fischer HP, Bode JG, Merfort I. Genome-wide comparison between IL-17 and combined TNF-alpha/IL-17 induced genes in primary murine hepatocytes. BMC Genomics. 2010;11:226. doi: 10.1186/1471-2164-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- Tamura T, Chiba J. STEAP4 regulates focal adhesion kinase activation and CpG motifs within STEAP4 promoter region are frequently methylated in DU145, human androgen-independent prostate cancer cells. International Journal of Molecular Medicine. 2009;24:599–604. doi: 10.3892/ijmm_00000270. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Kaneto H, Miyatsuka T, Yamamoto K, Yoshiuchi K, Yamasaki Y, Shimomura I, Matsuoka T-A, Matsuhisa M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocrine Journal. 2009;56:699–706. doi: 10.1507/endocrj.k09e-051. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Matsumoto I, Iwanami K, Inoue A, Umeda N, Tanaka Y, Sugihara M, Hayashi T, Ito S, Sumida T. Six-transmembrane epithelial antigen of prostate 4 (STEAP4) is expressed on monocytes/neutrophils, and is regulated by TNF antagonist in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology. 2012a;30:99–102. [PubMed] [Google Scholar]
- Tanaka Y, Matsumoto I, Iwanami K, Inoue A, Minami R, Umeda N, Kanamori A, Ochiai N, Miyazawa K, Sugihara M, et al. Six-transmembrane epithelial antigen of prostate4 (STEAP4) is a tumor necrosis factor alpha-induced protein that regulates IL-6, IL-8, and cell proliferation in synovium from patients with rheumatoid arthritis. Modern Rheumatology / the Japan Rheumatism Association. 2012b;22:128–136. doi: 10.1007/s10165-011-0475-y. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, Maneschi E, Serni S, Gacci M, Carini M, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. The Journal of Endocrinology. 2012;212:71–84. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. STAMPing out Inflammation. Cell. 2007;129:451–452. doi: 10.1016/j.cell.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Walter RMJ, Uriu-Hare JY, Olin KL, Oster MH, Anawalt BD, Critchfield JW, Keen CL. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14:1050–1056. doi: 10.2337/diacare.14.11.1050. [DOI] [PubMed] [Google Scholar]
- Wan C, Fu J, Wang Y, Miao S, Song W, Wang L. Exosome-related multi-pass transmembrane protein TSAP6 is a target of rhomboid protease RHBDD1-induced proteolysis. PloS One. 2012;7:e37452. doi: 10.1371/journal.pone.0037452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Knutson MD. Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology (Baltimore, Md.) 2013;58:788–798. doi: 10.1002/hep.26401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clinical Chemistry. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hodgkinson V, Zhu S, Weisman GA, Petris MJ. Advances in the understanding of mammalian copper transporters. Advances in Nutrition (Bethesda, Md.) 2011;2:129–137. doi: 10.3945/an.110.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. The Journal of Biological Chemistry. 2012a;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SB, Lei T, Zhou LL, Zheng HL, Zeng CP, Liu N, Yang ZQ, Chen XD. Functional analysis and transcriptional regulation of porcine six transmembrane epithelial antigen of prostate 4 (STEAP4) gene and its novel variant in hepatocytes. The International Journal of Biochemistry & Cell Biology. 2012b doi: 10.1016/j.biocel.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Han L, Wang Z, Ding W, Shang Y, Tang M, Li W, Zhang Y, Zhang W, Zhong M. Overexpression of STAMP2 suppresses atherosclerosis and stabilizes plaques in diabetic mice. Journal of Cellular and Molecular Medicine. 2014;18:735–748. doi: 10.1111/jcmm.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangensteen T, Akselsen H, Holmen J, Undlien D, Retterstol L. A common haplotype in NAPEPLD is associated with severe obesity in a Norwegian population-based cohort (the HUNT study) Obesity (Silver Spring, Md.) 2011;19:612–617. doi: 10.1038/oby.2010.219. [DOI] [PubMed] [Google Scholar]
- Weksler-Zangen S, Jorns A, Tarsi-Chen L, Vernea F, Aharon-Hananel G, Saada A, Lenzen S, Raz I. Dietary copper supplementation restores beta-cell function of Cohen diabetic rats: a link between mitochondrial function and glucose-stimulated insulin secretion. American Journal of physiology.Endocrinology and Metabolism. 2013;304:E1023–E1034. doi: 10.1152/ajpendo.00036.2013. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129:537–548. doi: 10.1016/j.cell.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlazlo N, van Greevenbroek MMJ, Ferreira I, Jansen EHJM, Feskens EJM, van der Kallen CJH, Schalkwijk CG, Bravenboer B, Stehouwer CDA. Iron metabolism is associated with adipocyte insulin resistance and plasma adiponectin: the Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care. 2013;36:309–315. doi: 10.2337/dc12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, Gu C, Cai G, Ouyang W, Sen G, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. The Journal of Experimental Medicine. 2015;212:1571–1587. doi: 10.1084/jem.20150204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HM, Cui YZ, Wang WG, Cheng HX, Sun YJ, Zhao HY, Yan YQ. Expression and clinical significance of obesity-associated gene STEAP4 in obese children. Genetics and Molecular Research: GMR. 2016;15 doi: 10.4238/gmr.15048705. [DOI] [PubMed] [Google Scholar]
- Yao Y-G, Kong Q-P, Wang C-Y, Zhu C-L, Zhang Y-P. Different matrilineal contributions to genetic structure of ethnic groups in the silk road region in china. Molecular Biology and Evolution. 2004;21:2265–2280. doi: 10.1093/molbev/msh238. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Cheong J, Kim HY. STAMPing into Mitochondria. International Journal of Biological Sciences. 2014;10:321–326. doi: 10.7150/ijbs.8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang CM, Chi X, Wang B, Zhang M, Ni YH, Chen RH, Li XN, Guo XR. Downregulation of STEAP4, a highly-expressed TNF-alpha-inducible gene in adipose tissue, is associated with obesity in humans. Acta Pharmacologica Sinica. 2008;29:587–592. doi: 10.1111/j.1745-7254.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 101.Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. The Journal of Biological Chemistry. 2010;285:32141–32150. doi: 10.1074/jbc.M110.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou J, Ye S, Fujiwara T, Manolagas SC, Zhao H. Steap4 Plays a Critical Role in Osteoclastogenesis in vitro by Regulating Cellular Iron/ROS Levels and CREB Activation. The Journal of Biological Chemistry. 2013 doi: 10.1074/jbc.M113.478750. [DOI] [PMC free article] [PubMed] [Google Scholar]