Abstract
Six-transmembrane epithelial antigen of the prostate 4 (STEAP4) is a metalloreductase that has been shown previously to protect cells from inflammatory damage. Genetic variants in STEAP4 have been associated with numerous metabolic disorders related to obesity, including putative defects in the acute insulin response to glucose in type 2 diabetes (T2D).
Purpose
We examined whether obesity and/or T2D altered STEAP4 expression in human pancreatic islets.
Methods
Human islets were isolated from deceased donors at two medical centers and processed for quantitative PCR. Organ donors were selected by status as non-diabetic or having T2D. Site1 (Edmonton): N=13 T2D donors (7M, 6F), N=20 non-diabetic donors (7M, 13F). Site2 (Virginia): N=6 T2D donors (6F), N=6 non-diabetic donors (3M, 3F).
Results
STEAP4 showed reduced islet expression with increasing BMI among all donors (P<0.10) and non-diabetic donors (P<0.05) from Site1; STEAP4 showed reduced islet expression among T2D donors with increasing HbA1c. Islet STEAP4 expression was also marginally higher in female donors (p<0.10). Among T2D donors from Site2, islet insulin expression was reduced, STEAP4 expression was increased, and white blood cell counts were increased compared to non-diabetic donors. Islets from non-diabetic donors that were exposed overnight to 5ng/ml IL-1β displayed increased STEAP4 expression, consistent with STEAP4 upregulation by inflammatory signaling.
Conclusions
These findings suggest that increased STEAP4 mRNA expression is associated with inflammatory stimuli, whereas lower STEAP4 expression is associated with obesity in human islets. Given its known protective role, downregulation of STEAP4 by chronic obesity suggests a mechanism for reduced islet protection against cellular damage.
Key terms: inflammation, islets, obesity, type 2 diabetes, cytokines, iron
Introduction
The intersection between low-grade inflammation and beta-cell dysfunction has gained increased interest in recent years as a key factor in the pathophysiology of type 2 diabetes (T2D). It has previously been shown that islets from T2D donors demonstrate inflammatory changes at the histological level [1–4], and an inflammatory milieu heightens the risk for T2D development in prospective clinical studies [5].
A potential player in this intersection is six-transmembrane epithelial antigen of the prostate 4 (STEAP4; also called STAMP2 [6] or TIARP [7]). A number of genome-wide association studies suggest that STEAP4 is involved with numerous disorders related to obesity and metabolism including insulin resistance [8,9], metabolic syndrome [10,11], and insulin secretion [12,13]. Beyond its known role as a metalloreductase [14], studies have shown that STEAP4 plays an anti-inflammatory or protective role from cellular damage in adipocytes [15,9], mesangial cells [16], hepatocytes [17–19], and synoviocytes [20]. In studies of mice, the STEAP4-/- phenotype includes the spontaneous development of obesity, insulin resistance, and eventual hyperglycemia [21], as well as signs of atherosclerosis [22], also suggesting a protective role for the protein.
The limited data in humans generated thus far have been conflicting. Human abdominal subcutaneous and omental white adipose tissue collected from insulin-resistant individuals was associated with increased STEAP4 levels in a pair of studies [23,24]. By contrast, several similar studies have demonstrated decreased STEAP4 expression in visceral adipose tissue with increased adiposity or body mass index (BMI) [25–28].
Our laboratory has recently demonstrated that STEAP4 is expressed in rodent islets and strongly upregulated in response to low-grade inflammation [13]. Further, single nucleotide polymorphisms in STEAP4 have been associated with differences in acute insulin response to glucose (AIRg) in humans [13], suggesting a role for STEAP4 in pancreatic islet function. In the present study, we compared STEAP4 expression in human islets collected from T2D donors and non-diabetic controls from two different sites to determine what factors are associated with differences in STEAP4 expression at the islet level.
Materials and Methods
Human islet isolations at University of Alberta (Edmonton)
Human islets were isolated from donor organs at the Alberta Diabetes Institute IsletCore (www.bcell.org/IsletCore.html) or the Clinical Islet Laboratory at the University of Alberta as described [29] and cultured as described [30]. Information on donor age, BMI, sex and HbA1c were provided.
Human islet isolation at University of Virginia (Virginia)
Human pancreatic islets were isolated at the University of Virginia cell processing facility. Under GMP conditions, pancreata were cleaned of extraneous tissue; inflated with Liberase MTF collagenase and digested using the Ricordi method. Digestate was washed and pelleted for purification. Purification was performed using a COBE 2991 cell processor with a continuous Biocoll gradient. Purified pancreatic islets were cultured in CMRL media supplemented with 10% human albumin, nicotinomide and glutathione. Cultured islets were considered high purity if purity was 85% or above.
Select medical data were available for human islets sourced from UVA consisting of basic demographic information and results of laboratory studies performed during hospital admission prior to death. The following serum studies were analyzed across the donor set: sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine, glucose, calcium, albumin, international standardized ratio (INR), lipase, white blood count, and hemoglobin. Several sets of values were available for each donor, as multiple iterations had been performed during hospitalization. The maximum, minimum, average, and final values of each study category were compiled and compared by scatter plot with islet STEAP4 expression values.
RNA isolation and qPCR of human islet samples
For the Edmonton samples, islets were hand-picked to >99% purity and RNA was extracted using TRIzol Reagent (ThermoFisher Scientific, Burlington, ON. Canada) and reverse transcription performed using 5X All-in-One RT Master Mix (Applied Biological Materials Inc, Richmond, BC. Canada). qPCR was performed on a 7900HT Fast Real-Time PCR System using Fast SYBR Green Master Mix (ThermoFisher Scientific) and primers listed below.
For the Virginia samples, RNA isolations were performed using an AllPrep DNA/RNA/Protein mini Kit from Qiagen GmbH (Hilden, Germany) and reverse transcription of 0.25-1μg RNA using a High Capacity cDNA kit (Life Technologies). qPCR was performed using iQ SYBR green SuperMix (Bio-Rad, Hercules, CA, USA) on an iCycler (MyiQ Optical Module) Bio-Rad system. mRNA expression was quantified based on the HPRT1 house-keeping gene, using the Relative Expression Software Tool [31]. Outliers were removed based on their relation to the interquartile range (IQR). Specifically, gene expression less than [First Quartile (Q1) – 1.5*IQR] or greater than [Third Quartile (Q3) + 1.5*IQR] were considered outliers. The following key primer sets were used: for hHPRT1, forward (5′-ATGGACAGGACTGAACGTCT-3′) and reverse (5′-TCCAGCAGGTCAGCAAAGAA-3′); for insulin, forward (5′-TGCGGGGAACGAGGCTTCTTCTA-3′) and reverse (5′-AGGGACCCCTCCAGGGCCAAG-3′); for STEAP4, forward (5′-GCGCCTCTCCCTCAGTTATG-3′) and reverse (5′-GGTCTTCTGGGGGTTTCGAC-3′). A full list of primers is included as Supplemental Table S1.
Note that STEAP4 values were normalized to HPRT1 gene for both the Edmonton and Virginia data sets. STEAP4 expression patterns in the Edmonton group were also normalized to 18s, but similar results were observed with either control. For the data reported here, STEAP4 expression was normalized to HPRT1 unless otherwise stated.
Statistics
Correlations between STEAP4 and chart data were made by Pearson correlation. GraphPad Prism software was used to determine p-values for linear regression analysis. A two-tailed t-test was used for all other comparisons. P-values < 0.05 were considered significant.
Results
Site-related differences in STEAP4 expression
The same primers were used at both islet isolation sites to measure STEAP4 mRNA and HPRT1 control mRNA, however, STEAP4 expression among all donors from the Edmonton site was 4.1+/-0.2 fold over the HPRT1 values versus 2.1+/-0.4 for the Virginia site (P=0.0003). Due to this disparity between data sets, we analyzed the data from each site separately. We have also provided the results of analysis using the combined data sets from both sites in Supplemental Table S2 for comparison.
Donor Characteristics from University of Alberta (Edmonton)
We examined islets from n=20 non-T2D donors and n=13 T2D donors isolated at the University of Alberta (Edmonton). As shown in Table 1, no differences in age or body mass index (BMI) among T2D vs non-T2D donors were observed. Glycosylated hemoglobin (HbA1c) was significantly higher among donors with T2D.
Table 1. Donor Information: University of Alberta.
| Age | Male | Female | BMI HbA1c | |
|---|---|---|---|---|
| T2D | 54.3 +/- 2.7 | N=7 | N=6 | 31.2 +/- 1.7 7.4 +/- 0.4 |
| Non-T2D | 46.9 +/- 2.9 | N=7 | N=13 | 29.2 +/- 1.5 5.7 +/- 0.1 |
| P-value | 0.11 | 0.43 <0.001 |
Age, male and female donor number, BMI and HbA1c values for T2D and non-T2D donors from University of Alberta (Edmonton, Site 1).
T2D donors tended to be older than non-T2D donors, but differences did not reach statistical significance. Scatter plot analysis across all ages produced a correlation coefficient of R2=0.002 with STEAP4 expression (not significant). In addition, among donors 60 years of age and over (the highest quartile), STEAP4 expression levels were 4.0+/-0.6 fold of the HPRT1 house-keeping gene, whereas STEAP4 levels for donors 40 years of age or under (lowest quartile) were 3.8+/-0.5 (p=0.72). Thus, STEAP4 values do not appear to differ with age.
Decreased STEAP4 expression is associated with increased BMI (Edmonton)
Initial observations showed no statistically significant differences between islets isolated from non-T2D donors (4.1+/-0.3, n=20) and T2D donors (4.0+/-0.4, n=13). We next analyzed BMI as a categorical variable and grouped T2D and non-T2D donors as lean (BMI≤25), overweight (25>BMI≤30), or obese (BMI>30). In this analysis among all donors from Site 1, STEAP4 expression was marginally reduced (P<0.10) in obese compared to lean donors (Figure 1A). This relationship was more apparent among non-T2D donors, where islets from obese donors had significantly reduced STEAP4 expression compared to lean and overweight donors (Figure 1B). These findings are consistent with several studies showing that STEAP4 levels in adipose tissue decrease with increasing adiposity [25–27]. Surprisingly, no significant changes in STEAP4 expression were observed between lean, overweight, and obese T2D islet donors, as shown in Figure 1C. When evaluated as a continuous variable, BMI was significantly correlated with reduced STEAP4 levels for all donors combined (Figure 1D), for non-T2D donors (Figure 1E), but not for T2D donors (Figure 1F).
Figure 1.

STEAP4 is negatively correlated with BMI. (A) STEAP4 expression levels for all donors from Site 1 (Edmonton) divided into lean (BMI≤25, N=9), overweight (25>BMI≤30, N=11), and obese (BMI>30, N=13). (B) STEAP4 expression levels for non-T2D donors divided into lean (N=7), overweight (N=6), and obese (N=7). (C) STEAP4 expression levels for T2D donors divided into lean (N=2), overweight (N=5), and obese (N=6). (D-F) Scatter plots for STEAP4 expression vs BMI for all donors (D) non-T2D donors (E) and T2D donors (F). *P<0.05, #P<0.10. R2 and p-values provided in lower left corner of (D-F).
STEAP4 expression is reduced with increasing HbA1C in T2D donors (Edmonton)
We next examined the relationship between STEAP4 expression and HbA1c. As shown in Figure 2A, HbA1c was elevated among T2D donors (see also Table 1), but there were no overall differences in STEAP4 expression levels between T2D and non-T2D donors. When examined by scatter plot, STEAP4 showed no relationship with HbA1c among all donor islets (P=0.17) or among non-T2D donors (Figure 2B). In contrast, STEAP4 was negatively correlated with HbA1C (P=0.01) among T2D donors, as shown in Figure 2C.
Figure 2.
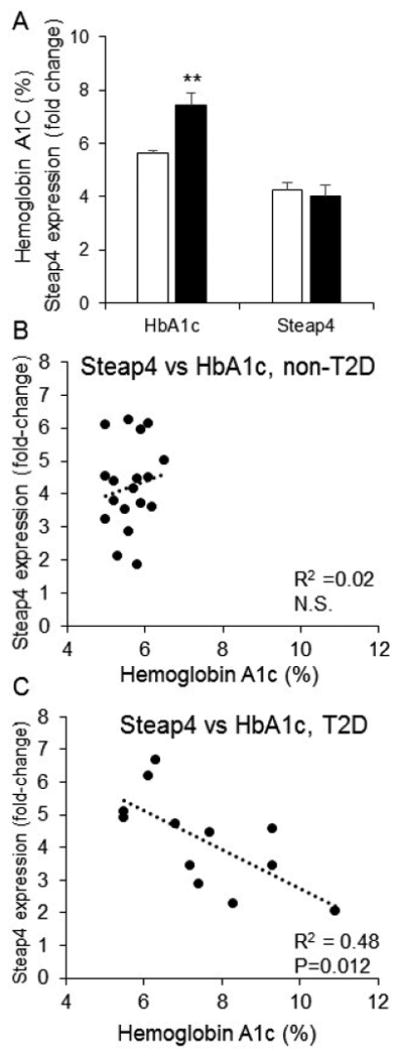
STEAP4 is negatively correlated with HbA1c in T2D donors. (A) Mean HbA1c (left columns) and STEAP4 gene expression (right columns) for non-T2D (white columns) and T2D (black columns) donors (**P<0.01). (B-C) Scatter plots for STEAP4 expression vs HbA1c for non-T2D donors (B) and T2D donors (C). R2 and p-values provided in lower right corner of (B) and (C).
STEAP4 expression is higher in female donors compared to male donors (Edmonton)
We also examined STEAP4 expression for possible differences between male and female donors. Combining both T2D and non-T2D donor islets in the Edmonton group, the fold change for STEAP4 expression over the housekeeping gene HPRT1 in islets from female donors (4.4 +/-0.3, n=19) was marginally higher than in islets from male donors (3.6 +/-0.4, n=14, P<0.10). STEAP4 levels were slightly higher in islets from female donors compared to male donors for both non-T2D and T2D subsets, but these differences were not significant. Higher STEAP4 levels were also observed in islets from female donors in the Virginia group (Female: 2.6+/-0.5, n=8 vs. Male: 0.7+/-0.2, n=3, P<0.01, see also Table 2) and when both data sets from Site 1 and Site 2 were combined (P<0.10, see Supplemental Table S2). STEAP4 expression levels thus appear to be consistently higher in islets from females compared to males, although the significant of these differences is marginal (p-values of 0.05-0.10 in most comparisons).
Table 2. Donor Information: University of Virginia.
| Donor | STEAP4 | Age | BMI | WBC Count (×1000) | Sex | Cause of Death |
|---|---|---|---|---|---|---|
| Donors with Type 2 Diabetes | ||||||
| VP163* | 26.6 | 67 | 23 | 14.8 | F | CVA |
| VP168 | 5.1 | 56 | 28 | 22.2 | F | CVA |
| VP093 | 4.5 | 42 | 29 | 23.4 | F | CVA |
| VP173 | 2.5 | 59 | 24 | no data | F | Anoxia |
| VP141 | 2.3 | 56 | 29 | 32.2 | F | CVA |
| VP143 | 1.4 | 54 | 53 | 20.8 | F | CVA |
| Average | 3.1+0.7 | 53 ± 3 | 33±5 | 24.7+2.6 | ||
| Donors without Type 2 Diabetes | ||||||
| VP169 | 3.0 | 27 | 18 | 9.3 | F | CNS Tumor |
| VP164 | 1.1 | 58 | 20 | no data | F | CVA |
| VP179 | 1.0 | 47 | 40 | 14 | F | CVA |
| VP151 | 1.0 | 59 | 27 | 11.4 | M | Cervical Fracture |
| VP166 | 0.7 | 59 | 20 | 18.7 | M | CVA |
| VP183 | 0.4 | 42 | 26 | 9.8 | M | CVA |
| Average | 1.21 ±0.37 | 49±5 | 25 ± 3 | 12.6 ± 1.7 | ||
| P-value | 0.03 | 0.48 | 0.24 | 0.005 | ||
Age, BMI, average white blood cell (WBC) count, sex, cause of death, and islet STEAP4 expression levels for donors diagnosed with or without T2D from University of Virginia (Virginia, Site 2). N/A = lab results were not available.
indicates STEAP4 value was listed but was removed as an outlier for all statistical comparisons.
Donor characteristics from the University of Virginia and STEAP4 correlations (Virginia)
Studies were also performed on islet STEAP4 expression levels with a donor base from Virginia. Clinical chart data from hospitalizations prior to death were available for most tissue donors from Virginia. A summary of key demographic information is shown in (Table 2). Tissue samples were obtained from a total of N=7 females and N=3 males. Notably, all five donors from the T2D group were female. The cause of death for the majority of donors was cerebrovascular accident (CVA). Anoxia, central nervous system (CNS) tumor, and cervical fracture accounted for the deaths of the three other donors, respectively. There were no significant differences in age or BMI between the two groups.
Analysis of chart data yielded a number of significant differences. STEAP4 expression levels correlated strongly to maximum serum sodium (R2 = 0.6279, p = 0.006) and maximum serum chloride (R2 = 0.704, p = 0.002) as shown in Figure 3A and Figure 3B. “Maximum” refers to the highest value recorded among multiple measures while in hospital. A borderline significant correlation to STEAP4 was also observed for mean sodium (R2 = 0.3801, p = 0.06) and mean chloride (R2 = 0.3794, p = 0.06). This correlation may indicate STEAP4 upregulation related to the acute patient condition in hospital.
Figure 3.
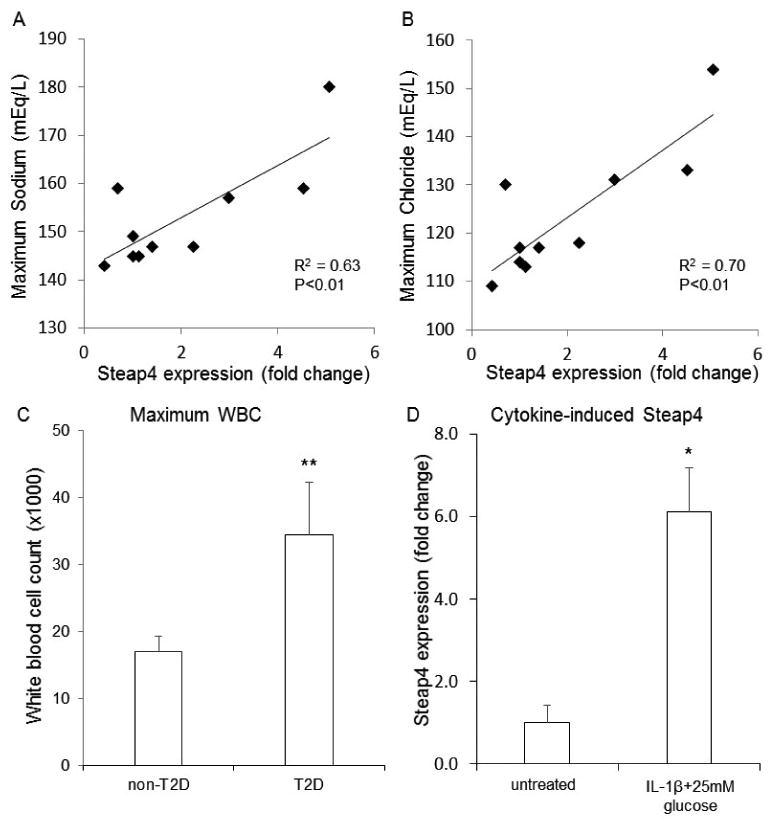
STEAP4 associations with sodium levels, chloride levels, and inflammatory status. (A-B) Scatter plots for STEAP4 expression vs maximum sodium (A) and maximum chloride (B) for islet donors recorded during hospitalization (N=10). R2 and p-values provided in lower right corner. (C) White blood cell counts in non-T2D (N=5) and T2D donors (N=4). (D) STEAP4 expression in islets isolated from N=3 donors treated overnight (24 hours) with 5ng/ml interleukin-1beta in 25mM glucose or untreated. *P<0.05, **P<0.01.
Mean white blood cell (WBC) counts differed markedly between T2D and non-T2D donor populations, as shown in Table 2. Maximum WBC count (p = 0.004, Figure 3C) and last WBC (p = 0.004) also differed between the two groups, with higher counts found in the T2D group.
STEAP4 is upregulated by exposure to interleukin 1-beta (Virginia)
Since STEAP4 is highly sensitive to cytokine-induced expression and inflammatory signaling [7,13,32], we examined whether exposure to proinflammatory conditions could directly increase STEAP4 expression in human islets. We exposed islets from 3 non-T2D donors to 5 ng/ml IL-1β + 25mM glucose conditions overnight and then collected mRNA to measure STEAP4 expression levels. As shown in Figure 3D, STEAP4 expression was markedly upregulated compared to untreated controls. In contrast to chronic obesity or hyperglycemia downregulating STEAP4 expression, these data suggest an increase in inflammation or immune response may be responsible for the increased STEAP4 expression. Note that 25mM glucose exposure could be regarded as a hyperglycemic trigger of STEAP4 in addition to IL-1β. We showed in rodent islets, however, that chronic high glucose exposure has little impact on STEAP4 levels [13], especially compared to cytokine effects.
STEAP4 gene expression in comparison to key markers of islet function (Virginia)
Expression levels of the following known pancreatic islet genes were quantified in relation to the HPRT1 housekeeping gene: STEAP4, genes related to secretory hormones (insulin, glucagon), genes related to maintenance of normal islet function (GLUT1, GLUT2, PDX1, NKX6-1, KCNJ11), genes related to insulin signaling pathways (GLP1R, INSR, IRS2, IGF1R), and genes related to metabolism (CITED2, ACC1, ACC2, FASN, SCD1). The fold change in mRNA expression between donors with T2D and donors without T2D are shown in Figure 4A.
Figure 4.
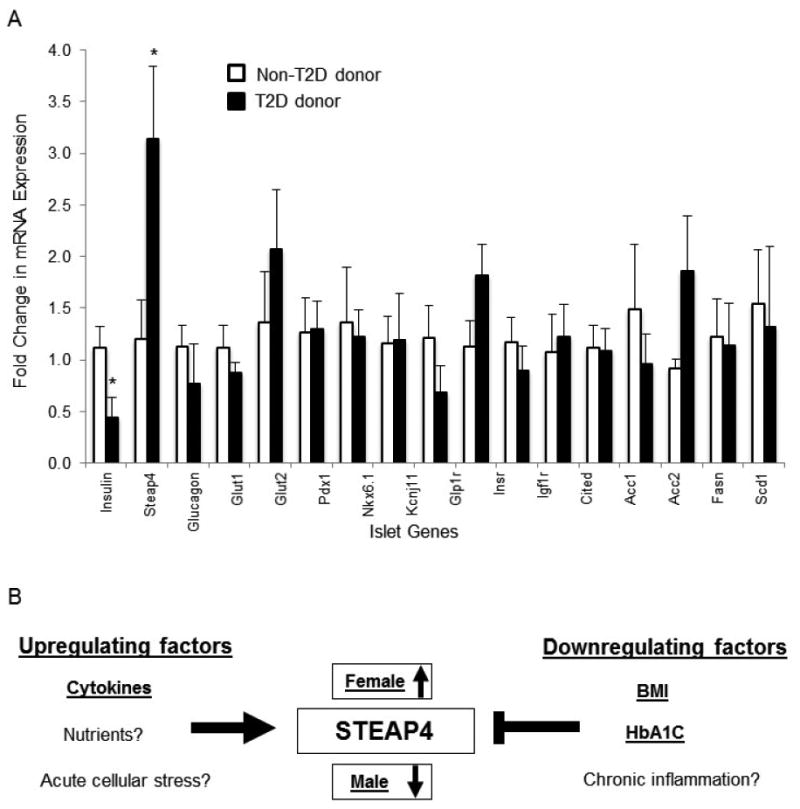
(A) Gene expression differences in islets from non-T2D (N=5) vs. T2D donors (N=5) for a total of 17 genes related to islet function. *P<0.05. (B) Depiction of factors regulating STEAP4 expression in human islets.
Among all 17 genes examined, only insulin (p = 0.04) and STEAP4 (p = 0.03) showed significant differences between donor populations. Islets from donors with T2D expressed significantly lower levels of insulin (expected for individuals with T2D) and significantly higher levels of STEAP4 compared to islets from non-diabetic donors. However, there was a strong relationship between STEAP4 expression and insulin expression (R2 = 0.90, p = 0.01, n=5) in the T2D population, with low levels of insulin correlated with low levels of STEAP4 expression in islets. Since loss of insulin is related to hyperglycemia, the reduced STEAP4 levels with low insulin is consistent with the lower STEAP4 with increased HbA1c observed in the Edmonton data. Notably, one outlier from the T2D group was included in Table 2 but excluded from all analysis due to exceptionally high STEAP4 expression (fold change of 26.61); the high expression did not appear to correlate with unusual values in any other measured parameter.
Discussion
This is the first study to show that STEAP4 is expressed in the human pancreatic islet. Our data suggest that multiple regulatory factors contribute to increased or decreased STEAP4 expression, as depicted in Figure 4B. Known and putative upregulating factors include pro-inflammatory cytokines [7,13,32,33], nutrient intake [21], and acute cellular stress [13,21]. Downregulating factors of STEAP4 include obesity [25–28], HbA1c/diabetic condition [26], and chronic inflammation. We discuss below how these putative competing regulatory factors may impact islet STEAP4 expression.
STEAP4 expression in obesity
The relationship between STEAP4 and obesity is not entirely clear due to conflicting findings in the literature. A pair of studies showed that STEAP4 levels in adipocytes and white adipose tissue were increased in obesity [23,24], whereas other studies showed that STEAP4 was downregulated in obese people [25–28]. In yet another study, STEAP4 gene expression in both fat and muscle was reduced among the most insulin resistant individuals independent of BMI [34].
With regard to islets, we showed a clear and significant decrease in STEAP4 with increasing BMI, especially among non-T2D donors (Edmonton, Site 1). T2D donors in the Edmonton group were skewed toward higher BMI with only N=2 lean donors (BMI <25), thus potentially masking a relationship. STEAP4 levels in T2D donors were decreased with increasing HbA1c, suggesting an inverse relationship between STEAP4 expression and severity of hyperglycemia. For the Virginia data (site 2), determining a relationship between STEAP4 expression BMI was not statistically feasible due to the smaller sample size and relatively tight distribution of BMI values (10 of 12 donors were non-obese, (<30 BMI)). Even with these limitations, trend lines pointed to an inverse relationship between STEAP4 expression and BMI for both T2D and non-T2D donors, thus supporting the Edmonton findings.
STEAP4 expression and acute vs. chronic inflammation
Because obesity is associated with chronic low-grade inflammation and therefore elevated cytokine levels, reduced STEAP4 expression with increased obesity seems counterintuitive to previous research showing upregulation of STEAP4 in the presence of cytokines [7,13,32,33]. Our data show that direct overnight exposure to pro-inflammatory cytokines (5 ng/ml IL-1β) increases expression of STEAP4 in human islets from non-T2D donors, supporting previous studies [7,13,32,33]. Note that glucose was also elevated for the overnight cytokine treatment, but high glucose alone does not appear to increase STEAP4 expression in islets [13]. We also showed that T2D donors from the Virginia cohort had increased WBC counts compared to non-T2D controls, which may be indicative of an acute immune response. Sodium and chloride values were also associated with These data are consistent with an increase in STEAP4 in acute inflammatory circumstances.
The reduced STEAP4 levels with elevated BMI may be due to chronic physiologic aspects of obesity, such as chronic low-grade inflammation, causing STEAP4 downregulation. Of interest, STEAP4 levels are lower in circulating macrophages of women with metabolic syndrome compared to controls [35], suggesting downregulation of STEAP4 in inflammatory cells in chronic metabolic diseases. Thus, differences in STEAP4 expression may be due, at least in part, to differences between chronic and acute inflammation with regard to cytokine dose, duration, and milieu [36].
Male vs. Female
Islets from female donors in the Edmonton cohort generally showed higher STEAP4 expression compared to male donors. All Virginia T2D donors were female, while the non-T2D donors had equal male and female inclusion (n=3 each). It should be noted that the three lowest STEAP4 values among all n=11 donors from the Virginia cohort were all the male samples (See Table 2). Therefore, sex differences could explain, in part, why the STEAP4 expression in the Virginia T2D donors was higher than in non-T2D donors.
The underlying physiological reason for differences in islet STEAP4 expression between sexes is not known. It is significant to note that women with metabolic syndrome (MetS) show down regulation in STEAP4 expression compared to non-MetS, while men show no significant differences in expression between those with MetS vs. those without [35]. Other regulating factors to consider are hormones such as testosterone, which upregulates the expression of STEAP4 [37].
Additional Considerations and Limitations
The differences in STEAP4 expression between the Edmonton site and Virginia site warrant additional discussion. A limitation of our study is that donor data sets were not equivalent. Thus, analysis and data presentation for each site were performed separately and were never combined. For example, WBC count was known for Virginia, but not Edmonton, thus complicating analysis of the role of inflammation in both cohorts. The Virginia cohort did not provide sufficient statistical power to account for sex, BMI, or hemoglobin A1c. In addition, therapeutic interventions between donors groups, disease management of donors, medical status at death, and even differences in health care systems between countries [38] could contribute to the observed differences in STEAP4 expression. What we have attempted to do is to identify factors for which we could find a strong correlation with STEAP4 expression, while acknowledging other potential confounding factors.
The islet isolation procedures between sites also differed, resulting in potential differences in islet viability and function. Importantly, islets from Edmonton were hand-picked >99% purity, whereas the Virginia islets were purified to >85%, resulting in different levels of acinar tissue in the samples that could impact gene expression levels. Note that the islets themselves are composed predominantly of insulin-secreting beta-cells, glucagon-secreting alpha-cells, and somatostatin-secreting delta-cells [39,40], but the proportions of these cell types may differ from donor to donor, which could also impact expression. While differences in islet purity may contribute to differences in results between sites, we observed more similarities than differences when comparable between sites, as discussed above.
Conclusions
Several studies suggest that STEAP4 plays a protective role in the face of inflammatory stress in models of metabolic disorders [9,21,22,41,42]. Thus, if obesity results in reduced STEAP4 expression levels, this may depress a normal compensatory responses to cellular stress. Moreover, reduced STEAP4 could directly impact insulin secretory function. Genetic variants in STEAP4 have been associated differences in the AIRg (also called first phase) in a diabetes-prone population [13]. Our overall findings suggest that STEAP4 expression is downregulated by factors such as chronic obesity (BMI) and hyperglycemia (HbA1c) but upregulated by acute inflammation or cytokine exposure at the level of the pancreatic islet (see Figure 4B). These findings point to complex regulatory inputs to STEAP4, a protein that is increasingly associated with a protective role in metabolic disorders. Continued work toward elucidating what regulates STEAP4 and what role STEAP4 plays in metabolic disorders is warranted.
Supplementary Material
Acknowledgments
Support for this work was provided by R01 DK089182 and the Ohio University Diabetes Institute to CSN; PEM holds a 2016/2017 Killam Professorship; funding for CEM from VA Grant 5I01BX001733 and R01 DK093954. We thank the Human Organ Procurement and Exchange (HOPE) program (Edmonton, Canada) and the Trillium Gift of Life Network (TGLN; Toronto, Canada) for assistance with procurement of donor pancreas for research. We thank Mr. James Lyon for human islet isolations performed at the Alberta Diabetes Institute IsletCore, supported by funding from the Alberta Diabetes Foundation, and Drs. Tatsuya Kin and James Shapiro for human islet isolations performed at the University of Alberta Clinical Islet Laboratory. We thank Dr. Tatsuyoshi Kono at Indiana University School of Medicine for technical assistance. We also thank The UVA Department of Surgery and Human Islet Isolation GMP Facility.
Funding Sources: NIH DK089182 to CSN and Alberta Diabetes Foundation to PEM.
Footnotes
Compliance with Ethical Standards: I certify that neither I nor my co-authors have any conflict of interest with regard to the subject matter or materials included in this Work. All studies were approved by the Human Research Ethics Board at the University of Alberta (Pro00001754, Pro00013094) for donors from Site 1 and by University of Virginia Institutional Review Board approved protocol #14904 for donors from Site 2. Informed consent of the donor and their family, including for use of organs for research, was obtained by the local organ procurement agency prior to organ retrieval.
References
- 1.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 2.Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B, Serradas P. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55:1625–1633. doi: 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):152–158. doi: 10.1111/dom.12168. [DOI] [PubMed] [Google Scholar]
- 4.Butcher MJ, Hallinger D, Garcia E, Machida Y, Chakrabarti S, Nadler J, Galkina EV, Imai Y. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57:491–501. doi: 10.1007/s00125-013-3116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkmaz CG, Korkmaz KS, Kurys P, Elbi C, Wang L, Klokk TI, Hammarstrom C, Troen G, Svindland A, Hager GL, Saatcioglu F. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene. 2005;24:4934–4945. doi: 10.1038/sj.onc.1208677. [DOI] [PubMed] [Google Scholar]
- 7.Moldes M, Lasnier F, Gauthereau X, Klein C, Pairault J, Feve B, Chambaut-Guerin AM. Tumor necrosis factor-alpha-induced adipose-related protein (TIARP), a cell-surface protein that is highly induced by tumor necrosis factor-alpha and adipose conversion. J Biol Chem. 2001;276:33938–33946. doi: 10.1074/jbc.M105726200. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhu C, Ji C, Zhao Y, Zhang C, Chen F, Gao C, Zhu J, Qian L, Guo X. STEAP4, a gene associated with insulin sensitivity, is regulated by several adipokines in human adipocytes. Int J Mol Med. 2010;25:361–367. doi: 10.3892/ijmm_00000353. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Huang Z, Zhou B, Wang H, Jia G, Liu G, Zhao H. STEAP4 and insulin resistance. Endocrine. 2014;47:372. doi: 10.1007/s12020-014-0230-1. [DOI] [PubMed] [Google Scholar]
- 10.Nanfang L, Yanying G, Hongmei W, Zhitao Y, Juhong Z, Ling Z, Wenli L. Variations of six transmembrane epithelial antigen of prostate 4 (STEAP4) gene are associated with metabolic syndrome in a female Uygur general population. Arch Med Res. 2010;41:449–456. doi: 10.1016/j.arcmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Qi Y, Yu Y, Wu Y, Wang S, Yu Q, Shi J, Xu Z, Zhang Q, Fu Y, Fu Y, Kou C. Genetic Variants in Six-Transmembrane Epithelial Antigen of Prostate 4 Increase Risk of Developing Metabolic Syndrome in a Han Chinese Population. Genet Test Mol Biomark. 2015;19:666. doi: 10.1089/gtmb.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miot A, Maimaitiming S, Emery N, Bellili N, Roussel R, Tichet J, Velho G, Balkau B, Marre M, Fumeron F, Group DS. Genetic variability at the six transmembrane protein of prostate 2 locus and the metabolic syndrome: the data from an epidemiological study on the Insulin Resistance Syndrome (DESIR) study. J Clin Endocrinol Metab. 2010;95:2942–2947. doi: 10.1210/jc.2010-0026. [DOI] [PubMed] [Google Scholar]
- 13.Sharma PR, Mackey AJ, Dejene EA, Ramadan JW, Langefeld CD, Palmer ND, Taylor KD, Wagenknecht LE, Watanabe RM, Rich SS, Nunemaker CS. An Islet-Targeted Genome-Wide Association Scan Identifies Novel Genes Implicated in Cytokine-Mediated Islet Stress in Type 2 Diabetes. Endocrinology. 2015;156:3147. doi: 10.1210/en.2015-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han L, Tang MX, Ti Y, Wang ZH, Wang J, Ding WY, Wang H, Zhang Y, Zhang W, Zhong M. Overexpressing STAMP2 Improves Insulin Resistance in Diabetic ApoE(-/-)/LDLR(-/-) Mice via Macrophage Polarization Shift in Adipose Tissues. PloS One. 2013;8:e78903. doi: 10.1371/journal.pone.0078903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang CT, Guh JY, Lu CY, Wang YT, Chen HC, Chuang LY. Steap4 attenuates high glucose and S100B-induced effects in mesangial cells. J Cell Mol Med. 2015;19:1234. doi: 10.1111/jcmm.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SB, Lei T, Zhou LL, Zheng HL, Zeng CP, Liu N, Yang ZQ, Chen XD. Functional analysis and transcriptional regulation of porcine six transmembrane epithelial antigen of prostate 4 (STEAP4) gene and its novel variant in hepatocytes. Int J Biochem Cell Biol. 2012 doi: 10.1016/j.biocel.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, Cho HK, Yoo SK, Cheong JH. Hepatic STAMP2 decreases hepatitis B virus X protein-associated metabolic deregulation. Exp Mol Med. 2012;44:622–632. doi: 10.3858/emm.2012.44.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HY, Park SY, Lee MH, Rho JH, Oh YJ, Jung HU, Yoo SH, Jeong NY, Lee HJ, Suh S, Seo SY, Cheong J, Jeong JS, Yoo YH. Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and insulin resistance. J Hepatol. 2015;63:477. doi: 10.1016/j.jhep.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y, Matsumoto I, Iwanami K, Inoue A, Minami R, Umeda N, Kanamori A, Ochiai N, Miyazawa K, Sugihara M, Hayashi T, Goto D, Ito S, Sumida T. Six-transmembrane epithelial antigen of prostate4 (STEAP4) is a tumor necrosis factor alpha-induced protein that regulates IL-6, IL-8, and cell proliferation in synovium from patients with rheumatoid arthritis. Mod Rheumatol Jpn Rheum Assoc. 2012;22:128–136. doi: 10.1007/s10165-011-0475-y. [DOI] [PubMed] [Google Scholar]
- 21.Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129:537–548. doi: 10.1016/j.cell.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freyhaus HT, Calay ES, Yalcin A, Vallerie SN, Yang L, Calay ZZ, Saatcioglu F, Hotamisligil GS. Stamp2 Controls Macrophage Inflammation through Nicotinamide Adenine Dinucleotide Phosphate Homeostasis and Protects against Atherosclerosis. Cell Metab. 2012;16:81–89. doi: 10.1016/j.cmet.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arner P, Stenson BM, Dungner E, Naslund E, Hoffstedt J, Ryden M, Dahlman I. Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. J Clin Endocrinol Metab. 2008;93:2249–2254. doi: 10.1210/jc.2008-0206. [DOI] [PubMed] [Google Scholar]
- 24.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Six-transmembrane epithelial antigen of prostate 4 and neutrophil gelatinase-associated lipocalin expression in visceral adipose tissue is related to iron status and inflammation in human obesity. Eur J Nutr. 2012 doi: 10.1007/s00394-012-0464-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CM, Chi X, Wang B, Zhang M, Ni YH, Chen RH, Li XN, Guo XR. Downregulation of STEAP4, a highly-expressed TNF-alpha-inducible gene in adipose tissue, is associated with obesity in humans. Acta Pharmacol Sin. 2008;29:587–592. doi: 10.1111/j.1745-7254.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Navarrete JM, Ortega F, Serrano M, Perez-Perez R, Sabater M, Ricart W, Tinahones F, Peral B, Fernandez-Real JM. Decreased STAMP2 expression in association with visceral adipose tissue dysfunction. J Clin Endocrinol Metab. 2011;96:E1816–25. doi: 10.1210/jc.2011-0310. [DOI] [PubMed] [Google Scholar]
- 27.Ozmen F, Ozmen MM, Gelecek S, Bilgic I, Moran M, Sahin TT. STEAP4 and HIF-1alpha gene expressions in visceral and subcutaneous adipose tissue of the morbidly obese patients. Mol Immunol. 2016;73:53. doi: 10.1016/j.molimm.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Xu HM, Cui YZ, Wang WG, Cheng HX, Sun YJ, Zhao HY, Yan YQ. Expression and clinical significance of obesity-associated gene STEAP4 in obese children. Genet Mol Res GMR. 2016;15 doi: 10.4238/gmr.15048705. [DOI] [PubMed] [Google Scholar]
- 29.Kin T, Shapiro AMJ. Surgical aspects of human islet isolation. Islets. 2010;2:265–273. doi: 10.4161/isl.2.5.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyon J, Manning Fox JE, Spigelman AF, Kim R, Smith N, O'Gorman D, Kin T, Shapiro AMJ, Rajotte RV, MacDonald PE. Research-Focused Isolation of Human Islets From Donors With and Without Diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology. 2016;157:560. doi: 10.1210/en.2015-1562. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kralisch S, Sommer G, Weise S, Lipfert J, Lossner U, Kamprad M, Schrock K, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta is a positive regulator of TIARP/STAMP2 gene and protein expression in adipocytes in vitro. FEBS Lett. 2009;583:1196–1200. doi: 10.1016/j.febslet.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Chambaut-Guerin AM, Klein J, Paschke R. Interleukin-6 is a positive regulator of tumor necrosis factor alpha-induced adipose-related protein in 3T3-L1 adipocytes. FEBS Lett. 2004;560:153–157. doi: 10.1016/S0014-5793(04)00096-1. [DOI] [PubMed] [Google Scholar]
- 34.Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes. 2011;60:1019–1029. doi: 10.2337/db10-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZH, Zhang W, Gong HP, Guo ZX, Zhao J, Shang YY, Feng JB, Zhang Y, Zhong M. Expression of STAMP2 in monocytes associates with cardiovascular alterations. Eur J Clin Invest. 2010;40:490–496. doi: 10.1111/j.1365-2362.2010.02288.x. [DOI] [PubMed] [Google Scholar]
- 36.Nunemaker CS. Considerations for Defining Cytokine Dose, Duration, and Milieu That Are Appropriate for Modeling Chronic Low-Grade Inflammation in Type 2 Diabetes. J Diabetes Res. 2016;2016:2846570. doi: 10.1155/2016/2846570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maneschi E, Morelli A, Filippi S, Cellai I, Comeglio P, Mazzanti B, Mello T, Calcagno A, Sarchielli E, Vignozzi L, Saad F, Vettor R, Vannelli GB, Maggi M. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol. 2012;215:347–362. doi: 10.1530/JOE-12-0333. [DOI] [PubMed] [Google Scholar]
- 38.Ridic G, Gleason S, Ridic O. Comparisons of health care systems in the United States, Germany and Canada. Mater Socio-Medica. 2012;24:112. doi: 10.5455/msm.2012.24.112-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunemaker CS, Satin LS. Episodic hormone secretion: a comparison of the basis of pulsatile secretion of insulin and GnRH. Endocrine. 2014;47:49–63. doi: 10.1007/s12020-014-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waki H, Tontonoz P. STAMPing out Inflammation. Cell. 2007;129:451–452. doi: 10.1016/j.cell.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Lindstad T, Jin Y, Wang L, Qu S, Saatcioglu F. STAMPs at the crossroads of cancer and nutrition. Nutr Cancer. 2010;62:891–895. doi: 10.1080/01635581.2010.509836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


