Abstract
Artificial pancreas (AP) systems provide automated regulation of blood glucose concentration (BGC) for people with type 1 diabetes (T1D). An AP includes three components: a continuous glucose monitoring (CGM) sensor, a controller calculating insulin infusion rate based on the CGM signal, and a pump delivering the insulin amount calculated by the controller to the patient. The performance of the AP system depends on successful operation of these three components.
Many APs use model predictive controllers that rely on models to predict BGC and to calculate the optimal insulin infusion rate. The performance of model-based controllers depends on the accuracy of the models that is affected by large dynamic changes in glucose-insulin metabolism or equipment performance that may move the operating conditions away from those used in developing the models and designing the control system. Sensor errors and missing signals will cause calculation of erroneous insulin infusion rates. And the performance of the controller may vary at each sampling step and each period (meal, exercise, and sleep), and from day to day.
Here we describe a multi-level supervision and controller modification (ML-SCM) module is developed to supervise the performance of the AP system and retune the controller. It supervises AP performance in 3 time windows: sample level, period level, and day level. At sample level, an online controller performance assessment sub-module will generate controller performance indexes to evaluate various components of the AP system and conservatively modify the controller. A sensor error detection and signal reconciliation module will detect sensor error and reconcile the CGM sensor signal at each sample. At period level, the controller performance is evaluated with information collected during a certain time period and the controller is tuned more aggressively. At the day level, the daily CGM ranges are further analyzed to determine the adjustable range of controller parameters used for sample level and period level.
Thirty subjects in the UVa/Padova metabolic simulator were used to evaluate the performance of the ML-SCM module and one clinical experiment is used to illustrate its performance in a clinical environment. The results indicate that the AP system with an ML-SCM module has a safer range of glucose concentration distribution and more appropriate insulin infusion rate suggestions than an AP system without the ML-SCM module.
Keywords: Artificial Pancreas, Sensor Error Detection, Controller Performance Assessment, Controller Retuning, Type 1 Diabetes
Graphical abstract
1. INTRODUCTION
1.1 Overview of Artificial Pancreas and performance assessment
Artificial pancreas (AP) systems regulate blood glucose concentration (BGC) of people with type 1 diabetes (T1D) and those without a functional pancreatic beta cell population. An AP relies on a continuous glucose monitoring (CGM) sensor to provide BGC information at a high frequency (sampling time of 5 minutes), a controller to calculate the insulin infusion rate based on the CGM signal (BGC estimate) and an insulin pump that infuses the insulin calculated by the controller to a subcutaneous port on the body.
The performance of the AP relies on correct operation of all the three components, the CGM sensor, controller, and insulin pump. Various control strategies, ranging from proportional-integral-derivative (PID) control(Kovatchev et al., 2009; Renard et al., 2010; Ruiz et al., 2012; Sherr et al., 2013; G. Steil et al., 2006; G. M. Steil et al., 2011) to model-based techniques such as model predictive control(Harvey et al., 2014; Kovatchev et al., 2009; Luijf et al., 2013; Magni et al., 2007), generalized predictive control (GPC)(El-Khatib et al., 2014; Eren-Oruklu et al., 2009; Turksoy et al., 2014b), and knowledge-based systems with fuzzy logic(Atlas et al., 2010; Mauseth et al., 2010)have been used in developing the control algorithms for the AP. All strategies except the fuzzy logic approach use mathematical models that estimate BGC and insulin in formulating the control algorithms. The fuzzy logic controllers use rules derived from experiential knowledge and medical facts.
One of the challenges for AP systems is variation in BGC dynamics among different people with T1D and also from day to day, even minute to minute, for the same person. Factors such as meals and exercise will influence BGC dynamics. Various AP control systems have been proposed to address the effects of these factors and improve AP system.(Dassau et al., 2008; Perfect et al., 2012; Turksoy et al., 2015; Turksoy et al., 2014b; Turksoy et al., 2016) In a fully automated AP system, no announcement of meals or exercise is provided by patients as to meals or activities, and the controller needs to adapt to the dynamic changes in the human body by updating its model and/or insulin dose calculation algorithm. GPC uses a recursively updated data-driven model when a new CGM signal value is available.(El-Khatib et al., 2014; Eren-Oruklu et al., 2009; Turksoy et al., 2014b) Various modules that identify specific conditions such as meal detection module(Lee et al., 2009; Turksoy et al., 2016) and exercise module(Turksoy et al., 2015) provide additional information for making more accurate control decisions. However, the controller performance is often limited by the size of the database and richness of the historical information for training and tuning the controllers. For a new patient with different BGC dynamics (e.g., different insulin sensitivity), unexpected conditions (e.g., large meal, different types of exercise) or illness, the AP system may not able to regulate the BGC. Besides the challenges of the BGC dynamics, the performance of AP system also relies on the accuracy of CGM values reported. Sensor failures such as signal bias and outliers, and missing data will also affect insulin infusion rate calculation and may endanger the safety of the patient. (Baysal et al., 2014; Del Favero et al., 2014; Facchinetti et al., 2016) In this paper, we focus on the detection of controller performance deterioration caused by changes in glucose and insulin concentration dynamics and sensor failures, the diagnosis of the cause for the deterioration, and the retuning of the controller.
A multi-level supervision and modification (ML-SCM) module is developed to supervise the performance of the AP system and modify it to adapt to the patient’s current state and mitigate the impact of CGM sensor faults. The ML-SCM module contains three sub-modules based on different time scales: sample level supervision module (SLSM), period-level supervision module (PLSM) and 24-hour day level supervision module (DLSM). In previous work, an index based online controller performance assessment (CPA) module (Feng, Turksoy, & Cinar, 2016) and a hybrid CGM sensor error detection and functional reconciliation (SED&FR) module(Feng, Turksoy, Samadi, et al., 2016) based on outlier-robust Kalman filter (ORKF) and locally-weighted partial least square (LW-PLS) were developed for a GPC based AP(Turksoy et al., 2014a; Turksoy et al., 2014b). These two modules were capable of retuning the controller and reconciling the CGM signal values at each sampling time. In the ML-SCM module, the performance of the AP will not only be assessed at the sample level but also at period-level and day level. The CPA module is enhanced with more indexes to track different aspects of controller performance and combined with the SED&FR module to formulate the SLSM. The dynamic changes in the body triggered by meal or physical activity will last for many sampling times. Hence, the ML-SCM module should accommodate the effects of different situations over different time periods. The PLSM based on linear quadratic Gaussian (LQG) control-based tradeoff curve, and DLSM based on daily BG distribution analysis are developed to assess the AP performance in longer time scales.
1.2 Generalized predictive controller for the AP system
We have already developed an AP control system based on adaptive constrained weighted recursive identification methods and generalized predictive controller (GPC) (Figure 1), GPC model-based adaptive control.(Turksoy et al., 2014a) We have enhanced recursive time series modeling to assure the stability of every multi-input single-output model developed, used these models in our GPC, and introduced rules that improve its performance in presence of physical activity. (Turksoy et al., 2014a; Turksoy et al., 2014b; Turksoy et al., 2016) We also integrated a meal detection module to improve the performance against post-meal high glucose level for an AP without any meal announcement. (Turksoy et al., 2016)
Figure 1.
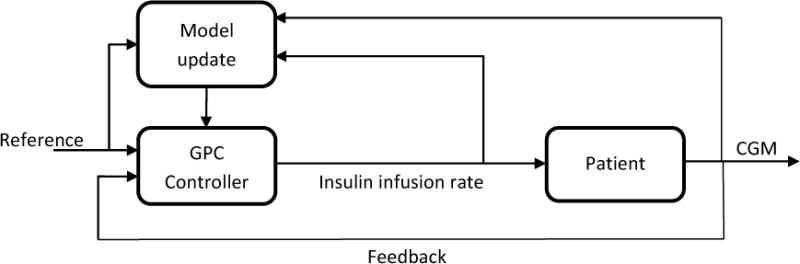
AP system with GPC algorithm
A single-variable version of this controller is used to conduct simulations with the UVa/Padova simulator. An autoregressive moving average model with exogenous inputs (ARMAX) is used to predict glucose concentration (GC) measured with a CGM.(Turksoy et al., 2014a) The insulin infusion rates computed in previous steps and past and current CGM readings are the inputs to the model. Model parameters are updated recursively at each sampling time and then the updated model provides GC predictions to the controller. The controller then computes the insulin dose to be infused.
In this paper, we outline the components of the controller that are relevant to the ML-SCM module and focus on the objective function for model estimation and the objective function of the GPC.
The optimum coefficients of the recursive ARMAX model are obtained by minimizing the objective function:
| (1) |
where is the vector of estimated model coefficients, N is the number of samples, λ is the forgetting factor, and e denotes the modeling error. The recursive model is updated by giving different weights to previous data based on part of the data that is more important to adjust the model. λ is the critical parameter that adjusts the relative importance of GC data collected and the effective size of the moving window for the input data.
Another important component of the controller is its objective function. The constrained controller signal I(k) is calculated by minimizing the objective function J (N1,N2,Nu w):
| (2) |
where N1 and N2 are the first and last time instants of the modeling horizon and Nu is the control horizon. I denotes the insulin infusion rate suggestions and only the first element I (k)of is implemented. GCpred (k+j|k) is the predicted CGM reading at step k + j in the future based on the previous data at and before step k, r is the reference value, ΔI represents the changes of insulin infusion, and wj is the jth diagonal element of weight matrix w(k) for ΔI. Insulin delivery is constrained by both the ability of the insulin pump and the maximum value calculated by the model to prevent hypoglycemia, and insulin value cannot be negative.
At step k, the equations for r (k+j) and w(k)are
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
where BW is the body weight and r0 = 100 mg/dl is the constant “reference”, the desired GC trajectory(Turksoy et al., 2014a) and ./ denotes elementwise division. For Eq. 2 - 8, μ (between 0-1) is the parameter that determines the speed of approximation of the reference trajectory to r0 such that as μ is reduced, the reference trajectory becomes smoother. The weight wj in (2) is also influenced by μ and as μ is reduced, more weight is put on the insulin efficiency term. In other words, μ determines the aggressiveness of the controller in bringing the BGC closer to the reference r0, μ is closer to 1 for more aggressive control action. A constant value is assigned to μ according to the age group and insulin resistance level of the subject (Table 2). (Turksoy et al., 2014a)
Table 2.
Value setting for μ
| Sensitive | Normal | Resistant | |
|---|---|---|---|
| Adolescent | 0.2 | 0.3 | 0.55 |
| Adult | 0.2 | 0.3 | 0.55 |
| Child | 0.45 | 0.7 | 0.9 |
This GPC-based AP is used to illustrate the performance of the ML-SCM. The detailed algorithm of failure detection and modification of AP system according to the ML-SCM is described in section 2. Simulations and results are reported in section 3. Discussion of the results is presented in section 4. Conclusions are provided in section 5.
2. METHOD
The ML-SCM includes three sub-level supervision modules: SLSM at sample level, PLSM at period level and DLSM at day (24-hour) level. The SLSM has an online CPA sub-module based on the computation and interpretation of several indexes, and a hybrid online sensor error detection and functional redundancy (SED&FR) sub-module. At each sampling time, the performance of the different components of the controller and the CGM sensor signal are evaluated and AP controller is modified conservatively. In PLSM, the controller performance during a specific time period is evaluated by the tradeoff curve method and the AP controller is modified more aggressively since more information has been collected and evaluated compared to SLSM. In DLSM, the BG variation within a 24-hour period is analyzed and the range of controller parameter retuning and insulin correction in SLSM and PLSM are modified.
2.1 Sample Level Supervision Module
The SLSM has two sub-modules, a CPA and a SED&FR. In previous work(Feng, Turksoy, & Cinar, 2016), an online CPA module with six indexes to track different aspects of a model-based controller was developed. The indexes used in the previous CPA module included model prediction error index (IMPE), model error elimination speed index (IMEES), dangerous change potential index (IDCP), dangerous change index (IDC), insulin constraints limitation index (IICL), and weight ratio index (IWR). Three different types of controller failures, model prediction error, insulin constraints error, and weight ratio error, are detected and the controller is modified accordingly. The equations of these six indexes, controller failure detection and controller modification based on those indexes are described in the part I of appendix. Two additional indexes: idle index(Hägglund, 1999) (Ii) and performance watchdog(Rhinehart, 1995) (IPW) are added to the CPA sub-module to detect sluggish control and offset from set-point (reference trajectory), respectively.
The Ii describes the relation between times of positive and negative correlation between the control signal (insulin infusion rate suggested) ΔI and the controlled variable (CGM signal) increments ΔG:
| (9) |
| (10) |
| (11) |
where k indicates the time steps with 5-minutes sampling time, and ΔI(k) is the change between two consecutive samples (G (k) − G (k − 1)). The positive values of Ii (k) >0.4 indicate sluggish control.(Horch, 2000)
The performance watchdog index IPW (k) represent the ratio of the controlled variable (CGM) variance, calculated in two different ways:
| (12) |
| (13) |
| (14) |
The variance represents the normalized accumulated deviation of sample k from the reference value (r0). Variance indicates the normalized distance between two consecutive samples. In this case, the averaged absolute consecutive samples difference is used for normalization . The forgetting factor αi is introduced in both Ii and IPW to make them adaptive to the time-varying characteristics of the human body. IPW >3 indicates that the CGM measurement is steady at a value which is far from the reference value (large offset).(Horch, 2000)
When Ii and IPW indicate sluggish control or large offset, the parameter μ, which determines the aggressiveness of the controller, will be increased according to:
| (15) |
| (16) |
| (17) |
| (18) |
Instead of a constant value related only to the insulin sensitivity and age group of the patient (Table 2), μ is updated at each sampling time based on the current conditions of the AP. The values listed in Table 2 now become the initial value of μ (μ(1)). μ is increased only when CGM is larger than reference value. When sluggish control or large offset was detected based on the accumulated time of error, μ is increased towards its upper bound (μmax). When large offset is detected by IPW, a correction insulin bolus (Icor) needs to be delivered in order to bring the GC back to the reference value. Icor is based on ISF:
| (19) |
where ISF (k,1,1) denotes the first element of(ISF(k)) More correction insulin need to wait until the effect of previous correction insulin bolus is cleared or it becomes clear that the previous correction is insufficient. A maximum constraint was defined to prevent potential hypoglycemia that may be caused by the correction bolus. The clearance time (Ncor) changes according to the type of rapid-acting insulin. For this experiment, Ncor =24 to simulate 2-hour insulin clearance time.
If CGM< r0 and may have potential towards hypoglycemia, then μ will be decreased towards the lower bound (μmin). To include this change, Eq. 18 is modified as:
| (20) |
| (21) |
In the CPA sub-module, μ is varied between μmin to μmax, in order to optimize the aggressiveness of the controller. The insulin infusion rate suggestion from the controller is modified when sluggish control or prediction error occurs (Eq. 19 and A.11), and these additional insulin doses are constrained by . The constraints μmin, μmax and may also be changed as more information is collected from the operation of the AP, as described in Section 2.3 (PLSM).
The hybrid sensor error detection and functional redundancy (SED&FR) module of the SLSM detects and mitigates the effects of abnormal CGM sensor behavior (Figure 2).(Feng, Turksoy, Samadi, et al., 2016) All CGM sensor signals are analyzed and reconciled in SED&FR module before transmission to other modules of the controller. This module uses two techniques, an outlier-robust Kalman filter (ORKF) and a locally-weighted partial least squares (LW-PLS) regression model, which leverage the advantages of automatic measurement error elimination with ORKF and data-driven prediction with LW-PLS. The module includes a nominal angle analysis (NAA) method to distinguish between signal faults and large changes in sensor values caused by real dynamic changes in glucose-insulin metabolism and insulin kinetics. The SED&FR module also smooths CGM signal jumps caused by CGM calibration with BGC measurements collected from finger sticks.
Figure 2.
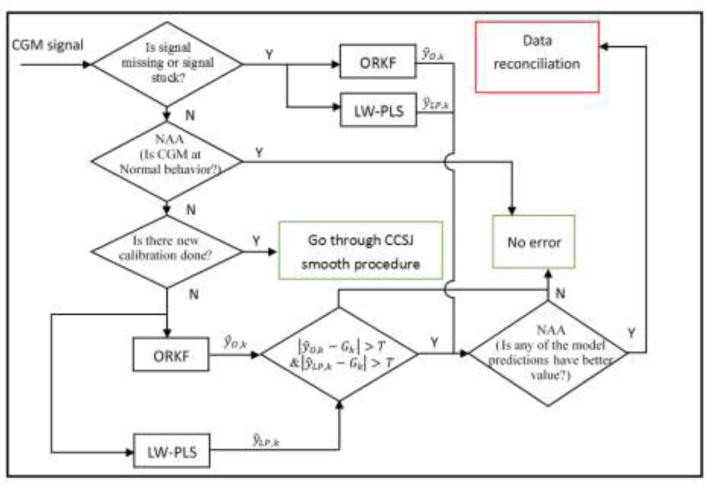
Flow diagram of SED&FR algorithm
At each CGM reading (5 min sampling time), the SLSM module (Figure 3) will first reconcile the CGM value in the SED&FR sub-module. The reconciled CGM value and the information from the controller will be sent to the CPA sub-module. CPA sub-module will generate 8 indexes to assess different aspects of controller performance and modify the controller parameters such as λ, insulin constraints, weight in controller objective function, and the aggressiveness parameter μ. GC prediction and insulin infusion rate suggestions will also be corrected based on the faults indicated by these indexes.
Figure 3.
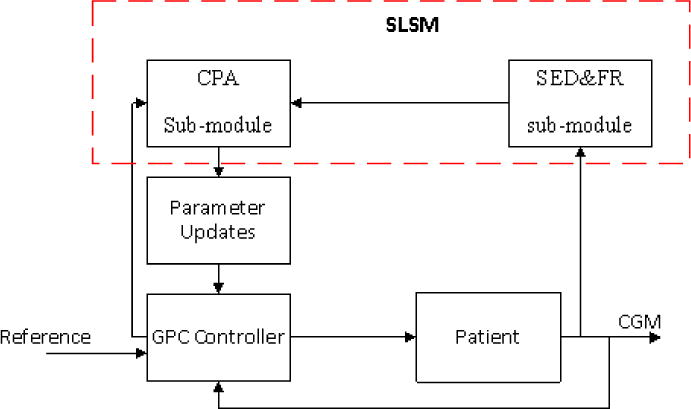
Flow diagram of SLSM procedure
2.2 Period-Level Supervision Module
The PLSM is developed based on LQG tradeoff curve to assess the performance of GC regulation during a specific period of time that may include various disturbances to GC such as meals and exercise.
For GPC objective function (Eq. 2) if N1 = 1, Nu = N2, N → ∞, the objective function converges to the LQG objective function(CLARKF et al., 1987):
| (22) |
The GPC control decisions optimized its objective function irrespective of the fact that only the first control move is actually implemented.(Huang, 1998) Hence, the LQG problem can be solved by the infinite GPC solution. The infinite value of N2 may cause a large calculation burden to solve the linear least squares problem. The model in GPC may not accurate enough to give a GC prediction over a long horizon. In practice, a finite value of N2 is usually enough to achieve the approximate infinite horizon LQG solution via the GPC approach(Huang, 1998). In this case, N1, N2, and Nu in LQG objective function are set to the same values used in the GPC objective function (Eq. 2).
Once the problem is formulated as an LQG problem, the tradeoff curve (Figure 4) can be calculated by varying the weight w. The weights used to generate the tradeoff curve are denoted as wt. w is varying at each time step (Eq. 1 to 8). In order to generate the tradeoff curve, wt is assigned values in the range 0.1 *w (k) to 2 * w (k) with increment of 0.1 * w (k). By using different wt, a set of E [GC − r f]2 and E [ΔI]2 are plotted as tradeoff curve at each time period (period length=Nu). The operation point is calculated based on previous data by using
| (23) |
| (24) |
Figure 4.
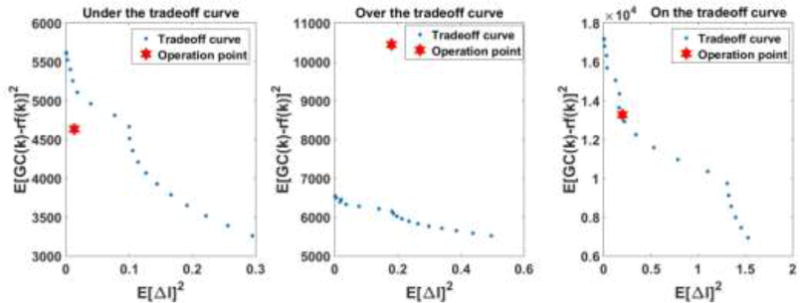
Example of tradeoff curve analysis
Comparing the tradeoff curve and the operation point positions, the performance of controller is as expected or better than expected if the operation point is at or below the tradeoff curve, respectively. If the operation point is above the tradeoff curve, the controller is not at its optimal condition. And if current GC(k) is close or at hypoglycemia or hyperglycemia thresholds (indicated by dangerous change index (IDC) in Eq A.4) the controller will be retuned through the following procedure:
| (25) |
| (26) |
When k mod Nu = 0, if operating point (EI (k), Ey (k)) is above the tradeoff curve:
| (27) |
hypo (k) and hyper(k) are denoting the hypoglycemia and hyperglycemia danger indicated by IDC, respectively. Once the operation point (EI (k), Ey (k)) is above the tradeoff curve if hypo =1 during every Nu samples, μ will be decreased by , and if no such hypo exists and hyper =1, μ will be increased by . The factor μ is still limited by upper and lower bounds, μmxa and μmin. and are the largest reduction or increase increments of μ in the past Nu steps, respectively. This way, PLSM has a more aggressive parameter retuning strategy than SLSM since more information is available to PLSM.
2.3 Day Level Supervision Module
BGC dynamic changes can be affected by many factors such as a meal, physical activity and sleep throughout the day. The performance assessment of AP based on just one time period is not informative about various changes that may occur at different times and cannot tune the controller for optimal performance over a day. For example, a large value is assigned to μ for post-meal hyperglycemia, will yield an aggressive controller during sleep and may drive the BGC into hypoglycemia. The day Level Supervision Module (DLSM) analyzes the performance of AP during past 24 hours and guides future controller retuning accordingly.
In the DLSM, first the CGM values are separated into 3 different categories and their frequency of occurrence is captured by parameters C1, C2, and C3 based on the values of GC. The classification procedure is:
When k mod 288 = 0, set C1, C2, and C3 equal to 0.
For j from k − 287 to k:
| (28) |
where, C1, C2, and C3 indicate the number of GC samples in hypoglycemia, euglycemia, and hyperglycemia range in the last 24 hours, respectively. For a sampling time of 5 minutes, there are 288 samples in 24 hours. If C1 > 0, hypoglycemia has occurred during the last 24-hours period and the adjustable range of the controller parameters and constraints for insulin need to be changed to be more conservative to reduce hypoglycemia potential in the future. The maximum and minimum GC within the previous 24 hours are denoted as GCmax and GCmin. The adjustable range of controller parameters and the constraints for insulin calculations are modified as
| (29) |
| (30) |
Hypoglycemia may cause more immediate treats than the hyperglycemia. Consequently, the constraints for aggressive control system modification are stricter when BGC is near the hypoglycemia limit. If there has been a hypoglycemia episode in the past 24 hours, the constraints for correction insulin and the upper bound of μ are reduced based on the distance between the minimum GC value and the reference value. And if GC values larger than 180mg/dl occurred in the past 24 hours and there were no hypoglycemia threat during that period, the constraints for correction insulin and the upper bound of μ are reduced based on the distance between the maximum GC value and the hyperglycemia threshold (180mg/dl). To prevent extreme cases causing large variation in and μmin, the absolute deviation of μmax and μmin is limited as 0.1 for each 24-hour period supervision and the constraints for the , μmax,, and μmin are set as [0.1, 3], [0.1 1], and [0.05 1], respectively. The initial values for , μmax, and μmin are 1, 0.65, and 0.4.
2.4 Multi-level Supervision Module
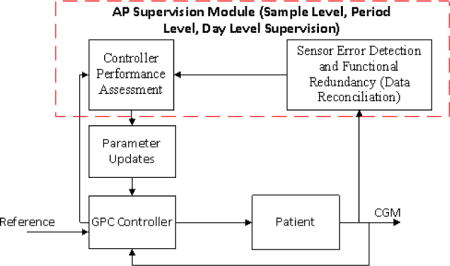
The ML-SCM (Figure 5) is developed to supervise the performance of AP system in different time scales by using and coordinating SLSM, PLSM, and DLSM. The ML-SCM is capable of detecting the errors in CGM readings reported to the AP, assessing the performance of the AP control system, and retuning it to improve its performance. The CGM signal is reconciled by SED&FR sub-module at each sampling time. The reconciled CGM signal is used by all other modules of the AP. SLSM and PLSM will modify the AP controller settings to suggest more accurate insulin infusion rate and these modifications are guided by DLSM.
Figure 5.
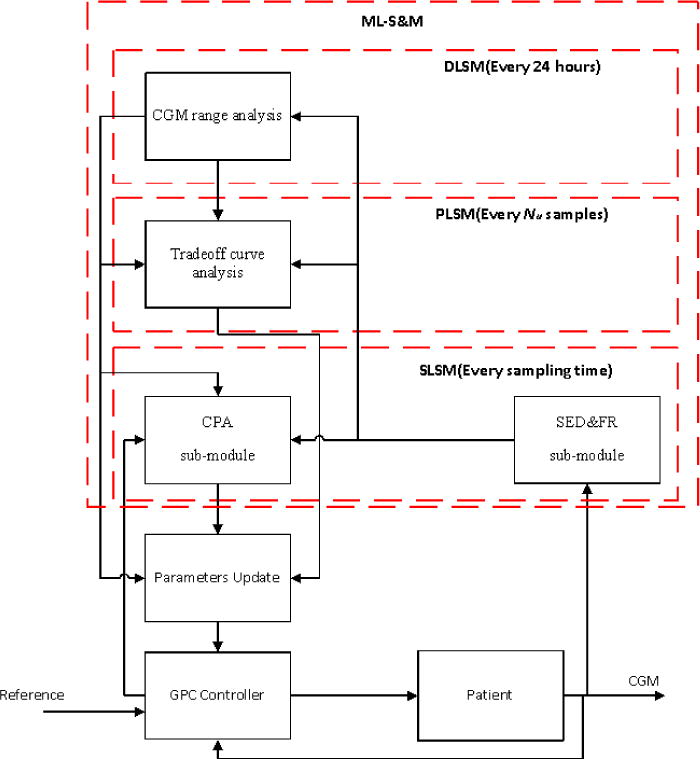
Flow chart of AP system with multi-level supervision module
The summary of parameters, index, and indicators used in the ML-SCM is described in Table 3.
Table 3.
Summary of parameters, index and indicators
| Indexes and indicators | Definition | Equation number for definition | |
|---|---|---|---|
| μ | Aggressiveness factor | 4 | |
| Ii | idle index | 9 | |
| Ipw | performance watchdog | 12 | |
| Islug | sluggish control indicator | 15 | |
| Ioff set | large offset indicator | 16 | |
| Icor | correction insulin bolus | 19 | |
| hypo | Hypoglycemia indicator | 25 | |
| hyper | Hyperglycemia indicator | 26 | |
|
|
Aggressiveness factor decrement | 27 | |
|
|
Aggressiveness factor increment | 27 | |
|
|
Maximum of correction insulin bolus | 29 | |
| μmax | Maximum of aggressiveness factor | 30 | |
| μmin | Minimum of aggressiveness factor | 30 |
3. RESULTS
3.1 Simulations
Thirty in silico subjects (10 adults (Adu), 10 adolescents (Ado), and 10 children (Chi)) in the UVa/Padova simulator were tested by using AP control systems with four different supervision conditions: without any supervision and modification (S&M), with SLSM, with SLSM and PLSM, and with ML-(including SLSM, PLSM and DLSM). Each patient was simulated 10 times with a 3-day scenario. For CGM seeds provided by the simulator. Different meal plans are given for different age groups (Table 4). The detailed result of AP system when different sub-modules of ML-SCM are operational is displayed in the part II of appendix. The comparison of average percent time in various ranges of BGC for AP systems for each age group is reported in Table A.5 (Detailed data of each subject is displayed in Table A.4). The number of severe hyperglycemia and hypoglycemia periods, maximum and minimum BGC, and insulin usage are listed in Table A.6 and Table A.7. The BGC percent time in each BGC concentration range on different days for AP systems with different ML-SCM modules is given in Table A.6.
Table 4.
Three-day meal scenarios for different age groups (Carbohydrate (g))
| Time | Adolescent | Adult | Children | |
|---|---|---|---|---|
| Day 1 | 09:45 | 48 | 60 | 36 |
| 13:30 | 47 | 59 | 35 | |
| 17:45 | 75 | 94 | 56 | |
| 21:30 | 31 | 39 | 23 | |
| Day 2 | 09:10 | 55 | 69 | 41 |
| 13:45 | 70 | 88 | 53 | |
| 18:00 | 65 | 81 | 49 | |
| 22:00 | 20 | 25 | 15 | |
| Day 3 | 09:00 | 40 | 50 | 30 |
| 14:00 | 68 | 85 | 51 | |
| 18:20 | 75 | 94 | 56 | |
| 22:30 | 25 | 31 | 19 | |
| Daily average | 206 | 258 | 155 | |
The performance of the AP system is consistently improving by using additional supervision modules (Tables A.5 to A.7). Overall, more BGC values are shown to be in the euglycemia range with ML-SCM, average the BGC values in range (70-180 mg/dl) have increased by 14% compared to the AP system without S&M. The AP system with the ML-SCM successfully modified its parameters to reduce serious hypo- and hyperglycemia (BGC>250 or BGC<50) (Table A.6). AP system with ML-SCM had better insulin efficiency for keeping BGC in target range. For the 3-day simulation, the total insulin use is reduced by about 2.3% on average compared to the AP system without S&M.
3.2 Clinical Assessment of the AP with ML-SCM
A clinical experiment was conducted to illustrate the performance of the AP with and without ML-SCM. A multivariable AP was used(Turksoy et al., 2014a). The same subject participated in two experiments using the AP with and without ML-SCM. Both experiments lasted for 56 hours (From first day 8:00 to third day 16:00). The AP suggested insulin boluses, and the subject had the same meal scenarios (Table 5) and basal insulin plan.
Table 5.
Meal scenarios for the clinical experiments
| Day | Day1 | Day2 | Day3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | 8:00 | 12:00 | 6:00 | 10:00 | 8:00 | 12:00 | 6:00 | 10:00 | 8:00 | 12:00 |
| Carb(g) | 67 | 82 | 89 | 24 | 67 | 82 | 89 | 24 | 67 | 82 |
Besides the meal, the subject may also eat additional carbohydrates, the so-called rescue carbohydrate (RC), when the BGC level is considered to be dangerous in order to prevent the occurrence of hypoglycemia. After the warning for RC is issued, consuming the RC is decided by the subject and the endocrinologist based on the CGM measurements and their experience. Since it is potentially dangerous to keep the subject in the low BGC range, the amount of RC is considered to evaluate the number of hypoglycemia episodes the subject may have during the experiment. The CGM measurements in the three days and insulin infusion rate of the two experiments are compared in Figure 6 along with the carbohydrate information.
Figure 6.
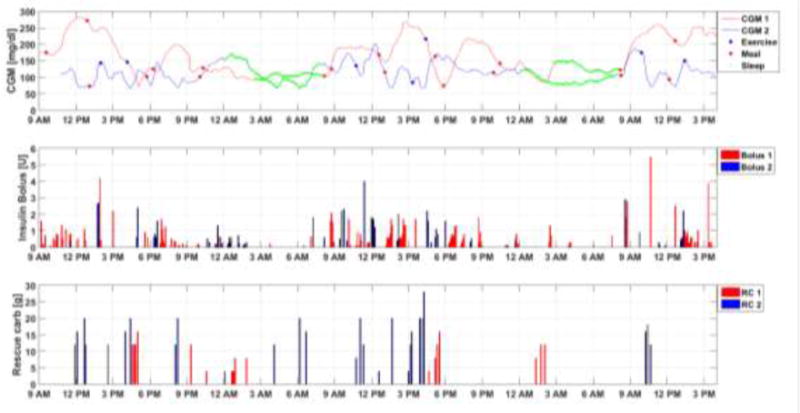
Performance comparison of AP system with and without ML-SCM (1: AP system with ML-SCM, 2: AP system without ML-SCM)
The CGM values are higher for the AP with ML-SCM for two reasons. First, the subject had much higher GC in the morning every day and then had breakfast and lunch but did not perform any exercise, challenging the AP controller. Second, the controller was modified to be less aggressive in order to reduce the reliance on RC for preventing hypoglycemia. The intent was to move away from the GC remaining close to hypoglycemia threshold because hypoglycemia is more dangerous in the short term to cause immediate harm to the subject (Cryer et al., 2003). For AP system without ML-SCM, the CGM values remain above the hypoglycemia limit but low even after taking RC based on predictive hypoglycemia alarms. Without taking RC, there would have been a large number of hypoglycemic events. During the three-day experiment, the subject consumed 184 g when the AP with ML-SCM was used compared to 402 g RC using the AP without ML-SCM. The AP without ML-SCM was too aggressive for this subject and the subject had to rely on taking additional carbohydrates as RC to maintain the CGM in range.
On the first day, since the initial CGM value is higher in CGM 1 (AP with ML-SCM) compared with CGM 2 (AP without ML-SCM), more small doses of insulin bolus were given to bring the CGM back to safe range (Figure 6). Around 5 PM on the first day a low CGM value appeared and the controller was modified to be less aggressive to prevent future hypoglycemia. For the CGM 2 trace (AP without ML-SCM), since the controller is not modified by the ML-SCM module, low CGM values appeared at 1 PM, 4:40 PM, 6:00 PM and 8:00 PM on the first day.
On the second day, CGM 2 still has multiple low CGM values including one after lunch (1:30PM). For CGM 1, only one CGM value is close to the hypoglycemia threshold because of exercise. On the third day, there was no exercise when the AP system with ML-SCM was used and the CGM 1 value is higher compared with CGM 2, and the controller is modified to be more aggressive. Hence, the insulin bolus amount is much larger on the third day compared to the case using the AP without ML-SCM.
4. DISCUSSION
The percentage of time in severe hyperglycemia (BGC>250 mg/dl) has increased for some subjects such as Adu3 and Adu10, when SLSM was used with respect to the AP without an ML-SCM (Table A.5). This is because the factors that influence the BGC dynamics, such as meals, often last much longer than one sampling time (5 minutes). The AP system modification based on one sample need to be more conservative to avoid a controller tuning that would be too aggressive. For example, as the BGC increases after a meal, the insulin infusion rate should be large enough to prevent hyperglycemia but not too large to cause hypoglycemia later. Since hypoglycemia may have more immediate threat to patient, the system is modified conservatively to reduce the potential for hypoglycemia.
By adding SLSM and PLSM, the improvement of BGC percentage between 180-70 mg/dl is almost the same in different days comparing with AP system without S&M (Table A.8). Improvement with ML-SCM is more significant in the second and third day than the first day, because DLSM has modified the adjustable range of the parameters in the AP system and constraints for correction insulin 24 hours after the beginning of the experiment. With data of a whole day, more information is available about the performance of the AP system, and the AP system is modified to reduce both hyperglycemia and hypoglycemia (Tables A.5, A.7, A.8).
In the PLSM, the horizon in LQG tradeoff curve is set the same as the GPC in the AP system, because there is no explicit information about the exact disturbance(s) (such as meal and sleep) to the glucose metabolism of the subject. If the information about the conditions were available, the range of the time period can be set to cover that condition from the beginning to end of the disturbance.
Besides meals, other factors such as exercise, stress and sleep may influence the dynamics of BGC. Because of the limitations of the simulator, the effects of exercise are not tested in this paper. In future work, different condition such as meal, exercise, and sleep need to be identified and the AP system should be modified to have a specified strategy to calculate insulin infusion rates for these various conditions.
5. CONCLUSION
A novel module for AP system performance assessment and modification based on multiple time scales is developed and tested. The ML-SCM integrates the CGM sensor error detection and signal reconciliation with controller performance assessment and modification. Three different levels of supervision modules are developed based on their time windows. The results indicate that the AP system with ML-SCM can improve its performance.
Table 1.
Acronyms List
| Acronyms | Description |
|---|---|
| AP | Artificial Pancreas |
| BGC | Blood Glucose Monitoring |
| T1D | Type 1 Diabetes |
| CGM | Continuous Glucose Monitoring |
| PID | Proportional-Integral-Derivative |
| MLSM | Multi-level Supervision and Modification |
| SLSM | Sample-level Supervision Module |
| PLSM | Period-level Supervision Module |
| DLSM | Day-level Supervision Module |
| SED&FR | Sensor Error Detection and Functional Reconciliation |
| ORKF | Outlier-Robust Kalman Filter |
| LW-PLS | Locally-Weighted Partial Least Squares |
| LQG | Linear Quadratic Gaussian |
| GPC | Generalized Predictive Control |
| ARMAX | Autoregressive Moving Average Model with Exogenous Inputs |
| CPA | Controller Performance Assessment |
| NAA | Nominal Angle Analysis |
Highlights.
A multi-level supervision module is developed for model-based control systems.
Sensor error detection and controller performance assessment are integrated.
The performance of artificial pancreas is evaluated in different time scales.
The controller is retuned by adjusting controller parameters and constraints.
Acknowledgments
This work is supported by the National Institutes of Health (NIH) under grants 1DP3DK101077-01 and 1DP3DK101075-01 and the Juvenile Diabetes Research Foundation International (JDRF) under grant 17-2013-472. The experimental work is partially supported by the University of Chicago Diabetes Research and Training Center (DRTC) funded by NIDDK P30DK020595. The authors are grateful to Gail Gannon, APN and Nancy Devine, BSN, RN for the management of clinical experiments.
APPENDIX
Part I: Index based controller failure detection and controller modification
Table A.1.
Summary of all indexes and indicators in CPA
| Indexes and indicators | Definition | Equation number for definition |
|---|---|---|
| IMPE | Model prediction error index | A.1 |
| IMEES | Error elimination speed index | A.2 |
| IDCP | Dangerous change potential index | A.3 |
| IDC | Dangerous change index | A.4 |
| IICL | Insulin constraints limitation index | A.5 |
| IWR | Weight ratio index | A.6 |
| IPE | Prediction error indicator | A.7 |
| IICE | Insulin constraints error indicator | A.8 |
| IWRE | Weight ratio error indicator | A.9 |
Index generation:
| (A.1) |
| (A.2) |
| (A.3) |
| (A.4) |
| (A.5) |
| (A.6) |
Controller failure detection (Binary numbers 1 and 0 are used to indicate the presence or absence of such errors):
| (A.7) |
| (A.8) |
| (A.9) |
Controller retuning based on indexes:
| (A.10) |
| (A.11) |
| (A.12) |
| (A.13) |
| (A.14) |
Table A.2.
Value for parameter ISF
| Adult | Adolescent | Child | |
|---|---|---|---|
| Sensitive | 0.8 | 0.8 | 0.4 |
| Normal | 1 | 1 | 0.5 |
| Resistant | 1.2 | 1.2 | 0.6 |
Table A.3.
Summary of parameters and their values in CPA
| Parameters | Definition | Equation number | Value | |
|---|---|---|---|---|
| MEmax | Threshold of IMPE to determine IMEES | A.2 | 10 | |
|
|
Threshold of IDCP to determine IDC | A.4 | 4 | |
|
|
Threshold of IMPE to determine IPE | A.7 | 20 | |
|
|
Threshold IMEES of to determine IPE | A.7 | 30 | |
|
|
Threshold IICL of to determine IICE | A.8 | 40 | |
|
|
Threshold of IMPE to tuning λ | A.10 | 30 | |
| α | Parameter for tuning I′ | A.11 | 270 | |
| β | Proportional feedback constant for | A.12 | 0.35 | |
| γ | Filter constant determines how fast the insulin constraint is increasing | A.13 | 1.1 | |
| den | Parameter for tuning I′ | A.10 | 60 (adults, adolescents) 90 (children) |
Part II: Comparison of AP performance under different conditions
Table A.4.
Comparison of performance between AP system without S&M, with SLSM, SLSM and PLSM, and with ML-SCM (percent time in each concentration range. The insulin sensitivity level (ISL): S sensitive, N normal, and R resistant. A: <250 mg/dl, B: 250-180 mg/dl, C: 180-70 mg/dl, D: 70-50 mg/dl, E: >50 mg/dl)
| Subject Type |
ISL | BW (kg) |
BGC without S&M | BGC with SLSM | BGC with SL&PLSM | BGC with ML-SCM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | |||
| Ado 1 | S | 68.7 | 0 | 3 | 83 | 8 | 5 | 0 | 4 | 84 | 8 | 4 | 0 | 2 | 88 | 6 | 4 | 0 | 0 | 91 | 5 | 4 |
| Ado 2 | R | 51 | 22 | 30 | 48 | 0 | 0 | 20 | 27 | 53 | 0 | 0 | 16 | 22 | 62 | 0 | 0 | 12 | 15 | 73 | 0 | 0 |
| Ado 3 | N | 44.8 | 28 | 33 | 38 | 1 | 0 | 26 | 33 | 40 | 1 | 0 | 25 | 28 | 47 | 0 | 0 | 24 | 23 | 53 | 0 | 0 |
| Ado 4 | N | 49.6 | 14 | 20 | 64 | 1 | 1 | 13 | 20 | 66 | 0 | 1 | 13 | 19 | 67 | 1 | 0 | 10 | 11 | 78 | 1 | 0 |
| Ado 5 | S | 47.1 | 17 | 26 | 57 | 0 | 0 | 16 | 23 | 61 | 0 | 0 | 13 | 20 | 67 | 0 | 0 | 10 | 14 | 76 | 0 | 0 |
| Ado 6 | N | 45.4 | 4 | 8 | 85 | 1 | 2 | 4 | 7 | 86 | 1 | 2 | 4 | 6 | 89 | 1 | 0 | 4 | 5 | 90 | 1 | 0 |
| Ado 7 | N | 37.9 | 37 | 40 | 23 | 0 | 0 | 35 | 37 | 28 | 0 | 0 | 31 | 33 | 36 | 0 | 0 | 26 | 24 | 50 | 0 | 0 |
| Ado 8 | R | 41.2 | 36 | 39 | 25 | 0 | 0 | 35 | 36 | 29 | 0 | 0 | 32 | 33 | 35 | 0 | 0 | 29 | 26 | 45 | 0 | 0 |
| Ado 9 | R | 43.9 | 42 | 41 | 17 | 0 | 0 | 41 | 40 | 19 | 0 | 0 | 40 | 38 | 22 | 0 | 0 | 38 | 28 | 34 | 0 | 0 |
| Ado 10 | N | 47.4 | 10 | 17 | 73 | 0 | 0 | 10 | 16 | 74 | 0 | 0 | 9 | 15 | 76 | 0 | 0 | 5 | 7 | 88 | 0 | 0 |
| Mean | 47.7 | 21 | 26 | 51 | 1 | 1 | 20 | 24 | 54 | 1 | 1 | 18 | 22 | 59 | 1 | 0 | 16 | 15 | 68 | 1 | 0 | |
| Adu 1 | S | 102.3 | 0 | 3 | 86 | 5 | 7 | 0 | 5 | 84 | 5 | 6 | 2 | 4 | 86 | 5 | 3 | 2 | 4 | 89 | 3 | 2 |
| Adu 2 | S | 111.1 | 0 | 1 | 84 | 8 | 7 | 0 | 3 | 84 | 7 | 6 | 2 | 3 | 88 | 3 | 4 | 1 | 4 | 92 | 2 | 1 |
| Adu 3 | S | 81.6 | 2 | 6 | 74 | 18 | 0 | 4 | 9 | 74 | 13 | 0 | 4 | 7 | 77 | 12 | 0 | 0 | 3 | 88 | 9 | 0 |
| Adu 4 | S | 63 | 1 | 3 | 78 | 10 | 7 | 2 | 4 | 79 | 9 | 6 | 3 | 2 | 80 | 9 | 6 | 3 | 3 | 82 | 9 | 3 |
| Adu 5 | R | 94.1 | 6 | 10 | 74 | 8 | 2 | 6 | 10 | 76 | 6 | 2 | 6 | 9 | 77 | 6 | 2 | 6 | 7 | 79 | 8 | 0 |
| Adu 6 | N | 66.1 | 5 | 3 | 24 | 1 | 67 | 2 | 3 | 41 | 1 | 53 | 3 | 4 | 49 | 5 | 39 | 2 | 3 | 80 | 6 | 9 |
| Adu 7 | S | 91.2 | 2 | 7 | 86 | 2 | 2 | 2 | 5 | 89 | 2 | 2 | 2 | 4 | 90 | 2 | 2 | 2 | 5 | 92 | 1 | 0 |
| Adu 8 | N | 102.8 | 0 | 3 | 84 | 7 | 7 | 1 | 3 | 84 | 6 | 6 | 1 | 4 | 84 | 6 | 5 | 0 | 1 | 88 | 5 | 5 |
| Adu 9 | R | 74.6 | 1 | 4 | 91 | 3 | 1 | 1 | 4 | 92 | 2 | 1 | 1 | 2 | 94 | 2 | 1 | 0 | 1 | 99 | 0 | 0 |
| Adu 10 | S | 73.9 | 0 | 3 | 76 | 16 | 5 | 2 | 4 | 78 | 12 | 4 | 2 | 3 | 81 | 10 | 4 | 2 | 4 | 82 | 9 | 4 |
| Mean | 86.1 | 2 | 4 | 76 | 8 | 11 | 2 | 5 | 78 | 6 | 9 | 3 | 4 | 81 | 6 | 7 | 2 | 4 | 87 | 5 | 2 | |
| Chi 1 | S | 34.6 | 40 | 43 | 17 | 0 | 0 | 39 | 39 | 22 | 0 | 0 | 38 | 37 | 25 | 0 | 0 | 37 | 28 | 35 | 0 | 0 |
| Chi 2 | N | 28.5 | 21 | 30 | 47 | 2 | 1 | 20 | 29 | 49 | 2 | 0 | 13 | 20 | 65 | 2 | 0 | 11 | 14 | 74 | 1 | 0 |
| Chi 3 | S | 41.2 | 11 | 19 | 69 | 1 | 0 | 10 | 18 | 71 | 1 | 0 | 9 | 13 | 77 | 1 | 0 | 7 | 11 | 82 | 0 | 0 |
| Chi 4 | S | 35.5 | 0 | 5 | 82 | 7 | 6 | 0 | 4 | 84 | 7 | 5 | 3 | 4 | 87 | 4 | 2 | 3 | 6 | 88 | 3 | 0 |
| Chi 5 | N | 37.8 | 14 | 24 | 62 | 0 | 0 | 13 | 21 | 66 | 0 | 0 | 13 | 19 | 68 | 0 | 0 | 10 | 13 | 77 | 0 | 0 |
| Chi 6 | N | 41 | 4 | 8 | 74 | 9 | 5 | 4 | 7 | 77 | 8 | 4 | 4 | 8 | 77 | 7 | 4 | 4 | 6 | 80 | 8 | 2 |
| Chi 7 | R | 45.5 | 6 | 14 | 76 | 2 | 2 | 5 | 10 | 82 | 2 | 1 | 4 | 8 | 85 | 2 | 1 | 4 | 8 | 85 | 2 | 1 |
| Chi 8 | R | 23.7 | 3 | 8 | 89 | 0 | 0 | 2 | 8 | 90 | 0 | 0 | 2 | 5 | 93 | 0 | 0 | 2 | 3 | 95 | 0 | 0 |
| Chi 9 | R | 35.5 | 9 | 15 | 75 | 0 | 1 | 9 | 13 | 78 | 0 | 0 | 8 | 13 | 79 | 0 | 0 | 8 | 10 | 82 | 0 | 0 |
| Chi 10 | N | 35.2 | 42 | 44 | 14 | 0 | 0 | 40 | 38 | 22 | 0 | 0 | 33 | 33 | 34 | 0 | 0 | 28 | 24 | 48 | 0 | 0 |
| Mean | 35.9 | 15 | 21 | 61 | 2 | 2 | 14 | 19 | 64 | 2 | 1 | 13 | 16 | 69 | 2 | 1 | 11 | 12 | 75 | 1 | 0 | |
| Overall | 56.6 | 13 | 17 | 62 | 4 | 4 | 12 | 16 | 65 | 3 | 3 | 11 | 14 | 70 | 3 | 3 | 10 | 10 | 76 | 2 | 1 | |
Table A.5.
Comparison of performance between AP system without S&M, with SLSM, SLSM and PLSM, and with ML-SCM. Average percent time for each population group in each concentration range (A: <250 mg/dl, B: 250-180 mg/dl, C: 180-70 mg/dl, D: 70-50 mg/dl, E: >50 mg/dl)
| Subject Type |
BW (kg) |
BGC without S&M | BGC with SLSM | BGC with SL&PLSM | BGC with ML-SCM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | ||
| Ado | 47.7 | 21 | 26 | 51 | 1 | 1 | 20 | 24 | 54 | 1 | 1 | 18 | 22 | 59 | 1 | 0 | 16 | 15 | 68 | 1 | 0 |
| Adu | 86.1 | 2 | 4 | 76 | 8 | 11 | 2 | 5 | 78 | 6 | 9 | 3 | 4 | 81 | 6 | 7 | 2 | 4 | 87 | 5 | 2 |
| Chi | 35.9 | 15 | 21 | 61 | 2 | 2 | 14 | 19 | 64 | 2 | 1 | 13 | 16 | 69 | 2 | 1 | 11 | 12 | 75 | 1 | 0 |
| Overall | 56.6 | 13 | 17 | 62 | 4 | 4 | 12 | 16 | 65 | 3 | 3 | 11 | 14 | 70 | 3 | 3 | 10 | 10 | 76 | 2 | 1 |
Table A.6.
Comparison of performance between AP systems with SLSM and without S&M
| Subject Type |
BGC without S&M | BGC with SLSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average daily hyper- glycemia episodes (GC>250 mg/dl) |
Average daily hypo- glycemia episodes (GC<50 mg/dl) |
Max GC (mg/dl) |
Min GC (mg/dl) |
Average total insulin use (U/3 days) |
Average daily hyper- glycemia episodes (GC>250 mg/dl) |
Average daily hypo- glycemia episodes (GC<50 mg/dl) |
Max GC (mg/dl) |
Min GC (mg/dl) |
Average total insulin use (U/3 days) |
|
| Ado 1 | 0.00 | 0.13 | 246 | 12 | 93 | 0.00 | 0.11 | 248 | 13 | 88 |
| Ado 2 | 2.38 | 0.00 | 326 | 98 | 148 | 2.14 | 0.00 | 307 | 98 | 151 |
| Ado 3 | 3.01 | 0.00 | 297 | 69 | 128 | 2.94 | 0.00 | 293 | 77 | 129 |
| Ado 4 | 1.63 | 0.17 | 349 | 25 | 151 | 1.31 | 0.17 | 348 | 32 | 150 |
| Ado 5 | 1.84 | 0.00 | 291 | 95 | 131 | 1.70 | 0.00 | 287 | 95 | 132 |
| Ado 6 | 0.45 | 0.43 | 271 | 17 | 91 | 0.45 | 0.43 | 271 | 30 | 91 |
| Ado 7 | 4.28 | 0.00 | 308 | 99 | 134 | 4.18 | 0.00 | 296 | 99 | 134 |
| Ado 8 | 4.10 | 0.00 | 424 | 100 | 218 | 3.86 | 0.00 | 423 | 100 | 219 |
| Ado 9 | 4.73 | 0.00 | 479 | 100 | 270 | 4.55 | 0.00 | 474 | 95 | 270 |
| Ado 10 | 1.17 | 0.00 | 271 | 95 | 143 | 1.15 | 0.00 | 269 | 95 | 147 |
| Mean | 2.36 | 0.07 | 326 | 71 | 151 | 2.23 | 0.07 | 322 | 73 | 151 |
| Adu 1 | 0.00 | 0.83 | 217 | 37 | 133 | 0.00 | 0.81 | 218 | 39 | 131 |
| Adu 2 | 0.00 | 0.80 | 239 | 29 | 140 | 0.00 | 0.77 | 245 | 30 | 140 |
| Adu 3 | 0.21 | 0.00 | 222 | 50 | 87 | 0.45 | 0.00 | 223 | 51 | 85 |
| Adu 4 | 0.11 | 0.67 | 262 | 32 | 110 | 0.22 | 0.64 | 263 | 33 | 110 |
| Adu 5 | 0.63 | 0.50 | 306 | 31 | 205 | 0.68 | 0.49 | 307 | 49 | 205 |
| Adu 6 | 0.52 | 1.07 | 291 | 7 | 76 | 0.20 | 0.99 | 289 | 8 | 94 |
| Adu 7 | 0.21 | 0.30 | 274 | 43 | 147 | 0.22 | 0.29 | 279 | 45 | 141 |
| Adu 8 | 0.00 | 0.47 | 238 | 31 | 146 | 0.11 | 0.47 | 239 | 29 | 146 |
| Adu 9 | 0.10 | 0.97 | 253 | 27 | 157 | 0.11 | 0.34 | 253 | 33 | 149 |
| Adu 10 | 0.00 | 0.70 | 242 | 41 | 106 | 0.22 | 0.70 | 251 | 43 | 105 |
| Mean | 0.18 | 0.63 | 254 | 33 | 131 | 0.22 | 0.55 | 257 | 36 | 131 |
| Chi 1 | 4.54 | 0.00 | 338 | 83 | 158 | 4.51 | 0.00 | 318 | 81 | 158 |
| Chi 2 | 2.32 | 0.50 | 373 | 23 | 115 | 2.30 | 0.43 | 353 | 30 | 112 |
| Chi 3 | 1.26 | 0.00 | 290 | 40 | 110 | 1.16 | 0.00 | 288 | 44 | 103 |
| Chi 4 | 0.00 | 0.50 | 249 | 28 | 86 | 0.00 | 0.39 | 250 | 37 | 85 |
| Chi 5 | 1.43 | 0.00 | 320 | 83 | 128 | 1.39 | 0.00 | 320 | 82 | 128 |
| Chi 6 | 0.43 | 0.93 | 318 | 19 | 128 | 0.43 | 0.92 | 330 | 26 | 128 |
| Chi 7 | 0.69 | 0.57 | 295 | 26 | 115 | 0.58 | 0.56 | 302 | 26 | 117 |
| Chi 8 | 0.31 | 0.00 | 283 | 80 | 59 | 0.24 | 0.00 | 279 | 80 | 60 |
| Chi 9 | 1.06 | 0.37 | 309 | 21 | 111 | 0.97 | 0.15 | 309 | 22 | 109 |
| Chi 10 | 4.22 | 0.00 | 341 | 96 | 157 | 4.58 | 0.00 | 337 | 96 | 162 |
| Mean | 1.63 | 0.30 | 312 | 50 | 117 | 1.62 | 0.25 | 309 | 52 | 116 |
| Overall | 1.39 | 0.33 | 297 | 51 | 133 | 1.35 | 0.29 | 296 | 54 | 133 |
Table A.7.
Comparison of performance between AP systems with SLSM and PLSM, and with ML-SCM
| Subject Type |
BGC with SLSM and PLSM | BGC with ML-SCM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average daily hyper- glycemia episodes (GC>250 mg/dl) |
Average daily hypo- glycemia episodes (GC<50 mg/dl) |
Max BGC (mg/dl) |
Min BGC (mg/dl) |
Average total insulin use (U/3 days) |
Average daily hyper- glycemia episodes (GC>250 mg/dl) |
Average daily hypo- glycemia episodes (GC<50 mg/dl) |
Max BGC (mg/dl) |
Min BGC (mg/dl) |
Average total insulin use (U/3 days) |
|
| Ado 1 | 0.00 | 0.11 | 248 | 14 | 82 | 0.00 | 0.07 | 249 | 22 | 75 |
| Ado 2 | 1.83 | 0.00 | 293 | 98 | 152 | 1.27 | 0.00 | 290 | 98 | 153 |
| Ado 3 | 2.60 | 0.00 | 283 | 90 | 129 | 2.51 | 0.00 | 283 | 96 | 129 |
| Ado 4 | 1.46 | 0.17 | 345 | 53 | 150 | 1.05 | 0.00 | 343 | 59 | 150 |
| Ado 5 | 1.44 | 0.00 | 279 | 94 | 133 | 1.02 | 0.00 | 273 | 94 | 134 |
| Ado 6 | 0.47 | 0.42 | 271 | 38 | 90 | 0.41 | 0.40 | 271 | 39 | 90 |
| Ado 7 | 3.62 | 0.00 | 293 | 99 | 135 | 2.63 | 0.00 | 292 | 99 | 140 |
| Ado 8 | 3.24 | 0.00 | 423 | 100 | 220 | 3.21 | 0.00 | 413 | 100 | 230 |
| Ado 9 | 4.47 | 0.00 | 459 | 91 | 273 | 4.25 | 0.00 | 403 | 68 | 275 |
| Ado 10 | 1.01 | 0.00 | 264 | 95 | 147 | 0.59 | 0.00 | 264 | 94 | 148 |
| Mean | 2.01 | 0.07 | 316 | 77 | 151 | 1.69 | 0.03 | 308 | 77 | 152 |
| Adu 1 | 0.21 | 0.63 | 255 | 41 | 130 | 0.23 | 0.50 | 263 | 41 | 125 |
| Adu 2 | 0.21 | 0.48 | 247 | 33 | 136 | 0.11 | 0.43 | 261 | 38 | 130 |
| Adu 3 | 0.43 | 0.00 | 232 | 51 | 82 | 0.00 | 0.00 | 237 | 51 | 80 |
| Adu 4 | 0.31 | 0.63 | 263 | 33 | 109 | 0.33 | 0.53 | 266 | 36 | 91 |
| Adu 5 | 0.68 | 0.17 | 308 | 52 | 204 | 0.63 | 0.00 | 309 | 56 | 204 |
| Adu 6 | 0.35 | 0.95 | 288 | 9 | 105 | 0.23 | 0.83 | 286 | 26 | 113 |
| Adu 7 | 0.24 | 0.27 | 282 | 46 | 133 | 0.22 | 0.10 | 290 | 49 | 122 |
| Adu 8 | 0.11 | 0.45 | 247 | 29 | 146 | 0.00 | 0.37 | 251 | 24 | 144 |
| Adu 9 | 0.11 | 0.23 | 252 | 33 | 148 | 0.00 | 0.00 | 249 | 78 | 145 |
| Adu 10 | 0.23 | 0.53 | 255 | 43 | 94 | 0.21 | 0.27 | 263 | 46 | 94 |
| Mean | 0.29 | 0.43 | 263 | 37 | 129 | 0.20 | 0.30 | 267 | 45 | 125 |
| Chi 1 | 3.87 | 0.00 | 313 | 80 | 156 | 4.02 | 0.00 | 302 | 76 | 155 |
| Chi 2 | 1.46 | 0.05 | 346 | 40 | 111 | 1.11 | 0.00 | 325 | 52 | 99 |
| Chi 3 | 1.06 | 0.00 | 286 | 47 | 101 | 0.78 | 0.00 | 279 | 63 | 100 |
| Chi 4 | 0.34 | 0.07 | 252 | 45 | 82 | 0.32 | 0.00 | 257 | 53 | 71 |
| Chi 5 | 1.35 | 0.00 | 321 | 77 | 129 | 1.18 | 0.00 | 325 | 70 | 130 |
| Chi 6 | 0.41 | 0.73 | 336 | 26 | 128 | 0.41 | 0.60 | 347 | 34 | 120 |
| Chi 7 | 0.48 | 0.56 | 335 | 26 | 124 | 0.44 | 0.37 | 349 | 42 | 136 |
| Chi 8 | 0.23 | 0.00 | 278 | 80 | 60 | 0.23 | 0.00 | 270 | 80 | 60 |
| Chi 9 | 0.89 | 0.05 | 310 | 37 | 107 | 0.93 | 0.00 | 310 | 87 | 101 |
| Chi 10 | 3.38 | 0.00 | 333 | 96 | 162 | 2.96 | 0.00 | 315 | 96 | 162 |
| Mean | 1.35 | 0.15 | 311 | 55 | 116 | 1.24 | 0.10 | 308 | 65 | 113 |
| Overall | 1.22 | 0.22 | 297 | 57 | 132 | 1.04 | 0.13 | 295 | 62 | 130 |
Table A.8.
Comparison BGC percent time in each concentration range for AP systems without S&M, with SLSM, SLSM and PLSM, and with ML-SCM on different days (percent time in each concentration range. A: >250 mg/dl, B: 250-180 mg/dl, C: 180-70 mg/dl, D: 70-50 mg/dl, E: <50 mg/dl)
| Time | BGC without S&M | BGC with SLSM | BGC with SL&PLSM | BGC with ML-SCM | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | A | B | C | D | E | ||
| Day1 | Ado | 20 | 29 | 50 | 1 | 0 | 19 | 28 | 52 | 1 | 0 | 17 | 26 | 56 | 1 | 0 | 17 | 26 | 56 | 1 | 0 |
| Adu | 2 | 4 | 83 | 8 | 3 | 2 | 5 | 83 | 8 | 2 | 4 | 4 | 86 | 6 | 0 | 4 | 4 | 86 | 6 | 0 | |
| Chi | 16 | 25 | 57 | 2 | 0 | 15 | 22 | 61 | 2 | 0 | 12 | 18 | 68 | 2 | 0 | 12 | 18 | 68 | 2 | 0 | |
| Mean | 12 | 19 | 64 | 4 | 1 | 12 | 18 | 66 | 3 | 1 | 11 | 16 | 70 | 3 | 0 | 11 | 16 | 70 | 3 | 0 | |
| Day2 | Ado | 22 | 24 | 52 | 1 | 1 | 21 | 23 | 55 | 1 | 0 | 19 | 20 | 61 | 0 | 0 | 17 | 10 | 73 | 0 | 0 |
| Adu | 2 | 5 | 72 | 7 | 14 | 3 | 6 | 75 | 5 | 11 | 3 | 5 | 77 | 5 | 10 | 3 | 4 | 87 | 4 | 2 | |
| Chi | 19 | 18 | 61 | 1 | 1 | 18 | 16 | 64 | 1 | 1 | 17 | 15 | 67 | 1 | 0 | 16 | 10 | 73 | 1 | 0 | |
| Mean | 14 | 16 | 62 | 3 | 5 | 13 | 15 | 65 | 3 | 5 | 13 | 13 | 69 | 2 | 3 | 12 | 8 | 78 | 2 | 1 | |
| Day3 | Ado | 22 | 24 | 51 | 2 | 1 | 20 | 22 | 55 | 2 | 1 | 19 | 20 | 59 | 1 | 1 | 16 | 8 | 74 | 1 | 1 |
| Adu | 1 | 3 | 75 | 6 | 15 | 1 | 4 | 76 | 6 | 13 | 1 | 3 | 79 | 7 | 10 | 1 | 3 | 89 | 4 | 3 | |
| Chi | 11 | 21 | 62 | 3 | 3 | 10 | 19 | 66 | 3 | 2 | 9 | 16 | 71 | 3 | 1 | 9 | 6 | 83 | 1 | 1 | |
| Mean | 11 | 16 | 63 | 4 | 6 | 10 | 15 | 66 | 4 | 5 | 10 | 13 | 70 | 4 | 4 | 9 | 6 | 81 | 2 | 1 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic artificial pancreas system. Diabetes Care. 2010;33:1072–1076. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal N, Cameron F, Buckingham BA, Wilson DM, Chase HP, Maahs DM, Bequette BW, Aye T, Clinton P, Harris BP. A novel method to detect pressure-induced sensor attenuations (PISA) in an artificial pancreas. Journal of diabetes science and technology. 2014;8:1091–1096. doi: 10.1177/1932296814553267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D, Mohtadi C, Tuffs P. Generalized predictive control Part II. Extensions and interpretations. Automatica. 1987;23:149–160. [Google Scholar]
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- Dassau E, Bequette BW, Buckingham BA, Doyle FJ. Detection of a meal using continuous glucose monitoring implications for an artificial β-cell. Diabetes Care. 2008;31:295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- Del Favero S, Monaro M, Facchinetti A, Tagliavini A, Sparacino G, Cobelli C. Real-time detection of Glucose Sensor and Insulin Pump Faults in an Artificial Pancreas. IFAC Proceedings Volumes. 2014;47:1941–1946. [Google Scholar]
- El-Khatib FH, Russell SJ, Magyar KL, Sinha M, McKeon K, Nathan DM, Damiano ER. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2014;99:1701–1711. doi: 10.1210/jc.2013-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren-Oruklu M, Cinar A, Quinn L, Smith D. Adaptive control strategy for regulation of blood glucose levels in patients with type 1 diabetes. Journal of process control. 2009;19:1333–1346. [Google Scholar]
- Facchinetti A, Del Favero S, Sparacino G, Cobelli C. Modeling transient disconnections and compression artifacts of continuous glucose sensors. Diabetes technology & therapeutics. 2016;18:264–272. doi: 10.1089/dia.2015.0250. [DOI] [PubMed] [Google Scholar]
- Feng J, Turksoy K, Cinar A. Prediction Methods for Blood Glucose Concentration. Springer; 2016. Performance Assessment of Model-Based Artificial Pancreas pControl Systems; pp. 243–265. [Google Scholar]
- Feng J, Turksoy K, Samadi S, Hajizadeh I, Cinar A. Hybrid Online Sensor Error Detection and Functional Redundancy for Artificial Pancreas Control Systems. IFAC-PapersOnLine. 2016;49:753–758. doi: 10.1016/j.jprocont.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund T. Automatic detection of sluggish control loops. Control Engineering Practice. 1999;7:p1505–1511. [Google Scholar]
- Harvey RA, Dassau E, Bevier WC, Seborg DE, Jovanovič L, Doyle FJ, III, Zisser HC. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes technology & therapeutics. 2014;16:348–357. doi: 10.1089/dia.2013.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch A. Condition monitoring of control loops 2000 [Google Scholar]
- Huang B. Multivariate statistical methods for control loop performance assessment. University of Alberta Alberta; Edmonton, Canada: 1998. [Google Scholar]
- Kovatchev BP, Breton M, Dalla Man C, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. Journal of diabetes science and technology. 2009;3:44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Buckingham BA, Wilson DM, Bequette BW. A closed-loop artificial pancreas using model predictive control and a sliding meal size estimator. Journal of diabetes science and technology. 2009;3:1082–1090. doi: 10.1177/193229680900300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijf YM, DeVries JH, Zwinderman K, Leelarathna L, Nodale M, Caldwell K, Kumareswaran K, Elleri D, Allen JM, Wilinska ME. Day and night closed-loop control in adults with type 1 diabetes a comparison of two closed-loop algorithms driving continuous subcutaneous insulin infusion versus patient self-management. Diabetes Care. 2013;36:3882–3887. doi: 10.2337/dc12-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni L, Raimondo DM, Bossi L, Dalla Man C, De Nicolao G, Kovatchev B, Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. Journal of diabetes science and technology. 2007;1:804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauseth R, Wang Y, Dassau E, Kircher R, Matheson D, Zisser H, Jovanovič L, Doyle FJ. Proposed clinical application for tuning fuzzy logic controller of artificial pancreas utilizing a personalization factor. Journal of diabetes science and technology. 2010;4:913–922. doi: 10.1177/193229681000400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect MM, Patel PG, Scott RE, Wheeler MD, Patel C, Griffin K, Sorensen ST, Goodwin JL, Quan SF. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35:81–88. doi: 10.5665/sleep.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010;33:121–127. doi: 10.2337/dc09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart R. A watchdog for controller performance monitoring; American Control Conference, Proceedings of the 1995; IEEE; 1995. pp. 2239–2240. [Google Scholar]
- Ruiz JL, Sherr JL, Cengiz E, Carria L, Roy A, Voskanyan G, Tamborlane WV, Weinzimer SA. Effect of insulin feedback on closed-loop glucose control: a crossover study. Journal of diabetes science and technology. 2012;6:1123–1130. doi: 10.1177/193229681200600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr JL, Cengiz E, Palerm CC, Clark B, Kurtz N, Roy A, Carria L, Cantwell M, Tamborlane WV, Weinzimer SA. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36:2909–2914. doi: 10.2337/dc13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil G, Rebrin K, Mastrototaro JJ. Metabolic modelling and the closed-loop insulin delivery problem. Diabetes research and clinical practice. 2006;74:S183–S186. doi: 10.1016/S0168-8227(06)70028-6. [DOI] [PubMed] [Google Scholar]
- Steil GM, Palerm CC, Kurtz N, Voskanyan G, Roy A, Paz S, Kandeel FR. The effect of insulin feedback on closed loop glucose control. The Journal of Clinical Endocrinology & Metabolism. 2011;96:1402–1408. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksoy K, Paulino TML, Zaharieva DP, Yavelberg L, Jamnik V, Riddell MC, Cinar A. Classification of Physical Activity Information to Artificial Pancreas Control Systems in Real Time. Journal of diabetes science and technology. 2015;9:1200–1207. doi: 10.1177/1932296815609369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksoy K, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive identification and control for artificial pancreas systems. IEEE Transactions on Biomedical Engineering. 2014a;61:883–891. doi: 10.1109/TBME.2013.2291777. [DOI] [PubMed] [Google Scholar]
- Turksoy K, Quinn LT, Littlejohn E, Cinar A. An integrated multivariable artificial pancreas control system. Journal of diabetes science and technology. 2014b:498–507. doi: 10.1177/1932296814524862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksoy K, Samadi S, Feng J, Littlejohn E, Quinn L, Cinar A. Meal Detection in Patients With Type 1 Diabetes: A New Module for the Multivariable Adaptive Artificial Pancreas Control System. IEEE journal of biomedical and health informatics. 2016;20:47–54. doi: 10.1109/JBHI.2015.2446413. [DOI] [PMC free article] [PubMed] [Google Scholar]


