Abstract
Purpose
We tested the hypothesis that clinical statokinetic dissociation (SKD, defined as the difference in sensitivity to static and kinetic stimuli) at the scotoma border in retinal disease is due to individual criterion bias and that SKD can be eliminated by equating the psychophysical procedures for testing static and kinetic stimulus detection.
Methods
Six subjects with glaucoma and six with retinitis pigmentosa (RP) were tested. Clinical procedures (standard automated perimetry [SAP] and manual kinetic perimetry [MKP]) were used to determine clinical SKD and the region of interest for laboratory-based testing. Two-way Method of Limits (MoL) was used to establish the isocontrast region at the scotoma border in glaucoma and RP subjects. Method of Constant Stimuli (MoCS) and a two-interval forced choice (2IFC) procedure then were used to present static or kinetic (inward or outward) stimuli at different eccentricities within the isocontrast region. The results were fitted with psychometric functions to determine threshold eccentricities.
Results
Clinical SKD was found in glaucoma and RP subjects, with variable magnitude among subjects, but significantly exceeding expected typical measurement variability. The resultant psychometric functions when using MoCS and 2IFC showed equal sensitivity to static and kinetic targets, thus eliminating SKD.
Conclusions
Clinical SKD found using clinical techniques is due to methodologic differences and criterion bias, and is eliminated by using an equated and more objective psychophysical task, similar to normal subjects.
Translational relevance
Eliminating SKD using a psychophysical approach minimizing criterion bias suggests that it is not useful to distinguish between normal and diseased fields.
Keywords: Goldmann perimetry, glaucoma, retinitis pigmentosa, statokinetic dissociation, standard automated perimetry
Introduction
Assessment of the visual field (VF) is an integral component of ophthalmic examination.1 Two modalities of VF assessment are static and kinetic perimetry, where the stimulus is either stationary or moving. Sensitivity results across the VF are reported differently, depending on the technique. Standard automated perimetry (SAP), which is the clinical standard of static perimetry for assessing conditions, such as glaucoma,2 is a technique that presents discrete light increments (of fixed size, Goldmann size III, and fixed duration of 200 ms) at discrete locations across the VF, and the sensitivity is determined by a thresholding procedure, such as a staircase method, whereby the luminance of the target is modulated until a threshold is reached. On the other hand, in kinetic perimetry, such as that using Goldmann manual perimetry, stimuli of fixed size and luminance typically are moved from a region of nonseeing, and the subject responds when the target is seen: a one-way Method of Limits (MoL).3 Thus, results are expressed as isopters or boundaries delineating regions of equal contrast sensitivity.
Previous studies have suggested discordance between the sensitivity results of static and kinetic perimetry, that is, that sensitivity to static versus kinetic stimuli is different. This has been referred to as “statokinetic dissociation” (SKD). Although SKD was first observed in patients with occipital lobe damage (Riddoch's syndrome),4 it was also later reported in normal observers (distinguished as physiologic SKD). When differences in results in static and kinetic perimetry results are observed in patients with retinal disease, including retinitis pigmentosa and glaucoma, and in other nonoccipital lesions beyond the retina, others have referred to this as SKD, not necessarily distinguishing between this and Riddoch's syndrome.5–14 In SKD, the discordance between the position of the kinetic perimetry isopter and the spatial position of a static target with the same contrast level has been taken to indicate differential levels of sensitivity: a relatively greater sensitivity to kinetic compared to static stimuli. Because of its presence in disease, SKD also has been proposed as a method or potential marker for detection of pathology.5,6 The phenomenon of SKD, when defined as the apparent differences in sensitivity to static and kinetic stimuli, appears to be widely described in ophthalmology textbooks, representing a concept ingrained in clinical practice.15,16
While previous research has accounted for SKD as being due to greater sensitivity to kinetic stimuli, our recent study has indicated that the phenomenon of SKD might be due to the different psychophysical methods used to quantify contrast detection between static and kinetic perimetric techniques. We showed that so-called physiologic SKD7,8,14 in normal subjects can be eliminated by equating the psychophysical procedure used to measure sensitivity to static and kinetic stimuli.17 Physiologic SKD is due to the differences in psychophysical procedures: MoL used in kinetic perimetry (which is affected by errors such as individual criterion bias and reaction time17,18) and staircase procedures used in static perimetry (affected by errors such as habituation, local adaptation,19 response variability,20–23 and attentional factors24–26) result in discordance between the isopter border and the threshold point where stimulus uncertainty is highest. Instead, a forced choice procedure that reduces criterion bias results in equal sensitivities to static and kinetic stimuli, thus eliminating physiologic SKD.17 The resolution of current perimetric techniques also may be inadequate for comparing static (e.g., fixed test grids in SAP) and kinetic perimetry (e.g., radial vectors) results, potentially providing confounding clinical information (Fig. 1); both techniques may be used in various ocular pathologies, including glaucoma.27
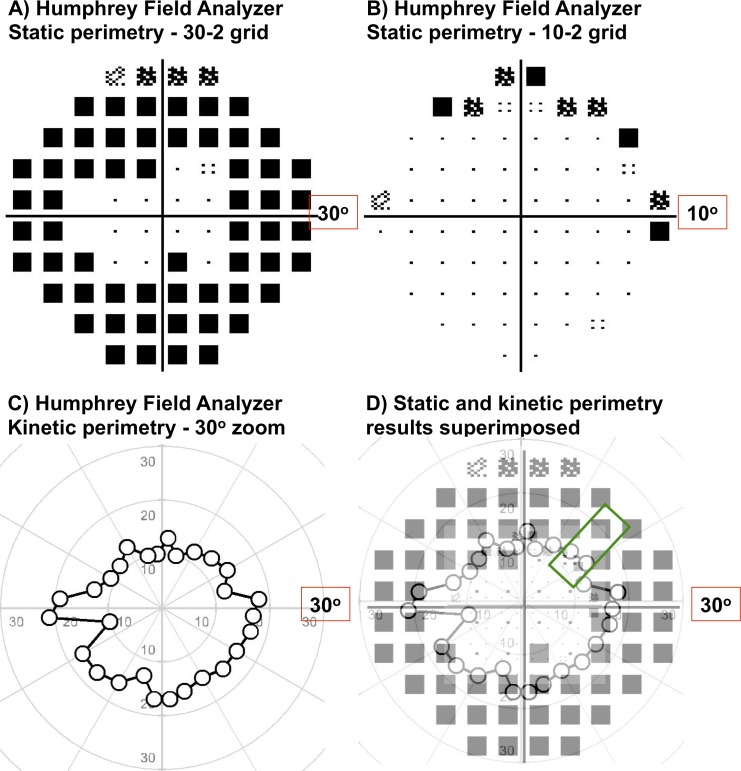
Figure 1.
Examples of static and kinetic perimetry results from one of the RP subjects in our study: Humphrey Field Analyzer 30-2 test grid (A), 10-2 test grid (B) and one-way MoL kinetic perimetry using a III4e target (C). In (A-C), all results have been magnified, but show different extents of the VF and different levels of detail, depending on the resolution of the test grid. These differences are made more apparent in (D), when scaled down to their respective grid sizes and superimposed (kinetic results at 100% transparency and static results at 30% transparency). An example region of interest tested in our study is highlighted by the green box.
Given the interest in SKD as a means for distinguishing disease from normal5,6 and our recent results in eliminating physiologic SKD,17 we sought to establish whether differences in sensitivity found using kinetic and static stimuli are actual phenomena in retinal disease by investigating their presence in subjects with glaucoma and retinitis pigmentosa (RP). To do this, we firstly measured the sensitivities of subjects with glaucoma and with RP using conventional clinical techniques, and also with a subjective two-way MoL to determine SKD. Then, a two-interval forced choice procedure (2IFC) and Method of Constant Stimuli (MoCS) was used to determine threshold eccentricity to static and kinetic stimuli of fixed contrast.
Methods
Participants
Six subjects with primary open-angle glaucoma (POAG) and six with RP were prospectively recruited for this study. All subjects had undergone comprehensive examination at the Centre for Eye Health, University of New South Wales to determine study eligibility.
Subjects were diagnosed with POAG based on the following clinical examination findings: characteristic glaucomatous optic nerve head (ONH) changes (thinning or notching of the neuroretinal rim, disc hemorrhage, enlarged cup-to-disc ratio, and/or peripapillary atrophy) with correlated thinning of the retinal nerve fiber layer on red-free viewing, correlating global and local structural findings measured using optical coherence tomography (OCT; Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA; and Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany), open and normal anterior chamber angles on gonioscopic examination, and with or without elevated intraocular pressure, as per our previous studies.22,26,28 Although VF defects do not necessarily need to be present for a diagnosis of glaucoma,29,30 we wished to examine subjects with VF defects in our study to examine the effect of SKD in regions with functional deficits, as per typical glaucomatous VF criteria.31 Therefore, the POAG subjects were required to have a VF defect on SAP testing (Humphrey Field Analyzer [HFA], 24-2 SITA-Standard test protocol) that correlated with the structural loss. All POAG subjects had early or moderate glaucoma as per their HFA mean deviation (MD) score (early, MD better than −6 dB; moderate, MD between −6 and −12 dB).31
Subjects with RP had characteristic retinal, electrophysiologic, and VF changes expected in RP.32 Retinal changes included bone spicule patterns (hyperpigmentary changes) in the mid-periphery of the fundus and waxy disc pallor, with thinning of the retinal layers on OCT, particularly at the outer nuclear layer (ONL) and retinal pigment epithelium (RPE).33 Subjects with cystoid macular edema were excluded from the study if the amount of edema exceeded the size of one sector on the Early Treatment of Diabetic Retinoscopy Study (ETDRS) grid.
For all subjects, spherical equivalent refractive errors were limited to between −6.00 and +6.00 diopters (D), with cylinder power not exceeding −3.00 diopters cylinder (DC). All subjects had best corrected visual acuities of 20/25 or better. All participants gave their signed informed consent before participating. Ethics approval was provided by the relevant University of New South Wales Ethics Committee, and the experiment followed the tenets of the Declaration of Helsinki.
Phase 1 – Clinical Visual Field Testing to Determine the Region of Interest for Testing
Clinical VF assessment was performed on all subjects to determine a baseline for subsequent laboratory-based psychophysical testing and also to identify the region of interest for further psychophysical testing. For RP and POAG subjects, the region of interest was the border of a scotoma which lay within 30° of fixation. The reason for using scotomata within 30° was because of limitations to the size of the test screen for the laboratory-based testing (see below section). Although many cases of Riddoch syndrome were initially described to occur mostly within the peripheral VF (>30° from fixation),4 subsequent studies reported it within the central VF (within 30°),5,7,11,12 and, thus, we examined this area as our region of interest. For RP subjects, the border of the scotoma was defined as the SAP test location at which there was a defect on the pattern deviation map at the P < 0.005 level. For glaucoma subjects, it was defined as the test location closest to fixation which was part of a cluster of at least three contiguous points of depression at least at the P < 0.05 level, one of which was at least P < 0.01, as per typical glaucomatous defect definitions.31 Using RP as a model to determine whether there was an effect of meridian or severity of VF defect upon the isocontrast zone, when we plotted isocontrast zone width as a function of isocontrast midpoint, there was a statistically significant nonzero slope (0.059 ± 0.020, P = 0.0032). However, there was a very poor fit to the data (R2 = 0.060), with the majority of widths within 8° (70.8%). Thus, we chose cardinal meridians that targeted regions within 30° of fixation but not temporally, in case the physiologic blind spot was accidentally tested.
For RP subjects, clinical perimetry consisted of SAP (HFA using the SITA-Standard paradigm using 30-2 and 10-2 test grids) and Goldmann kinetic perimetry. For POAG subjects, this included only SAP, as per typical clinical guidelines (HFA 24-2 SITA-Standard). Although the 24-2 test grid uses points with 6° spacing, a coarser grid compared to the 2° spacing of the 10-2, it served as a baseline for further psychophysical testing.
Stimulus and test screen
Testing conditions in the laboratory set-up were matched as closely as possible to clinical testing, namely, an achromatic stimulus of fixed size (Goldmann size III, 0.43° in diameter) was presented upon a white-gray background of constant luminance in the low photopic range (10 cd.m–2). Stimuli were generated using custom written software (MATLAB version 7 and Psychtoolbox version 3.0.11; MathWorks, Natick, MA) and were presented upon a linearized iMac 27-inch computer driven at a frame rate of 60 Hz. For all testing conditions, a black square (Weber contrast −0.2, 0.6° × 0.6°) was used as a fixation target for the subject. For most subjects, this target was situated in the middle of the screen, but for two subjects where the region of interest of testing was farther in the periphery, this was offset in the opposite direction by 10° to reduce the effect of the screen edge. A chin rest was used to ensure a constant viewing distance of 30 cm, and a trial frame with wide aperture lenses (38 mm) was used to correct for the subject's refractive error and to compensate for the working distance. Only one eye was tested with the other patched, and testing was conducted with natural pupils. For RP subjects, the better seeing eye (based on visual acuity and VF area) was chosen to maximize the possible test range; for POAG subjects, the eye with the more severe level of glaucoma (based on MD score) was chosen to access a deep enough scotoma for testing.
Phase 2A – Two-way Method of Limits for disease: defining the boundaries of the scotoma
Laboratory-based psychophysical testing consisted of two phases. In subjects with disease (POAG and RP), the aim was to compare the boundary of a scotoma measured using MoL and 2IFC. The first phase of testing consisted of a two-way MoL. As mentioned above, manual kinetic perimetry and SAP were used clinically to determine the initial region of interest and clinically-derived SKD. However, manual perimetry has potential errors including inconsistency in test speeds and examiner bias.34 Semiautomated perimetry is available on the HFA, but only a one-way MoL is available. Thus, we conducted an initial phase of testing on the laboratory-based system to determine the two-way MoL isopters for all subjects at the region of interested identified in Phase 1.
In this phase, the subjects were instructed to maintain fixation on the central fixation mark. Then, a beep signaled the start of the trial, after which a stimulus of constant luminance moved from the periphery towards fixation along a linear path (“inward” condition, Fig. 2A) at a rate of 4°/s.35 The origin of the stimulus always was at least 35° from fixation (i.e., beyond the 30-2 test grid). The subject was asked to press a button on a keyboard to signal when they first saw the stimulus. This was repeated 10 times to obtain the “inner” isopter. In a separate trial, the stimulus would move outward from the fixation mark along the same path as the inward condition, but this time, the subject was instructed to press a button when the stimulus first disappeared (“outward” condition, Fig. 2B) at the same speed. Again, this was repeated 10 times to obtain the “outer” isopter. Using these results, an “average” isopter location was obtained, which was the midpoint of the inward and outward conditions. As a practice run, the subjects underwent three trials before the data recording session. This then was used in Phase 2B of testing.
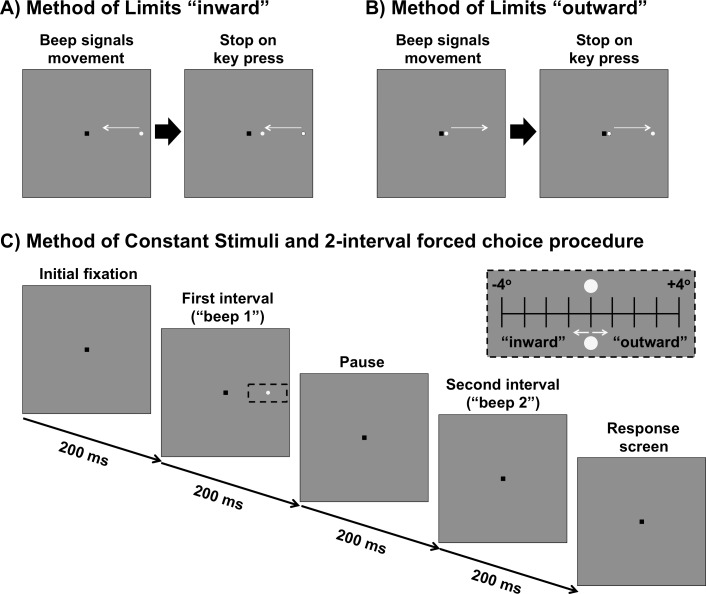
Figure 2.
(A) MoL for determining the “inner” isopter. The black square is the fixation mark for the subject. A stimulus moves in an inward direction (straight path along the meridian) at a constant speed and the subject responds when they first see the target. (B) MoL for determining the “outer” isopter. The stimulus moves outward along the meridian and the subject responds when the target disappears. (C) MoCS and 2-interval forced choice procedure. The fixation mark is shown. After 200 ms, there is a tone signaling the first interval, followed by a 200 ms pause, then another tone signaling the second interval. After both intervals are shown, the fixation mark is shown again as the program waits for a response from the first subject. During one of the two intervals, a stimulus is shown. The stimulus was randomly presented up to 4° inward or up to 4° outward in 1° steps around the midpoint of the isopters found in (A) and (B) (black dashed inset). The stimulus conditions included a static stimulus, inward moving stimulus or outward moving stimulus. All stimuli were shown for 200 ms.
For RP subjects, the contrast of the stimulus was the maximum output of the screen (approximately 375 cd.m–2). As there were no absolute scotomata (taken as a <0 dB result on the HFA) present in the VF results of the POAG subjects in our cohort, we used a stimulus with Weber contrast of 0.5 for testing in regions of deficit (equivalent to a 22 dB target on the HFA).
Phase 2B – 2-interval forced choice procedure: equating the psychophysical task
The next phase of psychophysical testing was the same for all subjects. MoCS was used to present stimuli at the midpoint of the inner and outer isopters obtained from phase one, and then at 1° intervals inward and outward up to 4° either side along the meridian (i.e., nine eccentricity levels). Although this represented a reasonable approximation of a location which was close to the point of subjective equivalence of the resultant psychometric function for some subjects, most subjects required refinement of the test locations to obtain a psychometric function that had a floor and plateau (approximately 0.5 and 1.0 proportion seen, respectively; also see Supplementary Fig. S1).
The contrast level of the stimulus was the same as that used in Phase 2A. In this experiment, the subject was asked to fixate on the central fixation mark. Then, a tone would signal the first interval (200 ms). This would be followed by a blank interval of the same duration (200 ms), and then by a second tone to signal the second interval (200 ms). Then, the response screen, consisting solely of the fixation mark, would be shown as the program awaited the subject's response. The stimulus would appear randomly in either the first or second interval, and the subject was asked to indicate in which interval they did see the target; if they could not see it in either interval, they were asked to guess (i.e., a 2IFC). After the response was recorded on the computer, the next trial would commence. There were 10 trials per eccentricity level, and, thus, each run consisted of 90 trials in total. Similar to Phase 2A, all subjects underwent one practice run for each condition before data recording to make sure they understood the task. One RP subject had to undergo two practice runs per condition due to poor initial understanding, where results were consistently at a guessing rate 0.5; the results of the second run also were excluded, with the results from subsequent runs recorded for analysis.
Three stimulus conditions were examined in this phase of testing: static, kinetic inward, or kinetic outward. For the static condition, the stimulus was presented at the location for 200 ms; that is, in the same fashion as in SAP. For the kinetic conditions, the stimulus also was shown for 200 ms, but moved either in an inward or outward direction at the same speed as in phase one (4°/s; i.e., a total travel distance of 0.8°, which is less than the 1° spacing between test locations). These conditions were tested separately and each subject underwent testing at least twice for each condition. The proportion of times seen was recorded for each eccentricity level.
The inner eccentricities (a negative relative eccentricity) represented regions that were suprathreshold for contrast detection in subjects with disease, as these were closer to fixation and were within regions of intact VF. The outer eccentricities (a positive relative eccentricity) represented regions that were infrathreshold, as these corresponded to region of the scotomas.
For the psychophysical paradigms used in our study, we did not directly measure fixation losses, false-positives or false-negatives using methods conventionally employed by perimeters, such as the HFA. Notably, all subjects had to have HFA results that were within manufacturer recommended reliability levels at <20% fixation losses and <15% false-positive rate. False-negatives were not used as an index as it is known to be elevated in disease. Although our subjects were highly experienced psychophysical observers, there were potential problems with fixation drift when the anticipated location of stimulus appearance was known (see Discussion for further comments).
Statistical Analysis
The proportion seen was plotted as a function of absolute eccentricity from fixation (in degrees) for each individual subject. The three conditions (static, inward, and outward) were plotted on the same figure. Psychometric functions were fitted to the data using a sigmoidal nonlinear regression function with variable slope (GraphPad Prism version 7; GraphPad Software, La Jolla, CA). The higher end of the psychometric function was allowed to float between 0.9 and 1.0, to allow for a degree of false-negative answers.36 We defined the eccentricity threshold as the EC50. The equations of the sigmoidal function are defined as: y = b + ([a – b] / [1 + 10(logEC50 – x) * HillSlope]), where b and a are the bottom and top values supplied by GraphPad Prism, respectively. The logEC50 and HillSlope parameters also are reported directly from GraphPad. Using this equation, we were able to solve for x, the eccentricity, for y = 0.625 and y = 0.875 to obtain the interquartile range (IQR). Dividing the IQR by 0.5 resulted in the slope parameter of the psychometric function.37,38 The slope parameter provides information about the threshold variance and decision-making of the responder to stimuli of various levels, whereby a steeper slope typically is regarded as greater certainty.25,26,39 The inner and outer isopter eccentricities determined by the two-way MoL also were plotted on the same figure for each subject. The midpoint of the isopters also was compared to the threshold eccentricity found using the psychometric function. Otherwise, relevant eccentricity values for each group were reported as median and IQR.
Results
Two-way MoL and the Isocontrast Zone Compared to SAP in Retinal Disease
The eccentricities of the inner and outer isopters measured at the border of the scotomata were significantly different across all RP subjects (P = 0.0312) and for all POAG subjects (P = 0.0312). The width of the isocontrast zone, defined as the difference between inner and outer isopter positions in degrees, was determined for both of the disease groups. For RP subjects, the median width of the isocontrast zone was 3.4° (IQR, 1.54°, 4.05°) and for POAG subjects, it was 1.55° (IQR, 0.93°, 4.24°). There was no significant difference between the width of the zones found in both patient groups (P = 0.3095).
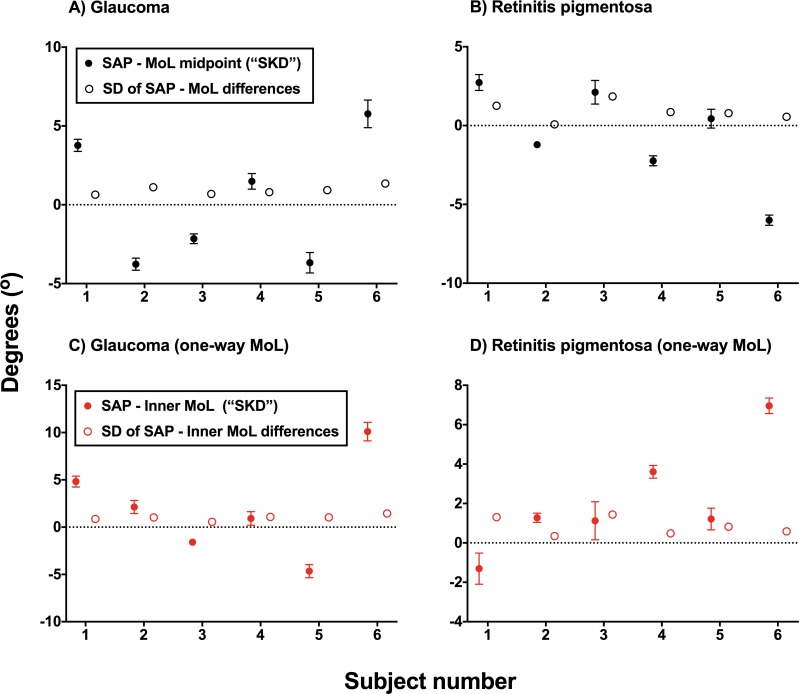
In clinical practice, differences in resolution of the instruments manifest as a difference between scotomata found when using static and kinetic perimetry measurements (so-called SKD). The magnitude of the value of SKD (magnitude was used due to the comparison with SD) was significantly greater than the SD of the MoL measurements (RP, P = 0.0250; glaucoma, P = 0.0299), which suggests that this would not be fully accountable by measurement variability alone (Fig. 3). This persisted when using a one-way MoL (inward isopter, which is more conventionally used clinically) for glaucoma subjects (P = 0.0220), but did not for RP subjects (P = 0.1444), which suggests a role of individual criterion bias for directional preference. There were no differences in the magnitude of SKD between glaucoma and RP groups (P = 0.8182). A one-sample t-test was used to examine the relative difference between SAP and MoL techniques, where the sign of the effect (as shown in Fig. 3) is preserved. There was a significant difference from 0 for the magnitude of SKD found within each glaucoma patient (all P < 0.0001, except subject 4 for the one-way MoL comparison at P = 0.0178) and all (average P = 0.0029) but one RP subject (subject 5 for the two-way MoL comparison at P = 0.1263). Overall, clinical SKD was demonstrated in the majority of subjects when comparing SAP and MoL techniques.
Figure 3.
A comparison of the difference between the midpoint of inner and outer isopters generated using the two-way MoL and the defect position found on SAP, typically regarded as statokinetic dissociation found using clinical instruments (SKD) for glaucoma (A) and RP (B) subjects. The relative difference (sign dependent, where a positive y-axis value indicates a more outward position obtained using the MoL) between SAP and MoL for each subject is shown by the black filled circle. Error bars: 95% CI. The standard deviations (SD) of the differences are also plotted for each subject (open circle). Similar figures are shown when the differences between one-way MoL (inward isopter, more conventionally used in practice) and SAP border (red filled circle; Error bars: 95% CI) and SD of the differences (red open circle) are considered (C, D). In all figures, the black dotted horizontal line indicates no disparity between SAP and MoL techniques, that is no clinical SKD found.
Equating the Psychophysical Procedure Eliminates Clinical SKD in RP and Glaucoma
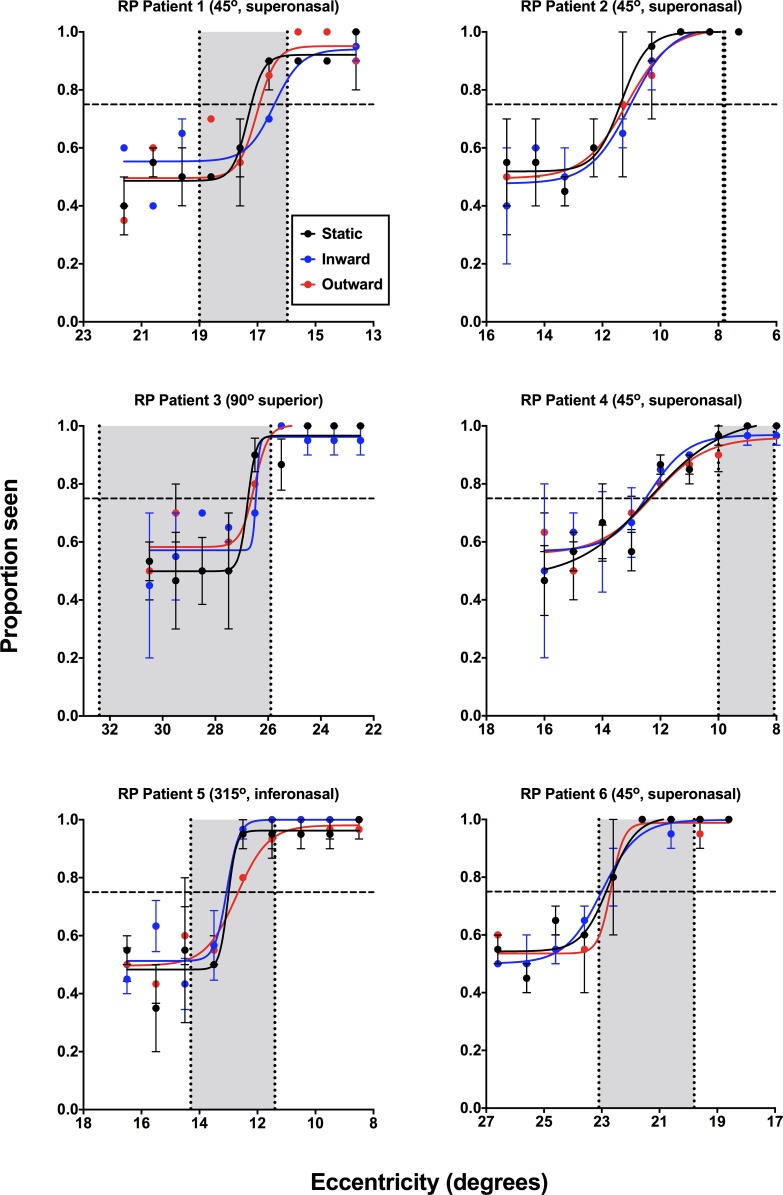
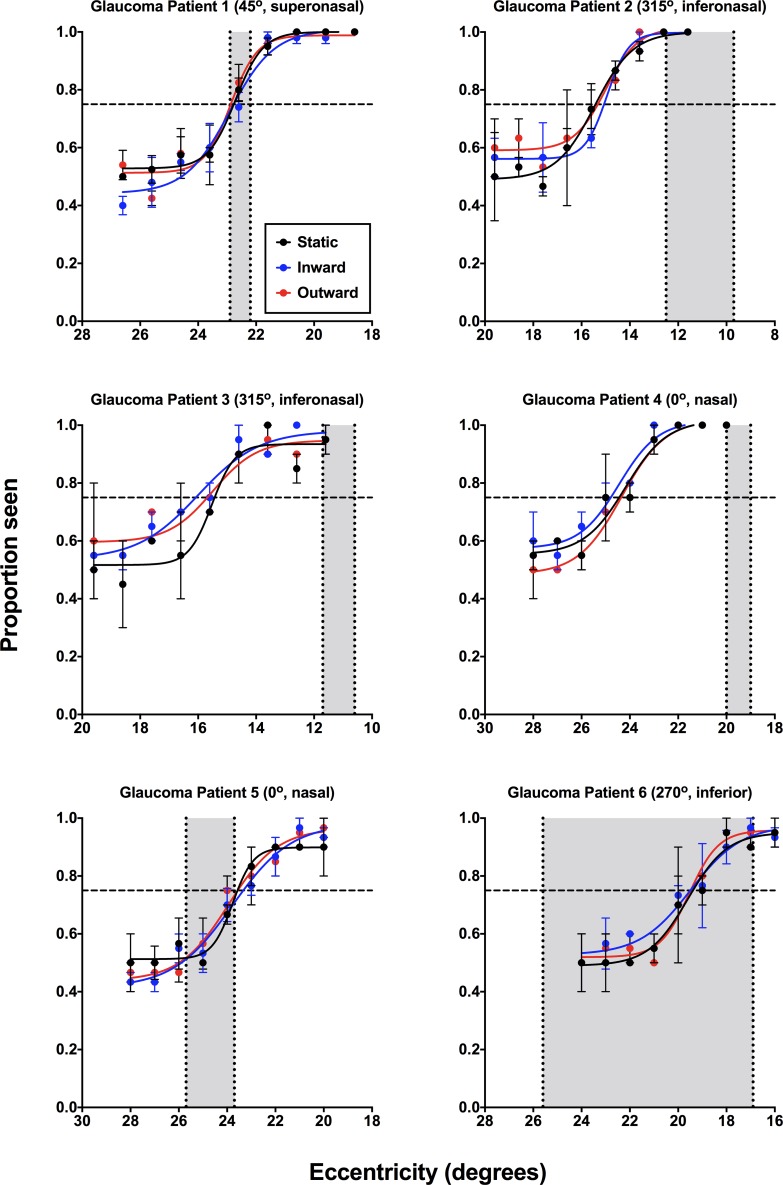
Psychometric functions for subjects with RP (Fig. 4) and glaucoma (Fig. 5) generated using the 2IFC and MoCS showed overlap between static, inward, and outward for all subjects, rather than the differences to static and kinetic stimuli expected in clinical SKD. In Figures 4 and 5, the results of the inner and outer isopters determined using the two-way MoL also are depicted. The overlap of these functions suggests similar performance for the three stimulus types.
Figure 4.
Psychometric functions (proportion seen as a function of eccentricity, in degrees) for the individual RP subjects (n = 6) for static (black), inward moving (blue), and outward moving (red) stimuli. Note that for consistency across all subjects the x-axis has been flipped, such that the lower eccentricity indicates a location closer to fixation. The cardinal visual field direction of testing has been noted for each patient (0° indicates the nasal direction, increasing in a counterclockwise direction). Each datum point represents the average of at least 20 responses. Error bars: 1 SEM. The horizontal dashed line indicates the threshold value (0.75). The vertical dashed lines indicate the inner and outer isopter positions measured using the laboratory-based two-way MoL, with the gray zone representing the isocontrast zone.
Figure 5.
Psychometric functions (proportion seen as a function of eccentricity, in degrees) for the individual glaucoma subjects (n = 6) for static (black), inward moving (blue), and outward moving (red) stimuli. Functions are plotted as per Figure 4.
There was no significant effect of stimulus condition (inward, outward, or static stimuli) for RP (H[3] = 3.739, P = 0.1718) or glaucoma (H[3] = 1.000, P = 0.7402) subjects upon relative eccentricity threshold determined using the psychometric functions. One-way analysis of variance (ANOVA) comparing the eccentricity thresholds of both RP (H[4] = 3.407, P = 0.3587) and glaucoma subjects (H[4] = 1.400, P = 0.7715) showed no significant difference between relative eccentricity threshold and the midpoint of the two-way MoL. This was likely due to a directional effect of the difference between the 2IFC and the MoL results (sometimes the threshold was more nasal while other times it was more temporal). Like the individual differences in SKD found in Figure 3, differences between the relative threshold eccentricity and midpoint of the two-way MoL were likely due to individual criterion bias.
Are There Slope Differences across Conditions Signifying Different Threshold Variability?
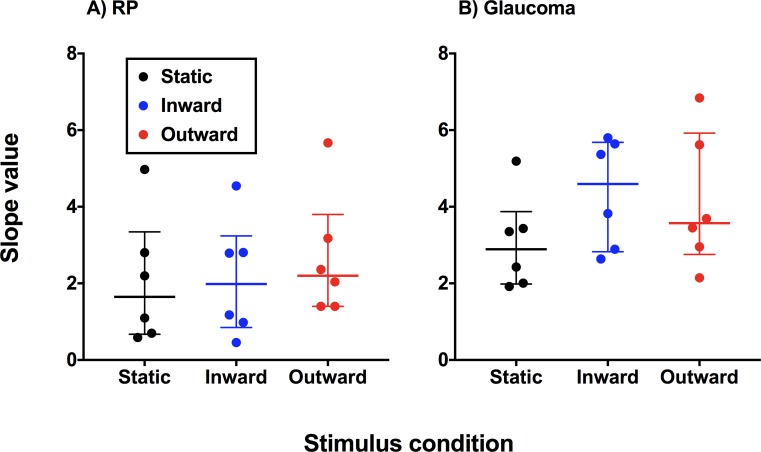
There was no significant difference in the slopes obtained using static, inward moving, and outward moving stimuli within each group (RP, P = 0.1840; glaucoma, P = 0.4297; Fig. 6). There was a significant difference between glaucoma and RP subjects in the overall magnitude of slope value (P = 0.0052).
Figure 6.
Slope values calculated by dividing the IQR of the psychometric function by 0.5 for each diagnostic category. Each datum point represents the result from one observer, and the horizontal bars indicate the median and IQR of slope values.
Discussion
Clinical SKD in Retinal Disease Obtained using Conventional Perimetric Techniques
Our results demonstrated differences between the position of static thresholds and kinetic perimetry isopters (described previously in the literature as SKD, which we continue to refer to in its clinical form in our study) when using conventional clinical techniques in patients with retinal disease. Some studies have likened SKD found in patients with cortical lesions to “blindsight,”40 and kinetic stimuli are purportedly more effective in localizing occipital lobe lesions compared to static stimuli.41 However, in relation to our report, studies in anterior visual pathway disease have suggested that SKD may be a marker for disease, and may be useful for early diagnosis. Studies demonstrating SKD in normal subjects also have shown a greater magnitude of dissociation in the presence of disease (e.g., Wedemeyer et al.11). Schiller et al.5 aimed to develop a method for quantifying SKD. Thus, SKD has been suggested to be useful to identify patients with anterior visual pathway anomalies.
To investigate the validity of SKD found using conventional clinical techniques, we performed two main investigations. We firstly identified and compared the magnitude of clinical SKD (SAP border compared to two-way MoL) identified using groups with different loci of anatomic loss or deficit to determine the amount of overlap and differences. The magnitude of difference in clinical SKD was small, with no systematic effect in the direction of the SKD. A potential explanation for this may be due to the precision by which clinically available techniques are able to measure SKD. The test grids typically used for glaucoma assessment may imprecisely define the border of scotomata.42 The relative scotomata present in glaucoma and the reliance upon statistically significant depressions on the pattern deviation analysis further confounds this. In contrast, scotomata in RP tend to be better defined, due to their sudden change in anatomy.33 Thus, resolution of clinical techniques may contribute towards clinical SKD.
Elimination of SKD by Equating the Psychophysical Procedure
Importantly, the lack of clear systematic effects in clinical SKD and overlap across groups found in our study, along with the significant interindividual variability found in our and in previous studies, suggested a phenomenon driven by individual criterion bias. In our second investigation, our paradigm reducing criterion bias by making the psychophysical procedure more objective eliminated differences between perception of static, inward moving, and outward moving kinetic stimuli in all subject groups, similar to our previous work in normal subjects.17 Differences between the isocontrast zone and the position of the psychometric functions along the VF meridian provide further evidence for individual criterion bias as a source of variability. Thus, while a larger magnitude of SKD may be expected in disease, it does not appear to be due to the disease process itself, but rather attributable to increased response variability in conventional procedures.
Interestingly, the slopes differed between glaucoma and the RP groups. One possible explanation for this is the gradient of structural change in each group. In glaucoma, the structural changes may occur gradually across different eccentricities, with greater loss within the scotoma.42 In comparison, the structural loss may be more abrupt in RP, as there is total obliteration of the detector elements within the affected area.32 At face value, the slope of the function might be flatter in glaucoma due to its gradient of structural change. However, as the slope is calculated using the psychometric function, it follows that there is a greater range at which perception of the stimulus lies within 0.5 and 1.0 in glaucoma, compared to RP, in which this range is narrower. Thus, the flatter slopes in RP subjects indicate the sharp delineation of the region of seeing and nonseeing.
The Structural Loss of SKD
Our results suggest that SKD measured using conventional perimetric procedures in retinal disease is not selective for any particular locus of structural deficit, but is due to individual criterion bias, highlighting a significant limitation of current perimetric procedures at characterizing borders of scotomata. Previous studies have suggested different etiologies for SKD. One proposal is separate processing streams of kinetic and static stimuli, which may be selectively damaged in different diseases14,43–46 (though this also has been debated in the literature47,48). The magnocellular (M) system is thought to be more sensitive to stimuli with high temporal and low spatial frequencies, and is more responsible for moving targets, while the parvocellular (P) system is more sensitive to low and high spatial frequency stimuli, and conveys color and contrast information of static targets.49–51 However, this theory of SKD is not fully compatible with other evidence suggesting overlap between the M and P systems,52 which is compounded by the equivocality of selective pathway dysfunction in diseases, such as glaucoma.47,48 Our results add to this debate by showing no difference in sensitivity between static and kinetic stimuli.
Limitations
Although we attempted to limit the subjects with disease to those with similar stages, it was more difficult to obtain a homogeneous sample of RP subjects. Given that elimination of SKD occurred in all of our subjects with little variability using our paradigm, this suggested compelling evidence for use of this technique.53 Further studies using subjects with a variety of stages of disease would be informative in characterizing variability of the isocontrast region. Structural measurements and comparisons were not made across the individuals at the region of the scotoma. Again, investigations at those locations would provide further information about the nature of SKD in retinal disease, and allow for examination of the structural locus of SKD.
As described in the methods, we did not obtain eye tracker results from our subjects and our paradigm did not include direct measurements of fixation. Patients with advanced stages of disease are more likely to present with fixation instability.54–56 In our study, the impact of fixation instability would likely manifest as inconsistencies in the position of isopters and eccentricity thresholds when using MoL and MoCS, respectively. Furthermore, instability would likely mask any effect of clinical SKD, due to increases in variability in gaze and fixation. Although the magnitude of clinical SKD found in our study overcame measurement variability using MoL (Fig. 3), a future study examining the effect of fixation instability upon SKD measurements would be informative.
Conclusions
Clinical SKD – the apparent difference in sensitivity to static and kinetic stimuli – in retinal disease may be apparent when using conventional clinical techniques, but is eliminated using similar, more objective psychophysical procedures. This shows that SKD is not a phenomenon of the disease process, but is due to methodologic differences in conventional perimetric techniques, which are affected by criterion bias; in translating this to clinical practice, SKD, therefore, may not be useful as a marker for disease as previously suggested. Examination of patients with cortical lesions, including the occipital lobe in which Riddoch's syndrome4 was first reported or the middle-temporal (MT) area57 (one of the major components of motion detection), using this modified paradigm would provide further insights into SKD.
Supplementary Material
Acknowledgments
Supported by the National Health and Medical Research Council of Australia (NHMRC #1033224). Guide Dogs NSW/ACT are partners in the NHMRC grant. JP is supported through a PhD scholarship provided by Guide Dogs NSW/ACT and an Australian Government Research Training Program PhD Scholarship.
Disclosure: J. Phu, None; M. Kalloniatis, None; H. Wang, None; S.K. Khuu, None
References
- 1.Phu J, Khuu SK, Yapp M, et al. The value of visual field testing in the era of advanced imaging: clinical and psychophysical perspectives. Clin Exp Optom. 2017;100:313–332. doi: 10.1111/cxo.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jampel HD, Singh K, Lin SC, et al. Assessment of visual function in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:986–1002. doi: 10.1016/j.ophtha.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Grobbel J, Dietzsch J, Johnson CA, et al. normal values for the full visual field, corrected for age- and reaction time, using semiautomated kinetic testing on the Octopus 900 perimeter. Transl Vis Sci Technol. 2016;5:5. doi: 10.1167/tvst.5.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddoch G. Dissociation of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain. 1917;40:15–57. [Google Scholar]
- 5.Schiller J, Paetzold J, Vonthein R, et al. Quantification of stato-kinetic dissociation by semi-automated perimetry. Vision Res. 2006;46:117–128. doi: 10.1016/j.visres.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Gandolfo E, Rossi F, Ermini D, et al. Early perimetric diagnosis of glaucoma by stato-kinetic dissociation assessment. In: Mills RP, Wall M, editors. Perimetry Update 1994/95 Proceedings of the XIth International Perimetric Society Meeting. Vol. 1994. Amsterdam/New York: Kugler Publications; pp. 271–276. [Google Scholar]
- 7.Hudson C, Wild JM. Assessment of physiologic statokinetic dissociation by automated perimetry. Invest Ophthalmol Vis Sci. 1992;33:3162–8. [PubMed] [Google Scholar]
- 8.Osako M, Casson EJ, Johnson CA, et al. Statokinetic dissociation: analysis of spatial and temporal characteristics by perimetry. In: Mills RP, Heijl A, editors. Perimetry Update 1990/91 Proceedings of the IXth International Perimetric Society Meeting. Vol. 1990. Amsterdam/New York: Kugler Publications; pp. 129–134. [Google Scholar]
- 9.Charlier JR, Defoort S, Rouland JR, et al. Comparison of automated kinetic and static visual fields in neuro-ophthalmology patients. In: Heijl A, editor. Perimetry Update 1988/89 Proceedings of the VIIIth International Perimetric Society Meeting. Vol. 1989. Amsterdam, Berkeley, Milano: Kugler & Ghedini Publications; pp. 3–8. editor. [Google Scholar]
- 10.Zappia RJ, Enoch JM, Stamper R, et al. The Riddoch phenomenon revealed in non-occipital lobe lesions. Br J Ophthalmol. 1971;55:416–20. doi: 10.1136/bjo.55.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedemeyer L, Johnson CA, Keltner JL. Statokinetic dissociation in optic nerve disease. In: Heijl A, editor. Perimetry Update 1988/89 Proceedings of the VIIIth International Perimetric Society Meeting. Vol. 1989. Amsterdam, Berkeley, Milano: Kugler & Ghedini Publications; pp. 9–14. editor. [Google Scholar]
- 12.Yabuki K, Sakai M, Suzumura H, et al. A comparison of kinetic and static perimetry for lesions in the visual pathway. In: Heijl A, editor. Perimetry Update 1988/89 Proceedings of the VIIIth International Perimetric Society Meeting. Vol. 1989. Amsterdam, Berkeley, Milano: Kugler & Ghedini Publications; pp. 15–9. editor. [Google Scholar]
- 13.Katsumori N, Bun J, Shirabe H, et al. Statokinetic dissociation in glaucomatous peripheral visual field damage. In: Mills RP, Heijl A, editors. Perimetry Update 1990/91 Proceedings of the IXth International Perimetric Society Meeting. Vol. 1990. Amsterdam/New York: Kugler Publications; pp. 503–507. [Google Scholar]
- 14.Safran AB, Glaser JS. Statokinetic dissociation in lesions of the anterior visual pathways. A reappraisal of the Riddoch phenomenon. Arch Ophthalmol. 1980;98:291–5. doi: 10.1001/archopht.1980.01020030287009. [DOI] [PubMed] [Google Scholar]
- 15.Rowe FJ. Boca Raton, USA: CRC Press, Taylor & Francis Group; 2016. Visual Fields via the Visual Pathway, 2nd ed. [Google Scholar]
- 16.Liu GT, Volpe NJ, Galetta SL. Liu, Volpe, and Galetta's Neuro-Ophthalmology E-Book: Diagnosis and Management. Amsterdam, Netherlands: Elsevier;; 2018. [Google Scholar]
- 17.Phu J, Al-Saleem N, Kalloniatis M, et al. Physiologic statokinetic dissociation is eliminated by equating static and kinetic perimetry testing procedures. J Vis. 2016;16:5. doi: 10.1167/16.14.5. [DOI] [PubMed] [Google Scholar]
- 18.Nowomiejska K, Vonthein R, Paetzold J, et al. Reaction time during semi-automated kinetic perimetry (SKP) in patients with advanced visual field loss. Acta Ophthalmol. 2010;88:65–69. doi: 10.1111/j.1755-3768.2008.01407.x. [DOI] [PubMed] [Google Scholar]
- 19.Stewart WC, Hunt HH. Threshold variation in automated perimetry. Surv Ophthalmol. 1993;37:353–361. doi: 10.1016/0039-6257(93)90065-f. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner SK, Swanson WH, Demirel S. The effect of limiting the range of perimetric sensitivities on pointwise assessment of visual field progression in glaucoma. Invest Ophthalmol Vis Sci. 2016;57:288–294. doi: 10.1167/iovs.15-18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner SK, Swanson WH, Goren D, et al. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology. 2014;121:1359–1369. doi: 10.1016/j.ophtha.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phu J, Khuu SK, Zangerl B, et al. A comparison of Goldmann III, V and spatially equated test stimuli in visual field testing: the importance of complete and partial spatial summation. Ophthalmic Physiol Opt. 2017;37:160–176. doi: 10.1111/opo.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rountree L, Mulholland PJ, Anderson RS, et al. Optimising the glaucoma signal/noise ratio by mapping changes in spatial summation with area-modulated perimetric stimuli. Sci Rep. 2018;8:2172. doi: 10.1038/s41598-018-20480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khuu SK, Kalloniatis M. Spatial summation across the central visual field: implications for visual field testing. J Vis. 2015. 15:15 1 6. [DOI] [PubMed]
- 25.Phu J, Kalloniatis M, Khuu SK. The effect of attentional cueing and spatial uncertainty in visual field testing. PLoS One. 2016;11:e0150922. doi: 10.1371/journal.pone.0150922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phu J, Kalloniatis M, Khuu SK. Reducing spatial uncertainty through attentional cueing improves contrast sensitivity in regions of the visual field with glaucomatous defects. Transl Vis Sci Technol. 2018;7:8. doi: 10.1167/tvst.7.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monter VM, Crabb DP, Artes PH. Reclaiming the periphery: automated kinetic perimetry for measuring peripheral visual fields in patients with glaucoma. Invest Ophthalmol Vis Sci. 2017;58:868–875. doi: 10.1167/iovs.16-19868. [DOI] [PubMed] [Google Scholar]
- 28.Kalloniatis M, Khuu SK. Equating spatial summation in visual field testing reveals greater loss in optic nerve disease. Ophthalmic Physiol Opt. 2016;36:439–452. doi: 10.1111/opo.12295. [DOI] [PubMed] [Google Scholar]
- 29.Jeong JH, Park KH, Jeoung JW, et al. Preperimetric normal tension glaucoma study: long-term clinical course and effect of therapeutic lowering of intraocular pressure. Acta Ophthalmol. 2014;92:e185–e193. doi: 10.1111/aos.12277. [DOI] [PubMed] [Google Scholar]
- 30.Kim KE, Jeoung JW, Kim DM, et al. Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol. 2015;159:160–168. doi: 10.1016/j.ajo.2014.10.010. e1–e2. [DOI] [PubMed] [Google Scholar]
- 31.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 32.Whatham AR, Nguyen V, Zhu Y, et al. The value of clinical electrophysiology in the assessment of the eye and visual system in the era of advanced imaging. Clin Exp Optom. 2014;97:99–115. doi: 10.1111/cxo.12085. [DOI] [PubMed] [Google Scholar]
- 33.Kalloniatis M, Nivison-Smith L, Chua J, et al. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review. Exp Eye Res. 2016;150:106–121. doi: 10.1016/j.exer.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Keltner JL, Johnson CA, Spurr JO, et al. Comparison of central and peripheral visual field properties in the optic neuritis treatment trial. Am J Ophthalmol. 1999;128:543–553. doi: 10.1016/s0002-9394(99)00304-9. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CA, Keltner JL. Optimal rates of movement for kinetic perimetry. Arch Ophthalmol. 1987;105:73–75. doi: 10.1001/archopht.1987.01060010079035. [DOI] [PubMed] [Google Scholar]
- 36.Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan BC, Tompkins JD, LeBlanc RP, et al. Characteristics of frequency-of-seeing curves in normal subjects, patients with suspected glaucoma, and patients with glaucoma. Invest Ophthalmol Vis Sci. 1993;34:3534–3540. [PubMed] [Google Scholar]
- 38.Strasburger H. Converting between measures of slope of the psychometric function. Percept Psychophys. 2001;63:1348–55. doi: 10.3758/bf03194547. [DOI] [PubMed] [Google Scholar]
- 39.Klein SA. Measuring, estimating, and understanding the psychometric function: a commentary. Percept Psychophys. 2001;63:1421–145. doi: 10.3758/bf03194552. [DOI] [PubMed] [Google Scholar]
- 40.Zihl J. “Blindsight”: improvement of visually guided eye movements by systematic practice in patients with cerebral blindness. Neuropsychologia. 1980;18:71–77. doi: 10.1016/0028-3932(80)90085-8. [DOI] [PubMed] [Google Scholar]
- 41.Wong AM, Sharpe JA. A comparison of tangent screen, Goldmann, and Humphrey perimetry in the detection and localization of occipital lesions. Ophthalmology. 2000;107:527–44. doi: 10.1016/s0161-6420(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 42.Wyatt HJ, Dul MW, Swanson WH. Variability of visual field measurements is correlated with the gradient of visual sensitivity. Vision Res. 2007;47:925–936. doi: 10.1016/j.visres.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quigley HA, Sanchez RM, Dunkelberger GR, et al. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28:913–920. [PubMed] [Google Scholar]
- 44.Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 45.Silverman SE, Trick GL, Hart WM., Jr Motion perception is abnormal in primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 1990;31:722–729. [PubMed] [Google Scholar]
- 46.Dandona L, Hendrickson A, Quigley HA. Selective effects of experimental glaucoma on axonal transport by retinal ganglion cells to the dorsal lateral geniculate nucleus. Invest Ophthalmol Vis Sci. 1991;32:1593–1599. [PubMed] [Google Scholar]
- 47.Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Morgan JE. Selective cell death in glaucoma: does it really occur? Br J Ophthalmol. 1994;78:875–879. doi: 10.1136/bjo.78.11.875. discussion 9–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987;7:3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 51.Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 52.Lee BB. Macaque ganglion cells and spatial vision. Prog Brain Res. 1993;95:33–43. doi: 10.1016/s0079-6123(08)60355-6. [DOI] [PubMed] [Google Scholar]
- 53.Smith PL, Little DR. Small is beautiful: In defense of the small-N design. Psychon Bull Rev. 2018] doi: 10.3758/s13423-018-1451-8. [published online ahead of print, March 10, [DOI] [PMC free article] [PubMed]
- 54.Amore FM, Fasciani R, Silvestri V, et al. Relationship between fixation stability measured with MP-1 and reading performance. Ophthalmic Physiol Opt. 2013;33:611–617. doi: 10.1111/opo.12048. [DOI] [PubMed] [Google Scholar]
- 55.Turano KA, Geruschat DR, Baker FH, et al. Direction of gaze while walking a simple route: persons with normal vision and persons with retinitis pigmentosa. Optom Vis Sci. 2001;78:667–675. doi: 10.1097/00006324-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Kameda T, Tanabe T, Hangai M, et al. Fixation behavior in advanced stage glaucoma assessed by the MicroPerimeter MP-1. Jpn J Ophthalmol. 2009;53:580–587. doi: 10.1007/s10384-009-0735-y. [DOI] [PubMed] [Google Scholar]
- 57.Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.