Abstract
Purpose
To characterize sources of inter- and intrasubject variability in quantitative foveal avascular zone (FAZ) metrics.
Methods
Two 3×3-mm optical coherence tomography angiography scans (centered on the fovea) were acquired in both eyes of 175 subjects. An image of the superficial plexus was extracted from each scan and segmented twice by a single observer. Four quantitative FAZ morphology metrics (area, axis ratio, acircularity, major horizontal axis angle) were calculated, and a variance components analysis was performed.
Results
Mean (±SD) age was 27.9 ± 11.9 years, and 55% were female. Area had the largest amount of variance resulting from intersubject differences (93.1%). In contrast, there was large interocular variance for axis ratio, acircularity, and major horizontal axis angle (55.0%, 53.7%, 70.7%, respectively), though only axis ratio showed significant asymmetry between fellow eyes (P < 0.05). Neither repeated images from the same eye nor repeated segmentation on the same image were significant sources of variance.
Conclusions
Metrics of FAZ morphology show excellent repeatability and reliability. Excluding FAZ area, there was a high amount of variance attributed to interocular differences for the other FAZ metrics; therefore, the fellow eye should not be considered a control for FAZ studies when using these metrics.
Translational Relevance
Vision scientists must be prudent when choosing FAZ metrics, as they display varying degrees of within-subject differences relative to between-subject differences. It seems likely that different metrics will be best suited for different tasks, such as monitoring small changes over time within a single subject or assessing whether a given FAZ is abnormal.
Keywords: foveal avascular zone, imaging, optical coherence tomography angiography, foveal morphology, variability, acircularity, axis ratio
The area devoid of retinal capillaries at the central macula defines the foveal avascular zone (FAZ). There is a variation in FAZ size across normal individuals,1–3 but it may be affected in a variety of diseases including diabetic retinopathy,4 sickle cell retinopathy,5 albinism,6 and foveal hypoplasia.7,8 Studies have reported a correlation between visual acuity and the enlargement of the FAZ in diabetic retinopathy and that vascular changes occur early in the disease course.9 This information suggests that FAZ metrics may serve as useful biomarkers in studying disease onset and progression not only in the eye but also in systemic diseases.10
Despite the ability to measure the FAZ with a plethora of imaging modalities for the last three decades,11–17 it is only recently, with the advent of optical coherence tomography angiography (OCTA), that imaging the FAZ has become highly practical in the clinic. Owing to its conceptual simplicity and the ease with which it can be measured, area has been the most studied metric for characterizing the FAZ.18–20 However, as with all laterally based measurements in retinal images, individual differences in ocular magnification and axial length need to be corrected in order to accurately compare area measurements between subjects or across studies.1,21 The need to measure axial length, as well as the large variation of area seen in normal individuals,1–3,19,22–25 makes it difficult to use area as a measure to compare small changes associated with disease progression between subjects or for identifying an FAZ as “abnormal.” While these limitations are less important when assessing repeatability of OCTA during a single visit,26 they represent important barriers to comparing data across different studies and populations.
Due to these limitations, other metrics have been introduced to assess the relative health of the FAZ. Acircularity index (acircularity), introduced in 2011,27 is defined as the perimeter shape of a given FAZ relative to a perfect circle with the same area of that FAZ. Axis ratio, introduced in 2017, is the ratio between the major and minor axis of an ellipse having the same normalized second central moments as the FAZ itself.28 Prior to the axis ratio, in 2016 the major horizontal axis angle (MHAA, defined as the difference between a horizontal line and the major axis of the aforementioned ellipse) was used in a diabetes study to differentiate between normal and diseased eyes.29 The major practical advantage of these proposed metrics is that axial length measurement and correction for ocular magnification is not required, making them easier to use in a clinical setting. However, the application of any metric to compare eyes in normal and diseased states requires a comprehensive understanding of the sources of variability underlying a given metric; this may help inform which metric may be more clinically useful. Sources of variability in a metric may include but are not limited to differences between subjects, interocular differences, segmentation differences, as well as differences between images of the same eye. However, there has been minimal direct comparison of different FAZ metrics regarding the factors that contribute to their respective variances. Therefore, this study was designed to assess the variance and interocular symmetry for four FAZ metrics (area, axis ratio, acircularity, and MHAA) in normal subjects.
Methods
Subjects
This study was approved by the Institutional Review Board of the Medical College of Wisconsin (PRO 23999 and PRO 17439) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained for all subjects once the nature and risks of the study were explained. An ocular health questionnaire (OHQ) was used to ascertain that subjects had no previous retinal disease. Inclusion criteria included 5 years of age or older, no major eye movement artifacts near the fovea, and subjectively good signal to noise ratio and overall image sharpness. Exclusion criteria included any previous history of ocular or systemic vascular disease, such as hypertension and diabetes.
Imaging
Axial length measurements were acquired using a partial-coherence interferometer (IOL Master; Carl Zeiss Meditec, Dublin, CA). Spectral domain optical coherence tomography (OCT) images were acquired from each subject (Cirrus HD-OCT; Carl Zeiss Meditec) to assess for any posterior segment anomaly and pathology by a single observer (MNM). If the images were not available, the structural OCT from the AngioVue OCTA system (Optovue, Inc., Fremont, CA) was examined instead (n = 9 subjects).
Subjects underwent OCTA imaging with the AngioVue OCTA system (Optovue Inc.). Two scans (one horizontal and one vertical), each consisting of 304 B-scans at 304 A-scans per B-scan, were acquired at the fovea of both eyes with a nominal scan size of 3 × 3 mm. The horizontal and vertical scans were then coregistered by the device (software version 2016.2.0.16) to minimize motion artifacts and create a single volume from which an image of the superficial plexus was extracted.30,31 The superficial plexus image was produced by integrating motion contrast data between 3 μm below the internal limiting membrane to 16 μm above the inner plexiform layer. Two such volumes for each eye of each subject were obtained for a total of four images per subject.
Quantitative FAZ Analysis
The FAZ in each superficial plexus image was manually segmented by a single observer (REL) using ImageJ software's multipoint tool.32 Each image was then segmented a second time, with the observer masked to their initial segmentation. The coordinates from each segmentation were entered into a custom MATLAB script similar to one previously described.1,12 In brief, the script used the function poly2mask to produce a mask defining the area and the perimeter of the FAZ. To accommodate the analysis required for this study, changes made to the script included calling for the major axis, minor axis, and MHAA (orientation) using the MATLAB function regionprops.12
The size of the FAZ is equal to the number of pixels included in the FAZ mask. The nominal image scale is taken as the nominal scan width (3000 μm) divided by the width of the image in pixels (304). The actual image scale was determined as previously described1 by multiplying the nominal image scale by the ratio of the subject's measured axial length to that assumed by the system (23.95 mm). This image scale was used to convert FAZ mask area from pixels to absolute retinal area in square millimeters. The perimeter was scaled in a similar manner and was used to derive the acircularity of the FAZ defined as ratio of the perimeter of a given FAZ to that of a perfect circle with the same area of the FAZ (will always be ≥1). The major axis, minor axis, and MHAA of the ellipse with the same normalized second central moments as the FAZ masked region were output by regionprops (Fig. 1). Axis ratio is simply the ratio of the major axis length to minor axis length (will always be ≥1). The MHAA is defined as the orientation of the major axis to a horizontal line through the origin of the ellipse (ranges between −90° and 90°).
Figure 1.
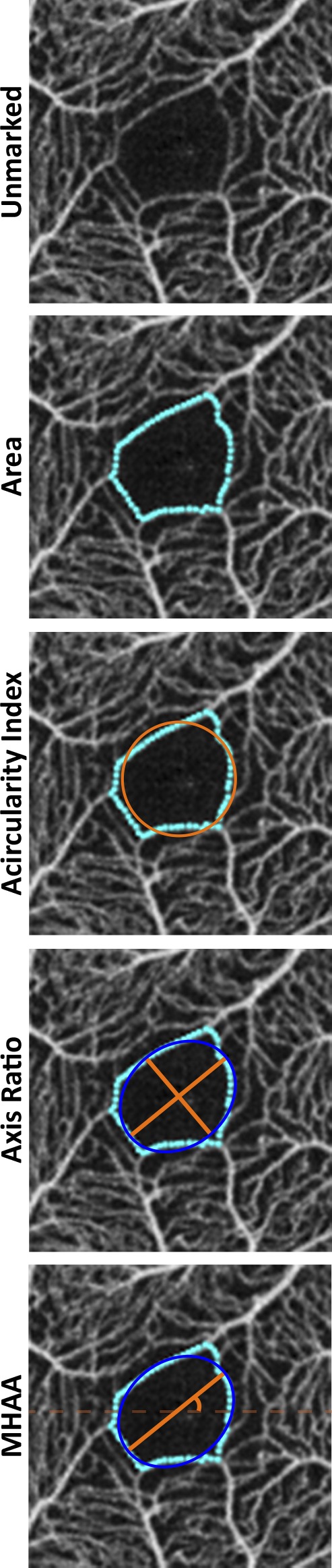
Finding each FAZ metric using our custom MATLAB script. All four metrics are found with a single segmentation shown in teal. Area is defined as the area within the FAZ segmentation boundary. Acircularity compares the perimeter of the FAZ segmentation to the perimeter of a perfect circle with the same area. For axis ratio and the MHAA, we use the ellipse (defined by regionprops) with the same normalized second central moments as the FAZ masked region. Axis ratio is ratio of the major to the minor axis of this ellipse (longest to shortest axis ratio), while the MHAA is the angle at which the major axis of the ellipse occurs relative to the horizontal axis.
Statistical Analysis
For each metric, a paired t-test or Wilcoxon matched-pairs signed rank test was used to evaluate interocular symmetry (Prism; GraphPad Software, La Jolla, CA). The choice of test was based on an analysis of normality (using P < 0.05 as the criterion) of the differences between eyes using the D'Agostino-Pearson normality test (Prism; GraphPad Software). In addition, a variance component model was used to estimate the subject effect (differences between subjects) and the effects of an eye within a subject (intraocular differences), image within a subject (differences between repeated images of the same eye), and trial within a subject (differences between repeated segmentations by the same observer) to the overall variance of each metric (SAS v9.4; SAS Institute, Cary, NC). As above, the data were analyzed for normality, and if normality could not be confirmed for a given metric, the variance component model was run on log-transformed data.
Results
We recruited 187 “normal” subjects for the study. However, upon review of their OHQs, as well as their structural OCT results, 12 subjects were excluded due to ocular or systemic abnormalities. Four of these subjects reported a history of hypertension (n = 2), diabetes (n = 1), or histoplasmosis (n = 1). Others were excluded based on OCT findings as follows: optic disc abnormalities (n = 3), foveal hypoplasia (n = 1), intraretinal hyperreflective speckle lesions (n = 1), microhole at the fovea (n = 1), posterior staphyloma (n = 1), and vitreous debris and retinal hemorrhage (n = 1). Our final database consisted of two images from 350 eyes of 175 subjects (96 females) with the average age (±SD) of 27.9 ± 11.9 years old (range, 5–77 years old).
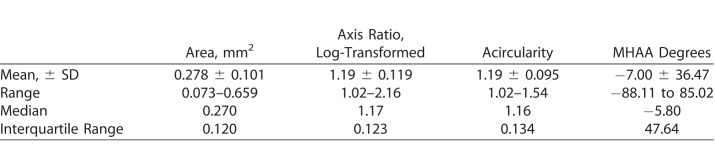
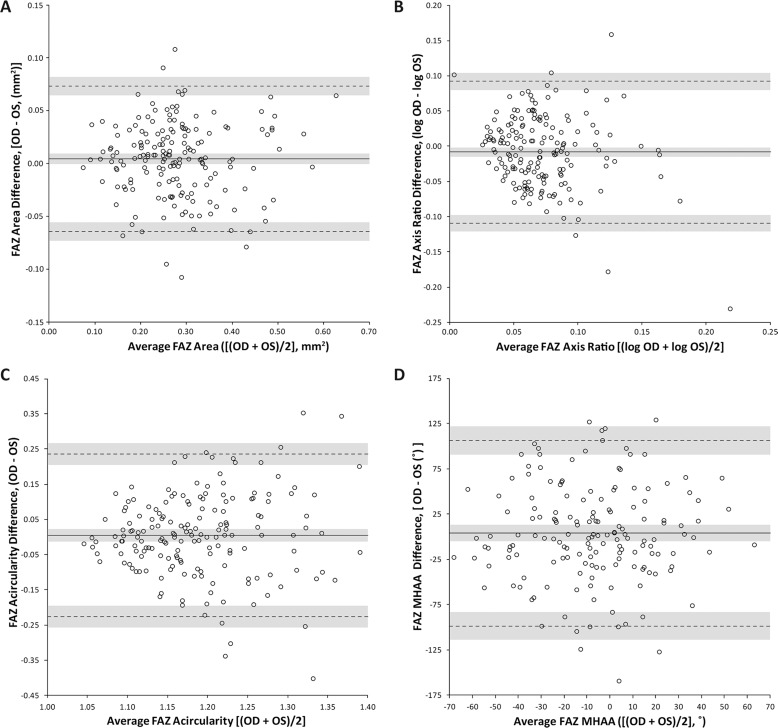
The population mean (±SD) and range for each metric is provided in Table 1. The difference between eyes for each metric was tested for normality with only the axis ratio failing. Because of this, axis ratio was log-transformed and the analysis was run on the transformed numbers. When assessing interocular symmetry of the FAZ (Fig. 2), area, acircularity, and MHAA showed no statistically significant difference between eyes (P = 0.11, P = 0.63, P = 0.35, respectively) with mean differences of 0.0043 mm2, 0.0044, and 3.71°, respectively. However, the log-transformed axis ratio resulted in statistically significant differences between eyes within a subject (P = 0.033), where the average ratio between eyes showed the right eye to be 3% larger than that of the left. However, even for the three metrics assessed as a having high degree of interocular symmetry, we found that true asymmetry for each metric can exist even in healthy patients as shown in Figure 3.
Table 1.
Summary of FAZ Metrics for 350 Eyes of 175 Subjects With Normal Vision
Figure 2.
Interocular symmetry of FAZ metrics. Data are expressed in Bland-Altman plots to show interocular differences for (A) area, (B) axis ratio, (C) acircularity, and (D) MHAA. Solid lines represent average difference (bias) between the eyes, while dotted lines represent limits of agreement (LOA). The gray shading represents the confidence intervals for the bias and LOAs for each metric.
Figure 3.
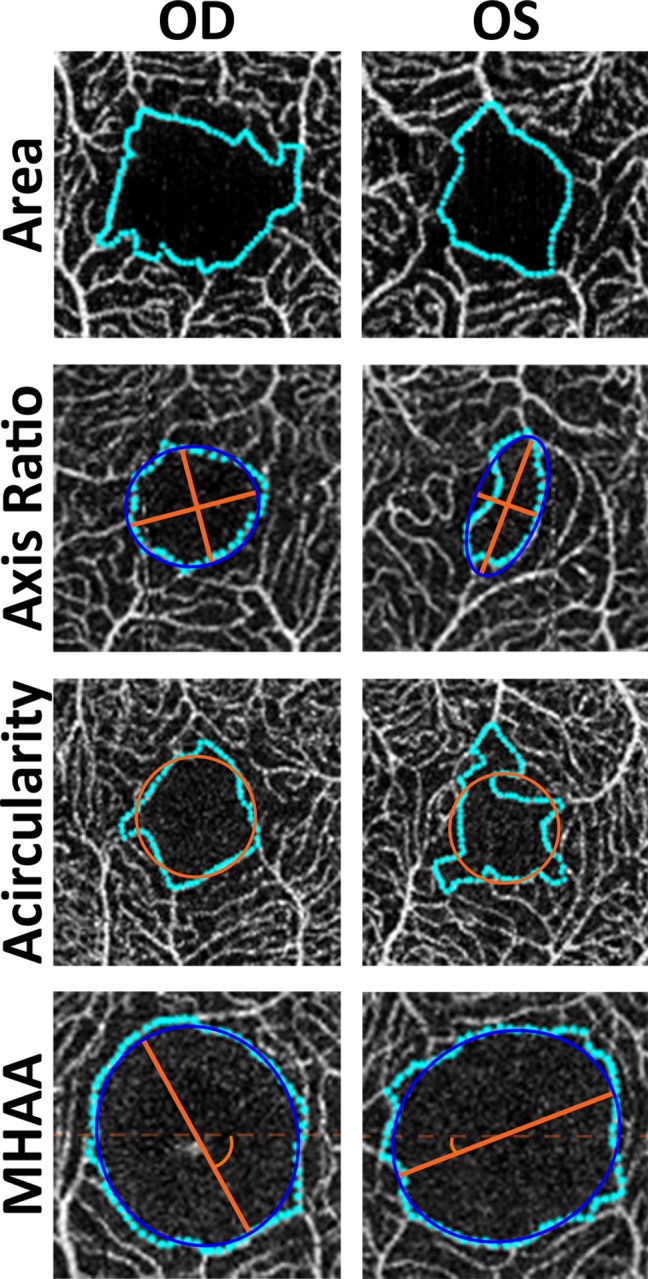
Examples of interocular asymmetry in FAZ morphology. From top to bottom, subject JC_11327 had an interocular difference of 0.11 mm2 for area, subject JC_10672 had an interocular difference of 0.59 for the log of the axis ratio (∼80%), subject JC_1246 had an interocular difference of 0.402 for acircularity, and subject JC_10579 had an interocular difference of 88.75° for the MHAA. Such large differences were not common but can occur, even in patients with no ocular pathology.
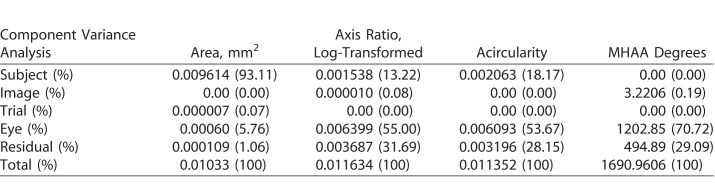
The results of the variance component analysis assessing the contributions to the variance for each of the four metrics are shown in Table 2. The axis ratio failed the normality test, thus the analysis was run on the log-transformed numbers. When comparing between the metrics, area had the largest contribution of variance due to subjects, whereas there was less contribution from subjects for the other three metrics. In addition, the area had significantly less variance caused by both interocular variability within subjects (between the two eyes of a subject) and residual component (variance not attributed to the other variables). In contrast, axis ratio, acircularity, and MHAA had greater than 50% of its variance between the fellow eyes. Also, the measurement error for axis ratio, acircularity, and MHAA varied between 20% and 30%. Variation arising from image segmentation and trial were negligible for all metrics. Thus, there was good repeatability between two images taken for each eye and reliability in segmentation of images. However, the repeatability and reliability for the MHAA can be decreased by small changes in head or eye positioning or rotation (Fig. 4), while the wide range of normal angles makes classifying angles as pathologically difficult (Fig. 5).
Table 2.
Component Variance Analysis for Each FAZ Metric With Amount of Variance Attributed to Each Possible Variable for Both Exact and Percentage
Figure 4.
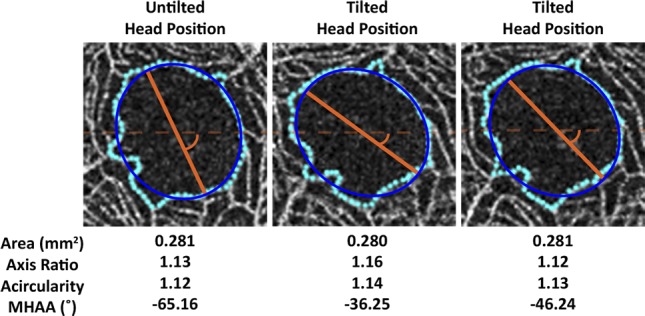
Illustrating the effect of head tilt on FAZ metrics. Shown on the left is an image from the right eye of a subject (JC_10567) acquired using the patient's normal head posture. The patient was then instructed to tilt the head, after which two additional images were acquired (middle and right panels). The area, axis ratio, and acircularity did not change substantially (less than 3%), whereas the MHAA changed by 44% (middle panel) and 29% (right panel).
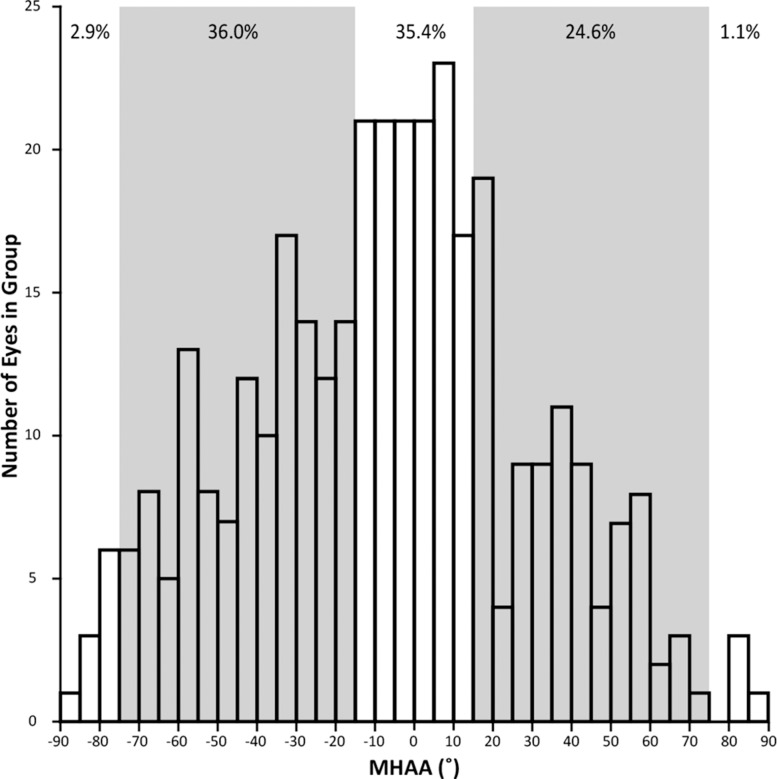
Figure 5.
Distribution of MHAA observed in 350 eyes with no known pathology. Freiberg et al.29 suggested that a normal MHAA for the FAZ should fall within 15° of either the horizontal (0°) or vertical (90°) axis. However, in our data, 60.6% of the eyes had angles that fell out of this proposed normal range (shown by the shaded gray regions in the histogram).
Discussion
Our large normative database allowed us to analyze four FAZ metrics (area, axis ratio, acircularity, and MHAA) that have been used in various OCTA studies.7,9,27–29,33–46 We evaluated whether there was interocular symmetry for each of the four metrics and assessed how different sources of variation contribute to the overall variance of each metric. These analyses are important for clinicians and imaging researchers to consider when deciding on the appropriate metric to be used as an imaging biomarker in a clinical setting or in research trials utilizing OCTA. It is important to note that our results are limited to the device used here (Optovue AngioVue). Other OCTA devices may yield different results, especially as the within-image distortions can be substantial in devices that scan the retina in only a single direction.47–50 Such distortions would be unique between repeated images of the same subject and thus would affect the absolute accuracy of the FAZ metric(s) being examined. This would also increase the contribution of the image to the overall variance of a given metric, thereby reducing the ability to detect real differences between subjects, between eyes, or between images of the same eye taken over time.
Area remains the most widely used and easily understood FAZ metric of the four studied here. The mean (±SD) area in our study of 0.278 ± 0.101 mm2 is very similar to that reported in other recent studies of 0.266 to 0.284 (±0.09–0.11) mm2.19,22–25,51 However, accuracy of the reported area is dependent on multiple factors, including the method used to segment the FAZ1,24,49,52 and whether or not the image scale was corrected for ocular magnification due to individual differences in axial length.1,21 Not correcting for axial length can result in errors in the estimated FAZ area of up to 51%.1,21 A limitation of our study is that the majority of subjects were Caucasian (78.9%). Area is known to be larger in African,53 Japanese,54 and Chinese51,55,56 subjects compared to Caucasian subjects. It seems likely that race/ethnicity-specific normative databases will be required to fully leverage area (and possibly other FAZ metrics) as biomarkers for detecting and monitoring retinal and systemic vascular diseases. Finally, while our study has a large age range (5–77 years), the majority of our subjects were 20 to 50 years old (87.4%). There is still debate on whether area changes naturally with aging, with some reports showing no change1,19,20,25 and others showing enlargement.3,56,57 Regardless, more work should be done to increase the number of younger and older subjects to better understand how these metrics may change among age groups, as it may also be necessary to utilize age-specific normative databases.
Acircularity and other shaped-based metrics have been proposed as potential biomarkers for diseases impacting the FAZ, as small capillary dropout at the FAZ can lead to large changes in the perimeter but not to the area.27,28,39 Researchers have shown that it is a more sensitive metric than area in detecting microvascular dropout.28,39 Conversely, this sensitivity itself may represent a potential liability as small errors in FAZ segmentation would have a more significant impact on the acircularity than on the area as well as cause a decrease in repeatability.1,51 Similar issues relating to metric sensitivity are seen with adaptive optics retinal images of the cone mosaic, where true cone degeneration is confounded by errors in cone identification. To fully characterize cone mosaic metrics, Cooper et al.58 simulated variable loss of cones from real images of the cone mosaic to monitor how different metrics respond to that loss, enabling a head-to-head comparison of the relative sensitivity of each metric. Similar simulation studies will be required to fully assess the relative sensitivity of each FAZ metric to capillary dropout and segmentation errors, which will help inform which metric may be most appropriate for a given study. It seems likely that a combination of metrics may, in fact, be the best approach.
For acircularity, we found no statistically significant difference between the eyes; yet when looking at our variance component model, over 53% of the variance for acircularity was attributed to differences between eyes. This apparent discrepancy is most likely due to the minimal amount of variance found in the metric itself across all subjects. This result is similar to that of Fang et al.51 as they found no statistically significant difference in FAZ circularity between eyes. However, they reported no significant correlation between eyes (Pearson r = 0.148, P = 0.411). While not our primary analysis, we also examined the interocular correlation and found a weak but statistically significant correlation (Spearman r = 0.274, 95% confidence interval = [0.1269–0.4098], P = 0.0002). The strength of correlation is comparable between our study and that of Fang et al.53; however, we had over five times as many subjects. When comparing FAZ area, both our study and that of Fang et al. found no significant difference between eyes; however, Fang et al. reported a larger mean FAZ area in their subjects. This is most likely due to difference in racial makeup of our populations, furthering the point that race and ethnicity should be considered when comparing FAZ metrics.
We identified an important confound in assessing MHAA, which has important implications for clinical use of this metric. Owing to the lack of an external anchor on which to base the measurement, small changes in head or eye positioning and rotation will manifest as real changes in the MHAA (Fig. 4). This means that FAZ images acquired over time should be registered to a baseline image to minimize this effect, though changes in the retinal vasculature and the FAZ itself may complicate this process. For comparing different subjects, however, it would be impossible to know if any difference in MHAA was due to differences in FAZ morphology or differences in relative head positioning of the two subjects during image acquisition. It is important to note that this does not impact the other three FAZ metrics.
Asymmetry of image-based measures of retinal and ocular anatomy has been shown to be a useful biomarker for retinal nerve fiber layer thickness,59 disc cupping,60 or axial length,61 as it can be an early indicator of pathology. While we found no significant asymmetry in area, acircularity, or MHAA, we did observe significant interocular asymmetry for axis ratio. This asymmetry may be caused by an oversimplification of the FAZ shape, since it requires an ellipse that represents the FAZ rather than using the true segmentation of the FAZ. Alternatively, it could be a more sensitive metric that shows true differences between eyes that the other metrics were not able to detect. Regardless, axis ratio has been suggested as a possible biomarker for diabetic retinopathy due to its ability to capture the enlargement of the FAZ occurring in retinopathies and does not require axial length.28 These factors make the axis ratio ideal for clinical practice, yet the significant asymmetry between fellow eyes in our normal population brings its clinical utility into question.
It is important to note that we used a single observer to manually segment the FAZs. We elected to use manual segmentation as prior studies in our lab demonstrated that manual segmentation had lower measurement error and better repeatability compared to two versions of automated segmentation provided by the device manufacturer.1 This is also reflected in the present study where the contribution of trial to the overall variance component model was near zero for all metrics (Table 2), indicating excellent repeatability of this observer. Our study was designed to examine interocular symmetry and relative between-subject variation in the four FAZ metrics used; having such highly repeatable segmentation allowed us to isolate these biological variables. Future studies seeking to characterize the effect of different algorithms or different manual graders will be needed to make generalized conclusions about a given method's ability to detect interocular or between-subject differences.
There is no ideal metric for assessing the FAZ, as all the metrics studied here have important limitations. While the variation in area was largely driven by real differences between subjects, it could be that the differences between eyes or images were too subtle to be picked up by such a high-level descriptor of the FAZ. Moreover, area must be scaled to axial length, a manual process that limits clinical applicability. Acircularity is a more sensitive measure when evaluating for minor changes in FAZ morphology. Yet, small differences in segmentation can produce unfounded, significant changes. In summary, each metric has beneficial and limiting characteristics. Therefore, clinicians and vision science researchers must use care when deciding which metric to use for a particular clinical application. A combination of metrics may be required to capture local and global aspects of the FAZ.
Acknowledgments
The authors thank Erin Curran, Mara Goldberg, Jamil Khan, Madia Russillo, Phyllis Summerfelt, Melissa Wilk, Vesper Williams, and Erica Woertz for their help recruiting and imaging subjects.
Supported in part by the National Eye Institute of the National Institutes of Health (NIH) under award numbers R01EY024969 and P30EY001931, and by the NIH National Institute of Aging under award number T35AG029793. This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program, Grant C06RR016511, from the National Center for Research Resources, NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Rachel Linderman is the recipient of a Fight for Sight–Nick Cacciola Summer Student Fellowship Award. Manickam Muthiah is the recipient of a Keeler–Royal College of Ophthalmologists Scholarship.
Disclosure: R.E. Linderman, None; M.N. Muthiah, None; S.B. Omoba, None; K.M. Litts, None; S. Tarima, None; A. Visotcky, None; J.E. Kim, None; J. Carroll, OptoVue (F), MeiraGTx (C)
References
- 1.Linderman R, Salmon AE, Strampe M, Russillo M, Khan J, Carroll J. Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling. Trans Vis Sci Technol. 2017;6:16. doi: 10.1167/tvst.6.3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chui TYP, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89:602–610. doi: 10.1097/OPX.0b013e3182504227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–5787. doi: 10.1167/iovs.16-20045. [DOI] [PubMed] [Google Scholar]
- 4.Tam J, Dhamdhere KP, Tiruveedhula P, et al. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom Vis Sci. 2012;89:E692–E703. doi: 10.1097/OPX.0b013e3182548b07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders RJ, Brown GC, Rosenstein RB, Magargal L. Foveal avascular zone diameter and sickle cell disease. Arch Ophthalmol. 1991;109:812–815. doi: 10.1001/archopht.1991.01080060076029. [DOI] [PubMed] [Google Scholar]
- 6.Wilk MA, McAllister JT, Cooper RF, et al. Relationship between foveal cone specialization and pit morphology in albinism. Invest Ophthalmol Vis Sci. 2014;55:4186–4198. doi: 10.1167/iovs.13-13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falavarjani KG, Iafe NA, Velez FG, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37:2289–2294. doi: 10.1097/IAE.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 8.Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology. 1999;106:1409–1413. doi: 10.1016/S0161-6420(99)00732-0. [DOI] [PubMed] [Google Scholar]
- 9.Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123:2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 10.McClintic BR, McClintic JI, Bisognano JD, Block RC. The relationship between retinal microvascular abnormalities and coronary heart disease: a review. Am J Med. 2010;123:374e1–374e7. doi: 10.1016/j.amjmed.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53:1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilk MA, Dubis AM, Cooper RF, Summerfelt P, Dubra A, Carroll J. Assessing the spatial relationship between fixation and foveal specializations. Vision Res. 2017;132:53–61. doi: 10.1016/j.visres.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley A, Applegate RA, Zeffren BS, van Heuven WA. Psychophysical measurement of the size and shape of the human foveal avascular zone. Ophthalmic Physiol Opt. 1992;12:18–23. [PubMed] [Google Scholar]
- 14.Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102:1286–1293. doi: 10.1001/archopht.1984.01040031036019. [DOI] [PubMed] [Google Scholar]
- 15.Wu LZ, Huang ZS, Wu DZ, Chan E. Characteristics of the capillary-free zone in the normal human macula. Jpn J Ophthalmol. 1985;29:406–411. [PubMed] [Google Scholar]
- 16.Nelson DA, Burgansky-Eliash Z, Barash H, et al. High-resolution wide-field imaging of perfused capillaries without the use of contrast agent. Clin Ophthalmol. 2011;5:1095–1106. doi: 10.2147/OPTH.S20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DY, Fingler J, Zawadzki RJ, et al. Noninvasive imaging of the foveal avascular zone with high-speed, phase-variance optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:85–92. doi: 10.1167/iovs.11-8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 19.Samara WA, Say EA, Khoo CT, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–2195. doi: 10.1097/IAE.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 20.Tan CS, Lim LW, Chow VS, et al. Optical coherence tomography angiography evaluation of the parafoveal vasculature and its relationship with ocular factors. Invest Ophthalmol Vis Sci. 2016;57:224–234. doi: 10.1167/iovs.15-18869. [DOI] [PubMed] [Google Scholar]
- 21.Sampson DM, Gong P, An D, et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:3065–3072. doi: 10.1167/iovs.17-21551. [DOI] [PubMed] [Google Scholar]
- 22.Samara WA, Shahlaee A, Sridhar J, Khan MA, Ho AC, Hsu J. Quantitative optical coherence tomography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol. 2016;166:76–83. doi: 10.1016/j.ajo.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;161:50–55. doi: 10.1016/j.ajo.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 24.La Spina C, Carnevali A, Marchese A, Querques G, Bandello F. Reproducibility and reliability of optical coherence tomography angiography for foveal avascular zone evaluation and measurement in different settings. Retina. 2016;37:1636–1641. doi: 10.1097/IAE.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 25.Ghassemi F, Mirshahi R, Bazvand F, Fadakar K, Faghihi H, Sabour S. The quantitative measurements of foveal avascular zone using optical coherence tomography angiography in normal volunteers. J Curr Ophthalmol. 2017;29:293–299. doi: 10.1016/j.joco.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen FK, Menghini M, Hansen A, Mackey DA, Constable IJ, Sampson DM. Intrasession repeatability and interocular symmetry of foveal avascular zone and retinal vessel density in OCT angiography. Trans Vis Sci Technol. 2018;7:6. doi: 10.1167/tvst.7.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam J, Dhamdhere KP, Tiruveedhula P, et al. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:9257–9266. doi: 10.1167/iovs.11-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krawitz BD, Mo S, Geyman LS, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res. 2017;139:177–186. doi: 10.1016/j.visres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–1058. doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraus MF, Potsaid B, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express. 2012;3:1182–1199. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus MF, Liu JJ, Schottenhamml J, et al. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed Opt Express. 2014;5:2591–2613. doi: 10.1364/BOE.5.002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye. 2005;19:322–326. doi: 10.1038/sj.eye.6701456. [DOI] [PubMed] [Google Scholar]
- 34.Gadde SGK, Anegondi N, Bhanushali D, et al. Quantification of vessel density in retinal optical coherence tomography angiography images using local fractal dimension. Invest Ophthalmol Vis Sci. 2016;57:246–252. doi: 10.1167/iovs.15-18287. [DOI] [PubMed] [Google Scholar]
- 35.Mastropasqua R, Toto L, Mastropasqua A, et al. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol. 2017;10:1545–1551. doi: 10.18240/ijo.2017.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugahara M, Miyata M, Ishihara K, et al. Optical coherence tomography angiography to estimate retinal blood flow in eyes with retinitis pigmentosa. Sci Rep. 2017;7:46396. doi: 10.1038/srep46396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Carlo TE, Salz DA, Waheed NK, Baumal CR, Duker JS, Witkin AJ. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46:611–616. doi: 10.3928/23258160-20150610-03. [DOI] [PubMed] [Google Scholar]
- 38.Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134:367–373. doi: 10.1001/jamaophthalmol.2015.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J, Kwon J, Shin JW, Lee J, Lee S, Kook MS. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One. 2017;12:e0184948. doi: 10.1371/journal.pone.0184948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rommel F, Siegfried F, Kurz M, et al. Impact of correct anatomical slab segmentation on foveal avascular zone measurements by optical coherence tomography angiography in healthy adults. J Curr Ophthalmol. 2018;30:156–160. doi: 10.1016/j.joco.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiihara H, Terasaki H, Sonoda S, et al. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci Rep. 2018;8:10143. doi: 10.1038/s41598-018-28530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz B, Nelis P, Rolfes F, et al. Effects of high-intensity interval training on optic nerve head and macular perfusion using optical coherence tomography angiography in healthy adults. Atherosclerosis. 2018;274:8–15. doi: 10.1016/j.atherosclerosis.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Simonett JM, Wang J, et al. Evaluation of automatically quantified foveal avascular zone metrics for diagnosis of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59:2212–2221. doi: 10.1167/iovs.17-23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrelli E, Lonngi M, Balasubramanian S, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2018] doi: 10.1097/IAE.0000000000002123. [published online ahead of print February 22, [DOI] [PubMed]
- 45.Kim K, Kim ES, Yu SY. Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. Br J Ophthalmol. 2017;102:1226–1231. doi: 10.1136/bjophthalmol-2017-311149. [DOI] [PubMed] [Google Scholar]
- 46.Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:5548–5555. doi: 10.1167/iovs.17-22036. [DOI] [PubMed] [Google Scholar]
- 47.Uji A, Balasubramanian S, Lei J, et al. Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography. Ophthalmology. 2017;124:944–952. doi: 10.1016/j.ophtha.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Pilotto E, Frizziero L, Crepaldi A, et al. Repeatability and reproducibility of foveal avascular zone area measurement on normal eyes by different optical coherence tomography angiography instruments. Ophthalmic Res. 2018;59:206–211. doi: 10.1159/000485463. [DOI] [PubMed] [Google Scholar]
- 49.Shiihara H, Sakamoto T, Yamashita T, et al. Reproducibility and differences in area of foveal avascular zone measured by three different optical coherence tomographic angiography instruments. Sci Rep. 2017;7:9853. doi: 10.1038/s41598-017-09255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munk MR, Giannakaki-Zimmermann H, Berger L, et al. OCT-angiography: a qualitative and quantitative comparison of 4 OCT-A devices. PLoS One. 2017;12:e0177059. doi: 10.1371/journal.pone.0177059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang D, Tang FY, Huang H, Cheung CY, Chen H. Repeatability, interocular correlation and agreement of quantitative swept-source optical coherence tomography angiography macular metrics in healthy subjects. Br J Ophthalmol. 2018] doi: 10.1136/bjophthalmol-2018-311874. [published online ahead of print June 2, [DOI] [PubMed]
- 52.Magrath GN, Say EA, Sioufi K, Ferenczy S, Samara WA, Shields CL. Variability in foveal avascular zone and capillary density using optical coherence tomography machines in healthy eyes. Retina. 2016;37:2102–2111. doi: 10.1097/IAE.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 53.Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52:625–634. doi: 10.1167/iovs.10-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujiwara A, Morizane Y, Hosokawa M, et al. Factors affecting foveal avascular zone in healthy eyes: an examination using swept-source optical coherence tomography angiography. PLoS One. 2017;12:e0188572. doi: 10.1371/journal.pone.0188572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, Chan S, Yang JY, et al. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:950109. doi: 10.1016/j.ajo.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56:3212–3217. doi: 10.1167/iovs.14-16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong D, Zou X, Zhang X, Yu W, Qu Y, Dong F. The influence of age and central foveal thickness on foveal zone size in healthy people. Ophthalmic Surg, Lasers Imaging Retina. 2016;47:142–148. doi: 10.3928/23258160-20160126-07. [DOI] [PubMed] [Google Scholar]
- 58.Cooper RF, Wilk MA, Tarima S, Carroll J. Evaluating descriptive metrics of the human cone mosaic. Invest Ophthalmol Vis Sci. 2016;57:2992–3001. doi: 10.1167/iovs.16-19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jee D, Hong SW, Jung YH, Ahn MD. Interocular retinal nerve fiber layer thickness symmetry value in normal young adults. J Glaucoma. 2014;23:125–131. doi: 10.1097/IJG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 60.Qiu M, Boland MV, Ramulu PY. Cup-to-disc ratio asymmetry in U.S. adults: prevalence and association with glaucoma in the 2005-2008 national health and nutrition examination survey. Ophthalmology. 2017;124:1129–1136. doi: 10.1016/j.ophtha.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Bao FJ. Interocular symmetry analysis of bilateral eyes. J Med Eng Technol. 2014;38:179–187. doi: 10.3109/03091902.2014.899401. [DOI] [PubMed] [Google Scholar]