Abstract
Stenotrophomonas maltophilia and Burkholderia cepacia complex (Bcc) have been increasingly recognized as relevant pathogens in hospitalized, immunocompromised and cystic fibrosis (CF) patients. As a result of complex mechanisms, including biofilm formation and multidrug resistance phenotype, S. maltophilia and Bcc respiratory infections are often refractory to therapy, and have been associated with a worse outcome in CF patients. Here we demonstrate for the first time that N-acetylcysteine (NAC), a mucolytic agent with antioxidant and anti-inflammatory properties, may exhibit antimicrobial and antibiofilm activity against these pathogens.
The antimicrobial and antibiofilm activity of high NAC concentrations, potentially achievable by topical administration, was tested against a collection of S. maltophilia (n = 19) and Bcc (n = 19) strains, including strains from CF patients with acquired resistance traits. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) ranged from 16 to 32 mg/ml and from 32 to >32 mg/ml, respectively. Sub-MIC concentrations (i.e., 0.25 × MIC) slowed down the growth kinetics of most strains. In time-kill assays, 2-day-old biofilms were more affected than planktonic cultures, suggesting a specific antibiofilm activity of NAC against these pathogens. Indeed, a dose- and time-dependent antibiofilm activity of NAC against most of the S. maltophilia and Bcc strains tested was observed, with a sizable antibiofilm activity observed also at 0.5 and 1 × MIC NAC concentrations. Furthermore, at those concentrations, NAC was also shown to significantly inhibit biofilm formation with the great majority of tested strains.
Introduction
N-acetylcysteine (NAC) has long been used in clinical practice for its mucolytic, antioxidant and anti-inflammatory properties [1]. In addition, in vitro studies have revealed that NAC may exhibit some intrinsic antimicrobial and antibiofilm activity against several clinically relevant pathogens (including Gram-positive and Gram-negative bacteria and yeasts), although knowledge on this topic remains limited and the underlying mechanisms are poorly understood ([2] and references therein, [3–7]). The concentrations at which the antimicrobial and antibiofilm activities of NAC have been observed were variable but usually higher than those achievable by systemic routes of administration (i.e., oral, intramuscular or intravenous), which can result in peak plasma concentrations of 0.2–1.2 mg/ml [8]. However, NAC can also be administered topically, either by nebulization or direct instillation [2,9], and reach at the site of infection the higher concentrations needed for the antimicrobial and antibiofilm activity. Despite initial concerns about the potential negative interaction of NAC on antibiotic activity [10], two recent articles have demonstrated that NAC does not negatively affect the activity of the major antibiotic classes, with the exception of carbapenems [11,12].
By virtue of its multiple beneficial effects and high tolerability, a renewed interest in the potential therapeutic efficacy of topical NAC has recently emerged, especially for the management of cystic fibrosis (CF) and other chronic respiratory diseases (e.g. chronic obstructive pulmonary disease, and non-CF bronchiectasis) [2].
With regard to the difficult-to-treat pathogens associated with these diseases, NAC was previously shown to exert some antimicrobial and antibiofilm activity against Pseudomonas aeruginosa [2,13], but its activity against Stenotrophomonas maltophilia and Burkholderia cepacia complex (Bcc) remains unexplored.
S. maltophilia and Bcc are ubiquitous environmental microorganisms that act as relevant opportunistic pathogens in immunocompromised and hospitalized patients (especially patients in high-risk wards, such as Intensive Care Units), and those affected by CF [14–21]. Respiratory infections by S. maltophilia and Bcc are often recalcitrant to antibiotic therapy, as a consequence of complex and still largely unexplored mechanisms, which involve also a wide range of intrinsic and acquired antimicrobial resistance mechanisms, and the propensity to grow as biofilms [22,23].
Despite the pathogenic role of S. maltophilia in CF individuals has long been a matter of debate, chronic lung colonization by this pathogen has been recently associated with an increased risk of pulmonary exacerbation, lung transplantation and death [14,19,23].
Bcc is a versatile group of 21 species, of which Burkholderia cenocepacia and Burkholderia multivorans show a higher prevalence in CF infections compared to other Bcc species [24]. B. cenocepacia has a well-recognised impact on post-transplant morbidity and mortality, representing a contraindication to lung transplantation [25].
In order to find new drugs and combinations to improve the outcome of chronic lung colonization by S. maltophilia and Bcc in CF patients, a renewed interest has been recently focused on inhaled route of administration, which allow to achieve higher drug concentrations in the lungs, whilst limiting systemic toxicity [16,17].
Here we demonstrate, for the first time, that NAC may exhibit antimicrobial activity against S. maltophilia and Bcc grown either in planktonic phase or in biofilms, at concentrations achievable by topical administration.
Materials and methods
Bacterial strains tested, identification and susceptibility testing
A total of 38 strains were investigated (S. maltophilia, n = 19; Bcc, n = 19), including CF isolates (Table 1). Identification was performed by MALDI-TOF MS, and Bcc strains were also identified by PCR/sequencing of the recA gene [26]. In addition, species identification and Multi Locus Sequence Typing (MLST) of the six Bcc strains selected for time-kill assays and biofilm experiments were further determined following whole genome sequencing (WGS). Bacterial DNA was extracted using the phenol:chloroform method [27], and it was subjected to WGS with a MiSeq platform (Illumina, Inc., San Diego, CA), using a 2 × 300 bp paired-end approach, and reads were assembled using SPAdes [28]. Draft genome assemblies were used for downstream analyses at the Oxford PubMLST site (https://pubmlst.org/) and at the Center for Genomic Epidemiology site (https://cge.cbs.dtu.dk/services/KmerFinder/). The same approach was also used for three of the six S. maltophilia strains selected for time-kill assays and biofilm experiments. New ST-types were identified for S. maltophilia (i.e., Z120, ST334; Z155, ST335) and Bcc (i.e., Z136, ST1396; Z138, ST1398). Antimicrobial susceptibility was determined using the reference broth microdilution method [29].
Table 1. Features of the 38 S. maltophilia and B. cepacia complex strains investigated.
| Straina | Species | Originb | Antibioticsc | N-acetylcysteine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MLST | MIC (μg/ml) | MIC (mg/ml) |
MBC (mg/ml) |
|||||||
| CAZ | MEM | LVX | SXT | MIN | ||||||
| Z63 | S. maltophilia | - | BSI | 2 | - | ≤0.25 | 0.5 | 0.125 | 16 | 32 |
| Z64 | S. maltophilia | - | BSI | 64 | - | 4 | 2 | 2 | 16 | >32 |
| Z65 | S. maltophilia | - | IAI | 64 | - | 2 | 1 | 1 | 32 | >32 |
| Z66 | S. maltophilia | - | LRTI | ≤1 | - | 1 | 0,5 | 0.25 | 16 | 32 |
| Z116 | S. maltophilia | - | LRTI | 16 | - | 2 | 0.5 | 0.25 | 32 | >32 |
| Z117 | S. maltophilia | - | LRTI | 64 | - | 0.5 | 0.5 | 0.25 | 16 | >32 |
| Z118 | S. maltophilia | ST162 | LRTI | 8 | - | 2 | 0.5 | 0.25 | 16 | >32 |
| Z119 | S. maltophilia | - | LRTI | 32 | - | 2 | 0.5 | 0.50 | 32 | >32 |
| Z120 | S. maltophilia | ST334 | LRTI | 32 | - | 1 | 0.5 | 0.5 | 16 | 32 |
| Z128 | S. maltophilia | - | LRTI | 4 | - | 1 | ≤0.25 | 0.25 | 16 | >32 |
| Z129 | S. maltophilia | - | LRTI | 4 | - | 1 | ≤0.25 | 0.25 | 16 | >32 |
| Z130 | S. maltophilia | - | IAI | 16 | - | 16 | 0.5 | 2 | 16 | >32 |
| Z131 | S. maltophilia | - | BSI | 64 | - | 32 | >8 | 1 | 16 | >32 |
| Z132 | S. maltophilia | - | LRTI | 2 | - | 16 | 1 | 1 | 32 | >32 |
| Z133 | S. maltophilia | - | LRTI | 2 | - | 1 | 1 | 0.25 | 32 | >32 |
| Z155 | S. maltophilia | ST335 | CF | 32 | - | 4 | >8 | 2 | 16 | >32 |
| Z156 | S. maltophilia | - | CF | 16 | - | 2 | 1 | 0.25 | 32 | 32 |
| Z157 | S. maltophilia | - | CF | 4 | - | 2 | 0.5 | 1 | 32 | >32 |
| Z158 | S. maltophilia | - | CF | 16 | - | 0.5 | ≤0.25 | 0.25 | 16 | >32 |
| Z136 | B. multivorans | ST1396 | CF | >64 | 8 | 64 | 4 | 8 | 32 | >32 |
| Z161 | B. multivorans | - | CF | >128 | 8 | 256 | 4 | 16 | 16 | >32 |
| LMG 16656 | B. cenocepacia | ST28 | CF | 128 | 32 | 8 | >8 | 16 | 16 | >32 |
| Z135 | B. cenocepacia | - | CF | 64 | 8 | >256 | 4 | 64 | 32 | >32 |
| Z139 | B. cenocepacia | - | CF | 8 | 4 | 4 | 1 | 16 | 32 | >32 |
| Z140 | B. cenocepacia | - | CF | 16 | 16 | >256 | > 8 | 8 | 16 | >32 |
| Z142 | B. cenocepacia | ST32 | CF | 2 | 4 | 32 | 8 | 8 | 16 | >32 |
| Z144 | B. cenocepacia | - | CF | 4 | 8 | 32 | 8 | 8 | 16 | >32 |
| Z146 | B. cenocepacia | - | LRTI | 16 | 16 | 128 | 8 | 4 | 32 | >32 |
| Z151 | B. cenocepacia | - | LRTI | 4 | 4 | 2 | 0.5 | 2 | 32 | >32 |
| Z160 | B. cenocepacia | - | CF | 16 | 16 | 32 | 8 | 4 | 16 | >32 |
| Z163 | B. cenocepacia | - | CF | >128 | 16 | 128 | 1 | 8 | 16 | >32 |
| Z141 | B. cepacia | - | CF | 8 | 8 | 128 | 4 | 8 | 32 | >32 |
| Z145 | B. stabilis | ST51 | CF | 128 | 8 | 32 | 8 | 4 | 16 | >32 |
| Z148 | B. stabilis | ST51 | LRTI | 4 | 1 | 2 | ≤0.25 | 1 | 16 | 32 |
| Z162 | B. stabilis | - | CF | 32 | 4 | 8 | 1 | 1 | 16 | 32 |
| Z137 | B. metallica | - | CF | 4 | 4 | 16 | 2 | 1 | 32 | >32 |
| Z138 | B. seminalis | ST1398 | CF | 2 | 2 | 64 | 2 | 2 | 16 | >32 |
| Z147 | B. contaminans | - | LRTI | 4 | 4 | 1 | 0.5 | 1 | 32 | >32 |
aThe 12 strains selected for planktonic time-kill assays and biofilm experiments are underlined.
bBSI, bloodstream infection; IAI, intra-abdominal infection; LRTI, lower respiratory tract infection; CF, cystic fibrosis.
cCAZ, ceftazidime; MEM, meropenem; LVX, levofloxacin; SXT, trimethoprim-sulfamethoxazole; MIN, minocycline.
Preparation of NAC-containing medium
N-acetylcysteine stock solutions (100 g/L) were prepared immediately before use, by dissolving N-acetylcysteine powder (Zambon, Bresso, Italy) in sterile double-distilled water, pH adjustment at 6.5–6.8 with NaOH, and filtering through a 0.22-μm membrane filter. All experiments were performed in CAMHB (Becton Dickinson, Milan, Italy), starting from an appropriately concentrated medium in order to avoid broth dilution when testing high N-acetylcysteine concentrations.
In vitro antimicrobial activity of NAC against planktonic cultures
Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) of NAC were determined by broth microdilution (range of NAC concentration tested, 0.25–32 mg/ml) [29]. The effect of sub-MIC NAC concentrations (i.e., 0.25 × MIC) on the growth kinetics was determined in duplicate by recording the optical density at 600 nm over 20 h, using CAMHB inoculated with ~1.5 × 106 CFU/ml.
Twelve strains (S. maltophilia, n = 6; Bcc, n = 6) (Table 1) were selected for time-kill assays with planktonic and biofilm cultures. The selected strains were representative of diverse origin (i.e., CF and non-CF LRTI), resistance phenotype (e.g., susceptibility to trimethoprim-sulfamethoxazole), and Bcc species. B. contaminans and B. metallica were not included at this stage, in order to test two B. cenocepacia belonging to diverse ST-types, and two B. stabilis strains belonging to the same ST-type, but showing diverse origin and resistance phenotype.
Planktonic time-kill assays were performed according to Clinical and Laboratory Standards Institute guidelines, in CAMHB [30]. Briefly, exponential phase bacterial cultures (OD600 ∼0.15) were diluted to ~5 × 105 CFU/ml (final volume 10 ml) and exposed to 16 and 32 mg/ml NAC (i.e. 1 × MIC and 2 × MIC concentrations for the selected strains) over 24 h. Viable cell counts were determined by plating method after 3, 6 and 24 h of incubation (detection limit 25 CFU/ml). Data were obtained from at least two independent experiments, with two replicates per condition per experiment.
In vitro antibiofilm activity of NAC
Biofilm susceptibility testing was performed using the Nunc-TSP lid system (Thermo Fisher Scientific, Waltham, MA, USA), as described previously [31]. Briefly, 2-day-old biofilms were challenged with daily refreshed NAC-containing medium (i.e., 8, 16, and 32 mg/ml) at 35°C under static conditions, and the effect of NAC was evaluated after 1 and 3 days of exposure. After the exposure time, biofilms were washed twice with 200 μl of phosphate-buffered saline (PBS) (Sigma Aldrich, Milan, Italy) to remove loosely adherent bacteria, and sessile cells were removed from pegs by sonication for 30 min (Elma Transsonic T 460, Singen, Germany) in 200 μl of tryptic soy broth (TSB) (Oxoid, Milan, Italy) supplemented with 0.1% Tween 20 (Sigma Aldrich) (i.e., the recovery medium). Mean viable cell count per peg (log CFU/peg) was then determined by plating 10 μl of appropriate dilutions of the recovery medium onto tryptic soy agar (TSA) (Oxoid) and incubating for 48 h at 35°C (detection limit, 1.3 log CFU/peg). Data were obtained from at least two independent experiments, with at least four replicates per condition per experiment.
The capability of NAC to affect biofilm formation was evaluated with biofilms grown for 72 hours in CAMHB in the presence of 0, 4, 8 and 16 mg/ml NAC concentrations in daily refreshed medium (at 35°C, static condition). After the incubation times, viable cells were counted as in eradication experiments (see above).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.0 (San Diego, CA, USA). D’Agostino-Pearson, Shapiro-Wilk and Kolmogorov-Smirnov normality tests were used to test for Gaussian distribution. Concerning biofilm experiments, for each time point multiple comparison tests were applied to assess differences of biofilms exposed to diverse NAC concentrations compared to controls. One-Way ANOVA with Dunnett’s correction and Kruskal-Wallis test with Dunn’s correction were performed in case of Gaussian or not Gaussian distribution, respectively. Unpaired t-test with Welch’s correction was used for growth curves analysis.
Results and discussion
In vitro activity of NAC against S. maltophilia and Bcc grown in planktonic phase
MICs of NAC for the tested S. maltophilia and Bcc strains were 16 or 32 mg/ml, whereas MBCs were 32 mg/ml for four strains (S. maltophilia, n = 2; Bcc, n = 2), and >32 mg/ml for the remaining ones (Table 1).
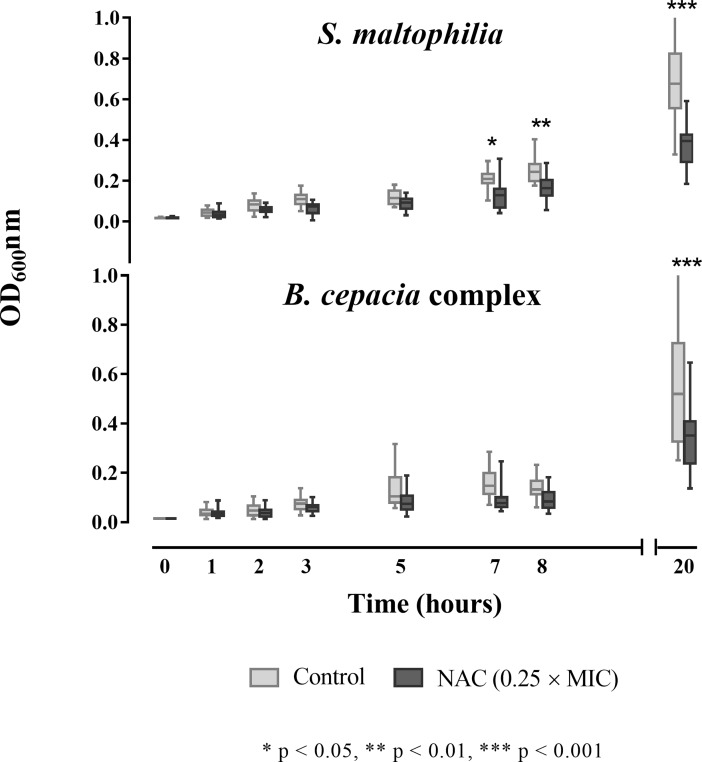
Sub-MIC NAC concentrations (i.e., 0.25 × MICs) were able to slow down the growth kinetics of most of the strains tested, with S. maltophilia being the most affected species (especially after 20 h of incubation) (Fig 1).
Fig 1. Boxplot representation of the effect of sub-MIC NAC concentrations on the growth kinetics of 19 S. maltophilia and 19 Bcc clinical isolates.
Data from two independent experiments. Boxes indicate from the 25th to the 75th percentiles, and whiskers indicate the minimum and maximum values.
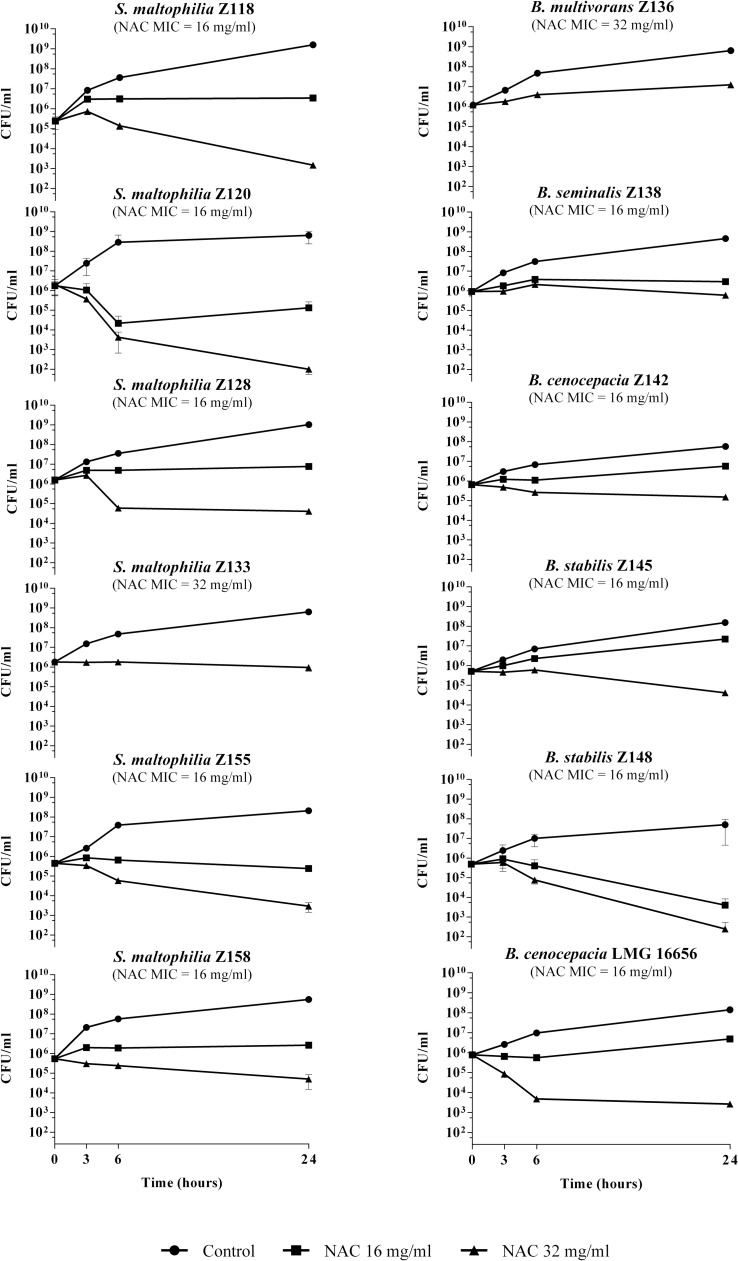
In time-kill assays performed with planktonic cultures, NAC at 1 × MIC did not exert bacterial killing against the strains tested, except for S. maltophilia Z120 (i.e., 1.3 log CFU/ml reduction after 24 h) and B. stabilis Z148 (i.e., 2.1 log CFU/ml reduction after 24 h) (Fig 2). At 2 × MIC concentrations, NAC was bactericidal (i.e., reduction of ≥3 log of the initial bacterial inoculum) for these two strains (i.e., 4.2 and 3.2 log CFU/ml reduction for S. maltophilia Z120 and B. stabilis Z148, respectively), and reduced of at least 1 log CFU/ml the viable cell counts for four additional S. maltophilia and two Bcc strains (range, 1.1–2.5 log CFU/ml) (Fig 2).
Fig 2. Time-kill assays of NAC for S. maltophilia and Bcc planktonic cultures.
Data from at least two independent experiments, with two replicates per condition per experiment. Mean values with standard deviation are plotted. The x axis is set at the limit of detection (i.e., 25 CFU/ml).
In vitro activity of NAC against S. maltophilia and Bcc grown in biofilms
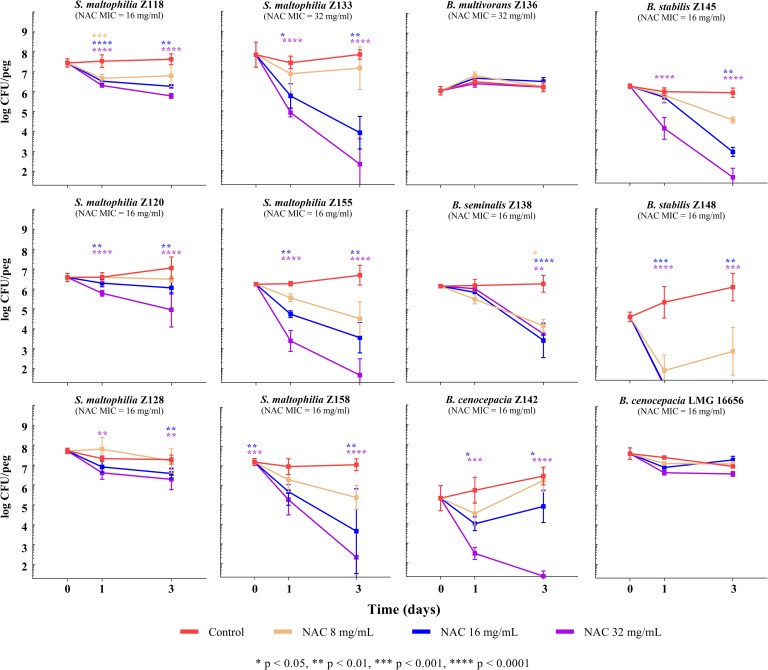
Time-kill assays performed with 2-day-old biofilms (average, 7.16 ± 0.63 and 5.98 ± 1.04 log CFU/peg for S. maltophilia and Bcc, respectively) revealed a dose- and time-dependent antibiofilm activity of NAC against most of the S. maltophilia and Bcc strains tested (except for B. multivorans Z136 and B. cenocepacia LMG 16656) (Fig 3). Interestingly, with six strains, including three S. maltophilia and three Bcc, a sizable antibiofilm activity was already observed at 0.5 × MIC and 1 × MIC NAC concentrations, which were found to determine a reduction of viable cells of ≥1 log CFU/peg and ≥2 log CFU/peg after a 3-days exposure, respectively (Fig 3). Considering the substantial lack of killing activity of 1 × MIC NAC concentrations against planktonic cultures of the same strains (Fig 2), these data would point towards a specific antibiofilm activity of NAC against these pathogens. Furthermore, at the highest concentration tested (i.e., 32 mg/ml), NAC exerted a bactericidal effect (i.e., reduction of ≥3 log CFU/peg) against half of the strains grown in biofilms (Fig 3).
Fig 3. Time-kill curves of NAC for 2-day-old biofilms of S. maltophilia and Bcc.
Data from at least two independent experiments, with at least four replicates per condition per experiment. Median values with standard deviation are plotted. The x axis is set at the limit of detection (i.e., 1.3 log CFU/peg).
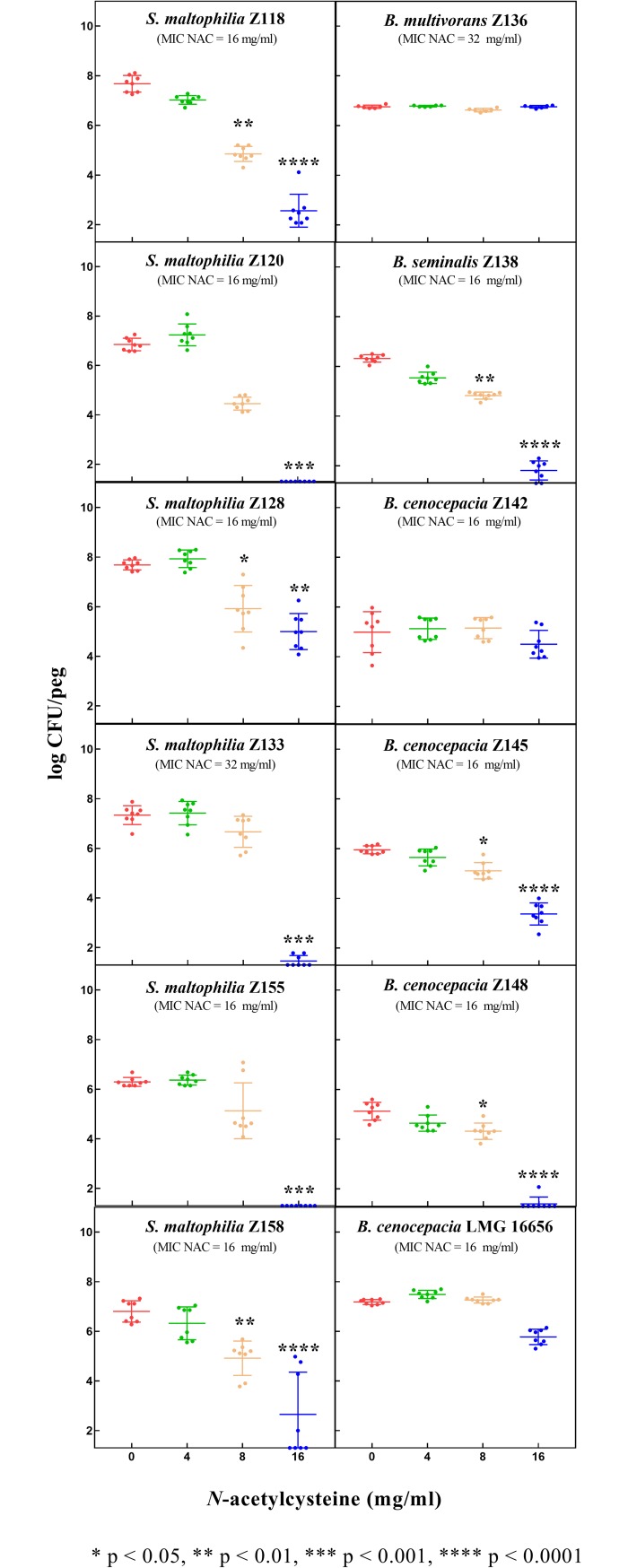
Finally, NAC at 0.5 × MIC or 1 × MIC concentrations was also shown to significantly affect biofilm formation of the great majority of tested strains (i.e., all except B. multivorans Z136 and B. cenocepacia Z142) (Fig 4). Results were overall consistent with those obtained in biofilm eradication experiments, except for B. cenocepacia Z142, for which NAC had a relevant antibiofilm effect on preformed biofilms, while no effect in inhibiting biofilm formation was observed (Figs 3 and 4).
Fig 4. Effect of NAC on S. maltophilia and Bcc biofilm formation (72-hours growth).
Data from at least two independent experiments, with at least four replicates per condition per experiment. Median values with standard deviation are plotted. The x axis is set at the limit of detection (i.e., 1.3 log CFU/peg).
The diverse response to NAC exposure observed in biofilm experiments among strains of the same species, exhibiting similar NAC MIC, suggests a strain-dependent antibiofilm activity of NAC against these pathogens. The reasons accounting for this phenomenon are difficult to hypothesize, since mechanisms underlying the antibiofilm activity of NAC remain still largely unknown. In addition, the different results obtained with B. cenocepacia Z142 in biofilm prevention and eradication experiments further suggest a complex and multifactorial antibiofilm activity of NAC.
Conclusions
The results of this study demonstrated for the first time that high NAC concentrations, achievable by topical administration (inhalation or direct instillation), may exert a relevant antimicrobial and antibiofilm activity against S. maltophilia and Bcc, including CF isolates with acquired resistance traits. These difficult-to-treat pathogens have been increasingly recognized as relevant pathogens in hospitalized, immunocompromised and CF patients, being associated with a worse outcome in CF patients [14–21]. Interestingly, the antibiofilm activity appeared to be only partially related to the antimicrobial activity, suggesting that NAC might act by inducing biofilm disgregation or be more active against biofilm-associated cells than planktonic cells. Further studies are needed to understand the mechanisms of such a phenomenon, considering that the antibiofilm properties of NAC have been hypothesized to be multifactorial (e.g. perturbation of cell physiology, direct interaction with crucial components of the matrix) [2], and have not been fully elucidated so far.
Although the low number of strains tested did not allow to speculate on potential associations between Bcc species and NAC susceptibility, the differences observed with the two B. cenocepacia strains (B. cenocepacia Z142 and LMG 16656) would rather suggest a strain-specific susceptibility. Based on the present findings, further studies aimed at expanding the number of strains and Bcc species tested and addressing the potential antibiofilm synergism of NAC plus conventional antibiotics are strongly encouraged.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by a research grant from Zambon S.p.A. FS is employed at Corporate Respiratory Medical Affairs, Zambon S.p.A. Zambon S.p.A. provided support in the form of salary for author FS, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the "author contributions" section.
References
- 1.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013; 1830:4117–4129. 10.1016/j.bbagen.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 2.Blasi F, Pave C, Rossolini GM, Pallecchi L, Matera MG, Rogliani P, et al. The effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infections. Respir Med. 2016; 117:190–197. 10.1016/j.rmed.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 3.Ferris RA, McCue PM, Borlee GI, Loncar KD, Hennet ML, Borlee BR. In vitro efficacy of nonantibiotic treatments on biofilm disruption of Gram-negative pathogens and an in vivo model of infectious endometritis utilizing isolates from the equine uterus. J Clin Microbiol. 2016; 54:631–639. 10.1128/JCM.02861-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eroshenko D, Polyudova T, Korobov V. N-acetylcysteine inhibits growth, adhesion and biofilm formation of Gram-positive skin pathogens. Microb Pathog. 2017; 105:145–152. 10.1016/j.micpath.2017.02.030 [DOI] [PubMed] [Google Scholar]
- 5.Domenech M, García E. N-Acetyl-l-Cysteine and cysteamine as new strategies against mixed biofilms of nonencapsulated Streptococcus pneumoniae and nontypeable Haemophilus influenzae. Antimicrob Agents Chemother. 2017; 61: 10.1128/AAC.01992-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volgers C, Benedikter BJ, Grauls GE, Hellebrand PHM, Savelkoul PHM, Stassen FRM. Effects of N-acetyl-L-cysteine on the membrane vesicle release and growth of respiratory pathogens. FEMS Microbiol Lett. 2017; 364: 10.1093/femsle/fnx087 [DOI] [PubMed] [Google Scholar]
- 7.Amaral EP, Conceição EL, Costa DL, Rocha MS, Marinho JM, Cordeiro-Santos M, et al. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016; 16:251 10.1186/s12866-016-0872-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiew AL, Isbister GK, Duffull SB, Buckley NA. Evidence for the changing regimens of acetylcysteine. Br J Clin Pharmacol. 2016; 81:471–481. 10.1111/bcp.12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mata AF, Sarnaik AA. Bronchoscopy with N-acetylcysteine lavage in severe respiratory failure from pertussis infection. Pediatrics. 2013; 132: e1418–1423. 10.1542/peds.2013-0912 [DOI] [PubMed] [Google Scholar]
- 10.Goswami M, Jawali N. N-acetylcysteine-mediated modulation of bacterial antibiotic susceptibility. Antimicrob Agents Chemother. 2010; 54:3529–3350. 10.1128/AAC.00710-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Beltrán J, Cabot G, Valencia EY, Costas C, Bou G, Oliver A, et al. N-acetylcysteine selectively antagonizes the activity of imipenem in Pseudomonas aeruginosa by an OprD-mediated mechanism. Antimicrob Agents Chemother. 2015; 59:3246–3251. 10.1128/AAC.00017-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landini G, Di Maggio T, Sergio F, Docquier J-D, Rossolini GM, Pallecchi L. Effect of high N-acetylcysteine concentrations on antibiotic activity against a large collection of respiratory pathogens. Antimicrob Agents Chemother. 2016; 60:7513–7517. 10.1128/AAC.01334-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010; 10:140 10.1186/1471-2180-10-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros. 2013; 12:482–486. 10.1016/j.jcf.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Abbott IJ, Peleg AY. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin Respir Crit Care Med. 2015; 36:99–110. 10.1055/s-0034-1396929 [DOI] [PubMed] [Google Scholar]
- 16.Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 2015; 6:893 10.3389/fmicb.2015.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam V, Shafiq N, Singh M, Ray P, Singhal L, Jaiswal NP, et al. Clinical and in vitro evidence for the antimicrobial therapy in Burkholderia cepacia complex infections. Expert Rev Anti Infect Ther. 2015; 13:629–663. 10.1586/14787210.2015.1025056 [DOI] [PubMed] [Google Scholar]
- 18.Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros. 2015; 14:293–304. 10.1016/j.jcf.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Barsky EE, Williams KA, Priebe GP, Sawicki GS. Incident Stenotrophomonas maltophilia infection and lung function decline in cystic fibrosis. Pediatr Pulmonol. 2017; 52:1276–1282. 10.1002/ppul.23781 [DOI] [PubMed] [Google Scholar]
- 20.Scoffone VC, Chiarelli LR, Trespidi G, Mentasti M, Riccardi G, Buroni S. Burkholderia cenocepacia infections in cystic fibrosis patients: drug resistance and therapeutic approaches. Front Microbiol. 2017; 8:1592 10.3389/fmicb.2017.01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Welsh SK, Budev M, Goldberg H, Noone PG, Zaas D, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia dolosa (Genomovar VI). Clin Transplant. 2018. March 12 10.1111/ctr.13236 [DOI] [PubMed] [Google Scholar]
- 22.Lewis ER, Torres AG. The art of persistence-the secrets to Burkholderia chronic infections. Pathog Dis. 2016; 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito A, Pompilio A, Bettua C, Crocetta V, Giacobazzi E, Fiscarelli E, et al. Evolution of Stenotrophomonas maltophilia in cystic fibrosis lung over chronic infection: a genomic and phenotypic population study. Front Microbiol. 2017; 8:1590 10.3389/fmicb.2017.01590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach E, Sant'Anna FH, Magrich Dos Passos JF, Balsanelli E, de Baura VA, Pedrosa FO, et al. Detection of misidentifications of species from the Burkholderia cepacia complex and description of a new member, the soil bacterium Burkholderia catarinensis sp. nov. Pathog Dis. 2017; 75. [DOI] [PubMed] [Google Scholar]
- 25.Snell G, Reed A, Stern M, Hadjiliadis D. The evolution of lung transplantation for cystic fibrosis: a 2017 update. J Cyst Fibros. 2017; 16:553–564. 10.1016/j.jcf.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 26.Payne GW, Vandamme P, Morgan SH, Lipuma JJ, Coenye T, Weightman AJ, et al. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl Environ Microbiol. 2005; 71:3917–3927. 10.1128/AEM.71.7.3917-3927.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, MacCallum P, Russell DW. Molecular Cloning: A Laboratory Manual, 3rd edn. New York: Cold Spring Harbor Laboratory Press, 2000. [Google Scholar]
- 28.Bankevich A, Nurk S, Antipov D et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 10th ed. M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA: 2015. [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. Methods for determining bactericidal activity of antimicrobial agents; approved guidelines (M26-A) Clinical and Laboratory Standards Institute, Wayne, PA: 1999. [Google Scholar]
- 31.Harrison JJ, Stremick CA, Turner RJ, Allan ND, Olson ME, Ceri H. Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat Protoc. 2010; 5:1236–1254. 10.1038/nprot.2010.71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.