Abstract
Background
The relationship between chronic obstructive pulmonary disease (COPD) and periprocedural complications of percutaneous coronary interventions (PCIs) is influenced by several factors. We aimed to investigate the association between COPD, its complication type and rate in patients undergoing PCI.
Methods
Data were prospectively collected using the Polish Cardiovascular Intervention Society national registry (ORPKI) on all PCIs performed in Poland between January 2015 and December 2016. COPD was present in 5,594 of the 221,187 patients undergoing PCI. We assessed the frequency and predictors of periprocedural complications in PCI.
Results
Patients with COPD were elder individuals (70.3 ± 9.9 vs. 67 ± 10.8 years; p < 0.05). We noted 145 (2.6%) periprocedural complications in the COPD group and 4,121 (1.9%) in the non-COPD group (p < 0.001). The higher incidence of periprocedural complications in the COPD patients was mainly attributed to cardiac arrest (p = 0.001), myocardial infarctions (p = 0.002) and no-reflows (p < 0.001). COPD was not an independent predictor of all periprocedural complications. On the other hand, COPD was found to be an independent predictor of increased no-reflow risk (odds ratio [OR] 1.447, 95% CI 1.085–1.929; p = 0.01), and at the same time, of decreased risk of periprocedural allergic reactions (OR 0.117, 95% CI 0.016–0.837; p = 0.03).
Conclusions
In conclusion, periprocedural complications of PCIs are more frequent in patients with COPD. COPD is an independent positive predictor of no-reflow and a negative predictor of periprocedural allergic reactions.
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) in the general population is estimated at 7.6% [1]. Diagnosis of COPD increases the risk of cardiovascular diseases and resulting mortality [2]. 2.4–10% of patients undergoing percutaneous coronary intervention (PCI) have been diagnosed with COPD [3,4]. More importantly, it has been observed that patients with COPD treated with PCI have more comorbidities and a greater extent of coronary artery disease. In addition, COPD is associated with an increased risk of repeated revascularization after PCI [5]. Data availability on the incidence of periprocedural complications in patients undergoing PCIs is limited. It has been demonstrated that the incidence of periprocedural complications in patients undergoing PCIs has decreased in previous years and is estimated at 1–3%. However, other studies have shown higher frequencies [6]. Several predictors of periprocedural complications, including advanced age, coronary plaque burden, chronic total occlusions (CTOs), coronary artery tortuosity, gender, PCI overall volume at one center and daily PCI volume have been demonstrated [7,8]. There are limited and conflicting data regarding periprocedural complications of PCI in patients with COPD. Some publications demonstrated that the overall complication rate was significantly lower in those with COPD. Whereas, patients with COPD had significantly higher incidences of death, major entry site complications and longer length of hospital stay [5].
The aim of this study was to assess the relationship between COPD, periprocedural complications and their predictors in patients treated with PCI.
Materials and methods
Study population, design and definitions
We analyzed prospectively collected national data from all patients who underwent PCIs in Poland between January 2015 and December 2016. Data on PCI practice in Poland were obtained from the ORPKI Polish National dataset which is coordinated nationwide by Jagiellonian University Medical College in cooperation with AISN PTK (Association of Cardiovascular Interventions–The Polish Cardiac Society). The method of collecting data in the ORPKI registry was presented in previously published works [9,10]. Consecutive patients with COPD were included. COPD was defined on the basis of previously established diagnosis. This fact was taken into account on the basis of existing medical records, including discharge cards and the typical treatment used for COPD. All indices recorded in the ORPKI database are based on periprocedural data uploaded by the operator after each procedure. Therefore, they do not include all in-hospital complications, mainly those which occurred after the procedure until discharge from the hospital. Also, we did not collect follow-up data after discharge. The diagnosis of all other periprocedural complications including death, cardiac arrest, puncture site bleeding, no-reflow, cerebral stroke, coronary artery perforation (CAP), coronary artery dissection, allergic reactions ultimately depended on the operators’ decisions. Periprocedural major adverse cardiac events (MACCE) were defined as the combination of all-cause deaths, myocardial infarction (MI) and cerebral stroke. The thrombolysis in myocardial infarction (TIMI) grade flow was used to estimate procedural angiographic effectiveness. PCI was considered effective when TIMI grade 3 was obtained after the procedure. The overall complication rate was presented as the number of patients with defined periprocedural complications, even if the particular patient presented more than one complication.
Statistical analysis
All continuous variables were evaluated with the Kolmogorov-Smirnov test for distribution. Continuous variables are presented as mean ± standard deviation and median ± interquartile range. Categorical variables are presented as numeric values and percentages. Continuous variables were compared using the two-tailed Student’s t-test and the Mann-Whitney U-test, whereas categorical variables via the χ2 test. ANOVA was used to compare data between following years. To identify predictors of all periprocedural complications and those more specific such as allergic reactions and no-reflow phenomenon in the overall group of patients undergoing PCI, univariate and multivariate analyses were performed. Both, univariate and multivariate regression models for MACCE were constructed. A model based on the retrograde correction method was created. In this analysis, the following variables were tested: age, gender, diabetes, previous cerebral stroke, MI, PCI, coronary artery by-pass grafting (CABG), smoking status, psoriasis, kidney disease, COPD, vascular access, fractional flow reserve, intravascular ultrasound, optical coherence tomography, thrombectomy, rotablation (RA), pharmacological treatment, baseline TIMI flow, contrast and radiation dose, gender, clinical presentation of coronary artery disease (CAD), type of coronary artery lesions including distribution, bifurcations and chronic total occlusion (CTO) procedures. A p value lower than 0.05 was considered to be significant. The statistical analyses were performed using Statistica 10.0 software (Dell Software, Inc, Round Rock, TX, USA).
Results
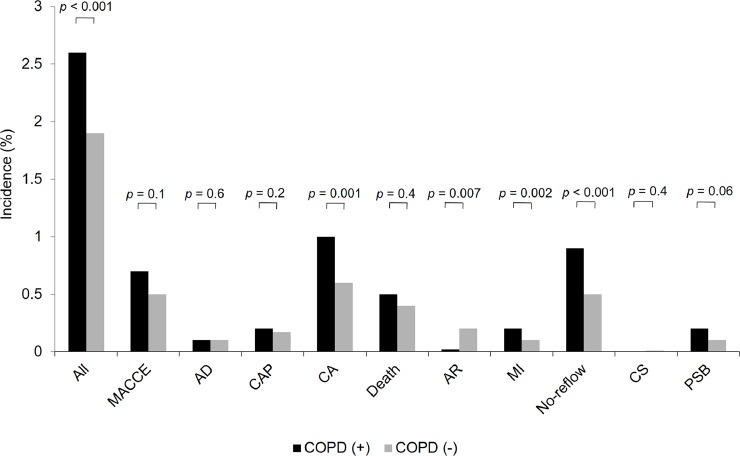
We analyzed 221,187 PCIs performed in Poland between January 2015 and December 2016. Among all PCI patients, 5,594 had COPD (2.5%). We found 145 overall periprocedural complications in 5,594 patients with COPD (2.6%) and 4,121 in 215,593 patients from the non-COPD group (1.9%) (p < 0.001) (Fig 1). It was mostly determined by the greater percentage of cardiac arrests (1% vs. 0.6%; p = 0.001), MIs (0.2% vs. 0.1%; p = 0.002), and no-reflows (0.9% vs. 0.5%; p < 0.001) in the COPD group as compared to the non-COPD group (Fig 1). Periprocedural allergic reactions were less frequent in the COPD group (0.02% vs. 0.2%; p = 0.007). Other periprocedural complications did not differ significantly between both groups (Fig 1).
Fig 1. The incidence of periprocedural complications in the COPD and non-COPD groups.
Data are presented as % unless otherwise indicated. Abbreviations: AD, arterial dissection; AR, allergic reactions; CA, cardiac arrest; CAP, coronary artery perforation; CS, cerebral stroke; MACCE, major adverse coronary and cerebrovascular events; MI, myocardial infarction; NS, not significant; PSB, puncture site bleeding.
Periprocedural complications and clinical presentation of CAD
ST-segment elevation MI (STEMI) occurred in 994 patients (17.7%), while non-ST-segment elevation MI (NSTEMI) took place in 1,384 patients (24.7%). More complications occurred in STEMI subgroup compared to NSTEMI, which was driven by a higher rate of cardiac arrests (p = 0.007), deaths (p = 0.02) and no-reflows (p < 0.001) (Table 1). The angiographic success was significantly lower in STEMI as compared to NSTEMI (p = 0.001). The usage rate of thrombectomy was higher in the STEMI group (p < 0.001) (Table 1). There were no significant differences in vascular access between those two groups. Differences in the location of culprit lesion in STEMI and NSTEMI patients is presented in Table 1. Stable angina (SA) occurred in 1,549 patients, while acute coronary syndrome (ACS) was noted in 3,980 individuals (72%). Among patients from the COPD group, complication rate was higher in patients with ACS compared to SA (1.2% vs. 3.1%; p < 0.001), with the most significant contribution of cardiac arrest (0.6% vs. 1.2%; p = 0.005), death (0% vs. 0.7%; p < 0.001) and no-reflow (0.5% vs. 1.1%; p = 0.04) (Table 1). This relationship was present despite the fact that PCI of CTOs (p = 0.01), bifurcations (p = 0.01) and RAs (p < 0.001) were more common in SA patients. The angiographic success expressed as TIMI grade 3 flow was higher in the SA group (p < 0.001). Femoral access (FA) was more common in STEMI patients (p = 0.02) (Table 1).
Table 1. Selected indices in COPD group according to clinical presentation of CAD (NSTEMI vs. STEMI) before PCI and SA vs. ACS.
|
COPD (+), n = 5,594 (100) |
P-value |
COPD (+), n = 5,529 (100) |
P-value |
|||
|---|---|---|---|---|---|---|
|
STEMI n = 994 (17.7) |
NSTEMI n = 1,384 (24.7) |
SA n = 1,549 (28) |
ACS n = 3,980 (72) |
|||
| Age, years |
70.3 ± 10.0 70 [63 ÷ 78] |
71.4 ± 9.6 71 [64 ÷ 79] |
0.006 |
69.5 ± 8.6 69 [63 ÷ 76] |
70.7 ± 9.5 70 [64 ÷ 78] |
<0.001 |
|
All complications |
62 (6.2) |
38 (2.7) |
<0.001 |
19 (1.2) |
122 (3.1) |
<0.001 |
|
Arterial dissection |
1 (0.1) |
3 (0.2) |
0.55 |
0 (0) |
5 (0.1) |
0.1 |
|
CAP |
3 (0.3) |
3 (0.2) |
0.68 |
4 (0.2) |
10 (0.2) |
0.9 |
|
Cardiac arrest |
26 (2.6) |
16 (1.1) |
0.007 |
6 (0.6) |
48 (1.2) |
0.005 |
|
Death |
17 (1.7) |
10 (0.7) |
0.02 |
0 (0) |
29 (0.7) |
<0.001 |
|
Allergic reactions |
0 (0) |
0 (0) |
- |
0 (0) |
1 (0.02) |
0.5 |
|
No-reflow |
28 (2.8) |
9 (0.6) |
<0.001 |
8 (0.5) |
43 (1.1) |
0.04 |
|
Cerebral stroke |
0 (0) |
0 (0) |
- |
0 (0) |
0 (0) |
- |
| Puncture site bleeding |
4 (0.4) |
3 (0.2) |
0.4 |
1 (0.06) |
9 (0.2) |
0.2 |
|
Chronic total occlusion |
38 (3.8) |
55 (4.0) |
0.85 |
91 (5.9) |
171 (4.3) |
0.01 |
|
Bifurcation |
51 (5.1) |
91 (6.6) |
0.14 |
122 (7.9) |
244 (6.1) |
0.01 |
|
Rotablation |
1 (0.1) |
4 (0.3) |
0.32 |
18 (1.2) |
11 (0.3) |
<0.001 |
|
Thrombectomy |
109 (10.9) |
27 (1.2) |
<0.001 |
- |
141 (3.5) |
- |
|
TIMI 3 after PCI |
841 (88.1) |
1,237 (92.2) |
0.001 |
1,438 (92.8) |
3,529 (91.8) |
0.2 |
|
Location of culprit lesion |
||||||
|
Left main coronary artery |
41 (4.1) |
95 (6.9) |
0.004 |
54 (3.5) |
206 (5.2) |
0.007 |
|
Left anterior descending |
372 (37.4) |
475 (34.3) |
0.11 |
512 (33.0) |
1,385 (34.8) |
0.2 |
|
Circumflex branch |
145 (14.6) |
379 (27.4) |
<0.001 |
391 (25.2) |
890 (22.4) |
0.02 |
|
Intermediate branch |
17 (1.7) |
20 (1.4) |
0.6 |
25 (1.6) |
52 (1.3) |
0.38 |
|
Right coronary artery |
377 (37.9) |
380 (27.4) |
<0.001 |
483 (31.2) |
1,256 (31.5) |
0.7 |
|
Saphenous vein graft |
9 (0.9) |
23 (1.7) |
0.11 |
14 (0.9) |
54 (1.3) |
0.2 |
|
Vascular access |
||||||
|
Right radial artery |
584 (58.7) |
820 (59.2) |
0.8 |
941 (60.7) |
2,343 (58.9) |
0.2 |
|
Left radial artery |
139 (14.0) |
220 (15.9) |
0.19 |
250 (16.1) |
611 (15.3) |
0.5 |
|
Femoral artery |
264 (26.5) |
344 (24.8) |
0.34 |
340 (21.9) |
987 (24.8) |
0.02 |
|
Others |
7 (0.7) |
18 (1.3) |
0.15 |
18 (1.2) |
39 (1.0) |
0.5 |
Data are presented as % unless otherwise indicated.
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; CAP, coronary artery perforation; NSTEMI, non-ST segment elevation myocardial infarction; SA, stable angina; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Periprocedural complications and distribution of coronary artery atherosclerosis
The COPD group consisted of 3,381 patients with single-vessel disease (SVD) (65.6%) and 1,771 individuals with multi-vessel disease (MVD) +/- left main coronary artery (LMCA) involvement and isolated LMCA disease (34.4%). The rate of MVD patients with LMCA involvement (p = 0.02), without LMCA involvement (p < 0.001) and isolated LMCA disease (p < 0.001) was significantly higher in the COPD group compared to the non-COPD group. Complication rate was almost two times higher in the MVD group compared to the SVD group (p < 0.001), which was mainly due to higher rate of MACCEs (p < 0.001), dissections (p = 0.01), cardiac arrests (p < 0.001), deaths (p < 0.001) and no-reflows (p = 0.04) (Table 2). In the MVD group, there were twice as many PCIs of bifurcations (p < 0.001), many more occurrences of LMCA PCIs (p < 0.001), significantly more PCIs of LAD (p < 0.001) and Cx (p < 0.001), while there were less PCIs of RCA (p < 0.001). We did not confirm any relationship between particular complications and location of the culprit lesion in specific coronary arteries. In the MVD group, there were significantly more patients with NSTEMI (p < 0.001) and STEMI (p = 0.02) presentation of CAD, while less with SA (p < 0.001). Differences in vascular access are presented in Table 2.
Table 2. Selected indices in the COPD group according to the distribution of coronary artery atherosclerosis and TIMI flow grade before PCI.
| COPD (+), n = 5,152 (100) |
P-value |
COPD (+), n = 5,392 (100) TIMI flow before PCI |
P-value |
|||
|---|---|---|---|---|---|---|
|
Non-SVD n = 1,771 (34.4) |
SVD n = 3,381 (65.6) |
0–1 n = 1,632 (30.3) |
2–3 n = 3,760 (69.7) |
|||
|
Age, years |
71.2 ± 9.5 71 [64 ÷ 77] |
69.8 ± 9.2 69 [63 ÷ 77] |
<0.001 |
70.2 ± 9.9 70 [63 ÷ 78] |
70.4 ± 8.9 70 [64 ÷ 77] |
0.35 |
|
All complications |
69 (3.9) |
68 (2.0) |
<0.001 |
76 (4.6) |
65 (1.7) |
<0.001 |
|
Arterial dissection |
5 (0.3) |
1 (0.03) |
0.01 |
3 (0.2) |
3 (0.1) |
0.37 |
|
CAP |
3 (0.2) |
9 (0.3) |
0.49 |
4 (0.2) |
10 (0.3) |
0.89 |
|
Cardiac arrest |
30 (1.7) |
21 (0.6) |
<0.001 |
34 (2.1) |
19 (0.5) |
<0.001 |
|
Death |
20 (1.1) |
9 (0.3) |
<0.001 |
22 (1.3) |
7 (0.2) |
<0.001 |
|
Allergic reactions |
0 (0) |
1 (0.03) |
0.46 |
0 (0) |
1 (0.03) |
0.51 |
|
Myocardial infarction |
6 (0.3) |
4 (0.1) |
0.08 |
6 (0.3) |
6 (0.2) |
0.13 |
|
No-reflow |
24 (1.3) |
26 (0.8) |
0.04 |
33 (2.0) |
19 (0.5) |
<0.001 |
|
Cerebral stroke |
0 (0) |
0 (0) |
- |
0 (0) |
0 (0) |
- |
|
Puncture site bleeding |
5 (0.3) |
5 (0.1) |
0.29 |
3 (0.2) |
5 (0.1) |
0.65 |
|
Chronic total occlusion |
86 (4.8) |
151 (4.4) |
0.52 |
7 (0.4) |
82 (2.2) |
<0.001 |
|
Bifurcation |
181 (10.2) |
158 (4.7) |
<0.001 |
126 (7.7) |
239 (6.3) |
0.06 |
|
Rotablation |
9 (0.5) |
18 (0.5) |
0.9 |
6 (0.4) |
20 (0.5) |
0.42 |
|
Thrombectomy |
56 (3.2) |
87 (2.6) |
0.22 |
114 (7.0) |
23 (0.6) |
<0.001 |
|
TIMI 3 after PCI |
1,599 (92.3) |
3,012 (92.4) |
0.89 |
1,327 (81.6) |
3,653 (97.4) |
<0.001 |
|
Location of culprit lesion |
||||||
|
LMCA |
209 (11.8) |
6 (0.2) |
<0.001 |
53 (3.2) |
204 (5.4) |
<0.001 |
|
Left anterior descent |
675 (38.1) |
1,097 (32.4) |
<0.001 |
504 (30.9) |
1,334 (35.5) |
0.001 |
|
Circumflex branch |
507 (28.6) |
658 (19.5) |
<0.001 |
357 (21.9) |
903 (24.0) |
0.08 |
|
Intermediate branch |
31 (1.7) |
39 (1.1) |
0.07 |
18 (1.1) |
55 (1.5) |
0.29 |
|
Right coronary artery |
479 (27.0) |
1,187 (35.1) |
<0.001 |
578 (35.4) |
1,129 (30.0) |
<0.001 |
|
Saphenous vein graft |
24 (1.3) |
42 (1.2) |
0.73 |
22 (1.3) |
46 (1.2) |
0.7 |
|
Vascular access |
||||||
|
Right radial artery |
1,023 (57.7) |
2,048 (60.6) |
0.05 |
957 (58.6) |
2,251 (59.8) |
0.39 |
|
Left radial artery |
266 (15.0) |
529 (15.6) |
0.55 |
249 (15.2) |
583 (15.5) |
0.81 |
|
Femoral artery |
461 (26.0) |
772 (22.8) |
0.01 |
411 (25.2) |
886 (23.6) |
0.2 |
|
Others |
21 (1.2) |
32 (0.9) |
0.4 |
15 (0.9) |
40 (1.1) |
0.62 |
|
Clinical presentation |
||||||
|
Stable angina |
370 (20.9) |
1,047 (31.0) |
<0.001 |
246 (15.1) |
1,243 (33.1) |
<0.001 |
|
Unstable angina |
522 (29.5) |
1,041 (30.8) |
0.32 |
326 (20.0) |
1,223 (32.6) |
<0.001 |
|
NSTEMI |
547 (30.9) |
742 (21.9) |
<0.001 |
433 (26.5) |
906 (24.1) |
0.059 |
|
STEMI |
310 (17.5) |
512 (15.1) |
0.02 |
604 (37.0) |
346 (9.2) |
<0.001 |
|
Others |
21 (1.2) |
37 (1.1) |
0.76 |
22 (1.3) |
36 (0.9) |
0.2 |
Data are presented as % unless otherwise indicated.
Abbreviations: CAP, coronary artery perforation; LMCA, left main coronary artery; MVD, multi-vessel disease; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SVD, single-vessel disease; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Gender and periprocedural complications
There were 4,045 males (72.3%) and 1,547 females (27.7%) in the COPD group (Table 3). All of the complications more frequently occurred in females compared to the males (2.3% vs. 3.3%, p = 0.04). This was mainly due to higher incidence of MI (0.1% vs. 0.4%, p = 0.01), which occurred despite the fact that more PCIs of bifurcations (p < 0.001) and CTOs (p < 0.001) were performed in males (Table 3). It seems, that the higher prevalence of complications in women can be mainly owed to the higher STEMI (p < 0.001) and lower SA (p < 0.001) incidences in individuals before PCI which resulted in the more frequent use of thrombectomy (p = 0.01) (Table 3).
Table 3. Selected indices in the COPD group according to gender before PCI.
|
Variable |
COPD (+), n = 5,592 (100) |
P-value |
|
|---|---|---|---|
|
Males n = 4,045 (72.3) |
Females n = 1,547 (27.7) |
||
|
Age, years |
69.8 ± 9.3 69 [63 ÷ 77] |
71.8 ± 9.1 72 [65 ÷ 79] |
<0.001 |
|
All complications |
94 (2.3) |
51 (3.3) |
0.04 |
|
Arterial dissection |
3 (0.09) |
3 (0.2) |
0.24 |
|
CAP |
11 (0.3) |
3 (0.2) |
0.6 |
|
Cardiac arrest |
35 (0.9) |
20 (1.3) |
0.14 |
|
Death |
19 (0.5) |
11 (0.7) |
0.26 |
|
Allergic reactions |
0 (0) |
1 (0.06) |
0.1 |
|
Myocardial infarction |
5 (0.1) |
7 (0.4) |
0.01 |
|
No-reflow |
36 (0.9) |
16 (1.0) |
0.61 |
|
Cerebral stroke |
0 (0) |
0 (0) |
- |
|
Puncture site bleeding |
6 (0.1) |
4 (0.2) |
0.38 |
|
Chronic total occlusion |
215 (5.3) |
52 (3.4) |
0.002 |
|
Bifurcation |
292 (7.2) |
77 (5.0) |
0.002 |
|
Rotablation |
22 (0.5) |
7 (0.4) |
0.67 |
|
Thrombectomy |
91 (2.2) |
52 (3.4) |
0.01 |
|
TIMI effectiveness (0-2/3) |
3,639 (94.0) |
1,384 (92.6) |
0.9 |
|
Location of culprit lesion |
|||
|
Left main coronary artery |
203 (5.0) |
60 (3.9) |
0.7 |
|
Left anterior descent |
1,389 (34.3) |
528 (34.1) |
0.88 |
|
Circumflex branch |
939 (23.2) |
355 (22.9) |
0.83 |
| Intermediate branch | 60 (1.5) | 18 (1.2) | 0.36 |
|
Right coronary artery |
1,234 (30.5) |
533 (34.4) |
0.004 |
|
Saphenous vein graft |
56 (1.4) |
12 (0.7) |
0.06 |
|
Vascular access |
|||
|
Right radial artery |
2,408 (59.5) |
913 (59.0) |
0.72 |
|
Left radial artery |
606 (15.0) |
263 (17.0) |
0.06 |
|
Femoral artery |
988 (24.4) |
356 (23.0) |
0.26 |
|
Other |
43 (1.1) |
15 (1.0) |
0.75 |
|
Clinical presentation |
|||
|
Stable angina |
1,176 (29.1) |
373 (24.1) |
<0.001 |
|
Unstable angina |
1,151 (28.5) |
450 (29.1) |
0.63 |
|
NSTEMI |
983 (24.3) |
400 (25.9) |
0.52 |
|
STEMI |
686 (17.0) |
308 (19.9) |
0.009 |
|
Others |
44 (1.1) |
14 (0.9) |
0.54 |
Data are presented as % unless otherwise indicated.
Abbreviations: CAP, coronary artery perforation; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Vascular access and periprocedural complications
FA was used in 1,344 individuals (24%), while radial access was implemented in 4,250 patients (76%). A trend towards a higher risk of periprocedural complications in patients with FA was observed (3.2% vs. 2.4%; p = 0.1). Similarly, when considering each complication separately, there were no significant differences. However, we have noticed discrepancies in several other features. Patients from the FA group were older (71.1 ± 8.9 years vs. 70.1 ± 9.3 years; p < 0.001) and underwent more PCIs with RA (1.0% vs. 0.3%; p = 0.002). They presented STEMI before PCI more often (19.6% vs. 10.1%; p = 0.03), while undergoing thrombectomy less often (1.7% vs. 2.8%, p = 0.02). They also presented SA before PCI less frequently (25.3% vs. 28.5%; p = 0.02). There were no differences in location of culprit lesion between femoral and radial access subgroups and angiographic success. No differences were found between PCI of CTOs and bifurcation rate as well.
Pre-PCI TIMI flow and periprocedural complications
Limited blood flow (TIMI 0–1) was found in 1,632 patients (30.3%), while preserved blood flow was noted in 3,760 individuals (69.7%) before PCI. All complication rates were higher in the TIMI 0–1 group compared to the TIMI 2–3 group assessed before PCI (p < 0.001). This was mostly caused by higher percentages of MACCE (p < 0.001), cardiac arrests (p < 0.001), deaths (p < 0.001) and no-reflows (p < 0.001) (Table 2). The number of patients with STEMI (p < 0.001) was greater in the group of patients with limited blood flow, whereas there were fewer patients with SA and UA (p < 0.001). The higher rate of complications in patients with the TIMI 0–1 grade flow seems to be mostly attributed to the higher rate of STEMI, despite the fact that there were more patients undergoing PCI of CTOs (p < 0.001) and LMCA (p < 0.001) in patients with preserved blood flow before PCI (Table 2).
Comparing 2015 with 2016
We observed an increase in the number of patients with COPD (2.4% vs. 2.6%; p = 0.006). In 2016, there were more patients with SA (23.9% vs. 31.3%; p < 0.001) but less with STEMI (26.3% vs. 23.3%; p = 0.009) and NSTEMI (21.3% vs. 14.6%; p < 0.001). The FA approach was more common in 2016 (57% vs. 61.5%; p < 0.001), whereas RA was less common (25.4% vs. 22.8%; p = 0.02). In 2016, we noticed an increase in complexity of PCIs in the COPD group. The rate of PCIs within bifurcations (3.3% vs. 9.6%; p < 0.001) and CTOs (3.3% vs. 6.1%; p < 0.001) increased as compared to 2015. Considering the type of implanted stents, in 2016 we noted an increased percentage of DES stents (79.4% vs. 87.0%; p < 0.001) and a decrease in BMS (8.1% vs. 2.6%; p < 0.001) and BVS stents (1.6% vs. 0.4%; p < 0.001). Radiation exposition and contrast dose also decreased significantly in 2016 compared to 2015 (p < 0.001).Considering these changes, the overall incidence of all complications in the COPD group decreased in 2016 as compared to 2015, but without statistical significance (2.9% vs. 2.3%; p = 0.15).
Predictors of all periprocedural complications, allergic reactions and no-reflows
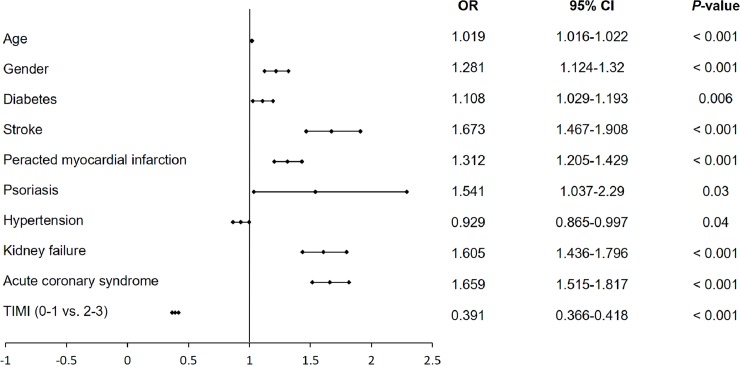
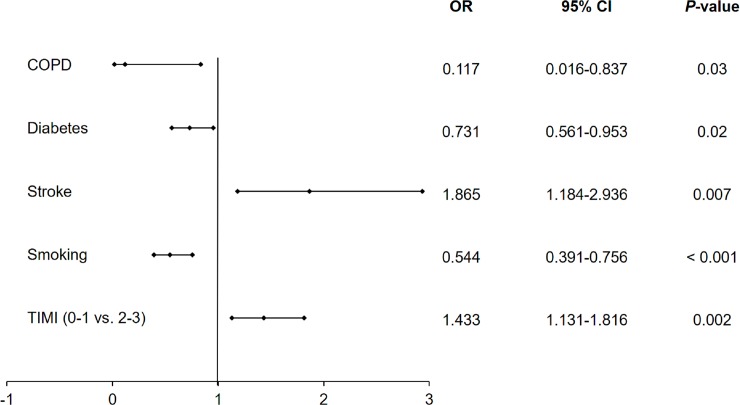
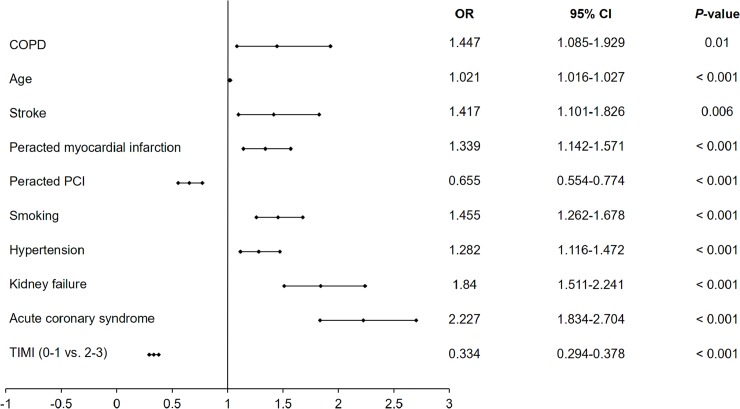
Among several independent predictors of all periprocedural complication rates estimated by multivariate analysis in the overall group of patients undergoing PCIs, COPD was not confirmed to be an independent predictor (Fig 2). Using multivariate analysis, we displayed that hypertension and preserved blood flow before PCI are predictors of lower periprocedural complication rates, while increased age, male gender, diabetes, previous stroke, previous MI, psoriasis, chronic kidney disease and ACS were predictors of increased periprocedural complication rates (Fig 2). We also noticed that smoking, COPD and diabetes were among the independent negative predictors of periprocedural allergic reactions assessed by multivariate analysis, while stroke and preserved blood flow assessed by TIMI score before PCI were among factors increasing the probability of periprocedural allergic reactions (Fig 3). Moreover, COPD alongside with age, stroke, MI, smoking, hypertension, chronic kidney disease and ACSs were independent positive predictors of periprocedural no-reflows, while previous PCI and baseline TIMI 2–3 grade flow decreased the probability of no-reflow (Fig 4).
Fig 2. Predictors of all periprocedural complications assessed by multivariate analysis in the overall group of patients undergoing PCI.
Abbreviations: CI, confidence interval; OR, odds ratio; TIMI, thrombolysis in myocardial infarction.
Fig 3. Predictors of allergic reactions during PCI assessed by multivariate analysis in the overall group of patients undergoing PCI.
Abbreviations: CI, confidence Interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; TIMI, thrombolysis in myocardial infarction.
Fig 4. Predictors of no-reflow complications during PCI assessed by multivariate analysis in the overall group of patients undergoing PCI.
Abbreviations: CI, Confidence Interval; COPD, chronic obstructive pulmonary diseases; OR, odds ratio; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Discussion
The main findings of the current study are that overall complication rates in patients with COPD are higher as compared to patients without COPD. Among all periprocedural complications, the rate of cardiac arrests, MIs and no-reflows was higher in patients from the COPD compared to the non-COPD group. Multivariate analysis did not confirm COPD as an independent predictor of periprocedural complications in the overall group of patients undergoing PCIs. Age, gender, diabetes, stroke, peracted MI, psoriasis, hypertension, kidney failure, ACS and TIMI flow before PCI were among the independent predictors of periprocedural complications confirmed by multivariate analysis. The third finding of the current study is that COPD and smoking along with diabetes were found to be a predictor of decreased periprocedural allergic reaction rate in patients undergoing PCI. The fourth significant finding of the presented study is that COPD and smoking were predictors of increased no-reflows in patients undergoing PCIs.
In the present study, the rate of periprocedural complications was comparable to the results published by other authors [5]. While our estimations indicate that the rate of periprocedural complications was significantly higher in the patients with rather than without COPD, the study published by Enriquez et al. reported a different proportion [5]. They estimated the rate of periprocedural complications in patients from the non-COPD group at 4.2%, which was significantly higher compared to the COPD group (2.6%; p = 0.004). In previously published studies, in-hospital mortality rate in the overall population ranges between 2.2%-2.83% and was significantly higher compared to those patients from the non-COPD groups [5,11]. Those death rates were higher compared to our results. However, we included only periprocedural deaths. Our analysis did not cover all hospital stays, which significantly underestimates the death rate. Previously published studies revealed higher rates of deaths in patients with COPD in the overall group of patients undergoing PCI and in patients with acute MI [5,12]. However, the study published by Sung et al. revealed a lower rate of in-hospital deaths in patients with COPD and STEMI undergoing primary PCI compared to non-COPD individuals. Furthermore they noticed an increased rate of recurrent MIs in the COPD group as compared to the non-COPD group (4.8% vs. 1.3%; p = 0.01) [13]. Although, their population of patients included more patients with MVD (59.7% vs. 34.4%) and baseline blood flow expressed as TIMI grade was 0–1 before PCI (80.6% vs. 30.3%) [13].
In the current study, we revealed a higher rate of periprocedural CAs, MIs and no-reflows in patients from the COPD group. The study published by Zhang et al. demonstrated an increased rate of in-hospital MIs in patients with rather than without COPD (16.3% vs. 4.6%) [14]. The study was conducted among a group of 2,362 patients undergoing PCI. Among them, 233 (9.8%) of patients were diagnosed with COPD. Since the cardiac mortality rate was zero in both groups, no differences were found between them. The important fact is that the authors did not mention any information about the distribution of CAD clinical presentation before PCI or the type of coronary atherosclerosis. Nevertheless, they also showed that the incidence of heart failure was greater in the COPD group as compared to the non-COPD group (22% vs. 9.2%) [14]. The increased rate of periprocedural MIs in patients with COPD as compared to the non-COPD group could be explained by more complex and disseminated coronary atherosclerosis as well as the difference in clinical presentation resulting from it. Whereas the increased rate of no-reflows and CAs could undoubtedly be related to the prothrombotic state attributed to COPD patients and the impeded return of blood flow through the vessel in STEMI patients related to it [15,16]. Our study confirmed that STEMI patients had a high rate of no-reflows (2.8%), CAs (2.6%), and the overall periprocedural complication rate was also high (6.2%) compared to other clinical presentations of CAD. Moreover, the relationship between no-reflows and the COPD as a prothrombotic state have been more widely discussed in two previously published manuscripts [17,18]. Furthermore, according to our knowledge, this is the first study verifying that among several confirmed predictors, smoking and COPD are independent risk factors of a higher incidence of no-reflows in patients undergoing PCI.
In contrast to our results, research conducted by other authors revealed that patients with COPD were more likely to experience major entry site complications than patients without COPD [16,19]. The leading entry site periprocedural complication was higher rate of bleeding that required transfusion in COPD patients. The study published by Enriquez et al. revealed that the rate of periprocedural access site bleeding requiring transfusion in patients with COPD was estimated at 2.3%, while in patients without COPD, it was at 1.4% (p = 0.03) [5]. We noted that puncture site bleeding rate was higher in patients with COPD compared to non-COPD patients but without statistical significance (0.2% vs. 0.15; p = 0.06). It was attributed mainly to age, gender and therapy implementing warfarin [16,19]. However, most studies maintain the thesis that the etiology of increased risk of periprocedural entry side bleeding is multifactorial [5].
Another worthy finding of the current study is that the rate of allergic reactions was higher in the COPD group as compared to the non-COPD group. Similarly, Mukherjee et al. observed a lower incidence of COPD in patients with respiratory allergy [20]. They noticed that among 550 participants, 18.9% of patients from the non-allergic population suffered from COPD, while 7.7% of allergic subjects had COPD [20]. Based on this, it could be concluded that patients with COPD and smokers present restrained allergic reactions. According to our knowledge, the present study is the first study which demonstrates that COPD and smoking are independent predictors of decreased periprocedural allergic reaction rate in the overall group of patients undergoing PCIs.
Limitations
The ORPKI database does not specifically define particular periprocedural complications and is based on the operators’ discretion. Similar concerns could be applied to periprocedural complications and their definitions usually remain in the hands of operators. Additionally, assignment to the COPD and non-COPD groups was based on previous diagnosis and/or typical treatment while no data on spirometry test results were collected. This could bring some false positive or false negative diagnosis of COPD and bias related to this issue. However this is a typical problem for the national registry. From the other side it presents the real life and the real frequency of COPD diagnosis confirmed in spirometry is close to the presented in the study. Patients with exacerbated COPD at admission could be treated in some cases with systemic glucocorticoids, which might modify the extent and frequency of procedural-related allergic reactions.
Conclusions
In the patients undergoing PCI, the periprocedural complication rate is higher in those from the COPD group than the non-COPD group. Cardiac arrests, myocardial infarctions and no-reflows may be found among the periprocedural complications with the largest contribution. COPD was not an independent predictor of periprocedural complications in the overall group of patients undergoing PCI, while COPD and smoking were found to be independent predictors of decreased allergic reaction periprocedural rate and increased rate of periprocedural no-reflows.
Data Availability
The data set for this study has been uploaded to a public repository and is available at: https://osf.io/397qv/?view_only=2fbfd66c75044c76ab26ebc42978dabf.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–21. 10.1183/09059180.00003609 [DOI] [PubMed] [Google Scholar]
- 2.Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. 10.1378/chest.128.4.2068 [DOI] [PubMed] [Google Scholar]
- 3.Hadi HA, Zubaid M, Al Mahmeed W, El-Menyar AA, Ridha M, Alsheikh-Ali AA, et al. Prevalence and prognosis of chronic obstructive pulmonary disease among 8167 Middle Eastern patients with acute coronary syndrome. Clin Cardiol. 2010;33:228–35. 10.1002/clc.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama K, Morimoto T, Furukawa Y, Nakagawa Y, Ehara N, Taniguchi R, et al. Chronic obstructive pulmonary disease—an independent risk factor for long-term cardiac and cardiovascular mortality in patients with ischemic heart disease. Int J Cardiol. 2010;143:178–83. 10.1016/j.ijcard.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Enriquez JR, Parikh SV, Selzer F, Jacobs AK, Marroquin O, Mulukutla S, et al. Increased adverse events after percutaneous coronary intervention in patients with COPD: insights from the National Heart, Lung, and Blood Institute dynamic registry. Chest. 2011;140:604–10. 10.1378/chest.10-2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Applegate R. J, Sacrinty M. T, Kutcher M. A, Kahl FR, Gandhi SK, Santos RM, et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1:317–26. 10.1016/j.jcin.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 7.Gayed M, Yadak N, Qamhia W, Daralammouri Y, Ohlow MA. Comorbidities and Complications in Nonagenarians Undergoing Coronary Angiography and Intervention. Int Heart J. 2017;58:180–4. 10.1536/ihj.16-083 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed B, Piper WD, Malenka D, VerLee P, Robb J, Ryan T, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2:423–9. 10.1161/CIRCINTERVENTIONS.109.860494 [DOI] [PubMed] [Google Scholar]
- 9.Januszek R, Siudak Z, Dziewierz A, Dudek D, Bartuś S. Predictors of in-hospital effectiveness and complications of rotational atherectomy (from the ORPKI Polish National Registry 2014–2016). Catheter Cardiovasc Interv 2017. 10.1002/ccd.27372 [DOI] [PubMed] [Google Scholar]
- 10.Siudak Z, Tokarek T, Dziewierz A, Wysocki T, Wiktorowicz A, Legutko J, et al. Reduced periprocedural mortality and bleeding rates of radial approach in ST-segment elevation myocardial infarction. Propensity score analysis of data from the ORPKI Polish National Registry. EuroIntervention. 2017;13:843–50. 10.4244/EIJ-D-17-00078 [DOI] [PubMed] [Google Scholar]
- 11.de Miguel-Díez J, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Bueno H, Puente-Maestu L, et al. Time trends in coronary revascularization procedures among people with COPD: analysis of the Spanish national hospital discharge data (2001–2011). Int J Chron Obstruct Pulmon Dis. 2015;10:2285–94. 10.2147/COPD.S92614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dziewierz A, Siudak Z, Dykla D, Rakowski T, Mielecki W, Dubiel JS, et al. Management and mortality in patients with non-ST-segment elevation vs.ST-segment elevation myocardial infarction. Data from the Malopolska Registry of Acute Coronary Syndromes. Kardiol Pol. 2009;67:115–20. [PubMed] [Google Scholar]
- 13.Sung PH, Chung SY, Sun CK, Yang CH, Chen SM, Hang CL, et al. Impact of chronic obstructive pulmonary disease on patient with acute myocardial infarction undergoing primary percutaneous coronary intervention. Biomed J. 2013;36:274–81. 10.4103/2319-4170.113373 [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Cheng YJ, Zheng WP, Liu GH, Chen HS, Ning Y, et al. Impact of Chronic Obstructive Pulmonary Disease on Long-Term Outcome in Patients with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Biomed Res Int. 2016;2016:8212459 10.1155/2016/8212459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessandri C, Basili S, Violi F, Ferroni P, Gazzaniga PP, Cordova C. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost. 1994;72:343–6. [PubMed] [Google Scholar]
- 16.Montalescot G, Salette G, Steg G, Cohen M, White HD, Gallo R, et al. Development and validation of a bleeding risk model for patients undergoing elective percutaneous coronary intervention. Int J Cardiol. 2011;150:79–83. 10.1016/j.ijcard.2010.02.077 [DOI] [PubMed] [Google Scholar]
- 17.Januszek R, Siudak Z, Dziewierz A, Rakowski T, Dudek D, Bartuś S. Chronic obstructive pulmonary disease affects the angiographic presentation and outcomes of patients with coronary artery disease treated with percutaneous coronary interventions. Pol Arch Intern Med. 2018;128:24–34. doi: 10.20452/pamw.4145 [DOI] [PubMed] [Google Scholar]
- 18.Januszek R, Siudak Z, Dziewierz A, Rakowski T, Dudek D, Bartuś S. Chronic obstructive pulmonary disease affects angiographic presentation and outcomes. Authors' reply. Pol Arch Intern Med. 2018;128:195–6. [DOI] [PubMed] [Google Scholar]
- 19.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, et al. ; National Cardiovascular Data Registry. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–9. 10.1161/CIRCINTERVENTIONS.108.846741 [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee S, Banerjee G, Das D, Mahapatra ABS. Occurrence of COPD in Patients with Respiratory Allergy: A Clinico-Spirometric Evaluation in a Tertiary Hospital, Kolkata. J Clin Diagn Res. 2017;11:CC11–CC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set for this study has been uploaded to a public repository and is available at: https://osf.io/397qv/?view_only=2fbfd66c75044c76ab26ebc42978dabf.