Abstract
Gelatinase B/matrix metalloproteinase-9 (MMP-9) triggers multiple sclerosis (MS) and the animal model of experimental autoimmune encephalomyelitis (EAE) by the breakdown of the blood-brain barrier. Interestingly, MMP-9 is beneficial in systemic autoimmunity caused by Fas-deficiency. Fas-deficient (faslpr) and Fas-ligand-deficient mice are protected against EAE. We here investigated the interaction between Fas and MMP-9 in the setting of induction of EAE and compared short- and long-term effects. We provoked EAE with myelin oligodendrocyte glycoprotein (MOG) peptide and compared EAE development in four genotypes (wild-type (WT), single knockout mmp-9-/-, faslpr, and mmp-9-/-/faslpr) and monitored leukocytes, cytokines and chemokines as immunological parameters. As expected, faslpr mice were resistant against EAE induction, whereas MMP-9 single knockout mice were not. In the double mmp-9-/-/ faslpr mice the effects on disease scores pointed to independent rather than interrelated disease mechanisms. On a short term, after EAE induction leukocytes infiltrated into the brain and cytokine and chemokine levels were significantly higher in all the four genotypes studied, even in the faslpr and mmp-9-/-/faslpr, which did not develop clinical disease. The levels of MMP-9 but not of MMP-2 were increased in the brain and in the peripheral organs after EAE induction. After 40 days all the animals recovered and did not show signs of EAE. However, the absence of MMP-9 in the remission phase suggested a protective role of MMP-9 in the late phase of the disease, because single mmp-9-/- mice presented a delayed remission in comparison with WT animals suggesting a phase-dependent role of MMP-9 in the disease. Nevertheless, the levels of some cytokines and chemokines remained higher than in control animals even 100 days after EAE induction, attesting to a prolonged state of immune activation. We thus yielded new insights and useful markers to monitor this activated immune status. Furthermore, MMP-9 but not MMP-2 levels remained increased in the brains and, to a higher extend, in the spleens of the WT mice even during the remission phase, which is in line with the role of MMP-9 as a useful marker and a protective factor for EAE in the remission phase.
Introduction
Multiple sclerosis (MS) is a devastating autoimmune disease in need of alternative pathophysiological insights and new pharmacological targets [1,2]. Despite major efforts, direct targeting of the adaptive arm of immunity by eliminating T or B cell clones has failed so far for MS treatment, whereas innate immune molecules, including interferons for substitution therapy, minocycline as protease inhibitor [3, 4, 5] and cell adhesion molecules as disease targets, are yielding gradually improved therapeutic schemes. The replacement of injectable drugs by oral ones is a major step forward [5,6]. Specifically, oral and inexpensive minocycline can be used when other drugs are excluded based on prescription and insurance requirements [5]. Targeting adaptive immune mechanisms indirectly with oral fingolimod, which blocks T and B cell egress, shows a better outcome for patients than parenteral interferon [6]. These examples illustrate that gradual improvements towards compliant therapies are possible and that the study of disease mechanisms remains essential [7].
The definition of genetic factors and their interactions in MS and animal models of experimental autoimmune encephalomyelitis (EAE) may give clues for the development of better diagnostics and therapeutics. One gene set that has been relatively neglected so far as a disease marker or therapeutic target encodes the apoptosis-inducing factors of the Fas system. Nevertheless, solid evidence exists that both Fas ligand (FasL/CD95L) as well as Fas receptor (Fas/CD95) are critical factors in determining the outcome of antigen-specific EAE [8,9]. Indeed, fas- and fasL-deficient mice are resistant to develop EAE. In addition, inflammatory lesions in fas-deficient lpr mice (faslpr) contain less apoptotic cells than those observed in wild-type lesions. Furthermore, in cell transfer experiments, Fas expression in recipient animals is important for EAE progression because faslpr recipients still develop lower disease scores despite transfer of faslpr or wild-type T lymphocytes. However, other factors, such as (auto)antigen generation and presentation, are necessary for EAE disease to occur and develop, because T cell receptor (TCR) transgenic mice for myelin antigen on an lpr background still produce EAE [10].
A natural way to generate autoantigens for MS and EAE is by extracellular proteolysis or other posttranslational protein modifications in an inflammatory context. Such extracellular proteolysis may be provided by host proteases such as MMP-9, abundantly induced by cytokines and chemokines in inflammation [11]. The focus on proteolysis in MS has been primarily on studies of the inducible matrix metalloproteinase-9 (MMP-9) [12]. This enzyme was first associated with MS [13]. Young mmp-9 knockout mice show resistance against EAE [14] and MMP inhibitors and double mmp-2/mmp-9 deficiency were found to be protective, also in adult mice [15,16]. Recently, targeting MMPs with minocycline as the most potent tetracycline inhibitor of MMP-9 [4], has yielded promising results in MS patients under monotherapy [5]. However, although MMP-9 activity at the blood-brain barrier has recently been shown to constitute a critical event in early MS lesion development [17], the MMP-9 gene has not been found as a disease susceptibility factor in genetic screenings for MS [18]. As a contrast, instead of a disease-causing function in organ-specific autoimmunity [19], a protective role has been described for MMP-9 in systemic autoimmune disease and also this role seems to depend on proteolytic antigen processing [20]. Indeed, when the MMP-9 gene was knocked out in fas-deficient lpr mice (faslpr), that are prone to develop systemic autoimmune disease, the animals were not resistant, but became instead more susceptible to develop lupus-like disease. In this phenotype, autoantibodies are generated against ubiquitous intracellular proteins, many of which are substrates of MMP-9 [20, 21].
Another often underestimated aspect when using EAE models is the duration of the experiments. Most often, EAE experiments are done over a time interval of only a few weeks. This interval is sufficient and usually employed to demonstrate beneficial aspects of gene knockouts or novel treatment schemes. However, and as we experienced in preliminary experiments studying therapeutic effects, gene knockout exploration and analysis of known drugs may fade out on longer duration.
We here addressed the questions (i) whether the “spontaneous” clinical effects of deletion of Fas (faslpr) and MMP-9 (mmp-9-/-), as previously observed [20], are similar or different after induction of autoimmunity with a known autoantigen and whether these are additive, synergistic or antagonistic (ii) which of both molecules, Fas and MMP-9, drives clinical outcome in the setting of an organ-specific autoimmune reaction (iii) which cellular and molecular mechanisms are behind the clinical outcomes and how can these be used in monitoring disease states and (iv) what is the long-term progression of EAE and the evolution of immune cells and molecules.
Materials and methods
Generation and maintenance of mice
To exclude confounding genetic influences of the genetic background we extensively backcrossed our original mouse line [14]. In the 10th backcross generation of MMP-9 knockout mice into C57/Bl6, the mice retained brown fur [20], suggesting that a dominant hair color determinant is located near the mouse MMP-9 gene. In addition, our knockout line has a severe subfertility phenotype [22]. For these reasons and in view of the increasing awareness about possible confounding influences imposed by differences in genetic backgrounds between wild-type and knockout mice [23,24] and by possible interference by differences in environmental conditions [25] and to avoid clinical bias on the basis of fur color, we further backcrossed our mmp-9 knockout mice (mmp-9-/-) till these became black [26]. We used in all forthcoming experiments black animals from the 13th generation backcross into C57Bl/6. Faslpr mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, ME, USA). Crossing of mmp-9-/- and faslpr mice yielded F1 heterozygotes that were mated to obtain mmp-9-/-/faslpr in the F2 generation. The genotype of each individual mouse was confirmed by PCR All mice were bred in specific pathogen-free (spf) insulators at the Rega Institute for Medical Research and under exactly the same environmental conditions (e.g. food, day night cycle, bedding) for all genotypes. At regular time intervals, we screened for the presence of a panel of common mouse microbes as a control for absence of infections. Induction and follow-up of EAE evolution were carried out with adult (8–10 week old) male and female mice under SPF housing conditions. During this period, the mice received appropriate nutrition and acidified drinking water without antibiotics. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Ethics Committee of KU Leuven (Licence number LA1210243, Belgium). All the animal facility staff as well as the research staff had followed the required courses and obtained certificates to be able to perform animal research.
Reagents
Mycobacterium tuberculosis strain H37Ra, Incomplete Freund’s Adjuvant (IFA) and Complete Freund’s Adjuvant (CFA) were purchased from Difco Laboratories (Detroit, MI, USA). Pertussis toxin was purchased from List Biological Laboratories (Campbell, CA, USA). Myelin oligodendrocyte glycoprotein peptide (MOG35-55) was produced by Fmoc (fluorenylmethoxycarbonyl) solid phase peptide synthesis, purified by reversed phase chromatography and peptide mass was confirmed by electrospray ion trap mass spectrometry [27]. Myelin Basic Protein (MBP) was purchased from Enzo Life Sciences, antiMBP polyclonal antibody was obtained from Sigma Aldrich (St. Louis, MO, USA).
Induction and clinical evaluation of EAE
For short-term experiments, several trials with equal numbers of animals were pulled and, as a result, 34 mice of each genotype were obtained. For long-term experiments 6 mice of each genotype were used. All the mice were anesthetized during EAE induction with ketamine 80–100 mg/kg IP and xylazine 10–12.5 mg/kg by intraperitoneal injection. EAE was induced in the four strains of mice with an identical scheme by injecting 50 μg of myelin oligodendrocyte glycoprotein (MOG)35-55 peptide (1 mg/ml in saline) emulsified in IFA containing 4 mg/ml of M. tuberculosis. On day 0, after anaesthesia, we injected subcutaneously 50 μl of the emulsion in each of the two hind footpads and immediately thereafter 100 ng pertussis toxin in 50 μl saline was intravenously (i.v.) administered in the tail vein. On day 2, a second dose of pertussis toxin was i.v. administered in the tail vein under anesthetized conditions.
Mice were evaluated daily for signs of clinical disease with the following grading system: grade 0, normal; grade 0.5, floppy tail; grade 1, tail paralysis and mild impaired righting reflex; grade 2, mild hind limb weakness and impaired righting reflex; grade 3, moderate to severe hind limb paresis and/or mild forelimb weakness; grade 4, complete hind limb paralysis and/or moderate to severe forelimb weakness; grade 5, quadriplegia or moribund; grade 6, death.
Mice were euthanized on day 30 or day 100 after EAE induction for short-and long-term experiments respectively with Dolethal (pentobarbital). Specifically, 200 mg/mL solution of pentobarbital in sterile saline was administered intraperitoneally at a dose of 40 mg/kg. Severely paralyzed mice or suffering animals were euthanized. Nine of the adult animals (7 months) died of natural and EAE symptoms during the complete duration of the experiments (30 or 100 days). Animals with severe symptoms of the disease where euthanized. Specifically, from the WT group 3 animals died before meeting criteria for euthanasia, from mmp-9-/- 5 died before meeting the criteria of euthanasia and 6 reached the endpoint criteria and they were euthanized. From mmp-9-/-/faslpr mice only one mouse died before meeting the criteria for euthanasia whereas in the faslpr group none of the animals had to be euthanized or died during the experiments.
Cell preparation from various organs
After euthanasia and perfusion of the mice, spleens were isolated, cut into small pieces and passed through cell strainers, to obtain single cell suspensions. Red blood cells were lysed by two incubations (5 and 3 min at 37°C) of the splenocyte suspensions in 0.83% NH4Cl solution. Remaining cells were washed two times with ice-cold PBS containing 2% FCS. For central nervous system (CNS) analysis, euthanized mice were gently perfused through the left cardiac ventricle with 50 ml ice-cold PBS to eliminate intravascular contaminating blood cells in the CNS. Spinal cords were removed by flushing the spinal canal with sterile PBS and brains were dissected. Brain and spinal cord cell fractions from individual mice were isolated according to the recent protocol by Legroux et al. [28]. Briefly, brains and spinal cords were digested with collagenase D and DNase for 15 min at 37°C. The products of the digestion were homogenized and filtered through a cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ, USA) and centrifuged in a single 37% PercollTM step (10 min, 300 g, 4°C). The cell fractions were then washed twice by adding Hank’s Balanced Salt Solution (HBSS1X).
Flow cytometry analysis
Single cell suspensions (0.5 x 106 cells) were incubated for 15 min with Fc-receptor-blocking antibodies anti-CD16/anti-CD32 (BD Biosciences Pharmingen, San Diego, CA, USA), washed with PBS supplemented with 2% FCS and then stained for 30 min with the indicated conjugated antibodies. Cells were washed twice and fixed with 0.37% formaldehyde in PBS. FITC-conjugated anti-CD19, PE-conjugated anti-CD3, APC-conjugated anti-CD4, BV410-conjugated anti-CD8, APC-conjugated anti-CD11b, BV711-conjugated anti-CD11c, FITC-conjugated anti-Gr-1 and PE-conjugated anti-F4/80, were purchased from eBioscience (San Diego, CA, USA). Cells were analysed in a FACS Fortessa flow cytometer and data were processed with the FlowJo software (Becton Dickinson Labware, Franklin Lakes, NJ, USA).
Zymography
Samples of tissue extracts were subjected to zymography analysis, as detailed previously. Briefly, to reduce background levels caused by contaminating glycoproteins, all samples were prepurified by gelatin-Sepharose affinity chromatography [29]. To allow quantitation and comparison of different substrate gels, all samples were spiked with a known amount of a recombinant deletion mutant of human proMMP-9 lacking the O-glycosylated and hemopexin domains, proMMP-9ΔOGHem [30]. The prepurified samples were loaded onto 7.5% polyacrylamide gels which contained 0.1% gelatin. After electrophoresis the gels were removed from the electrophoresis system and washed twice for 20 minutes with 2.5% Triton X-100. Then, the gels were incubated overnight at 37°C in 50 mM Tris-HCl pH 7.5, 10 mM CaCl2 for the development of gelatinolysis. Finally, the proteins in the gels were stained with Coomassie blue (GE Healthcare, Piscataway, NJ, USA) resulting in clear lysis zones which were analyzed with the ImageQuant TL software (GE Healthcare, Piscataway, NJ, USA). The concentrations of the gelatinase forms were calculated based on the density of the bands, on their relation with a known amount of the spiked internal standard sample of ΔOGHem proMMP-9 and on the comparison with a dilution series of a recombinant proMMP-9 standard mixture (including the ΔOGHem proMMP-9 that was used in the spiking) with known concentrations [31].
Multiplex ELISA
The protein levels of interferon (IFN)-α, IFN-γ, interleukin (IL-6), tumor necrosis factor (TNF)-α, keratinocyte chemoattractant (KC) (or (GRO)-α or CXCL1), monocyte chemoattractant protein (MCP)-1 (or CCL2), MCP-3 (or CCL7), IP-10 (or CXCL10), macrophage inflammatory protein (MIP)-1α (or CCL3) and MIP-1β (or CCL4) were measured in plasma with the use of a custom-made 12 multiplex Assay (ProcartaPlex, eBioscience). The protocol from the manufacturer was followed and quantitative data acquisition was with two different plate readers which gave similar results: Luminex plate platform provided by the Laboratory of Virology, (Rega Institute for Medical Research, KU Leuven, Belgium) and with Magpix (ProDigest, University of Gent, Belgium).
Statistical analyses
Statistical analysis were performed using GraphPad Prism 6 software. Differences in the clinical course of EAE were analysed with a non-parametric Kruskal-Wallis test with Dunn’s multiple comparison. Significant differences between groups were evaluated using a non-parametric ANOVA Kruskal-Wallis test. All p values of 0.05 or less were considered significant.
Results
EAE develops in WT and in mmp-9-/-, but less in single faslpr and mmp-9-/-/faslpr mice
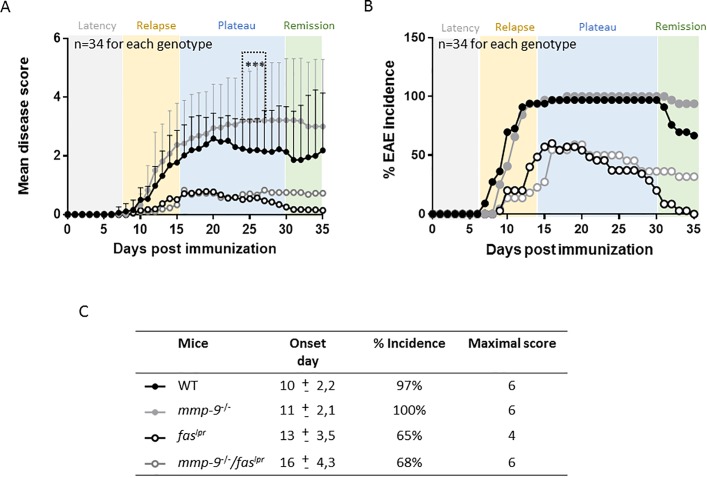
Previously, we documented the spontaneous disease phenotypes of single faslpr, mmp-9-/- mice and double mmp-9-/-/faslpr mice, all on C57Bl/6j genetic background and without any exogenous (auto)antigenic stimulus. These studies generated the concept that MMP-9 plays on long-term a protective role in lupus-like syndromes [20]. Thereafter, we started to investigate whether this concept might be broadened to the prototypic organ-specific autoimmunity animal model of EAE. We induced CNS disease with MOG peptide and compared EAE development in wild-type (WT), single faslpr, single mmp-9-/- mice and to define the interactions between both Fas and MMP-9, we used double mmp-9-/-/faslpr mice [20]. Disease read-outs during the first five weeks corroborated previous findings with single faslpr [32] and single mmp-9-/- mice [14]. These results were complemented with data about double mmp-9-/-/faslpr mice (Fig 1A, 1B and 1C and S1 Table). In Fig 1A we illustrate, up to 35 days, that adult mmp-9-/- mice developed EAE and even showed severed (P<0.001 compared with WT mice) disease scores after about four weeks. The faslpr mice were resistant against EAE development from two weeks onwards, as shown by Waldner et al. [32]. It is important to remark that mmp-9-/- animals showed higher mean disease scores that the WT cohort, while the disease scores of the WT animals increased slowly, in the mmp-9-/- group, the disease appeared more aggressively. At day 10 after EAE induction, 70% of WT mice had already EAE symptoms, whereas in the cohort of mmp-9-/- animals only 40% presented with symptoms, but the disease scores of the sick animals were higher in mmp-9-/- animals than in WT mice at day 10. In line with these results, the number of dead mice after EAE induction were 4 of 34 for WT mice, whereas for mmp-9-/- mice, 8 animals out of 34 had died, which suggested that lack MMP-9 resulted in a more aggressive disease. Data from the double mmp-9-/-/faslpr mice hinted to independent actions of Fas and MMP-9. Whereas MMP-9 attenuated the lymphoproliferation syndrome, observed on long term in the double deficient mice [20], it did not alter the EAE disease on the short term of 35 days (Fig 1A, 1B and 1C and S1 Table). Interestingly, mmp-9-/-/faslpr mice showed a delay in the disease onset (day 16) compared with the faslpr animals (day 13), (Fig 1C). In addition, lack of MMP-9 in the faslpr mice (mmp-9-/-/faslpr) suggestted a slightly longer remission phase. Faslpr mice had completely recovered from EAE symptoms at day 35, whereas in the mmp-9-/-/faslpr 25% of the animals still presented EAE symptoms (Fig 1A and 1B and S1 Table), suggesting different roles of MMP-9 in the initiation and the remission phases of the disease [19].
Fig 1.
Clinical evolution of EAE in WT, mmp-9-/-, faslpr and mmp-9-/-/faslpr on short-term. EAE was induced in the four mouse strains and disease scores were measured during 35 days. (A) Mean disease scores from 34 mice for each of the four mouse strains. Significant differences in the comparison between WT and mmp-9-/- are indicated with asterisks. In addition, the differences in mean diseases scores for all the data points from day 13 onwards till day 35 were significant for faslpr and mmp-9-/-/faslpr versus WT. Standard deviations from WT and MMP-9-/- are indicated. *p<0.05 versus WT by Kruskal-Wallis test with Dunn’s multiple comparison. (B) Incidence of the disease after EAE induction. (C) Averages of day-of-onset, percentages of the incidence and maximal disease scores. (mean ± SD).
Alterations in peripheral blood and CNS leukocyte subsets in the EAE model (short-term)
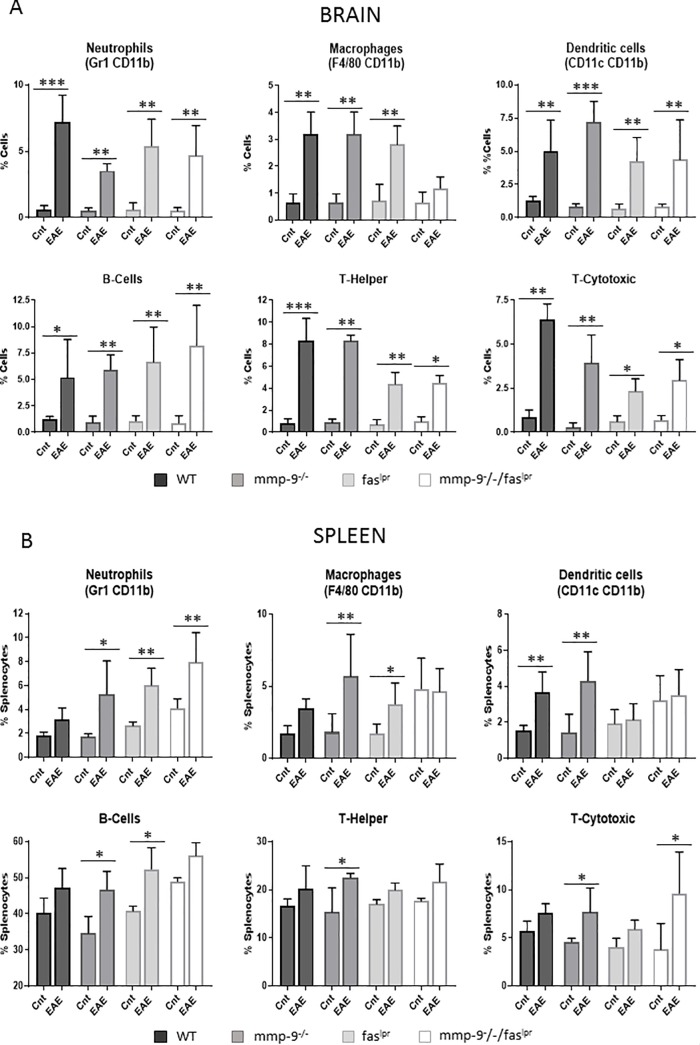
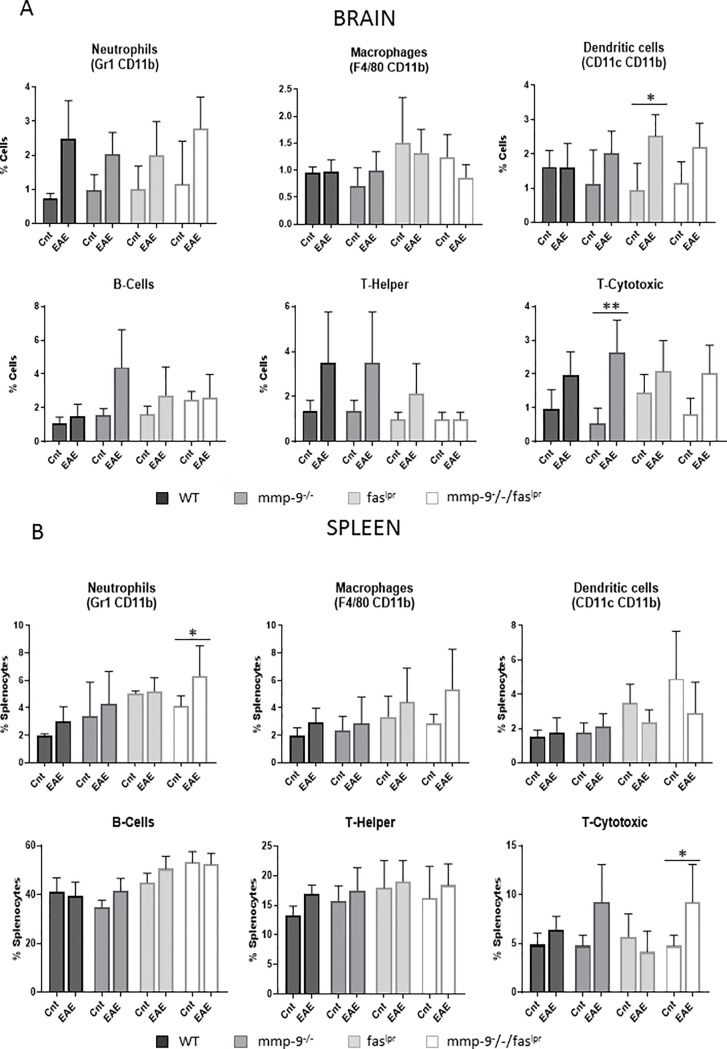
With the knowledge that the infiltration of inflammatory cells into the brain parenchyma leads to clinical effects [17], we measured CNS cell populations after extensive systemic perfusion of the mice to eliminate blood leukocytosis effects. CNS cells were isolated from individual mice [28] at 1 month of the EAE experiment and characterized by flow cytometry to detect neutrophils, macrophages, dendritic cells, B lymphocytes, CD4-positive T helper and CD8-positive cytotoxic (Fig 2A). In general, the basal brain parenchymal cell pools were analogous in mice of the 4 genotypes. After EAE induction, all tested leukocyte subsets were significantly increased (with the exception of macrophages in the faslpr/mmp-9-/- mice) in the central nervous system (CNS) for the four compared genotypes, including faslpr and mmp-9-/-faslpr mice, which presented lower incidence and diseases scores than WT and mmp-9-/- mice. Therefore, in these models no correlation existed between the clinical manifestations and the accumulation of the leukocytes into the CNS.
Fig 2.
Induction and alterations of leukocyte subsets in CNS and spleens after 1 month of EAE initiation. (A)- Cells purified from spinal cord and brain were analyzed by flow cytometry with the use of the indicated markers. Neutrophils (Gr1+, CD11b+), macrophages (F4/80+, CD11b+), dendritic cells (CD11c+, CD11b+), B-cells (CD19+), T-helper cells (CD3+, CD4+) and T-cytotoxic cells (CD3+, CD8+) were analyzed in total tissue cell suspensions. (B)- Flow cytometry analysis of splenocytes for the indicated markers were used to identify neutrophils (Gr1+, CD11b+), macrophages (F4/80+, CD11b+), dendritic cells (CD11c+, CD11b+), T-helper cells (CD3+, CD4+), T-cytotoxic cells (CD3+, CD8+) and B-cells (CD19+). P values were determined by ANOVA Kruskal-Wallis test. *p<0.05, **p<0.01 and ***p<0.001. The histogram bars represent the means (±SD) of all the data points. Cnt: Control. Control data were obtained from 3–4 mice. EAE data were obtained from 4–8 mice.
In addition, we monitored spleen leukocyte subsets at the end of the plateau phase (1 month, Fig 1) of inflammation. In Fig 2B, the percentages of the counts of neutrophils, macrophages, dendritic cells, B lymphocytes, CD4-positive T helper and CD8-positive T cytotoxic cells in spleens are represented. No mayor differences were observed between the four different genotype groups. In general, at 1 month after EAE induction, the relative abundancies of all subsets of splenic leukocytes were slightly increased. In particular, all the splenic leukocyte studied were significantly increased by EAE induction in the mmp-9-/- mice. In addition, neutrophils were significantly increased in faslpr and double mmp-9-/-/faslpr mice and macrophages and B lymphocytes in faslpr.
Altered levels of cytokines and chemokines after short-term EAE induction
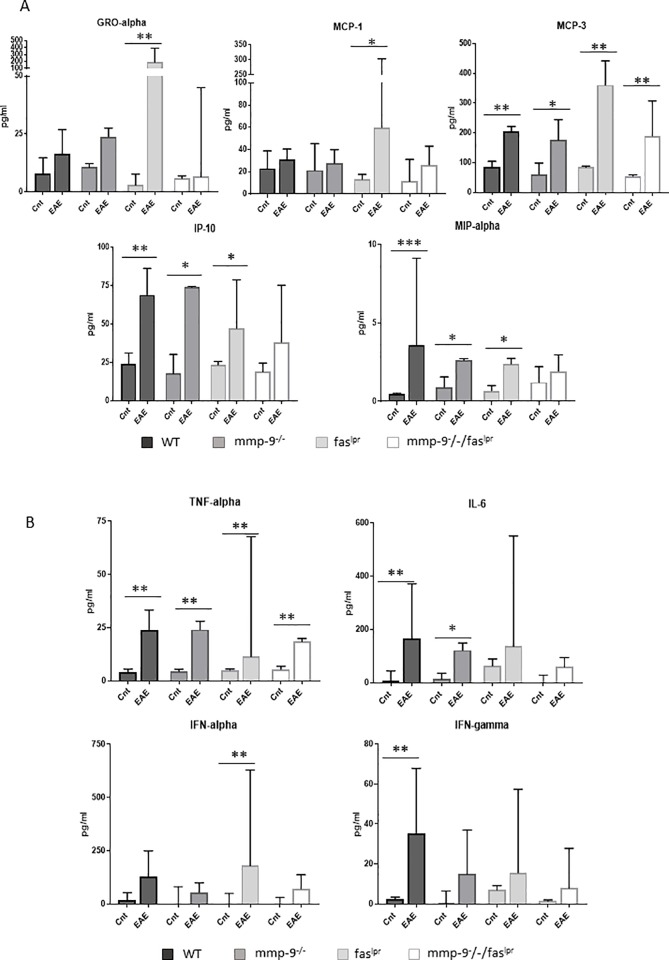
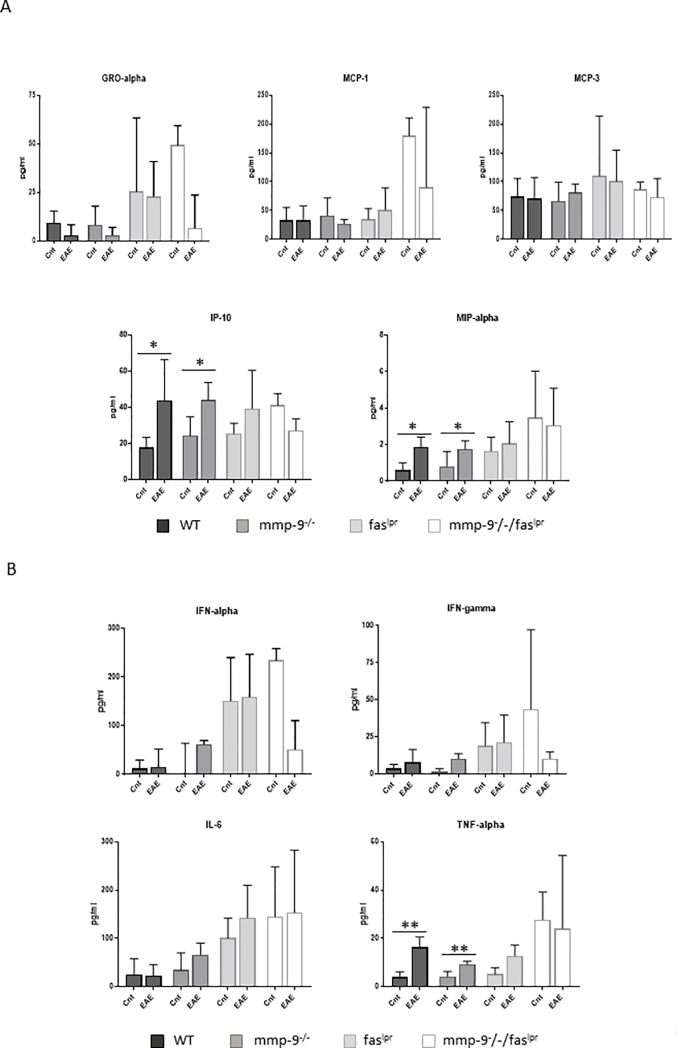
Cytokines, chemokines and their receptors play major roles in de progression of MS and EAE [12]. Therefore, we analyzed the plasma levels of the chemokines: GRO-alpha/KC (or CXCL1), MCP-1 (or CCL2), MCP-3 (or CCL7), IP-10 (or CXCL10) and MIP-1α (or CCL3) (Fig 3A) and the cytokines TNF-α, IL-6, IFN-α and IFN-γ (Fig 3B). The levels of many of the selected cytokines and chemokines were increased after 1 month of EAE induction but not all increases were found to be significant. A significant increase of TNF-α and MCP-3 was consistently detected in mice of all four genotypes 1 month after EAE induction. IFN-α, GRO-α and MCP-1 levels were significantly increased in faslpr mice. The levels of IP-10 and MIP-1α were increased significantly in WT, mmp-9-/- and faslpr, but not in the double mmp-9-/-/faslpr mice. IL-6 levels were only significantly increased in WT and mmp-9-/- animals. In the case of faslpr and double mmp-9-/-/faslpr mice, IL-6 levels had only a trend towards increases at 1 month after EAE induction.
Fig 3.
Plasma chemokine and cytokine levels at 1 month after EAE induction. ELISA analysis of the indicated chemokines (A) and cytokines (B) from plasma samples obtained after 1 month in control animals (Cnt) and animals with EAE (EAE). The histograms represent the means (±SD) of each group. P values were determined by ANOVA Kruskal-Wallis test. *p<0.05, **p<0.01 and ***p<0.001 versus control 1 month. Control data were obtained from 3–4 mice. EAE data were obtained from 4–8 mice.
Long-term EAE in WT, single mmp-9-/-, faslpr and double mmp-9-/-/faslpr knockout mice
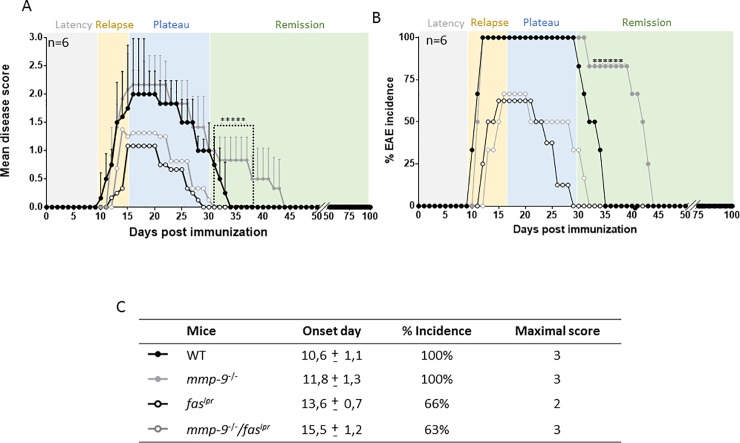
In a second type of experiment, we analyzed long-term evolution of EAE in smaller animal cohorts, and we compared the clinical evolutions in mice of the four genotypes up to 100 days, allowing all the animals to reach complete remission. As shown in Fig 4 all animals had complete clinical recovery after maximally 47 days. In the evaluations of the four disease phases (latency, relapse, plateau and remission), we remarked that mmp-9-/- and mmp-9-/-/faslpr mice animals had a slightly later onset of the disease than their respective controls WT and faslpr mice (day 10, 11 for WT and mmp-9-/-, and day 13, 15 for faslpr and mmp-9-/-/faslpr mice, respectively). Interestingly, the remission was significantly belated in the mmp-9-/- cohort. These animals needed more time to completely recover than the wild type animals (10 days more than WT mice). These data were suggestive for roles of MMP-9 in auto-antigen production (latency and relapse phases) and clearance of autoantigens and immune complexes (plateau and remission phases), the latter being as previously suggested for the SLE Fas/MMP-9 model [20].
Fig 4.
Clinical follow-up of EAE in WT, mmp-9-/-, faslpr and mmp-9-/-/faslpr mice for 100 days. EAE was induced in groups (n = 6) of mice of the four different genotypes and disease scores were measured during 100 days. (A) Significant differences between WT and mmp-9-/- are indicated with asterisks. Means ± SD from WT and MMP-9-/- are indicated. *p<0.05 versus WT by Kruskal-Wallis test with Dunn’s multiple comparison. (B) Percentages of EAE incidence after the induction of the disease. (C) Average day-of-onset, percentage of incidence and maximal score. (mean±SD).
Alterations in CNS and spleen leukocyte subsets in the EAE long-term model (100 days)
We then studied the CNS and spleen leukocyte subsets in the fading out of inflammation. Significant CNS inflammation was not observed anymore at 3 months. Nevertheless, there was still a trend towards an increase, albeit statistically insignificant in the levels of neutrophils and T-cytotoxic cells in the EAE induced mice from all the 4 genotypes studied compared with the controls (Fig 5A), which indicated that inflammation in the CNS was still present. Only significant increased numbers of cytotoxic T lymphocytes in the CNS of the single mmp-9-/- mice at 3 months were observed. This observation is in line with the prolonged disease phase before remission (Fig 4A and 4B) in the mmp-9-/- mice in comparison with the WT mouse cohort.
Fig 5.
Changes in leukocyte percentages in the CNS and spleens of mice after 100 days. Mice were induced to develop EAE or left untreated (Control, Cnt). Cells purified from spinal cord and brain (A) and spleens (B) were analyzed by flow cytometry with the use of the indicated markers. Neutrophils (Gr1+, CD11b+), macrophages (F4/80+, CD11b+), dendritic cells (CD11c+, CD11b+), B-cells (CD19+), T-helper cells (CD3+, CD4+) and T-cytotoxic cells (CD3+, CD8+) were analyzed in total tissue cell suspensions. P values were determined by ANOVA Kruskal-Wallis test. *p<0.05 and **p<0.01. The histogram bars represent the means ± SD of all the data points. Control data were obtained from 3–4 mice. EAE data were obtained from 4–8 mice.
At the end of the remission phase (3 months), splenic leukocyte populations in the WT mice and both single deficient mice (mmp-9-/- and faslpr) had returned to control levels (Fig 5B). However, neutrophils and CD8 T cells levels remained significantly increased in the mmp-9-/-/faslpr mice.
Altered levels of cytokines and chemokines in a long-term after EAE induction
Next we studied the levels of chemokines (Fig 6A) and cytokines (Fig 6B) after recovery at 100 days after EAE induction, thus in the absence of EAE symptoms. Interestingly, although the levels of most of the cytokines and chemokines decreased to control levels at 3 months after EAE induction, TNF-α, MIP-1α and IP-10 still remained significantly higher in comparison with healthy control animals for the WT and single mmp-9-/- genotypes, but not for the faslpr and the double mmp-9-/-/faslpr mice.
Fig 6.
Plasma chemokine and cytokine levels at 100 days after EAE induction. ELISA analysis of the indicated chemokines (A) and cytokines (B) from plasma samples obtained after 100 days in control animals (Cnt) and animals with EAE (EAE). The histograms represent the means ± SD of each group. P values were determined by ANOVA Kruskal-Wallis test. *p<0.05 versus control. Control data (Cnt) were obtained from 3–4 mice. EAE data were obtained from 4–8 mice.
EAE phase-specific changes of MMP-9 levels in WT and faslpr mice
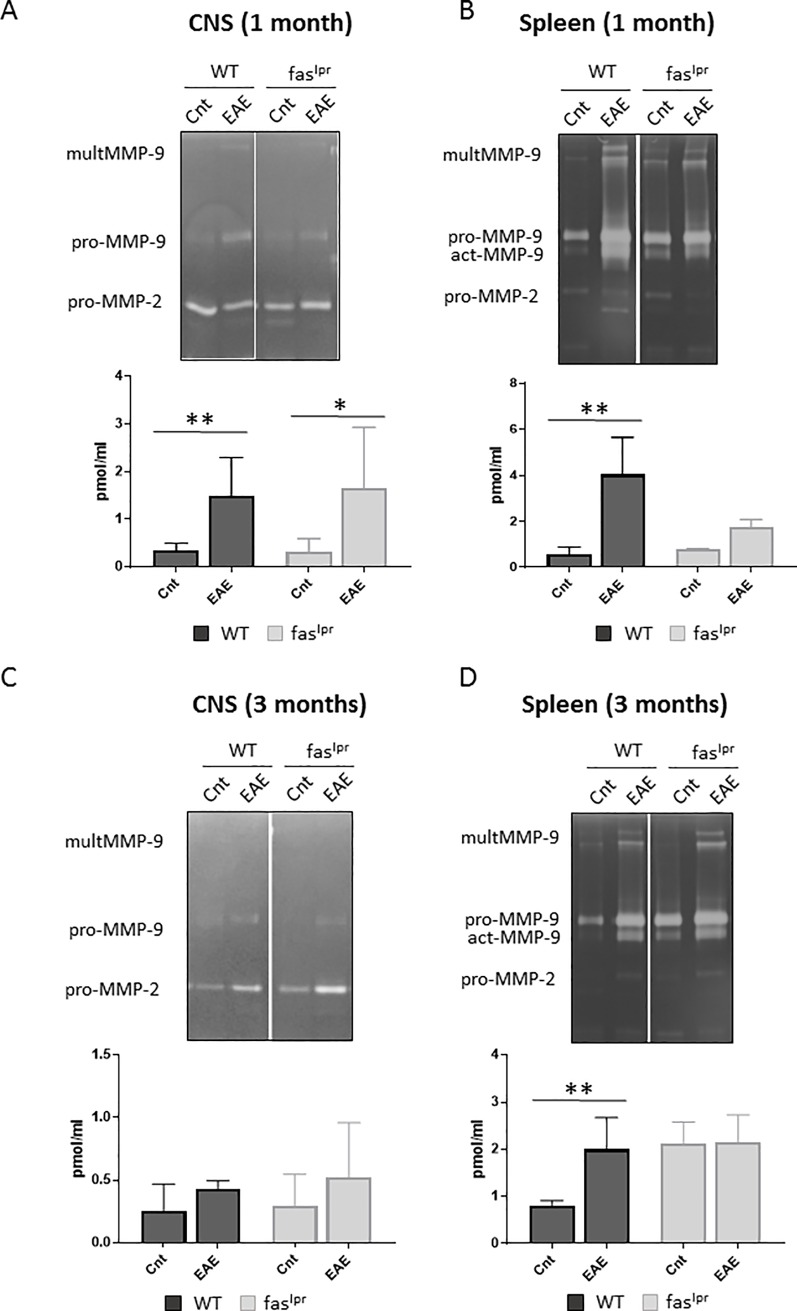
Because MMP-9 is a pivotal marker of inflammation in the CNS [12,17,33] and mmp-2/mmp-9-double knockout mice are resistant against EAE development [15,17], we evaluated the expression levels of the gelatinases MMP-2 and MMP-9 in the CNS and spleen (as a peripheral tissue) with optimized zymography analysis [31]. As previously demonstrated, by inducing EAE, MMP-9 levels were enhanced significantly in the CNS at 1 month (Fig 7A). Unexpectedly, in the immunized faslpr mice the MMP-9 levels in the brain were also significantly increased (Fig 7A), whereas these animals presented with significantly lower disease scores than WT mice. Furthermore, the levels of MMP-2 did not change significantly, neither by induction of EAE, nor by deletion of fas or mmp-9 (S1 Fig). This is in line with the view that MMP-2 is generally a constitutive enzyme in inflammation [12,24,34]. As a complementation, we evaluated MMP-9 tissue levels in the spleen as read-out of systemic organ effects (Fig 7B). MMP-9 and interestingly activated MMP-9 levels were increased significantly, at 1 month, by EAE induction in WT and in faslpr mice.
Fig 7.
MMP-9 upregulation in animals with EAE. Quantitative gelatin zymography analysis was used to analyze MMP-9 on tissue extracts from the central nervous system (CNS) (A) and spleens (B) after 1 month of EAE induction. Similar analyses were done at 3 months for CNS (C) and spleens (D). Volumes equivalent to 40 μl of tissue extracts were prepurified by gelatin-Sepharose. After purification, the samples were analyzed by gelatin zymography and the bands were quantified with the use of in-depth standardization. The histograms represent means ± SD of the quantification of MMP-9. P values were determined by ANOVA Kruskal-Wallis test. *p<0.05 and **p<0.01. Cnt 1m: Control at 1 month. 1m: EAE at 1 month. Cnt 3m: Control at 3 months. 3m: EAE at 3 months. Control data were obtained from 3–4 mice. EAE data were obtained from 4–8 mice.
We also analyzed and compared MMP-9 levels at 3 months after EAE induction (Fig 7C and 7D). In this analysis, we observed a decrease to basal levels locally in the CNS, both for WT and faslpr mice (Fig 7C). In contrast, MMP-9 levels remained high in the spleen of WT mice (Fig 7D). Although the WT animals had no longer signs of illness at that moment, the obtained data indicated that peripheral inflammation was still detectable.
MBP is degraded in immune complexes
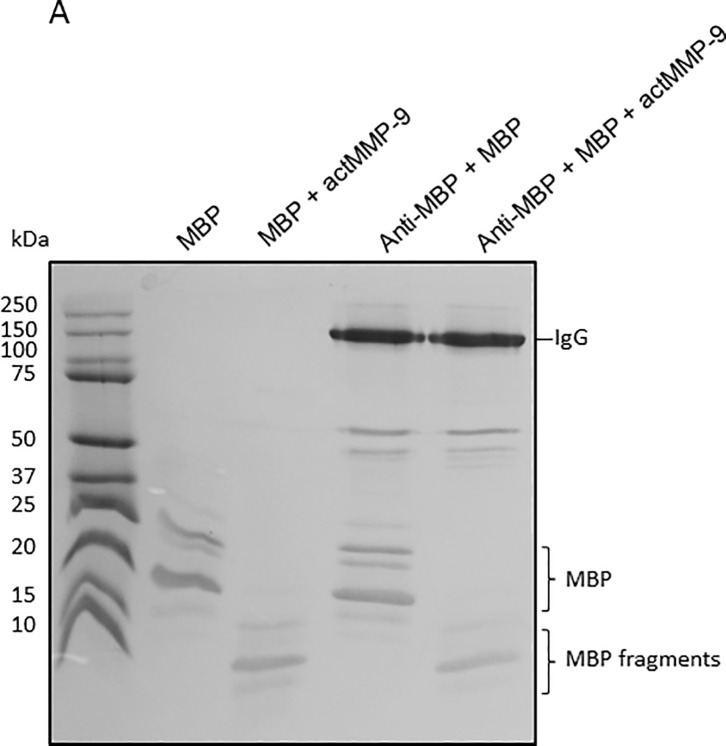
Mmp-9-/- animals showed increased disease scores and a delayed recovery in comparison with WT mice, thus suggesting that MMP-9 might play different roles in the pathogenesis of EAE, depending on an early or late disease phase [19]. Previously we showed that MMP-9 cleaves free MBP [35,36] and thus generates remnant epitopes of this auto-antigen, whereas independently and on long term MMP-9 might also contribute to the clearance of autoantigens [20]. We hypothesized that MMP-9 might clear immunogenic MBP peptides as such, but also might cleave MBP peptides caught within immune complexes (IC) between anti-MBP and MBP. Therefore, we analyzed the cleavage pattern of MBP in free form and in the form of IC. In Fig 8 it is shown that MBP was cleaved similarly when it was free or present in IC. These data are the first to indicate that MMP-9 is capable to destroy not only free autoantigens but also such autoantigens captured within IC.
Fig 8.
MBP and anti-MBP analysis. (A) Aliquots of 2 μg of MBP were incubated with anti-MBP antibody in an excess 1:4 molar ratio MBP:Ab during 15 minutes. Next, active MMP-9 was added at an enzyme:substrate ratio of 1:1000. After 16h, the reaction products of the incubations were analyzed by non-reducing SDS-PAGE and Coomassie blue staining. Immunoglobulin G (IgG), myelin basic protein isoforms (MBP) and MBP fragments are indicated. Whereas IgG remained intact, the free form and the immune-complexed IgG-bound form of MBP were cleaved into MBP fragments.
Discussion
Thanks to the use of preclinical studies with gene knockout mice and protease inhibitors, it has been suggested that MMPs, and MMP-9 in particular, may be therapeutic targets in MS. Although young mmp-9-/- mice are protected against EAE, adult mmp-9-/- mice develop EAE [14]. Therefore, an aggravating role for MMPs has been documented in EAE [15]. MMP-2 and other proteases in an inflammatory network [37,38] may act together to establish or control clinical disease, which can eventually be reversed with MMP inhibitors. An extreme phenotype was the observation of complete protection against EAE in mmp-2/mmp-9 double knockout mice [15]. However, in most preclinical studies only short-term effects of MMP-9 gene deletion or protein inhibition on EAE have been studied. Therefore, we performed, additionally, a long-term EAE study to analyze the role of MMP-9 after clinical remission.
MMP-9 plays a protective role in systemic autoimmunity in vivo, induced by fas deficiency [20] and single faslpr mice are protected against EAE [8–10, 32]. Whether the induction of EAE is extinguished or aggravated in double mmp-9-/-/faslpr knockout mice was not known. For that reason, we crossed faslpr mice, prone to systemic autoimmunity, with mmp-9-/- mice to dissect the net effect of MMP-9 on EAE development on short- and long-terms in an antigen-driven model of organ-specific autoimmunity.
In short-term EAE experiments, we showed that mmp-9-/- mice presented a slight delay in the disease onset but higher disease scores compared with WT mice, whereas, as expected, faslpr and mmp-9-/-/faslpr mice had minor disease symptoms and lower disease incidence. Interestingly, infiltration of leukocytes in the CNS and cytokine and chemokine levels were increased in the four genotypes studied, independently of the disease scores.
Analysis of the long-term EAE experiments, suggested a protective role of MMP-9 in the remission phase of the disease, thus mmp-9-/- mice needed longer time intervals to completely recover from the EAE symptoms. Furthermore, TNF-α, MIP-1α and IP-10 levels remained significantly higher in the WT and mmp-9-/- mice after 100 days of the EAE induction. It is important to remark the differences observed in the disease scores of WT and mmp-9-/- mice between the short and long term experiments, being higher in the short term. Beside the differences in the disease score levels, the effects produced by MMP-9 deficiency on EAE were similar in both experiments.
Due to the role of activated MMP-9 in breaking the blood brain barrier in MS [12,17], we expected a protective effect of deletion of MMP-9 on the induction of EAE in adult mice. In line with this expectation, disease onset was slightly delayed in mmp-9-/- mice, but in contrast, we observed in adult mice a significantly increased disease score during the plateau phase (Fig 1), which suggested that MMP-9 acted as a protective factor once the injury is made. This idea was reinforced by the observation of a delay in the remission of mmp-9-/- mice in comparison with WT mice (Fig 4A and 4B).
MMP-9-mediated processing of chemokines [39] plays an important role in cytokine and chemokine regulation in many pathologies and animal models of disease, including EAE. MMP-9 and MMP-2 selectively cleave chemokines and modulate their activity or availability [39,40]. In the context of the blood brain barrier, these proteases are also able to promote chemokine secretion by astrocytes after cleavage of Notch, which results in the inhibition of PTEN and the activation of the Akt/NF-kB pathway [41]. Such processes provide additional explanations why mmp-9-/- animals present a slight delay in the onset of the disease. The lack of MMP-9 will delay the expression of chemokines by astrocytes and, as a consequence, the migration of immune cells to the brain. Contrarily, MMP-9 could act as an inactivator of chemokines. For example, MMP-9 is able to cleave stromal-derived factor-1 (SDF-1/CXCL12), thereby inactivating this particular chemokine [42]. In the case of MCP-1/CCL2, the fragment generated after cleavage by MMP-2 behaves as a chemokine receptor antagonist [43]. The absence of MMP-9 will preserve the levels of intact chemokines and delay the inactivation of these specific chemokines and consequently maintain the disease course in the MMP-9 knockout mice, once it is initiated. These mechanisms might help to explain why mmp-9-/- mice presented a significantly prolonged remission phase compared with WT controls.
Why mmp-9-/- mice developed delayed but more exacerbated early EAE might also be explained by the overexpression of other MMPs [44]. For instance, Esparza and colleagues showed increased EAE in mmp-2-KO mice due to increased MMP-9 levels [45]. We tested the protein levels of MMP-2 in our mmp-9-/- mice and these were not significantly altered. In addition, by RNAseq analysis we found that basal levels of all mouse MMP mRNAs, except that of MMP-9, were not altered in our WT (n = 8) versus mmp-9-KO mice (n = 8) [26].
On long term, MMP-9 may also have beneficial effects, for example in the remyelination process [46] and it protects against systemic autoimmunity in faslpr mice [20]. For these reasons it was imperative to investigate what happens in an (auto-)antigen-driven condition in the presence or absence of MMP-9.
We hypothesized that MMP-9 helped in the clearance of (auto)antigens and immune complexes (IC). This hypothesis is supported by the in vitro experiments in which we show how MMP-9 is able to degrade MBP, even when MBP is in immune complexed form together with antiMBP-Ab (Fig 8). We choose on purpose to study MBP and not MOG for two reasons: (i) because we induced EAE with MOG35-55 peptide, the adaptive immune response might be skewed towards this peptide but not necessarily towards MBP and (ii) MBP is a water-soluble substrate of MMP-9 for which the remnant epitopes are well studied [35, 44].
Although the processing of MBP was discovered with MMP-9 as a prototypic MMP [35], also MMP-2, MMP-8, MMP-10, MMP-12, MT1-MMP and MT6-MMP are MMPs able to process MBP and its proteolysis was superior with MT6-MMP [44]. Several fragments of MBP including peptides 1–15, 68–86, 83–99, 84–104 and 87–99 are known autoantigens. Although MMP-9 is capable to generate the immunogenic peptide MBP1-15, it is also able to cleave at residues 91, 93, 97, 102, 109, 134, and 153, thus contributing to the degradation and clearance of the immunogenic peptides MBP83-99, MBP84-104 and MBP87-99. One might ask whether the generation of MBP remnant epitopes [11] becomes a next stimulus to induce a new autoimmune attack. Whereas this is perfectly possible and can not be excluded, it must be recognized that the autoantigen clearance remains an independent protective function. Indeed, those antibodies that capture the remnant epitopes autoantigens with the highest affinities will opsonize and eliminate these maximally, because the IgG remained intact, even after prolonged cleavage by MMP-9 (Fig 8). To understand the pathophysiology of and to better treat MS and other autoimmune diseases, it is thus critical to distinguish between the (early) phase of autoantigen generation, the awakening phase of the adaptive immune response and, as we here further document, the plateau and resolution phases [19]. By our finding of long-term persistence of innate cytokine and protease responses, we advocate that, for evaluations of novel treatments in preclinical settings, a long-term monitoring of cytokines, chemokines and MMP-9 will discriminate better between curative and symptomatic treatments than the earlier EAE studies, done with observations for only limited duration.
Another critical aspect, gaining more attention in recent research on MS and EAE, is the involvement of B-lymphocytes and antibodies. The oligoclonal antibody response in the cerebrospinal fluid has been used for a long time as a pathognomonic diagnostic test for MS, although the role of these immunoglobulins remains enigmatic. Nevertheless, antigen antibody complexes are pro-inflammatory by, for instance, activating the classical complement pathway. Destruction and resolution of immune complexes in the late phase of autoimmunity by proteolysis may thus be anti-inflammatory and beneficial.
Brändle et al. proved that distinct oligoclonal band antibodies in MS recognize ubiquitous intracellular self-proteins not specific to the brain, suggesting that the B-cell responses may be partially directed against intracellular autoantigen release during tissue destruction [47]. The oligoclonal antibodies that target intracellular proteins might be the result of a secondary immune reaction against cellular debris. These reactivities could constitute a more general characteristic of autoimmune and inflammatory diseases instead of being specific for MS. This viewpoint was already defined long ago by Grabar [48]. MMP-9 is able to degrade a broad range of intracellular proteins [49], thus the lack of this gelatinase might affect the amounts and persistence of intracellular antigens, thus increasing the levels of oligoclonal antibodies against these persistent proteins. This might explain why mmp-9-/- animals show higher disease scores, a prolonged plateau phase and a delayed remission in comparison with wild-type mice.
Mechanistically, the protective function of MMP-9 in the remission phase might be by autoantigen destruction and clearance, even for formed immune complexes. Whereas MMP-9 may be detrimental in the early phase of MS and EAE, once immune complexes with autoantigens have formed, MMP-9 becomes a protective factor by eliminating free and immune-complexed autoantigens.
As it was originally described, animals that lack FAS protein are protected to develop EAE [8–10, 32]. Surprisingly, the abundancies of CD4 and CD8-positive T cells, B cells, neutrophils, dendritic cells and macrophages were increased in the spleens and the central nervous system of these animals, even in those animals that did not show any disease symptoms. In addition, the MMP-9 levels in the brain and the spleen were increased suggesting that inflammation is present in these organs. The fact that these animals did not suffer from disease might be explained by lack of CNS cell apoptosis due the lack of FAS and disruption of the known neuronal apoptosis mechanism through Fas/Fas-ligand interaction [50,51]. A second explanation is the observation that only CNS leukocytes, which enter the brain parenchyma, cause disease symptoms. Leukocytes that are retained into vascular cuffs–which may become gigantic–do not cause disease symptoms [15, 17]. It is only when the blood-brain barrier is destroyed and the leukocytes penetrate through the barrier into the brain parenchyma that clinical neurological signs become evident [41]. This also explains why, irrespective of the persistence of significantly more cytotoxic T lymphocytes in the brains and spinal cords at 3 months in the single mmp-9-/- mice, no clinical disease was observed anymore.
MS patients show high cytokine levels even when they are in clinical remission phase, which implies that, although an association exists between specific cytokine profiles and the progression of the disease activity, MS patients have a constant and complex activation of the immune system [52]. Our data from long-term EAE studies were in line with this observation. We showed that the levels of TNF-α, IP-10, and MIP-1α remained at 100 days after EAE induction, significantly above basal levels in the groups with previously higher diseases scores (WT and mmp-9-/- mice), although the animals did not present anymore EAE symptoms. The latter situation may be correlated with high cytokine levels in the MS remission phase. Thus, although more studies need to be done, we suggest (i) that long-term studies after EAE induction represent a useful model to study the remission phase of MS patients and (ii) that therapy efficiency may be monitored with the analysis of such cytokines and chemokines, both preclinically and clinically.
In conclusion, whereas Fas is a strong driver molecule of EAE, MMP-9 is an independent and phase-specific effector molecule. In the plateau and early induction phase of EAE MMP-9 contributes to the generation of remnant epitopes and initiation of adaptive immune processes. In the later remission phase, MMP-9 becomes a protective factor when autoantigens in either free form or immune-complex form need to be eliminated and cytokines inactivated.
Supporting information
(TIF)
(TIF)
Acknowledgments
The present study was possible thanks to funding by the Geconcerteerde OnderzoeksActies (GOA 2013/015) and (C16/17/010), the Foundation for Scientific Research of Flanders (FWO-Vlaanderen) and the Charcot Foundation (Belgium).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present study was possible thanks to funding by the Geconcerteerde OnderzoeksActies (GOA 2013/015) and (C16/17/010), the Foundation for Scientific Research of Flanders (FWO-Vlaanderen) and the Charcot Foundation (Belgium).
References
- 1.Sospedra M & Martin R. Immunology of multiple sclerosis. Annu Rev Immuno. 2005;23: 683–747. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal SM & Yong VW. Immunopathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007; 79: 99–126. 10.1016/S0074-7742(07)79005-0 [DOI] [PubMed] [Google Scholar]
- 3.Garrido-Mesa N, Zarzuelo A & Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013; 169: 337–352. 10.1111/bph.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paemen L, Martens E, Norga K, Masure S, Roets E, Hoogmartens J & Opdenakker G. The gelatinase inhibitory activity of tetracyclines and chemically modified tetracycline analogues as measured by a novel microtiter assay for inhibitors. Biochem Pharmacol. 1996; 52: 105–111. [DOI] [PubMed] [Google Scholar]
- 5.Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M1 Cerchiaro G, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. New Engl J Med. 2017;376: 2122–2133. 10.1056/NEJMoa1608889 [DOI] [PubMed] [Google Scholar]
- 6.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple scleroosis. New Engl J Med. 2010;362: 402–415. 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 7.Loma I & Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011;9: 409–416. 10.2174/157015911796557911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabelko-Downes KA, Cross AH & Russell JH. Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. J Exp Med. 1999;189: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling P, Shang G, tLaval S, Menonna J, Cook S & Husar W. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med. 1996;184: 1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dittel BN, Merchant RM & Janeway CA. Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 1999;162: 6392–6400. [PubMed] [Google Scholar]
- 11.Opdenakker G & Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol Today. 1994;15: 103–107. [DOI] [PubMed] [Google Scholar]
- 12.Opdenakker G, Nelissen I & Van Damme J. Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol. 2003;2: 747–756. [DOI] [PubMed] [Google Scholar]
- 13.Gijbels K, Masure S, Carton H & Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992;41: 29–34. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104: 1507–1515. 10.1172/JCI6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G & Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203: 1007–1019. 10.1084/jem.20051342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Budde MD, Liang HF, Klein RS, Russell JH, Cross AH & Song SK. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis. 2006;21: 626–632. 10.1016/j.nbd.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 17.Gerwien H, Hermann S, Zhang X, Korpos E, Song J, Kopka K, et al. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci Transl Med. 2016;8: 364ra152 10.1126/scitranslmed.aaf8020 [DOI] [PubMed] [Google Scholar]
- 18.Vandooren J, Van den Steen PE & Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48: 222–272. 10.3109/10409238.2013.770819 [DOI] [PubMed] [Google Scholar]
- 19.Opdenakker G, Proost P & Van Damme J. Microbiomic and Posttranslational Modifications as Preludes to Autoimmune Diseases. Trends Mol Med. 2016;22: 746–757. 10.1016/j.molmed.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Cauwe B, Martens E, Sagaert X, Dillen C, Geurts N, Li S, et al. Deficiency of gelatinase B/MMP-9 aggravates lpr-induced lymphoproliferation and lupus-like systemic autoimmune disease. J Autoimmun. 2011;36: 239–252. 10.1016/j.jaut.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Cauwe B, Martens E, Proost P & Opdenakker G. Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integr Biol (Camb). 2009;1: 404–426. [DOI] [PubMed] [Google Scholar]
- 22.Dubois B, Arnold B & Opdenakker G. Gelatinase B deficiency impairs reproduction. J Clin Invest. 2010;106: 627–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanden Berghe T, Hulpiau P, Martens L, Vandenbroucke RE, Van Wonterghem E, et al. Mutations Confound Interpretation of All Genetically Modified Congenic Mice. Immunity. 2007;43: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Van den Steen PE, Sang QX & Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov.2007;6: 480–498. 10.1038/nrd2308 [DOI] [PubMed] [Google Scholar]
- 25.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479: 538–541. 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- 26.de Bruyn M, Breynaert C, Arijs I, De Hertogh G, Geboes K, Thijs G, et al. Inhibition of gelatinase B/MMP-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat Commun. 2017;8: 15384 10.1038/ncomms15384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berghmans N, Heremans H, Li S, Martens E, Matthys P, Sorokin L, et al. Rescue from acute neuroinflammation by pharmacological chemokine-mediated deviation of leukocytes. J Neuroinflammation. 2012;9: 243 10.1186/1742-2094-9-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legroux L, Pittet CL, Beauseigle D, Deblois G, Prat A & Arbour N. An optimized method to process mouse CNS to simultaneously analyze neural cells and leukocytes by flow cytometry. J Neurosci Methods. 2015;247: 23–31. 10.1016/j.jneumeth.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 29.Descamps FJ, Martens E & Opdenakker G. Analysis of gelatinases in complex biological fluids and tissue extracts. Lab Invest. 2002;82: 1607–1608. [DOI] [PubMed] [Google Scholar]
- 30.Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C, et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J Biol Chem. 2006;281: 18626–18637. 10.1074/jbc.M512308200 [DOI] [PubMed] [Google Scholar]
- 31.Vandooren J, Geurts N, Martens E, Van den Steen PE & Opdenakker G. Zymography methods for visualizing hydrolytic enzymes. Nat Methods. 2013;10: 211–220. 10.1038/nmeth.2371 [DOI] [PubMed] [Google Scholar]
- 32.Waldner H, Sobel RA, Howard E & Kuchroo VK. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol. 1997;159: 3100–3103. [PubMed] [Google Scholar]
- 33.Fainardi E, Castellazzi M, Bellini T, Manfrinato MC, Baldi E, Casetta I & et al. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler. 2006;12: 294–301. 10.1191/135248506ms1274oa [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg G. A. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8: 586–595. 10.1177/1073858402238517 [DOI] [PubMed] [Google Scholar]
- 35.Proost P, Van Damme J & Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 36.Gijbels K, Proost P, Masure S, Carton H, Billiau A & Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36: 432–440. 10.1002/jnr.490360409 [DOI] [PubMed] [Google Scholar]
- 37.Overall CM & Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25: 69–75. 10.1007/s10555-006-7890-0 [DOI] [PubMed] [Google Scholar]
- 38.Krüger A, Kates RE & Edwards DR. Avoiding spam in the proteolytic internet: future strategies for anti-metastatic MMP inhibition. Biochim Biophys Acta. 2010;1803: 95–102. 10.1016/j.bbamcr.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 39.Proost P, Struyf S, Van Damme J, Fiten P, Ugarte-Berzal E, Opdenakker G. Chemokine isoforms and processing in inflammation and immunity. J Autoimmun. 2017; 85: 45–57. 10.1016/j.jaut.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 40.Van den Steen PE, Proost P, Wuyts A, Van Damme J & Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96: 2673–2681. [PubMed] [Google Scholar]
- 41.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10: 1040–1054. 10.1016/j.celrep.2015.01.037 [DOI] [PubMed] [Google Scholar]
- 42.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I & Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276: 43503–43508. 10.1074/jbc.M107736200 [DOI] [PubMed] [Google Scholar]
- 43.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I & Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 44.Shiryaev SA, Savinov AY, Cieplak P, Ratnikov BI, Motamedchaboki K, Smith JW, & Strongin AY. Matrix metalloproteinase proteolysis of the myelin basic protein isoforms is a source of immunogenic peptides in autoimmune multiple sclerosis. PLoS One. 2009;4: e4952 10.1371/journal.pone.0004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esparza J, Kruse M, Lee J, Michaud M, & Madri JA. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J. 2004;18(14): 1682–91. 10.1096/fj.04-2445com [DOI] [PubMed] [Google Scholar]
- 46.Larsen PH, Wells JE, Stallcup WB, Opdenakker G & Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23: 11127–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brändle SM, Obermeier B, Senel M, Bruder J, Mentele R, Khademi M, et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci U S A. 2016;113: 7864–7869. 10.1073/pnas.1522730113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grabar P. "Self"and "not-self" in immunology. Lancet. 1974;1: 1320–1322. [DOI] [PubMed] [Google Scholar]
- 49.Cauwe B & Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2010;45: 351–423. 10.3109/10409238.2010.501783 [DOI] [PubMed] [Google Scholar]
- 50.D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184: 2361–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ethell DW. & Buhler LA. Fas ligand-mediated apoptosis in degenerative disorders of the brain. J Clin Immunol. 2003;23: 363–370. [DOI] [PubMed] [Google Scholar]
- 52.Kallaur AP, Oliveira SR, Colado Simão AN, Delicato de Almeida ER, Kaminami Morimoto H, Lopes J, et al. Cytokine profile in relapsing remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep. 2013;7: 1010–1020. 10.3892/mmr.2013.1256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.