Abstract
Background and objective
The involvement of the oral microbiota as a possible link between periodontitis, type 2 diabetes mellitus and obesity is still not well understood. The objective of the study was to investigate if glycemic control and obesity play a role in modulating the composition and diversity of the oral microbial ecology.
Material and methods
A cohort of patients with type 2 diabetes mellitus (n = 18) was recruited. Participants demonstrating improved glycemic control after 3 months (n = 6) were included in a second examination. A full mouth examination was performed to estimate periodontitis severity followed by sample collection (subgingival plaque and saliva). Generation of large sequence libraries was performed using the high-throughput Illumina MiSeq sequencing platform.
Results
The majority of participants (94.4%, n = 17) presented with moderate or severe forms of periodontitis. Differences in microbial composition and diversity between obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2) groups were statistically significant. Cross-sectional and longitudinal approaches failed to reveal statistically significant associations between HbA1c level and species composition or diversity.
Conclusions
Obesity was significantly associated with the oral microbial composition. The impact of glycemic control on oral microbiota, however, could not be assured statistically.
Introduction
There is mounting evidence that the intestinal microbiota may have an impact on both type 2 diabetes mellitus and obesity through alterations of metabolic processes in glucose and fatty acid metabolism pathways [1–4]. This impact is not only attributed to the composition of the intestinal microbiota, but also to a change in the bacterial diversity within the gut [3,5,6]. Interventional studies performed in the human and mouse model using fecal microbiota transplantation have shown that the gut microbiota plays a key role in weight regulation as well as in insulin sensitivity [7–9].
Periodontitis is the result of bacterially induced inflammation that extends into gum tissue leading to gradual destruction of connective tissues and alveolar bone [10]. Furthermore, periodontal inflammation has been linked to various systemic diseases with high prevalence, incidence, morbidity, and mortality; for example type 2 diabetes mellitus [11] and obesity [12]. In both cases, the pro-inflammatory milieu associated with both obesity and type 2 diabetes mellitus may play a key role in the mechanisms linking these two diseases with periodontal disease [13,14]. However, Shungin et al. argued that observational studies are sensitive to confounding, bias and reverse causality, and by employing Mendelian randomization causal analyses, concluded that total adiposity is unlikely to be causally related with periodontitis [15]. On the other hand, a 'two-way' interaction between type 2 diabetes mellitus and periodontitis is well-established [14], and implicates that not only is diabetes a risk factor for periodontitis, but periodontitis could have a negative effect on glycemic control.
Studies of the human microbiome and obesity have mainly focused on the distal gut and fecal microbiome samples, with less attention paid to the microbial composition in the upper gastrointestinal tract. Tsuda et al. [16] have demonstrated a high level of similarity, in both diversity and in composition, between the microbiotas of the oral cavity and the upper gastrointestinal tract. Interestingly, the fecal microbiota was shown to greatly differ from those of saliva and gastric fluid. Studies in mice have shown that there exists a causative link between oral pathogens and changes in the gut microbiota and in inflammatory status [17,18]. Hence, the aim of this study was to test the hypothesis that the composition and diversity of the oral microbiota may be associated with the glycemic state and body weight.
Methods
Study design and participants
This mono-centric, prospective cohort study was conducted at the University Hospital Dresden in the Department of Periodontology and in the Department and Outpatient Department of Medicine III. The target population included patients with poorly controlled type 2 diabetes mellitus in ambulant treatment with need of glycosylated haemoglobin (HbA1c) improvement. The inclusion criteria were: 18 to 80 years of age, type 2 diabetes mellitus as diagnosed following the recommendations of the American Diabetes Association (HbA1c ≥ 6.5%) [19], and at least 10 teeth and/or implants, wisdom teeth excluded. Patients were not included in the study when the following diseases and conditions were present: type 1 diabetes, endocarditis prophylaxis required, pregnancy or breastfeeding, incapability of assessing essence and possible consequences of the study, lack of compliance, consumption of more than five cigarettes per week, alcohol consumption exceeding 40 g/alcohol per day, requirement for medications known to influence the gingival condition (e.g. phenytoin, nifedipine, immune suppressive therapy, steroids, antiphlogistics), treatment with antibiotics three months prior to start of study, myocardial infarction and/or stroke within 2 years of start of study, current treatment of tumor disease, macroalbuminuria exceeding 300 mg/L albumin in urine and/or dialysis dependency, severe liver disorders (gamma-glutamyltransferase and alanin-aminotransferase activities exceeding 2 μmol/L x s), current participation in another study.
A total of 69 patients were questioned for participation. 51 patients either declined to participate or did not satisfy the necessary criteria. 18 participants were recruited for the study. Patients were motivated to exercise regularly and a healthy diet was promoted. Furthermore, oral antidiabetic medication and/or insulin were optimized. A significant improvement of glycemic control (IGC) was defined by an HbA1c reduction of at least 0.5% during the observation period of 3 months. Six participants demonstrated IGC and a follow up examination was performed 3 months after baseline. Comparisons were also made between obese and non-obese patients. Obesity was defined by body mass index (BMI) ≥ 30 kg/m2. Fasting blood samples were collected in the morning and processed within the same day. Fasting was defined as no caloric intake for ≥ 8 h. Laboratory parameters included fasting plasma glucose, HbA1c, triglyceride, total cholesterol, and LDL- and HDL-cholesterol concentrations.
All study participants received an appropriate description of the study protocol. Written informed consent was obtained before the study was performed, and the study protocol was approved by the Ethics Committee of the Technische Universitaet Dresden (EK 42022014) in accordance with the Declaration of Helsinki.
Oral examination and oral sample collection
Oral examination and sample collection was performed between 8:00 AM and 2:00 PM. All patients were asked to refrain from eating or tooth brushing for 1 h before oral examination and sample collection.
Participants underwent a complete periodontal examination. One trained and calibrated examiner (B.N.) conducted all periodontal measurements. Data collected included: periodontal probing depth (PD), clinical attachment loss (CAL) and bleeding on probing (BOP), all at six sites per tooth. A modification of the Silness-Löe plaque index (PI) was used to record plaque accumulation to determine oral hygiene status [20]. The diagnosis of periodontitis was assigned following recent consideration of diagnostic criteria for periodontal diseases [21,22]. Immediately after the oral examination, subgingival samples were collected at the deepest PD from each quadrant using sterile universal curettes after removal of supragingival plaque with a sterile swab and pooled together. Samples were transferred into sterilized microfuge tubes. Unstimulated whole saliva was collected by passive drooling into sterile plastic tubes and transferred into sterile microfuge tubes after collection of approximately 2 mL of whole saliva. All samples were immediately frozen at - 80°C.
DNA extraction and MiSeq sequencing
Bacterial chromosomal DNA extraction and purification was performed using the QIAamp DNA Stool Kit (Qiagen, Valencia, CA, USA). The DNA-isolation for Pathogen Detection Protocol was performed in accordance with the manufacturer’s guidelines with two modifications: (i) 0.7 mL of the ASL buffer was added to each sample instead of the recommended 1.4mL, and (ii) InhibitEX tablets were previously halved and added to each sample. DNA concentration and sample purity were estimated by Nanodrop (Thermo Scientific, San Diego, CA, USA). Bacterial 16S rRNA gene amplification, sequencing of the polymerase chain reaction products and subsequent diversity analysis were performed blinded at BGI Genomics Co, Ltd (Shenzhen, China). A region of approximately 469 base pairs encompassing the V3 and V4 hypervariable regions of the 16S rRNA gene was amplified using polymerase chain reaction and modified primers (341F: 5’-ACTCCTACGGGAGGCAGCAG-3’), 806R: 5’-GGACTACHVGGGTWTCTAAT-3’) [23]. The PCR conditions were as follows: 2 minutes of initial denaturation at 95°C followed by 30 cycles of denaturation at 95°C (20 s), annealing at 56°C (30 s), elongation at 72°C (45 s) and a final extension at 72°C for 7 minutes. Following purification of the amplicon pools using AMPure XT beads, sequencing was consequently performed on the Illumina MiSeq platform (San Diego, CA, USA) using the 300 PE MiSeq run.
Microbiome analyses
Overlapping paired-end reads were used to generate consensus sequences using Fast Length Adjustment of Short Reads (version 1.2.11) [24]. Clustering of tags into operational taxonomic units (OTUs) with a 97% threshold was obtained using UPARSE [25]. Chimeras were removed using UCHIME v.4.2.40 [26]. Taxonomic annotation was performed using the Ribosomal Database Project Classifier (version 2.2) [27] trained on the GreenGenes database (version 201305) [28] as well as the Human Oral Microbiome Database [29].
Statistical analysis
Statistical analyses were performed with the SPSS software version v22 (Statistical Package for the Social Sciences; SPSS, Chicago, IL). Alpha and beta diversity metrics were calculated to analyse within and between sample complexities. The non-parametric Wilcoxon-rank-sum test was utilized for comparing different sample groups as well as for comparisons between baseline and follow up examination. Correlation testing between diversity metrics and glycemic control or obesity was performed using Spearman's correlation analysis. The linear discriminant analysis (LDA) effect size (LEfSe) algorithm for high dimensional metagenomic biomarker discovery was used for identifying differentially abundant features between different conditions [30]. All analyses were run with LEfSe's α parameter for pairwise tests set to 0.05 and the threshold of the logarithmic score for LDA analysis set to 2.0.
Stringent multiple test correction was applied using the Benjamini Hochberg false discovery rate (FDR) [31]. Multidimensional scaling (MDS) was used for visualizing the level of similarity between species complexity before and after improved glycemic control.
Sample size calculation was performed based on a correlation analysis of glycemic control (HbA1c) with alpha and beta diversity parameters of OMB. The software package G*Power version 3.19.2, University Duesseldorf, Germany was used [32]. Assuming a moderate correlation, N = 16 plaque or saliva samples were necessary to detect a significant correlation between HbA1c and diversity indices (power = 0.8 and α = 0.05) [33].
Results
General findings
Characteristics of all study patients are summarized in Table 1. All of the participants in the study (n = 18) were prescribed oral antidiabetic medication and/or insulin. Of the 18 examined participants, 15 underwent statin therapy for management of blood cholesterol during the examination period. The majority of participants (94.4%, n = 17) presented with moderate or severe forms of periodontitis. The correlation between HbA1c and BMI was significant (r Spearman = 0.49, p = 0.039).
Table 1. Baseline demographic, periodontal, and metabolic data of the study population (mean ±SD) or number (%) of participants, n = 18.
| Male/female (number, %) | 10 (55.6)/ 8 (44.4) |
| Age (years) | 68.17 ± 6.83 |
| BMI (kg/m2) | 31.08 ± 6.09 |
| BMI ≥ 30 kg/m2 (number, %) | 6 (33.3) |
| Waist-hip ratio | 0.96 ± 0.06 |
| Oral antidiabetic medication (number, %) | 7 (38.9) |
| Insulin (number, %) | 3 (16.7) |
| Oral antidiabetic medication and Insulin (number, %) | 8 (44.4) |
| HbA1c (%) (minimum—maximum) | 8.22 ± 1.44 (6.8–13.5) |
| Total cholesterol (mmol/l) | 5.08 ± 2.23 |
| Low-density lipoprotein cholesterol (mmol/l) | 2.74 ± 0.97 |
| High-.density lipoprotein cholesterol (mmol/l) | 1.24 ± 0.31 |
| Triglycerides (mmol/l) | 3.14 ± 5.45 |
| Number of teeth (n) | 21 ± 5 |
| PI | 1.40 ± 0.61 |
| BOP (% sites) | 30.25 ± 19.82 |
| CAL (mm) | 3.39 ± 0.77 |
| PD (mm) | 2.67 ± 0.47 |
| CAL proximal ≥ 4 mm (% sites) | 34.78 ± 25.63 |
| CAL proximal ≥ 6 mm (% sites) | 6.25 ± 8.85 |
| PD ≥ 4 mm (% sites) | 15.89 ± 14.20 |
| PD ≥ 6 mm (% sites) | 1.44 ± 2.56 |
| Periodontitis n (%) | |
| Moderate periodontitisa | 7 (38.9%) |
| Severe periodontitis a | 10 (55.6%) |
| Generalized periodontitis b | 8 (44.4%) |
An average of 28139 valid tags per sample was obtained from 48 samples: saliva and pooled subgingival plaque samples at baseline n = 18 each, saliva and pooled subgingival plaque after IGC n = 6 each. In general, more valid tags were collected in saliva samples (28892 tags on average) than in plaque samples (27385 tags on average). Valid tags were clustered into OTUs at 97% similarity. A total of 386 distinct OTUs were identified in plaque and saliva samples. Of which, 358 were shared between both sample types; and 8 and 20 OTUs were exclusive to plaque and saliva respectively. Estimations of species richness and diversity in plaque and saliva by calculating alpha diversity metrics reported a statistically significant different distribution of species richness between plaque and saliva at baseline. On average, more OTUs were observed in saliva samples (Table 2). A difference in species diversity between plaque and saliva was found at least in trend (estimated by Shannon index and Simpson index, Table 2). Abundance values of all identified OTUs are presented in the supplemental material (S1 Table).
Table 2. Mean alpha diversities of plaque and saliva samples at baseline as calculated using different alpha diversity metrics (n = 18).
| Plaque | Saliva | P-value a | ||
|---|---|---|---|---|
| Observed OTUs | Mean ± SD | 165.72 ± 35.51 | 182.61 ± 46.27 | 0.025 |
| Median (IQR) | 165.00 (142.50; 195.00) | 194.50 (142.50; 222.00) | ||
| Chao1 | Mean ± SD | 186.07 ± 38.01 | 202.02 ± 48.64 | 0.022 |
| Median (IQR) | 180.02 (168.92; 213.80) | 208.57 (157.89; 243.54) | ||
| Shannon | Mean ± SD | 3.19 ± 0.62 | 3.41 ± 0.42 | 0.078 |
| Median (IQR) | 3.38 (2.58; 3.71) | 3.40 (3.16; 3.72) | ||
| Simpson | Mean ± SD | 0.11 ± 0.08 | 0.07 ± 0.03 | 0.022 |
| Median (IQR) | 0.09 (0.05; 0.19) | 0.07 (0.05; 0.09) |
IQR, Interquartile range
a Wilcoxon test
A total of 14 phyla was detected (Fig 1). In descending order, the six most dominant phyla present in plaque were Bacteroidetes (34.63%), Firmicutes (24.71%), Fusobacteria (22.40%), Proteobacteria (9.13%), Spirochaetes (3.42%) and TM7 (2.87%). Saliva samples, on the other hand, displayed different dominant phyla: (in descending order) Firmicutes (35.23%), Bacteroidetes (28.58%), Proteobacteria (17.59%), Fusobacteria (11.27%), TM7 (2.39%) and Spirochaetes (1.78%). Despite distinct hierarchal distributions of taxa in plaque and in saliva, the six most dominant phyla in this study population comprised approximately 96% of total detected phyla.
Fig 1. Comparison of the average taxonomy composition distribution at the phylum level in all plaque (outside) and saliva (inside) samples.
GN02, Elusimicrobia, Chloroflexi and Cyanobacteria have been classified as 'Other' due to low abundance levels <0.1%.
Glycemic status and composition of oral microbiome
No statistically significant associations between glycemic level and species composition or species diversity were found. Univariate Spearman's correlation analysis of the entire study population revealed no statistically significant correlation between HbA1c and community richness or evenness as measured by different alpha diversity indices (Table 3). While few bacterial taxa initially demonstrated a correlation with HbA1c, these were shown to not be significant after stricter testing corrections using FDR. Furthermore, LEfSe-analyses failed to report significantly discriminative features at different HbA1c levels.
Table 3. Spearman's correlation coefficients between HbA1c and alpha diversity indices in plaque and saliva samples (n = 18).
| Plaque | P-value | Saliva | P-value | |
|---|---|---|---|---|
| Sobs | - 0.150 | 0.552 | - 0.264 | 0.289 |
| Chao1 | 0.067 | 0.791 | - 0.282 | 0.256 |
| Shannon | - 0.237 | 0.344 | - 0.242 | 0.333 |
| Simpson | 0.220 | 0.380 | 0.195 | 0.437 |
Sobs, total observed species
Six of the 18 participants qualified for a second examination after demonstrating significant improvement of glycemic control after 3 months (Table 4). Five of these six patients had a baseline HbA1c value above the baseline median (HbA1c median = 8.05%). In summary, IGC participants demonstrated a mean HbA1c reduction of 1.57% (minimum = 0.8%, maximum = 3.6%). This IGC study population enabled a longitudinal approach for investigating relationships between microbial communities before and after improved glycemic control.
Table 4. Anthropometric and clinical data of patients demonstrating improved glycemic control at baseline and after 3 months (IGC population).
| Visit | IGC 1 | IGC 2 | IGC 3 | IGC 4 | IGC 5 | IGC 6 | |
|---|---|---|---|---|---|---|---|
| Sex | m | m | m | f | f | m | |
| Age (years) | 62 | 58 | 67 | 55 | 75 | 66 | |
| BMI (kg/m2) | Baseline | 34.60 | 31.41 | 25.22 | 46.23 | 29.05 | 35.92 |
| Visit 2 | 33.56 | 28.29 | 24.90 | 43.97 | 27.99 | 35.92 | |
| Waist-hip ratio | Baseline | 0.94 | 1.04 | 0.93 | 0.92 | 0.90 | 1.08 |
| Visit 2 | 0.95 | 1.01 | 0.97 | 0.93 | 0.89 | 1.06 | |
| RR systolic (mmHg) | Baseline | 180 | 144 | 122 | 156 | 148 | 153 |
| Visit 2 | 152 | 135 | 122 | 145 | 125 | 156 | |
| RR diastolic (mmHg) | Baseline | 96 | 81 | 85 | 88 | 71 | 77 |
| Visit 2 | 87 | 73 | 95 | 89 | 68 | 81 | |
| Triglycerides (mM) | Baseline | 1.71 | 1.46 | 1.00 | 2.85 | 1.37 | 1.77 |
| Visit 2 | 1.16 | 0.54 | 0.97 | 2.23 | 1.06 | 1.82 | |
| Total cholesterol (mM) | Baseline | 5.54 | 5.47 | 4.57 | 5.54 | 4.18 | 2.90 |
| Visit 2 | 5.42 | 4.39 | 4.47 | 4.66 | 3.32 | 2.82 | |
| LDL-C (mM) | Baseline | 3.91 | 3.71 | 2.94 | 3.32 | 2.66 | 1.41 |
| Visit 2 | 4.01 | 3.03 | 2.95 | 2.75 | 1.93 | 1.33 | |
| HDL-C (mM) | Baseline | 0.95 | 1.37 | 1.35 | 1.49 | 1.09 | 0.98 |
| Visit 2 | 0.99 | 1.47 | 1.37 | 1.40 | 1.07 | 1.02 | |
| HbA1c (%) | Baseline | 9.1 | 7.5 | 8.2 | 13.5 | 8.3 | 8.4 |
| Visit 2 | 7.8 | 5.9 | 6.9 | 9.9 | 7.5 | 7.6 |
HbA1c, glycosylated haemoglobin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; m, male; f, female, Visit 2: three month after baseline
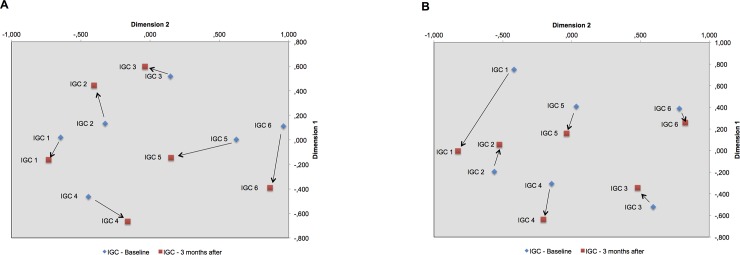
In both, plaque and saliva samples, all alpha diversity metrics failed to report a difference in richness or diversity between the two examinations (Wilcoxon rank sum p > 0.05) (Table 5). In addition, LEfSe-analysis as well as beta diversity analyses (Bray-Curtis-distance) also reported no differences. Using MDS based on Bray-Curtis-distance, the directional change of community composition following improved glycemic control was inconsistent and no uniform trend could be found (Fig 2).
Table 5. Comparison of alpha diversity measures (Mean ± SD) in participants demonstrating improved glycemic control (n = 6).
| Visit | Plaque | P-valuea | Saliva | P-valuea | |
|---|---|---|---|---|---|
| Sobs | Baseline | 169.00 ± 37.75 | 0.075 | 167.67 ± 56.96 | 0.753 |
| Visit 2 | 151.33 ± 57.33 | 161.33 ± 60.29 | |||
| Chao1 | Baseline | 194.76 ± 32.46 | 0.116 | 188.09 ± 59.52 | 0.463 |
| Visit 2 | 168.75 ± 56.45 | 181.92 ± 61.28 | |||
| Shannon | Baseline | 3.17 ± 0.74 | 0.753 | 3.27 ± 0.47 | 0.345 |
| Visit 2 | 3.26 ± 0.73 | 3.01 ± 0.47 | |||
| Simpson | Baseline | 0.13 ± 0.10 | 0.917 | 0.08 ± 0.03 | 0.345 |
| Visit 2 | 0.10 ± 0.07 | 0.11 ± 0.04 |
Sobs, total observed species; Visit 2, three month after baseline;
a Wilcoxon-rank-sum test
Fig 2.
Multi-dimensional scaling based on Bray-Curtis distance in plaque (A) and in saliva (B) before and after improvement of glycemic control.
Obesity and the composition of oral microbiome
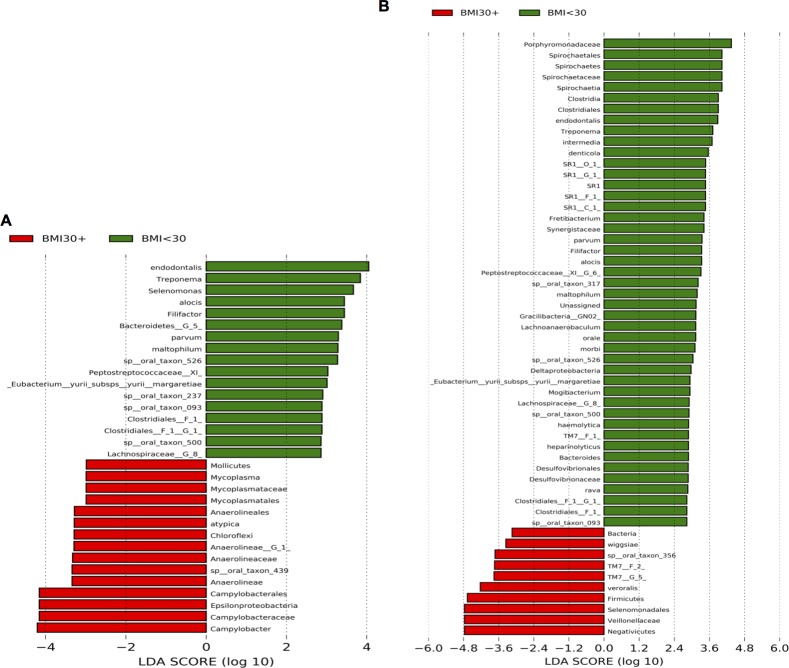
In order to analyse the potential association between body weight and the composition of the oral microbiome, the LEfSe analysis was repeated to search for significantly discriminative features between obese (BMI ≥ 30 kg/m2, n = 6) and non-obese patients with BMI < 30 kg/m2 (controls, n = 12). 32 significantly discriminative features were identified in plaque, and 55 in saliva (Fig 3). Especially in saliva, two interesting observations were made: (i) over four times as many discriminative features were found in the non-obese group, and (ii) the identification of the Firmicutes phylum as a significantly discriminative feature at over four orders of magnitude in patients with obesity.
Fig 3. Histogram of the computed LDA scores for features that are differential among conditions of interest with statistical and biological significance, ranked according to the effect size.
Conditions compared were (i) BMI < 30 kg/m2 (non-obese) and (ii) BMI ≥ 30 kg/m2 (obese, BMI +) in plaque (A) and in saliva (B).
Further, univariate Spearman's correlation analyses reported a statistically significant correlation between BMI and community richness/diversity as measured by alpha diversity metrics (Table 6). This observation was pronounced in plaque. An increase of BMI led to a significant reduction of Sobs, Chao1 and Shannon indices, and to a significant increase in the Simpson index. Thus, the conclusion can be made that BMI is negatively correlated with species richness and diversity in plaque. In saliva, measures of species richness (Sobs and Chao1) were found to be significantly inversely correlated with BMI.
Table 6. Spearman's correlation coefficients between BMI and different alpha diversity in plaque and saliva samples.
| Plaque | P-value | Saliva | P-value | |
|---|---|---|---|---|
| Sobs | - 0.595 | 0.009 | - 0.580 | 0.012 |
| Chao1 | - 0.517 | 0.028 | - 0.604 | 0.008 |
| Shannon | - 0.573 | 0.013 | - 0.461 | 0.054 |
| Simpson | 0.519 | 0.027 | 0.234 | 0.349 |
Sobs, total observed species
Discussion
In summary, the oral microbial composition was found to differ significantly between obese and non-obese subjects. Furthermore, a negative correlation between BMI and species diversity was observed in both subgingival plaque and in saliva. However, associations between the oral microbiome and glycemic level could not be replicated using cross-sectional or longitudinal approaches.
A large body of evidence supports the hypothesis that the gut microbiota may be associated with obesity. Interventional studies performed in the human and mouse model using fecal microbiota transplantation supported the causal effect of the gut microbiome on obesity [7–9]. The proposed pathophysiological mechanisms, with which the gut microbiota could contribute to the development of obesity, are not fully understood. Thus far, pathways describing (i) increased energy harvesting, (ii) the induction of inflammatory responses as well as (iii) a variety of metabolic and immune interactions by the gut microbiota have been of major interest. Bacteria specific pathways allow increased caloric uptake through microbial fermentation of otherwise indigestible dietary polysaccharides into absorbable monosaccharides [7,34]. Short chain fatty acids, specifically butyrate, acetate and propionate, are microbial-fermentation products directly involved in hepatic gluconeogenesis and cholesterol synthesis [35], and have also been shown to increase expression of the adipokine leptin [36,37]. Constant exposition to bacterial lipopolysaccharides (LPS) of gram-negative intestinal bacteria triggers a chronic low-grade inflammation, which has been shown to trigger body weight gain and insulin resistance [38]. In severe periodontitis, gram-negative anaerobes tend to dominate in the periodontal pocket [39]. The ulceration of the epithelium lining of periodontal pockets resulting from periodontitis represents a direct entry point for periodontal pathogens and bacterial products (e.g. LPS) into the systemic circulation. Thus, the oral microbiota could, together with intestinal bacteria, play a role in systemic inflammation and be involved in the microbiota-obesity-axis. Indeed, accumulating epidemiologic evidence has revealed a significant association between periodontitis and obesity [12,40].
Data from our study of the microbial composition within the oral cavity yielded some novel associations and also confirmed some previous findings. Our LEfSe analyses revealed distinct significantly discriminative features between two conditions (obese and non-obese patients). In subgingival plaque, LEfSe revealed a dominance of Bacteroidetes, Spirochaetes and Firmicutes in the population without obesity. In obese patients, Proteobacteria, Chloroflexi and Firmicutes were overrepresented, with the noted absence of representatives from the phylum Bacteroidetes. LEfSe analysis revealed a similar observation in saliva: in the control population, Bacteroidetes and Firmicutes, among others, were overly abundant. Notably, the phylum Firmicutes was identified in obese patients as an independent significantly discriminative feature with an abundance of over four orders of magnitude. These findings in the oral cavity mainly correspond to the hypothesis that the obesity-associated microbiota of the gut is characterized by reduced abundance of Bacteroidetes paralleled by an increased abundance of Firmicutes resulting in in lower ratios of Bacteroidetes to Firmicutes [3,34]. Importantly, the model that the Bacteroidetes/Firmicutes ratio alone may be linked to obesity might in fact be incomplete, as other factors may also be involved in the aetiology of this multifactorial metabolic disease. The first reported gut metagenomic analysis characterizing type 2 diabetes mellitus patients with obesity before and three months after bariatric surgery showed dramatic changes in the individual composition of the gut microbiota [4]. In particular, a substantial shift at the phylum level towards Proteobacteria with simultaneous decreases of both Firmicutes and Bacteroidetes after surgery was found. This shift in the microbial ecology of the gut was accompanied by marked reduction of BMI, significant improvement of the metabolic state and reduced inflammatory activity.
Beside different composition of oral microbiota in obese and non-obese subjects, reduced species diversity in the oral cavity of obese patients was pronounced in our study population. This result is in line with similar observations demonstrating reduced microbial diversity identified in the distal gut [3,5] as well as in the upper gastrointestinal tract [6] and which has been linked with obesity.
Although our results were limited to obesity within a cohort of patients with type 2 diabetes mellitus, it is plausible that obesity would also be associated with the oral microbiota in non-diabetic subjects. Studies mentioned above linking the microbiota of the gut and the upper digestive tract under non-diabetic conditions supports that hypothesis. In addition, the potential involvement of oral bacteria in obesity has also been investigated in healthy non-diabetes subjects. In saliva, Goodson et al. identified Selenomonas noxia, a representative of the Firmicutes phylum, to be a robust predictor of obesity [40]. The microbiotas of the oral cavity and the upper gastrointestinal tract are very similar [16], and oral pathogens may contribute to dysbiosis in the gut microbiota leading to impaired barrier function and systemic inflammation [17,18]. Obesity itself, is increasingly being associated with higher proportions of different periodontal pathogens [41,42].
Given the established inter-relationships between type 2 diabetes and periodontitis, it was anticipated and indeed observed that the study population of patients with type 2 diabetes mellitus presented with a high prevalence of periodontitis. The majority of participants (94.4%) were diagnosed with moderate or severe forms of periodontitis. An important consideration at this point is the inclusion criterion for the study: participants were required to have at least ten teeth (wisdom teeth excluded). The exclusion of edentulous patients or patients not meeting the inclusion criterion could have skewed the periodontitis prevalence. Given that the study population did not include an age-matched healthy population, comparisons with similar studies which included healthy, prediabetic and diabetic populations show that the results of the given study are likely in keeping with this consensus [43].
The effect of diabetes on the oral microbiome and its role in the aggravation of periodontitis remains unclear. There exist numerous studies demonstrating contrary results regarding a possible association between altered glucose metabolism and changes in the periodontal microbiome [14,44]. Currently, there is no consistent evidence of causal relation between glycemic state and periodontal microbial dysbiosis [44]. We were unable to replicate any associations between the oral microbiome and glycemic level. In the studies demonstrating a microbial shift as a result of diabetes [45–47], it is important to note that none of the three studies adjusted for body weight as a potential confounding factor. This may explain the contrary results as our study also demonstrates that body weight is associated with changes in the composition and diversity of the oral microbiota.
While our findings regarding obesity were largely in line with previous studies, this methodology presented with possible limitations that may have obscured the underlying relationship. The first limitation was the lack of a lean control group. In this case, a healthy weight group (18.5–25.0 kg/m2) was not included due to the study design. Our two groups only had a resolving power to differentiate between obese patients (BMI ≥ 30 kg/m2) and patients who were still overweight (BMI < 30 kg/m2). Secondly, the low resolving power as a result of the small sample size represents another potential limitation. It is well established that a reduction of HbA1c and blood pressure significantly reduces microvascular complications in patients with diabetes. A cross-sectional study from NHANES between 1988 and 2010 showed that while there appears to be a dramatic increase in patients with diabetes meeting the goals set by the ADA, there is still much room for improvement [48]. The authors also concluded that achieving the ADA recommendations may be biologically unattainable for some patients due to disease severity and other comorbidities. Glycemic control is also complicated in patients presenting with more severe ß-cell loss. Other factors including lack of management skills or lack of adherence to demanding self-care regiments, and an aversion to lifestyle changes are often attributed to complications of diabetes management. Given this, it was anticipated that only 4 of the 18 examined patients reached the ADA recommendation of HbA1c < 7.0%, despite many years of treatment with clinicians and diabetes educators. In the case of our study, only 6 participants demonstrated an improvement in glycemic control of at least of 0.5% HbA1c intensified diabetes treatment during the entire observation period of 3 months.
The oral cavity could represent a relevant surrogate representation of the gut microbiome [49]. Our study gives weight to previous findings that alterations in the oral microbiome have potential in sentinel diagnostic and prognostic application [49–51]. In conclusion, the results of the here presented study provide clues that oral bacteria may be involved in pathways leading to obesity, and this promising aspect warrants future examinations.
Supporting information
(XLSX)
Acknowledgments
We would like to thank Sigrid Nitzsche for her efforts in the isolation and purification of DNA.
Data Availability
Relevant data are within the paper and its Supporting Information files. Additional raw data are held in a public repository. The direct link is: http://www.ebi.ac.uk/ena/data/view/PRJEB28369.
Funding Statement
The authors acknowledge support by the Open Access Publication Funds of the SLUB/TU Dresden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crommen S, Simon MC. Microbial Regulation of Glucose Metabolism and Insulin Resistance. Genes 2017;9: 10 10.3390/genes9010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013;498: 99–103. 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature 2009;457: 480–484. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013;13: 514–522. 10.1038/tpj.2012.43 [DOI] [PubMed] [Google Scholar]
- 5.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500: 541–546. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 6.Lin SW, Freedman ND, Shi J, Gail MH, Vogtmann E, Yu G, et al. Beta-diversity metrics of the upper digestive tract microbiome are associated with body mass index: UGI Microbiome Beta-Diversity Associated with BMI. Obesity 2015;23: 862–869. 10.1002/oby.21020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444: 1027–1131. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 8.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101: 15718–15723. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143: 913–916. 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 10.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. The Lancet 2005;366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 11.Chapple ILC, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84: S106–112. 10.1902/jop.2013.1340011 [DOI] [PubMed] [Google Scholar]
- 12.Chaffee BW, Weston SJ. Association Between Chronic Periodontal Disease and Obesity: A Systematic Review and Meta-Analysis. J Periodontol 2010;81: 1708–1724. 10.1902/jop.2010.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6: 772–783. 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 14.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol 2013;40: S113–134. 10.1111/jcpe.12059 [DOI] [PubMed] [Google Scholar]
- 15.Shungin D, Cornelis MC, Divaris K, Holtfreter B, Shaffer JR, Yu YH, et al. Using genetics to test the causal relationship of total adiposity and periodontitis: Mendelian randomization analyses in the Gene-Lifestyle Interactions and Dental Endpoints (GLIDE) Consortium. Int J Epidemiol 2015;44: 638–650. 10.1093/ije/dyv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, et al. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol 2015;6: e89–e89. 10.1038/ctg.2015.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 2015;4 10.1038/srep04828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, et al. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLOS ONE 2015;10: e0134234 10.1371/journal.pone.0134234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33: S62–69. 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbott K, Mandel ID, Chilton NW. Reduction of baseline gingivitis scores with repeated prophylaxes. J Prev Dent 1976;4: 28–29. [PubMed] [Google Scholar]
- 21.Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. J Clin Periodontol 2005;32: 210–213. 10.1111/j.1600-051X.2005.00822.x [DOI] [PubMed] [Google Scholar]
- 22.Page RC, Eke PI. Case Definitions for Use in Population-Based Surveillance of Periodontitis. J Periodontol 2007;78: 1387–99. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- 23.Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014;2: 1 10.1186/2049-2618-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011;27: 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 2013;10: 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 26.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011;27: 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 2014;42: D633–642. 10.1093/nar/gkt1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol 2006;72: 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010;2010: baq013–baq013. 10.1093/database/baq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125: 279–284. [DOI] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39: 175–191. [DOI] [PubMed] [Google Scholar]
- 33.Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences vol. 5th Edition, Boston: Houghton Mifflin; 2003, p. 109. [Google Scholar]
- 34.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444: 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 35.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010;18: 190–195. 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 36.Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012;7: e35240 10.1371/journal.pone.0035240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 2004;101: 1045–1050. 10.1073/pnas.2637002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115: 1111–1119. 10.1172/JCI25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 2012;27: 409–419. 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodson JM, Groppo D, Halem S, Carpino E. Is Obesity an Oral Bacterial Disease? J Dent Res 2009;88: 519–523. 10.1177/0022034509338353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciel SS, Feres M, Gonçalves TED, Zimmermann GS, da Silva HDP, Figueiredo LC, et al. Does obesity influence the subgingival microbiota composition in periodontal health and disease? J Clin Periodontol 2016;43: 1003–1012. 10.1111/jcpe.12634 [DOI] [PubMed] [Google Scholar]
- 42.Silva-Boghossian CM, Cesario PC, Leao ATT, Colombo APV. Subgingival microbial profile of obese women with periodontal disease. J Periodontol 2018;89: 186–194. 10.1002/JPER.17-0236 [DOI] [PubMed] [Google Scholar]
- 43.Noack B, Aslanhan Z, Boué J, Petig C, Teige M, Schaper F, et al. Potential Association of Paraoxonase-1, Type 2 Diabetes Mellitus, and Periodontitis. J Periodontol 2013;84: 614–623. 10.1902/jop.2012.120062 [DOI] [PubMed] [Google Scholar]
- 44.Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol 2018;45: 150–166. 10.1111/jcpe.12803 [DOI] [PubMed] [Google Scholar]
- 45.Zhou M, Rong R, Munro D, Zhu C, Gao X, Zhang Q, et al. Investigation of the Effect of Type 2 Diabetes Mellitus on Subgingival Plaque Microbiota by High-Throughput 16S rDNA Pyrosequencing. PLoS ONE 2013;8: e61516 10.1371/journal.pone.0061516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casarin RCV, Barbagallo A, Meulman T, Santos VR, Sallum EA, Nociti FH, et al. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res 2013;48: 30–36. 10.1111/j.1600-0765.2012.01498.x [DOI] [PubMed] [Google Scholar]
- 47.Ganesan SM, Joshi V, Fellows M, Dabdoub SM, Nagaraja HN, O’Donnell B, et al. A tale of two risks: smoking, diabetes and the subgingival microbiome. ISME J 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People With Diabetes, 1988–2010. Diabetes Care 2013;36: 2271–2279. 10.2337/dc12-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodukula K, Faller DV, Harpp DN, Kanara I, Pernokas J, Pernokas M, et al. Gut Microbiota and Salivary Diagnostics: The Mouth Is Salivating to Tell Us Something. BioResearch Open Access 2017;6: 123–132. 10.1089/biores.2017.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Yao M, Lv L, Ling Z, Li L. The Human Microbiota in Health and Disease. Engineering 2017;3: 71–82. 10.1016/J.ENG.2017.01.008 [DOI] [Google Scholar]
- 51.Hall MW, Singh N, Ng KF, Lam DK, Goldberg MB, Tenenbaum HC, et al. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. Npj Biofilms Microbiomes 2017;3 10.1038/s41522-017-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Relevant data are within the paper and its Supporting Information files. Additional raw data are held in a public repository. The direct link is: http://www.ebi.ac.uk/ena/data/view/PRJEB28369.