Abstract
Opioid overdose deaths have been on the rise in the United States since 1999. Naloxone is a competitive opioid antagonist that rapidly reverses opioid overdose. The implementation of naloxone access laws and development of naloxone formulations that can be administered by laypersons have coincided with changes in the landscape of naloxone availability in the United States. Using data from IQVIA’s National Prescription Audit® we present the number of naloxone prescriptions dispensed quarterly from 2011 through the second quarter of 2017. The data demonstrate that nationwide naloxone dispensing increased nearly eight-fold from the fourth quarter of 2015 to the second quarter of 2017. Narcan® was the most commonly prescribed naloxone formulation as of the second quarter of 2017, accounting for 68% of prescriptions during that quarter followed by Evzio® (20%). There was considerable variability in the extent to which states experienced increases in naloxone dispensing, which may represent a general state-specific response to the opioid crisis, rather than direct association with opioid overdose death rates in a particular state. Although naloxone access laws continue to increase the amount of naloxone dispensed, cost remains a concern in terms of wide distribution of the life-saving medication.
Keywords: naloxone, opioid overdose, pharmacy, third-party prescribing, policy
1. Introduction
According to the Centers for Disease Control and Prevention (CDC, 2017), opioid overdose deaths have been on a steady incline since 1999. In fact, nationwide age-adjusted rates of opioid overdose deaths increased more than five-fold between 1999 and 2016. Naloxone, first approved by the Food and Drug Administration in 1971, is a competitive opioid antagonist that can rapidly reverse opioid overdose. Evidence suggests that communities with naloxone access have lower rates of opioid overdose deaths (Walley et al., 2013). In fact, the Surgeon General issued an advisory in April 2018 emphasizing the importance of increased awareness, understanding, and distribution of naloxone (Adams, 2018). Prior to the approval of the Evzio® autoinjector in April 2014 and Narcan® nasal spray in November 2015, the majority of naloxone prescriptions dispensed in the U.S. were off-label naloxone prefilled 2mg/ml syringes adapted for nasal administration (Jones et al., 2016).
Historically, access to naloxone for opioid overdose reversal has been achieved through community-based naloxone distribution programs (Wheeler et al., 2014), though as of 2014 only 8% of U.S counties had implemented Opioid Overdose Education and Naloxone Distribution programs (Lambdin et al., 2018). Beginning with New Mexico in 2001, third-party prescribing of naloxone has been implemented nationwide to increase access to naloxone (Davis and Carr, 2017, 2015). More recently, pharmacy-based policies have been deployed as a strategy to minimize barriers associated with obtaining the medication through traditional prescription-based methods (Davis and Carr, 2015).
Jones and colleagues (2016) analyzed national naloxone prescription trends from July 2010 through June 2015 and found that, although naloxone prescriptions increased by 11-fold between the fourth quarter of 2013 and second quarter of 2015, the total number of naloxone prescriptions dispensed in the U.S. remained low, with a national total of only 4,291 prescriptions dispensed in the second quarter of 2015. In the present study, we examine trends in rates of naloxone prescriptions by state and formulation to understand current patterns of naloxone dispensing in the context of expanded pharmacy access laws.
2. Methods
2.1. Data source
Data for naloxone prescriptions were obtained from IQVIA’s National Prescription Audit®, which provides nationwide estimates for prescriptions dispensed at the retail level, for the period of January 1, 2011 to June 30, 2017. The number of naloxone prescriptions dispensed (according to IQVIA’s national estimate) was plotted quarterly between 2011 and the second quarter of 2017. Based on classifications by Jones et al. (2016) naloxone formulations were categorized into the three following groups: 1) naloxone 2mg/2ml pre-filled syringes (syr) commonly used off-label as a nasal spray, 2) Evzio®, the autoinjector formulation (0.4mg/0.4ml and 2mg/0.4ml), and 3) naloxone 0.4mg/ml vials. A fourth group was added to include the FDA-approved nasal spray formulation (Narcan®). Bulk powder used for compounding is not included in any of the categories; at most, in the first quarter of 2011 it accounted for 17% (n=49 bulk powder) of prescriptions, but declined to less than 1% of prescriptions dispensed beginning in the 4th quarter of 2014. Trends in opioid overdose deaths, including heroin, natural and semisynthetic opioids, methadone, and non-methadone synthetic opioids, were obtained from the CDC’s report using data from the National Center for Health Statistics (CDC 2017). The CDC’s age-adjusted opioid overdose death rates (per 100,000 population) were plotted annually from 2011 to 2016. To illustrate the geographic and temporal changes in naloxone dispensing, the number of naloxone prescriptions dispensed in the second quarters of 2015 and 2017 were plotted as a rate (per 100,000 population) by state. Population estimates were taken from the U.S. Census Bureau.
3. Results
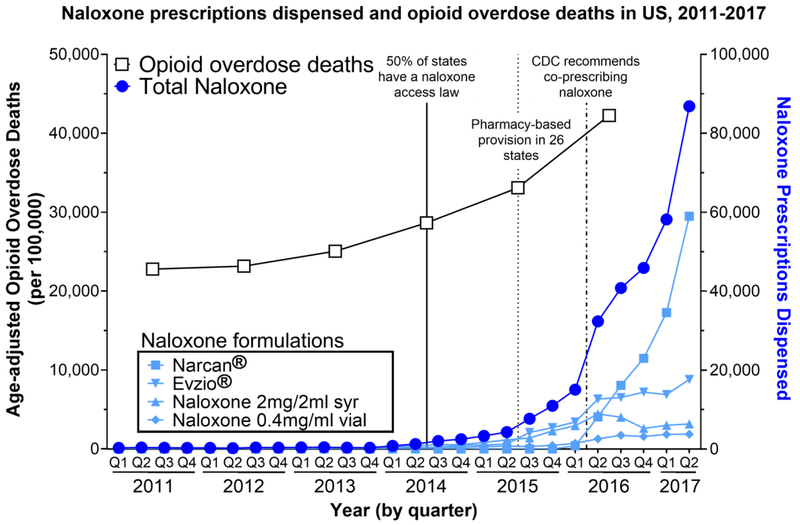
Nationwide, the number of naloxone prescriptions dispensed increased nearly eight-fold from the fourth quarter of 2015 to the second quarter of 2017 (Fig. 1, right y-axis). During the same time period, opioid overdose deaths continued to climb as they have since 1999 (Fig. 1, left y-axis). Since its approval in November 2015, Narcan® has become the most widely dispensed formulation, accounting for 68% of total naloxone prescriptions dispensed during the second quarter of 2017. Evzio® has been the second most widely dispensed, and accounted for 20% of naloxone prescriptions dispensed nationwide in the second quarter of 2017.
Figure 1.
Age-adjusted rates of opioid overdose deaths were obtained from the CDC through the National Center for Health Statistics. Number of naloxone prescriptions dispensed was obtained from IQVIA. Information regarding naloxone access laws is from pdaps.org.
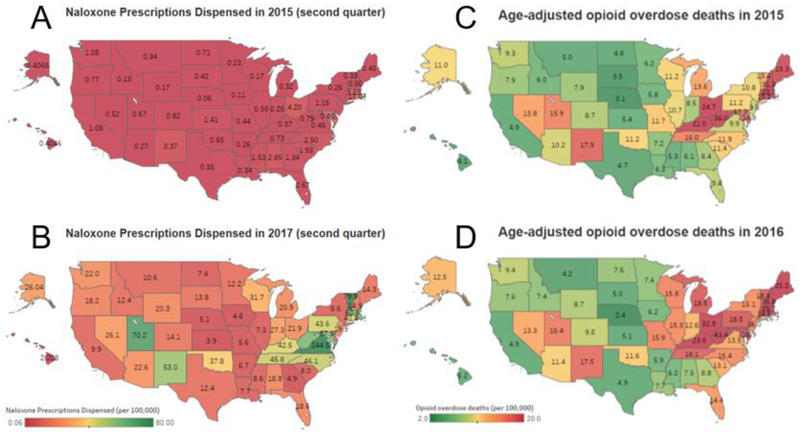
There was a nationwide surge in naloxone prescriptions dispensed after 2015, specifically over the second quarter of 2015 through 2017 (Fig. 2, panels A and B). However, rates differed widely by state as did rates for opioid overdose (Fig 2, panels C and D). For instance, in the second quarter of 2017, the lowest rate of naloxone dispensing was 2.2 prescriptions per 100,000 persons in Hawaii and the highest naloxone dispensing rate was 244 prescriptions per 100,000 persons in Virginia. Age-adjusted rates of opioid overdose in 2016 ranged from 2.4 per 100,000 persons in Nebraska to 43.4 per 100,000 persons in West Virginia.
Figure 2.
Panels a and b: Rates (per 100,000) of naloxone prescriptions dispensed by state during the second quarters of 2015 (a) and 2017 (b). Data source: IQVIA National Prescription Audit. Panels c and d: Rates (per 100,000) of age-adjusted opioid overdose deaths by state for 2015 (c) and 2016 (d). Data source: Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999-2016 on CDC WONDER Online Database, released December, 2017. Data are from the Multiple Cause of Death Files, 1999-2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/mcd-icd10.html on May 24, 2018. Data are provisional and subject to change.
4. Discussion
Naloxone is a life-saving treatment to reverse opioid overdose and, thus, is a critical part of the public health response to the ongoing opioid epidemic. Since the end of 2015, naloxone dispensing nationwide has increased dramatically, driven largely by increases in dispensing of Narcan®. Our results are consistent with a recent report on the number of naloxone prescriptions dispensed between the third quarter of 2010 and the second quarter of 2015, which revealed large (up to 10-fold) increases in naloxone dispensing during that period (Jones et al., 2016). The report also foreshadowed the rise in the number of Evzio® prescriptions dispensed, which increased 14-fold between the second quarters of 2015 and 2017. The fact that, of the two FDA-approved naloxone products, Narcan® has captured the greatest proportion of the retail market share is expected given the cost differences between the two products ($150 per two pack of the Narcan® intranasal devices and $4500 per two pack of the Evzio® autoinjector; Gupta et al., 2016). Although vials are the least expensive option and have historically been utilized by many opioid overdose education and community naloxone distribution programs, retail pharmacy dispensing of naloxone has gravitated toward the FDA-approved products specifically designed for bystander administration (Kerensky and Walley, 2017). Notably, the cost of all formulations remains a significant concern to public health officials who cite cost as a barrier to larger scale dissemination (Gupta et al., 2016).
It would be reasonable to presume that states with the highest opioid overdose deaths rates would have the highest rates of naloxone prescribing as a harm reduction approach. Interestingly, however, state naloxone dispensing rates do not appear to correlate with state rates of opioid-related overdoses. For example, of the states with the highest rates of naloxone dispensing in the second quarter of 2017 – Virginia, Vermont, Utah, Massachusetts, and West Virginia– only West Virginia ranked in the top five states based on age-adjusted opioid overdose death rates. Figure 2 demonstrates the relative mismatch in several states between opioid overdose death rates and naloxone dispensing suggesting that improved processes to increase dispensing to areas of need may be of significant public health benefit.
The sharp increase in naloxone dispensed across the U.S. is not uniform and may reflect a variety of factors that vary by state such as the amount of state and local attention given to escalating overdose deaths, influence of the CDC’s 2016 recommendation regarding naloxone coprescription with high-dose opioids and concurrent benzodiazepine use (Dowell et al., 2016), and adoption of policies that specifically facilitate pharmacy-based access to naloxone (Davis and Carr, 2015). For example, Virginia’s rate of prescribing in the second quarter of 2017 was three times higher than Vermont, which had the second highest rate of naloxone prescriptions dispensed during this time. Both states authorized pharmacists to dispense naloxone via standing order in late 2016 (Commonwealth of Virginia, Office of the Governor, 2016; Vermont General Assembly, 2013), but Virginia amended opioid prescribing regulations to require co-prescribing of naloxone for any patient who has risk factors for overdose, including prior overdose, substance misuse, opioid doses in excess of 120 MME/day, or concomitant use of benzodiazepines (Virginia Register of Regulations, 2017).
Similarly, naloxone access policies and advocacy efforts may have been an important factor for the higher rates of naloxone dispensing observed in the remaining three states in the top five - Utah, Massachusetts, West Virginia. For example, Utah, which had the 3rd highest rate of naloxone prescribing in the second quarter of 2017 authorized pharmacists to dispense naloxone by standing order in 2016 and has made significant efforts to promote retail pharmacy distribution of naloxone through Utahnaloxone.org, which lists and maps retail pharmacies that stock naloxone kits (Utahnaloxone.org). Massachusetts, which authorized prescriber standing orders for pharmacy-based access to naloxone in 2014, has a long history of promoting access to naloxone rescue kits to reduce opioid overdose deaths, beginning in 2006 with its community overdose prevention education programs (Davis et al., 2015). As noted, West Virigina was in the top five states for rates of both opioid overdose deaths and naloxone dispensing, so this in case the severity of the problem may be driving naloxone prescribing.
The data we present here reveal a sharp increase in naloxone dispensing after 2015, consistent with the diffusion of pharmacy-based naloxone access laws. Future work should evaluate the impact of naloxone access policies on opioid overdose deaths in the context of the translational impact of the policies on naloxone availability and the type of policy. Indeed, recent work suggests that states with a standing-order or third-party provisions experienced a 79% increase in naloxone prescriptions dispensed relative to states without these laws (Xu et al., 2018), however the impact of specific policies on opioid overdose deaths remains unknown. Other possible factors contributing to overdose mortality that should be considered in future research include the growing availability of highly potent illicit opioids (e.g., fentanyl), baseline rates of opioid use disorder, and access to effective medication treatment for opioid addiction. Particularly, as concern over the effectiveness of naloxone for fentanyl and other highly potent synthetic opioids grows (Fairbairn et al., 2017), future work will need to elucidate the impact of naloxone prescribing characteristics in the context of the distribution of these opioids. Similar to the association of prescription drug monitoring programs on opioid overdose deaths, the impact of greater naloxone access on the opioid epidemic is likely temporally dissociated from the implementation of policies (Pauly et al., 2018). Based on the recent surge in naloxone dispensing, it is likely that the impact of naloxone access laws on the opioid crisis is yet to be fully realized.
Highlights.
Opioid overdose deaths have been on the rise in the US since 1999.
States have implemented laws to increase access to the opioid antagonist, naloxone.
Number of naloxone prescriptions dispensed in US has risen considerably since 2015.
State rates of dispensed naloxone prescriptions are highly variable.
Cost remains a concern in terms of wide distribution of the life-saving medication.
Acknowledgements
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IQVIA. All Rights Reserved.
Author Disclosures
Role of Funding Source
This work was funded, in part, by NIDA T32 DA016176 and CTSA UL1TR001998.
Footnotes
Disclaimer
The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities.
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, 2018. Increasing naloxone awareness and use: The role of health care practitioners. JAMA 319, 2073–2074. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2017. NCHS Data Brief, Number 294. Drug Overdose Deaths in the United States, 1999–2016. Available at https://www.cdc.gov/nchs/data/databriefs/db294_table.pdf#page=4. (Accessed 16 May, 2018).
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, 2017. About Multiple Cause of Death 1999–2016. Available at http://wonder.cdc.gov/mcd-icd10.html. (Accessed 16 May, 2018).
- Commonwealth of Virginia, Office of the Governor, 2016. Opioid addiction crisis declared a public health emergency in Virginia. [Press Release]. Available at https://governor.virginia.gov/newsroom/newsarticle?articleId=18348. (Accessed 16 May, 2018).
- Davis CS, Carr D, 2017. State legal innovations to encourage naloxone dispensing. J. Am. Pharm. Assoc. 57, S180–S184. [DOI] [PubMed] [Google Scholar]
- Davis CS, Carr D, 2015. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alcohol Depend. 157, 112–120. [DOI] [PubMed] [Google Scholar]
- Davis CS, Walley AY, Bridger CM, 2015. Lessons learned from the expansion of naloxone access in Massachusetts and North Carolina. J. Law Med. Ethics, 43, Suppl.19–22. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Morb. Mortal. Wkly. Rep. Recomm. Rep 65 (No. RR-1), 1–49. doi: http://dx.doi.org/10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- Fairbairn N, Coffin PO, Walley AY, 2017. Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: Challenges and innovations responding to a dynamic epidemic. Int. J. Drug Policy 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Shah ND, Ross JS, 2016. The rising price of naloxone—risks to efforts to stem overdose deaths. N. Engl. J. Med. 375, 2213–2215. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lurie PG, Compton WM, 2016. Increase in naloxone prescriptions dispensed in US retail pharmacies since 2013. Am. J. Public Health 106, 689–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerensky T, Walley AY, 2017. Opioid overdose prevention and naloxone rescue kits: What we know and what we don’t know. Addict. Sci. Clin. Pract 12,4 10.1186/s13722-016-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Davis CS, Wheeler E, Tueller S, Kral AH, 2018. Naloxone laws facilitate the establishment of overdose education and naloxone distribution programs in the United States. Drug Alcohol Depend. 188, 370–376. [DOI] [PubMed] [Google Scholar]
- Pauly NJ, Slavova S, Delcher C, Freeman PR, Talbert J, 2018. Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depend. 184, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utah Naloxone. Pharmacies with naloxone rescue kits. http://www.utahnaloxone.org/utah-pharmacies-with-naloxone-rescue-kits. (Accessed 13 July, 2018).
- Vermont General Assembly, 2013. 18 V.S.A. 4240. Prevention and treatment of opioid-related overdoses. https://legislature.vermont.gov/statutes/section/18/084/04240. (Accessed 23 May, 2018).
- Virginia Register of Regulations, Vol. 33 Iss. 16 (Emergency Regulation) 18VAC85–21, 2017. Regulations Governing Prescribing of Opioids and Buprenorphine. Available at http://register.dls.Virginia.gov/details.aspx?id=6295. (Accessed 16 May, 2018).
- Walley AY, Xuan Z, Hackman HH, et al. , 2013. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: Interrupted time series analysis. BMJ 346, f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ, Centers for Disease Control and Prevention, 2015. Opioid overdose prevention programs providing naloxone to laypersons – United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 64, 631–5. [PMC free article] [PubMed] [Google Scholar]
- Xu J, Davis CS, Cruz M, Lurie P, 2018. State naloxone access laws are associated with an increase in the number of naloxone prescriptions dispensed in retail pharmacies. Drug Alcohol Depend. 189, 37–41. [DOI] [PubMed] [Google Scholar]