ABSTRACT
Introduction: The skin acts as a barrier and prevents transcutaneous delivery of therapeutic agents. Transfersomes are novel vesicular systems that are several times more elastic than other vesicular systems. These are composed of edge activator, phospholipids, ethanol, and sodium cholate and are applied in a non-occlusive manner.
Areas covered: This article covers information such as merits/demerits of transfersomes, regulatory aspects of materials used in preparation, different methods of preparation, mechanism of action, review of clinical investigations performed, marketed preparations available, research reports, and patent reports related to transfersomes.
Expert opinion: Research over the past few years has provided a better understanding of transfersomal permeation of therapeutic agents across stratum corneum barrier. Transfersomes provides an essential feature of their application to variety of compositions in order to optimize the permeability of a range of therapeutic molecules. This is evidenced by the fact that there are several Transfersome products being processed in advanced clinical trials. It is noteworthy that a number of Transfersome products for dermal and transdermal delivery will gain a global market success in near future.
KEYWORDS: Transfersomes, transdermal drug delivery, phospholipid, actinic keratoses, basal cell carcinoma, squamous cell carcinoma, melanoma, Kaposi’s sarcoma
1. Introduction
Cancer is one of the lead factors responsible for deaths worldwide, caused by persistent tissue injury, host environment relations, etc. The frequent contact of carcinogens such as tobacco, ultraviolet light, and infections leads to various genetic (mutations), epigenetic (loss of heterozygosity), and worldwide transcriptome changes (via inflammation pathways) and is linked with increased cancer risk [1]. Owing to increased rate of cancer and global prevalence during the last decade, it has posed a great challenge to health-care professionals. World Health Organization (WHO) statistics suggest a concerning 45% boost in global cancer deaths by 2030, of which 70% would be contributed from emerging countries such as India [2]. With constant progression in the field of science and technology, the need to address the practical problems associated with the drug therapies increased proportionately. Cutaneous melanoma is the most violent skin cancer, accounting for 75% of all deaths [3].
In 2012, the most common causes of cancer death worldwide (for both sexes) were:
Lung cancer (19% of all cancer deaths; 1.6 million people).
Liver cancer (9% of all cancer deaths; 745,000 people).
Stomach cancer (9% of all cancer deaths; 723,000 people).
Colorectal cancer (9% of all cancer deaths; 694,000 people).
Breast cancer (6% of all cancer deaths; 522,000 people).
Cancer of the esophagus (5% of all cancers diagnosed; 400,000 people).
Pancreas cancer (4% of all cancers diagnosed; 330,000 people).
In 2012, the most common causes of cancer death worldwide (for males and females) were:
Among males: lung, liver, stomach, colorectal, and prostate.
Among females: breast, lung, colorectal, cervical, and stoma
During 2016, a predicted population of around 1,685,210 consisted new cases of cancer which were diagnosedin the United States out of which around 595,690 people were died from the disease. In 2016, the most prevailing forms of cancer were; breast cancer, lung and bronchus cancer, prostate cancer, colon and rectum cancer, bladder cancer, melanoma of the skin, Non-Hodgkin lymphoma, thyroid cancer, kidney and renal pelvis cancer, leukemia, endometrial cancer, and pancreatic cancer.[4].
1.1. Transfersomes
The newly presented novel drug carriers are highly deformable vesicles, i.e. transfersomes, which can carry large molecules across intact mammalian skin. A transfersome, in the widest sense of the word, is a tool that can pass instinctively through a skin and transfer drugs from the application to the target site [5–7]. The use of lipid vesicles as a drug delivery system for skin treatment has gained increasing attention this year, but it remains controversial; mainly relevant reports cite the localization effect of liposomes, with the transport processes reported in a few cases, depending on the formulation [8,9]. To resolve these issues, a novel type of highly deformable lipid vesicle known as transfersome has been reported recently to go through intact skin, if applied non-occlusively, since non-occlusive conditions are essential to generate a transepidermal osmotic gradient, which acts as the driving force for elastic transport into the skin. The osmotic gradient is caused by the difference in water concentration between the skin surface and skin interiors. Transfersomes are highly deformable, and this property assists in their quick penetration through the intercellular lipid pathway of the subcutaneous tissue. Some of the exploratory findings reported on the existence of misdeeds inside the intercellular lipid packing of murine subcutaneous tissue, which acts as the virtual channel through which transfersomes can penetrate [6,10]. Transfersomes have been defined as specially designed vesicular particles consisting of at least one inner aqueous compartment enclosed by lipid vesicles; liposomes in morphology, but, functionally, transfersomes are suitably deformable to go through pores much smaller than their own size. A schematic diagram of the structure of transfersomes is presented in Figure 1.
Figure 1.
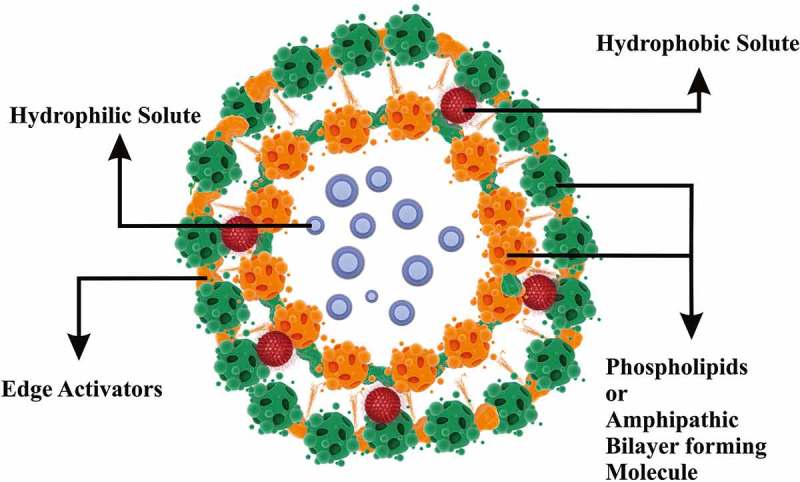
Structure of transfersomes.
Introduced in 1992, Cevc and Blume second-generation vesicular carriers, named Ultradeformable liposomes or Transfersomes®, possess slighter vesicular size (typically <300 nm) and higher elasticity (typically five–eight times higher compared with conventional liposomes) [10–12].
1.2. Historical background
The transfersome term was first introduced by Cevc [10] and has been the subject of several patents and literature information since the 1990s (Transfersomes, a trademark of IDEA AG, Munich, Germany), and it represents the first generation of ultradeformable vesicles. The skin permeation and penetration of these elastic vesicles result from a synergic mechanism among the carrier properties and the access enrichment ability. Transfersomes are ultradeformable lipid bundles of aggregates in supramolecular form constructed with a minimum of one interior aqueous segment encircled by a lipid bilayer exhibiting adapted properties, which are appropriate under the presence of surfactants in the vesicular membrane (edge activator (EA)) [13–18]. Even if it is generally accepted that the permeation of, usually, liposomes is limited to the outer layer of the stratum corneum, thus providing a drug or cosmetic localizing effect within the skin, transfersomes are claimed to infuse as intact vesicles through the skin layers to the complete circulation. Non-steroidal anti-inflammatory drug (NSAID) ketoprofen was successful on a validation basis and was immensely popular in the market. Ketoprofen was authorized by a Swiss regulatory agency (Swiss Medic) in 2007; the trade name was ‘Ketoprofen transdermal’, manufactured by ‘IDEA AG’ pharmaceuticals Pvt. Ltd.
2. Merits/demerits
Transfersomes defeat the skin obstacle by opening extracellular pathways among the cells in the organ and then deforming to fit into such passages. In the process, transfersomes go through a series of stress-dependent adjustments of the local carrier symphony to minimize the struggle of motion through the otherwise confining channels. This process allows transfersomes to convey the drugs associated into and diagonally across the skin easily and very reproducibly. This happens at a rate largely higher than that achieved by more predictable formulations and offers an excellent means for controlling drug distribution in the skin [19,20]. Transfersomes have been used as carriers for different therapeutic agents, including proteins, insulin [21,22], DNA [23], gap junction protein [24], peptides [25], albumin [15], nutraceuticals [26], corticosteroids [27], antigens [28], analgesics [29], sex hormones [30], and anesthetics [31], and have been proven to augment significantly the amount of drug permeated through the skin [10]. The topical application of transfersome-entrapped anticancer drug is described by a few research groups. Skin delivery of 5-fluorouracil (5-FU) [32,33,34], methotrexate, and bleomycin has been evaluated by many researchers. Edge activators are often single chain surfactant that destabilizes lipid bilayers of the vesicles and provides a flexible membrane, eventually making transfersomes highly flexible. The role of sodium deoxycholate, Spans, and Tween on the skin penetration and deposition is discussed in [35–37]. The application of transfersomes does not engage any intricate procedure and they can be applied by a non-occluded process, whereby they pass throughout the multilayered lipid matrix of the stratum corneum as an outcome of the hydration or osmotic force within the skin [38]. One main drawback of these vesicles corresponds to the difficulty of loading hydrophobic drugs into the vesicles without compromising their deformability and elastic properties [39]. Transfersomes, by virtue of their enhanced elasticity in contrast to standard liposomes, are more amenable to the transport of therapeutic agents across the human skin. [40].
2.1. Mechanism of action
The present investigation indicates that the transfersomes are drug mover systems that can penetrate across intact skin. It is believed that the unimpeded passage of such carriers is based on two key factors: the high elasticity (deformability) of the vesicle bilayers and the reality of an osmotic gradient across the skin. Because of their high deformability, transfersomes with the help of EAs generate a transepidermal osmotic gradient; and further squeeze among the stratum corneum cells and carry drug across the whole skin [10]. The transpore hydrostatic force difference is liable for the penetration or passage of transfersomes intact throughout the stratum corneum, i.e. the penetration of transfersome is an outcome of hydrotaxis and the permeation is governed by principles of elastomechanics [41]. When a transfersome reaches a pore, it is capable of changing its membrane work reversibly as an effect of its self-optimizing deformability. To go throughout the pore, the mechanism of the transfersome liable for its deformability starts accumulating at the site of tension, whereas the less elastic mechanism experiences dilution, which significantly reduces the active rate of membrane deformation and allows the highly elastic particles to go throughout the pores. The passage of transfersomes through the skin and the epithelial obstacle is greatly prejudiced by the flexibility of their membrane, which can be achieved via a suitable ratio of surfactants. The flexibility of the transfersomal membrane decreases the risk of complete vesicle rupture in the skin and permits the ultradeformable transfersomes to change their membrane composition locally and reversibly when they are pressed against or attracted into a narrow pore. This dramatically lowers the energetic cost of membrane deformation and permits the resulting highly flexible particles first to enter and then to pass through the pores rapidly and efficiently [42]. A schematic diagram of the mechanism of action of transfersomes is presented in Figure 2.
Figure 2.
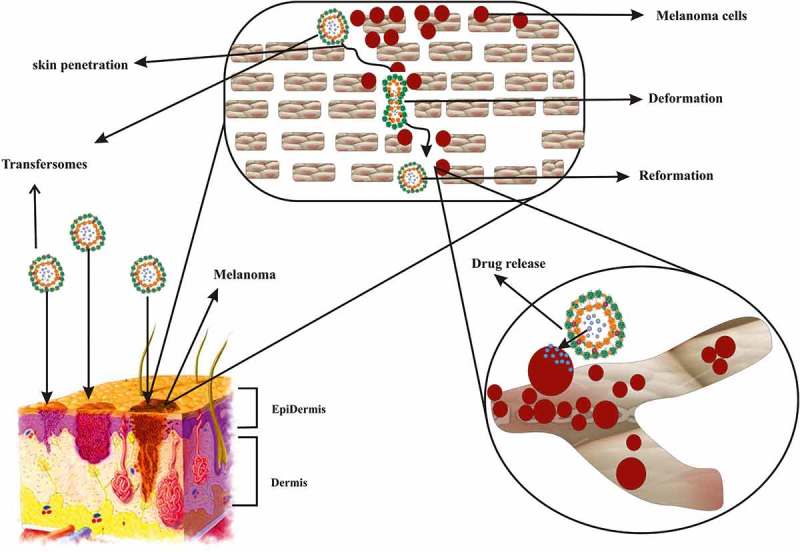
Schematic presentation of mechanism of action of transfersome in transdermal delivery of anticancer agent.
The first mechanism proposes that vesicles can act as drug carrier systems, whereby intact vesicles enter the stratum corneum carrying vesicle-bound drug molecules into the skin under the influence of the naturally occurring in vivo transcutaneous hydration gradient [13].
3. Regulatory aspects
Recently, advances in pharmaceutical science and skill have made available a range of new excipients, such as lipids, surfactants, and solvents; though, of late, there have been reservations within the scientific community regarding the dullness of excipients and that they in some capacity have unfavorable effects. Selection of an excipient throughout the research of a transfersome-based formulation is limited by safety and toxicity concerns associated with these excipients. Hence, a small range of excipients are obtainable for planning any highly porous drug deliverance system. Thus, inert excipients are usually measured when developing a transfersome-based formulation and these are used as vesicle-forming agents, surfactants, EAs, and solvents. Mitigating the safety concerns, a narrow range of excipients are obtainable for crafting any highly porous drug delivery system, such as a transfersome [43]. Different national regulatory agencies (WHO, International Pharmaceutical Excipients Council, US Food and Drug Administration (FDA), Japanese Ministry of Health and Welfare, and International Conference on Harmonisation of Technical necessities for muster of Pharmaceuticals for Human Use) have maintained a confidential list of excipients as ‘Generally Regarded as Safe’ (GRAS), which have been clinically categorized not to be toxic. The FDA keeps a record entitled ‘Inactive Ingredient Guide’, which includes a catalogue of permitted excipients. This documentation provides information about the excipients with a value of their utmost dosage stage by a fastidious route of direction or dosage form [44]. Phospholipid is a crucial element for the formation of a transfersome-based drug delivery system. It is also roughly always true that the fluid-chain vesicles with a rather elastic bilayer promote drug transport across skin obstruction better than the more rigid liposomes [45]. Therefore, nearly all the common phosphatidylcholine (PC) used to organize stretchy liposomes is unsaturated PC (i.e. soybean phosphatidylcholine (SPC) or egg phosphatidylcholine (EPC)). SPC is a GRAS-listed phospholipid and also complies with specifications of the Food Chemicals Codex (http://www.NutriScienceUSA.com). Edge activator is generally a kind of surfactant which destabilizes the lipid bilayer of the elastic liposomes and increases elasticity of the bilayer concurrently. Amid EAs, sodium cholate, sodium deoxycholate, Span-80, Tween-80 and Tween-20 were normally used. Biju et al. recommended that some chemical penetration enhancers such as oleic acid can be used as well as EA to replace the normally used surfactant [14]. The survival of mixed micelles also leads to lower drug trap due to their higher inflexibility and smaller size [46,47].
Edge activator plays an important role in determining the skin permeation behavior of elastic liposomes. An overview of the differences among EAs is helpful for the selection of an ideal EA for optimal formulation. Sodium deoxycholate is a water-soluble ionic surfactant. Valsartan-loaded elastic liposomes containing sodium deoxycholate as the EA were then investigated [48]. Similarly, sodium cholate, which is used as an EA, is reported to be non-toxic but has been kept in the hazardous category as it causes skin and eye irritations as well as respiratory sensitization. Surfactants can cause severe gastrointestinal discomfort when used above certain concentrations; the maximum safe limit of surfactant concentration is 10–25%. Ethanol is known to act as an efficient skin-penetration enhancer. It can interact with the polar head group region of the lipid molecules, resulting in a reduction of the melting point of the stratum corneum lipids, thereby increasing their fluidity and cell membrane permeability.
4. Method of preparation
4.1. Rotary film evaporation method
This method is also known as the hand-shaking process, which was initially invented by Bangham [49]. In this process, the quantity needed of phospholipids and surfactants (as EAs) is essential to organize a thin film [50,51]. It is largely worn for the research of multilamellar vesicles. A solution of phospholipids and EAs is organized in a crude solvent such as a combination of chloroform and methanol. The prepared solution is transferred to a round-bottomed flask which is rotated at constant temperature (above the glass transition temperature of lipids) and reduced pressure. A film of lipids and EA is formed on the walls of the flask. The twisted film is then hydrated using aqueous media containing drug. This causes lipids to swell and form bilayer vesicles. Vesicles of desired size can be obtained by extrusion or by sonication of the superior vesicles [52].
4.2. Reverse-phase evaporation method
At this point, the scheme will alter to a viscous gel followed by the arrangement of vesicles. The non-encapsulated material and residual solvents can be indifferentiable using dialysis or centrifugation [52]. In this method, lipids dissolved in organic solvents are collected in a round-bottomed flask. Aqueous media containing EAs is added under nitrogen purging. The drug can be added to the lipid or aqueous medium based on its solubility character. The system formed is then sonicated, awaiting its conversion into a standardized dispersion, and should not separate for at least 30 min after sonication. The organic solvent is then removed under low pressure.
4.3. Vortexing sonication method
In the vortexing sonication method, mixed lipids (i.e. phosphatidylcholine, EA and the therapeutic agent) are blended in a phosphate buffer and vortexed to attain a milky suspension. The suspension is sonicated, followed by extrusion through polycarbonate membranes [53]. Cationic transfersomes have also been set by this method, which involves mixing cationic lipids, such as DOTMA, with PBS to attain a concentration of 10 mg/ml followed by a count of sodium deoxycholate (SDC). The blend is vortexed and sonicated, followed by extrusion through a polycarbonate (100 nm) filter.
4.4. Ethanol injection method
In this process, the aqueous solution containing drug is heated with unremitting stirring at constant temperature. Ethanolic solution of phospholipids and EAs is injected into aqueous solution dropwise. As the solution comes into contact with aqueous media the lipid molecules are precipitated and form bilayered structures. This process offers assorted advantages over other methods, which include simplicity, reproducibility, and scale-up [54,55].
4.5. Freeze–thaw method
This method includes the exposure of multilamellar vesicles to alternate cycles of very low temperature for freezing followed by exposure to very high temperature. The geared-up suspension is transferred to a tube and dipped in a nitrogen bath (−30°C) for 30 s. After freezing, it is exposed to a high temperature in a water bath. This course is repeated eight–nine times [56]. An application of transfersomes in the delivery of various therapeutic agents is summarized in Table 1.
Table 1.
Examples of research reports on using transfersomes as carriers for the delivery of therapeutic agents.
| Therapeutic agent | Therapeutic category | Conventional topical available (market) formulations | Investigation | Lipid and surfactant used | Observations/conclusions | Reference |
|---|---|---|---|---|---|---|
| Ovalbumin and saponin |
Anti-OVA antibody titer in serum | – | Developed effective vesicular formulations including liposomes, transfersomes and ethosomes of ovalbumin and saponin. The effect of composition of formulations on protein encapsulation and the best one for each type of vesicular formulation was selected for their stability assay and in vivo transdermal immunization in mice | Soy phosphocholine, cholesterol, bovine serum albumin, sodium cholate, Tween-20 | The results clearly indicated that all nano lipid vesicular formulations increase peptide permeation into the skin as compared with negative control with ethosome formulation giving the highest serum antibody titers. In particular, size aging analysis demonstrated that only ethosome was stable both in size and polydispersity over a 2-month storage |
[57] |
| Diclofenac sodium | Non-steroidal anti-inflammatory drug (NSAID) | Voltaren® gel | Prepared diclofenac sodium loaded conventional liposomes and transfersomes and tested for their integrity and controlled release properties after subcutaneous administrations by liquid jet injector | Soy phosphatidylcholine, Polysorbate-80, ethanol | A result of the new approach was an improvement of both the efficacy and the safety of localized therapy combining the performance of painless liquid injection devices | [58] |
| Osthole | Anti-fibrotic, anti-inflammatory | – | In this paper, designed and prepared osthole-loaded liposome, ethosome and transfersome and evaluated for their physicochemical properties, in vitro skin permeation and in vivo plasma concentration | Soya phosphatidylcholine, Tween-80, methanol | In vitro study showed that osthole ethosome provided an enhanced transdermal flux of 6.98 ± 1.6 μg/cm2/h and a decreased lag time of 2.45 h across porcine ear skin. Data from in vivo pharmacokinetic studies showed that AUC and Cmax of the osthole-loaded ethosome were remarkably increasing compared with the other formulations | [59] |
| Itraconazole | Antifungal | Sporanox® | Itraconazole-loaded nanotransfersomes with three different types of surfactant in varying concentrations were prepared and characterized. The optimized transferosomal formulations were co-spray dried with mannitol and the aerosolization efficiency and aerodynamic properties of dry powders were determined | Lecithin, Span-60 | Optimized nanotransfersomes with lecithin:Span®60 in the ratio of 90:10 was a narrow size distribution pattern. Different types of surfactant did not influence the particle size significantly. Aerosolization evaluation of co-spray dried formulations with different amounts of mannitol indicated that a 2:1 ratio of mannitol:transfersome (w:w) showed the best aerosolization efficiency | [60] |
| Timolol maleate | Non-selective β-adrenergic receptor antagonist | Timoptol-XE gel | This work was to examine the deformability properties of unlike timolol maleate (TM)-loaded transfersomes by extrusion | Tween-20, egg L-α phosphatidylcholine, cholesterol, sodium deoxycholate, stearylamine | Results showed that TM-loaded transfersomes may improve the corneal transmittance and get better bioavailability of conventional TM delivery | [61] |
| Piroxicam | NSAID s |
PX-TRS gel | Studied the optimization and ex vivo study of piroxicam-loaded transethosomal gel using the central composite design | Soya phosphatidylcholine, ethanol, Span-80 | Improved stability and highest elasticity in its gel formulation | [62] |
| Asenapine maleate | Antipsychotic drug | Saphris® | Transfersomes of asenapine maleate were prepared by the thin film hydration method. Various chemical enhancers were screened for skin permeation enhancement of asenapine maleate. In vivo pharmacokinetic study was performed in rats to assess bioavailability by transdermal route against oral administration | Soyphosphatidylcholine, sodium deoxycholate, triethanolamine | Ethanol (20% v/v) showed greater skin permeation enhancement. The cumulative amount of asenapine maleate permeated after 24 h (Q24) by the individual effect of ethanol and transfersome. In vivo pharmacokinetic study revealed significant increase in bioavailability on transdermal application compared with the oral route | [63] |
| Ketoprofen | NSAIDs | Fastum gel | They conducted a randomised, double-blind, controlled Phase II study to compare the effect of ketoprofen in Transfersome® gel versus oral ketoprofen and ketoprofen and drug-free Sequessome™ vesicles in reducing pain from muscle soreness in the calves of healthy individuals after exercise consisting of walking down stairs | – | The results showed that Transfersome® gel and ketoprofen and drug-free Sequessome™ vesicles were superior to oral ketoprofen in reducing muscle soreness following exercise. Furthermore, oral ketoprofen delayed recovery from muscle soreness but ketoprofen and drug-free Sequessome™ vesicles and Transfersome® gel did not. Treating osteoarthritis-related joint pain with ketoprofen and drug-free Sequessome™ vesicles is known to be as effective as treatment with an oral NSAID | [64] |
| Emodin | Purgative, laxative | Regalia® | Nano emodin transfersome (NET) was prepared by the film-ultrasonic dispersion method. In this investigation 60 male rats were selected. After an 8-week treatment, fasting blood glucose and serum lipid levels were determined. The adipose tissue section and the cellular diameter and quantity of adipocytes were evaluated by light microscopy. The mRNA expression of ATGL and G0S2 from the peri-renal fat tissue was assayed by reverse transcription polymerase chain reaction | Lecithin, deoxycholic acid, sodium salt, cholesterol | NET might decrease body weight, pathological change of fatty liver, reduce the peripheral fat content, increase serum HDL-C, and reduce TG level and adipocyte mass, and this outcome was allied with the downregulation of G0S2 protein expression in the adipose tissue of obese rats and upregulation of ATGL protein expression. These mutually antagonistic effects work together to reduce the body weight of obese rats | [65] |
| Capsaicin | Antiarthritic agent | Zostrix cream | In this study, capsaicin-loaded transfersome lipid vesicles were prepared and the antiarthritic efficacy was evaluated in rat models. The results of the test formulation were compared with standard reference formulation, Thermagel (Marketed gel) | Phosphatidylcholine, ethanol and Tween-80 | It was observed that the prepared formulation showed better inhibitory activity (in reducing arthritis and associated inflammations) than the marketed Thermagel formulation, which could probably be due to the reduced penetrability of Thermagel across the skin, compared with the specially designed transfersomal delivery system | [66] |
| Diclofenac sodium | NSAIDs | Cambia | In this study to improve transdermal permeation of diclofenac sodium, a poorly water-soluble drug, employing conventional liposomes, ethosomes, and transfersomes. The prepared vesicular systems were incorporated into 1% Carbopol 914 gel | Soya lecithin, Span-80 cholesterol, ethanol, and Carbopol 914 | The transfersomes and ethosomes provided a significantly higher amount of cumulative permeation, steady state flux, permeability coefficient, and residual drug into skin compared with the conventional liposomes, conventional gel, or hydroethanolic solution. Stability tests indicated that the vesicular formulations were stable over 3 months. Results revealed that both ethosome and transfersome formulations were acting as a drug reservoir in skin and extending the pharmacologic effects of diclofenac sodium | [67] |
| Terbinafine | Antifungals | Lamisil Dermgel | Researcher investigated the mechanisms underlying the in vitro activity of terbinafine in Transfersome (TDT 067) by comparing the effects of TDT 067 and conventional terbinafine on the morphology of T. rubrum (the predominant cause of onychomycosis) in vitro using different microscopic tools, including white-light microscopy, scanning electron microscopy and transmission electron microscopy (TEM) |
– | Exposure of T. rubrum hyphae to TDT 067 led to rapid and extensive ultrastructural changes. After 24 h there was complete disruption of hyphae as compared with conventional terbinafine. Lipid droplets were observed under TEM following 30 min of exposure to TDT 067, which after 24 h had filled the intracellular space. These effects were confirmed in vivo in subungual debris from patients with onychomycosis who received topical treatment with TDT 067 | [68] |
| Cinnamic acid | Anti-inflammatory, antioxidant | – | In this study cinnamic acid-loaded transfersomes were prepared and dermal microdialysis sampling was used in Sprague–Dawley rats. The amount of drug released into the skin using transfersomes as transdermal carriers compares with that released from conventional liposomes | Phosphatidylcholine, sodium deoxycholate | An in vivo microdialysis sampling method revealed that the dermal drug concentrations from transfersomes applied on skin were much lower than those required with conventional liposomes. After the administration of drug-containing transfersomes and liposomes on abdominal skin regions of rats for a period of 10 h, the Cmax of cinnamic acid from the compared liposomes was 3.21 ± 0.25 mg/ml and that from the transfersomes was merely0.59 ± 0.02 mg/ml | [69] |
| Terbinafine | Antifungal | Terbinex | Ultradeformable lipid vesicles to facilitate release of terbinafine to the nail and surrounding tissue. TDT 067 (terbinafine in transfersome) is the only such therapy in development for onychomycosis, and we review published preclinical and clinical studies on this formulation | TDT 067 | This translated into eminent rates of mycological cure and evidence of clinical effect in a study of TDT 067 administered twice daily for 12 weeks in patients with onychomycosis. An ongoing Phase III trial involving more than 700 patients treated for 48 weeks is investigating the effectiveness and safety of TDT 067 | [70] |
| Ketoconazole | Antifungal agent | Nizoral Topical | Investigated the potential of transfersome for transdermal delivery of ketoconazole (KTZ), which was formulated by the lipid film hydration technique using a rotary vacuum evaporator using suitable essential oils acting as natural permeation enhancers. The transfersomes were converted into a suitable gel formulation and are evaluated for their gel characteristics such as pH, viscosity, spreadability, extrudability, homogeneity, drug content, etc. | Lecithin, Tween-80 | Study proved that addition of suitable permeation enhancers to the transfersomal formulation improved the release and permeation of KTZ, which showed that the permeation enhancers modify the barrier to penetration present in skin without itself undergoing any change | [71] |
| Curcuma longa extract | Photoprotective | – | Formulated creams containing Curcuma longa extract-loaded novel vesicular systems (liposomes, ethosomes, and transfersomes) and studied their photoprotective effect by assessment of skin hydration (Cutometer) and sebum content (Sebumeter) | Ethyl alcohol, soya phosphatidylcholine, cholesterol, ethanol, sodium deoxycholate | The results showed that extract-loaded transfersomes are better for improving skin properties than ethosomes and liposomes. Photoprotective herbal extract-loaded vesicles incorporated in the creams could be highly beneficial as photoprotectives with enhanced skin hydration and sebum level | [72] |
| Meloxicam | Non-steroidal anti-inflammatory | Meloxicam 3% gel | Prepared and evaluated the liposome and transfersome vesicles in the transdermal drug delivery of meloxicam (MX) and studied the effect of three surfactants that differ in length of carbon chains that were used for the preparation of transfersomes: sodium oleate (NaO, C18), sodium cholate (NaChol, C24), and dicetylphosphate (DCP, C32) | Phosphatidylcholine, cholesterol, sodium cholate, sodium oleate, dicetylphosphate | The use of surfactants containing medium-length carbon chains, including NaO (C18) and NaChol (C24), in the transfersomes resulted in a high entrapment efficiency. Transfersomes provide greater MX skin permeation than liposome and MX suspensions | [73] |
| Ketoprofen | NSAIDs | Orudis KT | Long-lasting studies have been in print to show safety and efficacy for long-term use of topical NSAIDs. Diractin (formerly IDEA-033) is a viscous, aqueous formulation for epicutaneous application of ketoprofen based on ultradeformable, self-regulating carrier (transfersome) | Sodium heparin | Diractin provided adequate pain relief with a good safety and tolerability profile when used for up to 18 months (72 weeks) | [74] |
| Ketoprofen | NSAIDs | Vopac | In the article, compared in vivo transport and biodistribution of ketoprofen applied on the skin in ultradeformable carriers (Diractin) or a conventional topical gel (Gabrilen) with oral drug (Oruvail) | Carbomer, methylparabene, benzyl alcohol, ethanol, glycerol, phosphatidylcholine, polysorbate | Ketoprofen from Diractin achieved more desirable biodistribution and clearance, debatably due to spontaneous carrier-mediated drug transport across the skin, which ensures local and fairly long-lasting drug evidence in periphery | [75] |
| Tanshinone | Anti-hypertensive | – | The transfersomes were prepared by the film dispersion method followed by sonication, and stability and deformability were studied | Lecithin, sodium cholate | The results of the study showed that transfersomes have good entrapment efficiency and stability. The vesicles possess high deformability in relation to the molar ratio of sodium cholate to lecithin and the external pressure | [76] |
| 18β-glycyrrhetic acid |
Dermatitis | – | Prepared elastic vesicular formulation to enhance the skin permeation of a poorly water-soluble 18β-glycyrrhetic acid (GA) and treat dermatitis | Soybean phospholipid, sodium deoxycholate, cholesterol |
After non-occlusive application to mice ear skin, deposition of GA increased immediately and reached Cmax at 3 h (1.95 ± 0.32 µg/cm2) and was still detected even 16 h after GA removed. The results of an in vivo anti-inflammatory activity study showed that GA elastic vesicles had a significant reduction in ear thickness and mass (25.52 and 49.23%) (p < 0.05) as compared with a positive control group (triamcinolone acetonide and econazole nitrate cream in the market) | [77] |
| Catechin | Antioxident | Veregen® | In this study were compared catechin-loaded conventional liposomal, deformable conventional liposomal, and deformable liposomes prepared by the reverse-phase evaporation (REV) method | L-α-phosphatidylcholine, cholesterol, sodium deoxycholate |
Results suggested that all liposomal formulations exhibited a prolonged catechin release. Compared with deformable liposomes, the REV deformable liposomes showed a greater deposition of (+)-catechin while catechin solution did not permeate into the porcine ear skin | [78] |
| Dipotassium glycyrrhizinate |
Anti-inflammatory agent |
– | Studied the possibility of elastic liposomes for skin release of dipotassium glycyrrhizinate (KG) for treatment of acute and chronic dermatitis | Soya lecithin (PC) or hydrogenated soya lecithin | Dipotassium glycyrrhizinate interacted with liposomes disrupting and fluidizing the lipid bilayer, skin deposition increased 4.5-fold compared with aqueous solution while KG was formulated in liposomes | [79] |
| Ketotifen | Antihistaminic | Zaditor ketotifen | In this study were investigated possible mechanisms of deformable liposomes and ethosomes for improving skin delivery of ketotifen under non-occlusive conditions | Phosphatidylcholine, Tween-80 |
Results suggested that both the penetration-enhancing effect and the intact vesicle permeation into the stratum corneum might play a role in improving skin delivery of drugs by deformable liposomes, under non-occlusive conditions, and that the penetration-enhancing effect was of greater importance in the case of ketotifen | [80] |
| Quercetin and resveratrol |
To reduce subcutaneous fat |
– | In this study, proposed quercetin and resveratrol-containing SDC-elastic liposomes as a new approach for dissolving the subcutaneous fat | Soya phosphatidylcholine cholesterol, stearylamine, sodium deoxycholate | Results showed optimized elastic quercetin and resveratrol-loaded liposomes with suitable physicochemical properties and kinetic drug profiles for subcutaneous injection | [81] |
| Tetanus | Vaccine | Adacel (Tdap) | In the present study, elastic vesicle transfersomes, non-ionic surfactant vesicles (niosomes) and liposomes were used to study their relative potential in non-invasive delivery of tetanus toxoid (TT) | Soya phosphatidylcholine, sodium deoxycholate, Span-85 |
In vivo study revealed that topical delivery of TT containing transfersomes, after secondary immunization, could elicit an immune response (anti-TT-IgG) that was equivalent to one that was produced following intramuscularly alum-adsorbed TT-based immunization. In comparison with transfersomes, niosomes and liposomes elicited a weaker immune response. Thus transfersomes hold promise for effective non-invasive topical delivery of antigen(s) | [82] |
| Metronidazole | Anti-amoebic | MetroLotion | Deformable liposomes composed of egg phosphatidylcholine (EPC) and various surfactants (sodium deoxycholate (SDCh), Tween-80 or Span-80) were prepared with and without metronidazole for vaginal administration. Additionally, a freeze–thaw method was applied to both classes of vesicles (liposomes) containing the drug to improve its trapping capacity and characterized in terms of size, polydispersity, zeta potential, entrapment efficiency and their permeability on a Caco-2 cell monolayer | Egg phosphatidylcholine, sodium deoxycholate, Tween-80, Span-80 | Results showed that deformable EPC/SDCh liposomes were found to enhance the permeability of metronidazole more effectively than the conventional liposomes based on the in vitro model of the epithelial barrier | [83] |
5. Applications
5.1. Actinic keratosis
Actinic keratosis (AK) is an ordinary skin ailment caused by long-standing sun exposure, and classically forms on the face, neck, balding scalp, chest, shoulders, and the back of arms and hands of adults, 75% of all reported lesions existing on the head, neck, and forearms. Actinic keratosis is characterized by the shape of keratotic macules, papules, or plaques with superficial scales on a red base. Lesions are frequently asymptomatic, but they can be painful or itchy. Owing to the swelling nature of the condition, the occurrence of AK increases with age and is an ordinary condition in the adult populace aged over 50 years [84]. Treatment of AK depends on the medical appearance of the lesions: it may be fought at exact lesions (lesion directed) or at numerous lesions over a large area (field directed), and occasionally both treatment approaches are used. Therapy options include cryosurgery, curettage, excision surgery, photodynamic therapy (PDT), and topical treatments (5-FU cream, diclofenac gel, imiquimod cream, and ingenol mebutate gel).
A study in England reported a prevalence rate of 15.4 and 5.9% in men and women, respectively. This rate amplified to 34.1 and 18.2% in men and women aged above 70 years [85]. The study set up to investigate the occurrence of AK was better in individuals with red hair and freckles, which indicates Fitzpatrick skin type I. There was a similar, but extra-marked, boost reported in Australia; prevalence rates of AK were 22 and 8% for men and women aged 30–39 years, which increased to 83 and 64%, respectively, in adults aged 60–69 years [86]. One reason for the greater incidence rate in males might be that it is more possible for them to labor outdoors and have more sun contact.
5.1.1. Topical delivery of 5-FU for the treatment of AK and non-melanoma skin cancer
Unfortunately, 5-FU showed poor percutaneous permeation, thus reducing its anticancer effectiveness after topical administration. The in vivo results concluded that vesiculization of 5-FU not only improves the topical delivery, but also enhances the cytotoxic effect of 5-FU [87]. An instance of transfersomal gel containing 5-FU provided efficient results against the treatment for AK and non-melanoma skin carcinoma, which showed up to a twofold increase of transdermal release in contrast to other marketed formulations [88].
5.2. Basal cell carcinoma
The frequency of basal cell carcinoma shows clear environmental variation. The age-standardized occurrence of basal cell carcinoma in South Wales was predictable at 114 per 100,000 population in 1998 [89]. The common age and sex-standardized annual occurrence in Minnesota, USA, was reported at 146 per 100,000. In Australia, the rate is ahead at 726 per 100,000 [90]. These statistics are likely to be underestimates, as basal cell carcinoma tends to be under-reported to the cancer registries. In white populations in North America, the frequency has improved more than 10% a year, foremost to a lifetime risk of 30% of growing a basal cell carcinoma [91]. With an ever increasing elderly population, the disease is likely to become more of a problem in the future. Certainly, the prevalence of this cancer will probably be greater than that of all other cancers mutually. The age-standardized occurrence of basal cell carcinoma in white populations is generally between 18 and 40% higher in men (British and Australian data) [92]. Basal cell carcinoma is extremely uncommon in dark-skinned races. Skin type I (always burns, never tans), red or pale hair, and blue or green eyes have been shown to be risk factors for the development of basal cell carcinoma, with an estimated odds ratio of 1.6 [93]. Advance of basal cell carcinoma is reported to be more frequent after freckling in childhood and also after frequent or severe sunburn in childhood [94,95]. This is in contrast to a history of sunburn as an adult, which does not seem to be associated with the development of basal cell carcinoma [95].
Other non-ultraviolet ecological exposures that have been connected with amplified risk of basal cell carcinoma include ionizing radiation, high dietary energy (especially fat), low intake of vitamins, and various chemicals and dust. Introduction to arsenic predisposes one to multiple basal cell carcinomas [96,97].
Patients on an immunosuppressive cure also have an increased risk of basal cell carcinoma. A study in the Netherlands showed that the occurrence of basal cell carcinoma in transplant recipients was 10 times higher than in the general population [97].
Fadel et al. [98] reported that indocyanine green was encapsulated in a vesicular colloidal nanocarrier (transfersomes) for potential application as a photosensitizer in topical PDT of basal cell carcinoma.
5.3. Kaposi’s sarcoma
Kaposi’s sarcoma (KS) was first described in 1872 by the Hungarian dermatologist Moritz Kaposi. At that time, before human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS), KS remained a rare tumor. While the majority of the cases seen in Europe and North America have occurred in aged men of Italian or Eastern European Jewish ancestry, the neoplasm also occurs in some other distinct populations: young black African adult males, prepubescent children, renal allograft recipients, and other patients receiving immunosuppressive therapy. The disseminated, fulminate form of KS associated with HIV disease is referred to as epidemic KS to distinguish it from the classic, African, and transplant-related varieties of the neoplasm. In addition, KS has been acknowledged in homosexual men apart from the HIV disease epidemic [99]. In the 1950s, KS was known as a comparatively ordinary neoplasm endemic in inhabitant populations in equatorial Africa and comprised approximately 9% of all cancers seen in Ugandan males. During 1969, the first case of KS in connection with immunosuppression in a renal transplant patient was described. At that time, a number of renal and additional organ allograft recipients who received conventional prednisone and azathioprine developed KS shortly after the onset of immunosuppressive therapy [100]. Pathak et al. [101] developed deformable nanovesicles of paclitaxel capable of being used in dermal chemotherapy, especially deep into the dermal areas of AIDS-related KS. An i n vitro cytotoxicity study on KSY-1 cell lines revealed higher IC50 (≤17) for transfersome against IC50 ≤ 19 for transfersome gel. Confocal laser scanning microscopy confirmed the penetrating potential of transfersomes via transfersome gel to the dermal layers of skin, the proposed target site [101].
5.4. Melanoma
A melanoma is an atrocious tumor that arises from melanocytes, dendritic cells that create melanin, a tincture that protects the body from destructive ultraviolet (UV) radiation. Melanocytes use tyrosine to synthesize melanin. A cluster of melanocytes form nevi (pigmented lesions or moles), and melanoma results when these melanocytes undergo a malignant transformation. Melanocytes may be created in various areas of the body; nevertheless, they are mainly situated in the epidermis, and more than 90% of all melanomas have cutaneous incidence rates and epidemiology. The frequency of melanoma varies, with the peak rates in Northern Europe, New Zealand, Australia, and North America [102,103]. Melanoma has one of the fastest-growing rates in the US. The US occurrence rate increased from 7.9 to 17.7 per 100,000 persons between 1975 and 2000. The American Cancer Society estimated that, in 2012, about 76,250 new melanoma cases (44,250 men, 32,000 women) would be diagnosed and about 9,180 individuals (6,060 men, 3,120 women) would die from the disease [104]. Lin et al. [105] formulated 5-aminolevulinic acid (5-ALA)-loaded DPPC liposomes in melanoma treatment. The results found that 5-ALA/DPPC formulation reduced cell viability and mitochondria membrane potential, and enhanced intracellular ROS accumulation as compared with 5-ALA alone in melanoma cells. Furthermore, the 5-ALA/DPPC formulation also had better skin penetration ability as compared with the 5-ALA in our ex vivo data by assaying 5-ALA converted into protoporphyrin IX (PpIX) in the skin of the mice that were experimented on. In melanoma xenograft models, 5-ALA/DPPC enhanced PpIX accumulation only in tumor tissue, not normal skin [105]. siRNAs have potential therapeutic applications in various dermatological diseases such as psoriasis, atopic dermatitis, and cancer. Dorrani et al. [106] prepared a series of liposome compositions that contained various concentrations of EA in their structures and then complexed them with siRNA at different ratios to generate a small library of liposome–siRNA complexes (lipoplexes) with different physicochemical properties. Quantitative imaging analysis showed effective permeation of lipoplexes through the skin layers and deposition at the upper dermis. The ability of the formulated lipoplexes to internalize into melanoma cells, knockdown the expression of the B-raf murine sarcoma viral oncogene homolog B1 (BRAF) protein, and induce cell death in melanoma cells was studied by fluorescent microscopy, in-cell immunofluorescence assay, and WST-1 cell proliferation assay [106].
5.5. Squamous cell carcinoma
Squamous cell carcinoma (SCC) is an epithelial malignancy that occurs in organs that are usually enclosed with squamous epithelium which includes numerous diverse anatomic sites, counting the skin, lips, mouth, esophagus, urinary tract, prostate, lungs, vagina, and cervix. SCC represents the most common cancer capable of metastatic spread in the US and worldwide [107]. Tobacco smoking and human papilloma virus (HPV) are carcinogenic causes for all four sub-types. In total, quite a lot of risk factors are public amid the chief SCC types. Fair-skinned persons who burn and never tan are at a much higher risk for on-the-rise skin SCC than those with darker skin, and it has been confirmed that both past sun exposure and strong sun exposure appear to heavily predispose the population to skin cancer [108,109].
Furthermore, HPV may be involved in the multistep procedure of skin carcinogenesis as a co-factor with UV radiation, especially in patients with poor resistance, such as limb transplant recipients and smoking tobacco, which may double the risk of skin cancer [110,111].
Gupta et al. [112] designed protransfersome for local delivery of cisplatin in cutaneous epithelial malignancies. The presence of a fluorescence marker in the skin showed better skin penetration ability of the protransfersome. The results of i n vivo performance of the system showed an increase in the therapeutic efficacy of the drug with less systemic toxicity [112]. Applications of transfersomes in the delivery of various anticancer agents are presented in Table 2. In addition to all this information, studies, and roles, a number of patents have been filed and granted, brief details of which, representing various formulation aspects and potential of transfersomes, are summarized in Table 3.
Table 2.
Examples of research reports on using transfersomes as carriers for the delivery of anticancer agents.
| Anticancer drugs | Conventional topical available formulation (market) | Investigation | Lipid and surfactant used | Observations/conclusions | Ref |
|---|---|---|---|---|---|
| Doxorubicin hydrochloride (DOX) | DOXIL | A novel hyaluronic acid modified transfersome was prepared to deliver drugs to lymphatics through the transdermal route. Hyaluronic acid effectively improved the uptake of drug-loaded nanocarriers by tumor cells | Sodium deoxycholate, lecithin |
Results revealed that DOX-loaded HA-GMS-T was able to penetrate efficiently into the deep skin tissue, leading to enhanced absorption by lymphatics and decreased organ toxicity. This study provides a new angle for tumor metastasis therapy through lymphatic drug delivery with transdermal nanomedicine | [113] |
| 5-Fluorouracil | Fluroplex | Different formulation of tranfersome was prepared using Tween-80 and Span-80 as edge activators. 5-FU containing transfersome loaded 1% Carbopol 940 used for deeper penetration into skin tumors and to compare its anticancer efficacy with its marketed formulation for the treatment of skin cancer | Tween-80, Span-80, edge activators | The results showed that Tween-80 seems to be a better edge activator than Span-80 on the basis of vesicle size and entrapment efficiency. The transfersomal gel was able to improve both in vitro skin permeation and skin deposition of 5-FU compared with the marketed formulation. Transfersomes showed maximum skin deposition (81.3%) and comparable transdermal flux of 21.46 mg/cm2/h | [61] |
| Gemcitabine | Gemzar | Investigated as supramolecular vesicular aggregates (SVAs) prepared by self-assembling liposomes and polyasparthydrazide copolymers conjugated to folic acid molecules as potential active targeting formulation for anticancer drug delivery | 1,2-dipalmitoyl-sn-glycero 3-phospocholine monohydrate (DPPC) and N-(carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3- phosphoethanolamine (DSPE-MPEG2000), cholesterol |
The results showed that chemotherapeutic activity of gemcitabine was increased extensively during in vivo experiments on NOD-SCID mice bearing MCF-7 human xenograft models after its entrapment inside the folate-targeted SVAs. Both the volume and weight of the tumor masses were decreased if compared with those obtained by treating animal models with gemcitabine-loaded mPEG-SUVs and the free form of gemcitabine | [114] |
| 5-Fluorouracil (5-FU) | Efudex® Cream | In this study, constructed transfersomes, liposomes, and niosomes of 5-FU for topical application for the treatment of actinic keratosis and non-melanoma skin cancer. Transfersomes were prepared by the solvent evaporation method, whereas liposomes and niosomes were constructed by the reverse-phase evaporation method. Cytotoxicity study was carried out on HaCaT cells | Dimiristoylphosphatidylcholine,dipalmitoyl-phosphatidylcholine, cholesterol, sodium cholate |
The IC50 value of transfersomes (1.02 μmol/l), liposomes (6.83 μmol/l), and niosomes (9.91 μmol/l) was found to be far less than 5-FU (15.89 μmol/l) at 72 h. 5-FU-loaded transfersomes were found to be most cytotoxic on the HaCaT cell line in comparison with liposomes and niosomes. The results concluded that vesiculization of 5-FU not only improves the topical delivery, but also enhances the cytotoxic effect of 5-FU | [115] |
| Raloxifene hydrochloride | – | In this study a researcher developed and optimized raloxifene hydrochloride-loaded nanotransfersomes for transdermal delivery, in order to overcome the poor bioavailability of the drug. A response surface methodology experimental design was applied for the optimization of transfersomes, using Box-Behnken experimental design | Phospholipon® 90G, sodium deoxycholate | Raloxifene hydrochloride-loaded transfersomes proved significantly superior in terms of amount of drug permeated and deposited in the skin, with enhancement ratios of 6.25 ± 1.50 and 9.25 ± 2.40, respectively, when compared with conventional liposomes, and an ethanolic solution. Differential scanning calorimetry study revealed a greater change in skin structure, compared with a control sample, during the ex vivo drug diffusion study. Further, confocal laser scanning microscopy proved an enhanced permeation of coumarin-6-loaded transfersomes, to a depth of approximately160 µM, as compared with rigid liposomes | [116] |
| Celecoxib | Celcoxib topical | Three kinds of celecoxib-loaded vesicular formulations have been investigated as drug carriers, liposomes containing a surfactant, or transfersomes and ethosomes containing suitable edge activators |
Tween-20, ethanol | All vesicular formulations markedly (p < 0.001) improved the drug amount that penetrated into the skin with respect to an aqueous suspension, from 2.0 to 6.5, up to 9.0-fold for liposomes, transfersomes, and ethosomes, respectively. In particular, ethosomes containing Tween-20 as edge activator enabled the highest increase in drug penetration through the skin, probably due to the simultaneous presence in their composition of ethanol and Tween-20, both acting as permeation enhancers | [117] |
| Vinblastine | – | In this study vinblastine liposomes were prepared from lipids dimiristoylphosphatidylcholine and dipalmitoylphosphatidylcholine with cholesterol and transfersomes with sodium cholate were prepared by the thin film hydration method. The drug encapsulation, stability, drug release and in vitro human cell lines were performed | Dimiristoylphosphatidylcholine,dipalmitoyl-phosphatidylcholine, cholesterol, sodium cholate |
The results showed that encapsulation of vinblastine into liposomes was higher than 98% at a drug/phospholipid molar ratio from 0.17 to 0.18, while encapsulation of vinblastine into transfersomes varied from 50 to 80% at a drug/phospholipid molar ratio from 0.05 to 0.09. The retention of drug in liposomes and in transfersomes was found to be time-dependent. The results of cell line study showed that the liposomes were found to exhibit 20-fold less activity as compared with the free vinblastine | [118] |
Table 3.
Examples of patent reports on transfersome drug delivery systems.
| S. No. | Patent Application No. (year of issue/publication/CPC classification, etc.) |
Inventor (s) | Drug molecule and formulation system | Case study/comments details | Reference |
|---|---|---|---|---|---|
| 1. | US6165500 A (2000) | GregorCevc | Edge active | Creation to state the properties of novel arrangements which are suitable for the mediation of rapid transport of diverse agents and other substances from side to side permeability barriers and constriction. Transfersomes in said medium on to the skin of said mammal such that an effectual dose of said lipid, said surfactant, or a further medical agent associated with said transfersomes is captivated into said creature | [119] |
| 2. | US20020048596 A1 (2002) | GregorCevc | Diclofenac, ibuprofen phosphatidyl choline from soybeans, distearoyl-glycerolphosphoethanolaminetriazopolyethylene glycol |
The copyright claims the incorporation of active agents such as NSAIDs in transfersomes for transport through natural barriers and constriction of skin. The transfersomes comprise at the smallest amount two carrier components whose solubilities in the suspension medium differ by a factor of 10 | [120] |
| 3. | US20060105955 A1 (2006) | Nicholas Perricone | Palmitate, lipoic acid, oxytocin, vasopressin, insulin, somatotropin, calcitonin, chorionic gonadotropin, menotropins, follitropins, somatostatins, progestins | Claimed relates to compositions and methods for transdermal drug delivery comprising formulating a phosphatidylcholine carrier composition containing the drug and applying the composition to the skin. Claim comprising crystallized phosphatidylcholine and polypeptide drug molecules entrapped within the phosphatidylcholine for transdermal delivery of the polypeptide drug molecules | [121] |
| 4. | US7175850 B2 (2007) | GregorCevc | Hydrocortisone, dexamethasone and triamcinolone acetonide | Disclosed the administration of corticosteroids (hydrocortisone, dexamethasone, and triamcinolone acetonide) via transfersomes on mice skin for oedema repression action and to be veteran against profitable orientation ointment | [122] |
| 5. | US20070042030 A1 (2007) | GregorCevc | Edge active, actrapid, insulin | Claimed that the dermal application of insulin through transfersomes for non-invasive and painless therapy of type 2 diabetes mellitus resulted in >90% of the practical drug amount attainment in the destined organ of the body | [123] |
| 6. | US7591949 B2 (2009) | Gregor, Cevc, Holger Richardsen, Andrea Weiland-Waibel | Peptide, protein | Claimed that kit and a device for controlling the flux of penetrants across a flexible semi-permeable absorbent barrier, the method comprising the steps of: preparing a formulation by suspending or dispersing said penetrants in a polar liquid in the form of fluid droplets surrounded by a membrane-like coating of one or several layers | [124] |
| 7. | WO2010/090654A1 | Henry William, Kroon Henk-Andre, Summerton Linda | Terbinafme, Tween-80, Polysorbate-40 | Claimed relates to formulations of an antimicrobial agent, a lipid, and optionally a surfactant, and uses thereof for reducing the proliferation and viability of microbial agents. Claim antifungal manager is absorbed by the phospholipid membranes of the Spitzenkorper or Polarisome regions of the hyphae of the alleged mycotic agent | [125] |
| 8. | US7867480 B1 (2011) | GregorCevc, Amla Chopra | IL-4, IL-3, IL-2, TGF, IL-6, IL-7, TNF, IL-1a and/or IL-1b, IL-12, IFN-g, TNF-b, IL-5, IL-10, a type I interferon, IFN-alpha, or IFN-b | Claimed relates to methods for the vaccination of mammals for obtaining a defensive or therapeutic resistant response. Claims novel vaccine for non-invasive transdermal administration of antigens via transfersomes that also enclose a cytokine-inducing multiple and a chemical irritant | [126] |
| 9. | CA2919971 A1 (2015) | Richard Wolf Garraway, William Henry | PEG, Tween, soy phosphatidylcholine | Claimed relates to vesicular formulations for employing in the current administration of a therapeutic, metabolic, cosmetic or structural agent of interest (AOI) and methods of administering an AOI. Claim vesicular formulation comprising a lipid, a surfactant and an AOI, wherein the AOI is bonded to a component of the vesicle such that a portion of each AOI molecule is on the outside of the vesicle and is outer to the vesicular casing | [127] |
| 10. | US20150157728 A1 (2015) | ModiPankaj | Botulinum toxin, hyaluronic acid, chitosan, chondroitin sulfate, alginate, carboxymethylcellulose | Innovation related to a novel stabilized and solubilized current formulation for make-up improvements and the use of the topical formulation in a link with the providing of cosmetic improvements in people. Claim a stabilized, low-viscosity protein composition for topical application and transdermal rescue of an active agent for therapeutic use or cosmetic improvement in humans | [128] |
6. Concluding remarks
Transfersomes are personally designed vesicles capable of responding to external stress by squeezing themselves through skin pores that are many times narrower than they are, leading to increased transdermal flux of the therapeutic agents. It is clear that transfersomes can deliver enhanced amounts of both small and large therapeutic agents into and through the skin. The exact mechanism by which transport occurs remains to be elucidated, and evidence for transport of intact vesicles beyond the stratum corneum is lacking. There are increasing applications of enhanced delivery by transfersome formulations. However, there are only two transfersome-based formulations currently available in the market and the reported clinical studies mainly involve ketoprofen and insulin. The application of transfersomes in cancer presents treatment of AKs, basal cell carcinoma, SCC, melanoma, and KS. The transfersomes can be a good carrier option for delivering the drug into the skin layers and hence useful for the treatment of skin cancers. It is likely that a number of transfersome products for dermal and transdermal applications will be developed in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Lieu CH, William WN, Lippman SM.. Cancer chemoprevention In: Hidalgo M, Eckhardt SG, Garrett-Mayer E, et al, editors. Principles of anticancer drug development. New York: Springer Science; 2010. p. 1–18. [Google Scholar]
- [2]. WHO Cancer-fact sheets. WHO; [cited 2017. January 2]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/index.html
- [3]. Sabitha M, Sanoj Rejinold NS, Nair A, et al. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr Polym. 2013;91:48–57. [DOI] [PubMed] [Google Scholar]
- [4]. Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2016;62:95–102. [DOI] [PubMed] [Google Scholar]
- [5]. Cevc G. Material transport across permeability barriers by means of lipid vesicles In: Lipowsky R, editor. Handbook of physics of biological systems. Vol. I. Vol. 9 Amsterdam: Elsevier Science; 1994. p. 441–466. [Google Scholar]
- [6]. Cevc G, Blume G, Schatzlein A, et al. The skin: a pathway for the systemic treatment with patches and lipid-based agent carriers. Adv Drug Deliv Rev. 1996;18:349–378. [Google Scholar]
- [7]. Cevc G, Schatzleinand A, Blume G. Transdermal drug carriers: basic properties, optimization and transfer-efficiency in the case of epicutaneously applied peptides. J Control Release. 1995;36:3–16. [Google Scholar]
- [8]. Mezei M, Gulasekharn V. Liposomes a selective drug delivery system for topical route of administration, 1. Lotion dosages form. Life Sci. 1980;26:1473–1477. [DOI] [PubMed] [Google Scholar]
- [9]. Fresta M, Puglisi G. Application of liposomes as potential cutaneous drug delivery system: in vitro in vivo investigation with radioactivity labeled vesicles. J Drug Target. 1996;4:95–101. [DOI] [PubMed] [Google Scholar]
- [10]. Cevc G, Blume G. Lipid vesicles penetrate into skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992;1104:226–232. [DOI] [PubMed] [Google Scholar]
- [11]. Song CK, Balakrishnan P, Shim CK, et al. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B Biointerfaces. 2012;92:299–304. [DOI] [PubMed] [Google Scholar]
- [12]. Shashank J, Niketkumar P, Mansi KS, et al. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2016;116:1–23. [DOI] [PubMed] [Google Scholar]
- [13]. Cevc G, Blume G. New highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, transfersomes. Biochimica Et Biophysica Acta. 2001;1514(2):191–205. [DOI] [PubMed] [Google Scholar]
- [14]. Biju SS, Talegaonkar S, Mishra PR, et al. Vesicular system an overview. Indian J Pharm Sci. 2006;68:141–153. [Google Scholar]
- [15]. Dayan N, Touitou E. Carriers for skin delivery of Trihexiphenidyl HCl ethosomes vs liposomes. Biomaterials. 2000;21:1879–1885. [DOI] [PubMed] [Google Scholar]
- [16]. Hofer C, Hartung R, Gobel R, et al. New ultradeformable drug carriers for potential transdermal application of interleukin-2 and interferon-alpha theoretic and practical aspects. World J Surg. 2000;24:1187–1189. [DOI] [PubMed] [Google Scholar]
- [17]. Manosroi A, Jantrawuta P, Manosroi J. Anti-inflammatory activity of gel containing novel elastic niosomes entrapped with diclofenac diethylammonium. Int J Pharm. 2008;360:156–163. [DOI] [PubMed] [Google Scholar]
- [18]. Zheng W, Fang Xia Wang L, Zhang Y. Preparation and quality assessment of itraconazole transfersomes. Int J Pharm. 2012;436:291–298. [DOI] [PubMed] [Google Scholar]
- [19]. Cevc G, Blume G. Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, transfersome. Biochim Biophys Acta. 2003;1614:156–164. [DOI] [PubMed] [Google Scholar]
- [20]. Ahad A, Aqil M, Kohli K, et al. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomed Nanotechnol Biol Med. 2012;8:237–249. [DOI] [PubMed] [Google Scholar]
- [21]. Jain S, Mishra D, Kuksal A, et al. Vesicular approach for drug delivery into or across the skin: current status and future prospects. Pharm Online. 2006;1:1–32. [Google Scholar]
- [22]. Morrow DIJ, McCarron PA, Woolfson AD, et al. Innovative strategies for enhancing topical and transdermal drug delivery. Open Drug Deliv J. 2007;1:36–59. [Google Scholar]
- [23]. Zaafarany GME, Awad GAS, Holayel SM, et al. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397:164–172. [DOI] [PubMed] [Google Scholar]
- [24]. Jain S, Jain P. Transferosomes: a novel vesicular carrier for enhanced transdermal delivery: development, characterization and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–1026. [DOI] [PubMed] [Google Scholar]
- [25]. Bhardwaj V, Shukla V, Singh A, et al. Transferosomes ultra flexible vesicles for transdermal delivery. Int J Pharm Sci Res. 2010;2:12–20. [Google Scholar]
- [26]. Schatzlein A, Cevc G. Non-uniform cellular packing of the stratum corneum and permeability barrier function of intact skin: a high resolution confocal laser scanning microscopy study using highly deformable vesicles (transfersomes). Brit J Dermatol. 1998;138:583–592. [DOI] [PubMed] [Google Scholar]
- [27]. Planas ME, Gonzalez P, Rodriguez L, et al. Noninvasive percutaneous induction of topical analgesia by a new type of drug carrier, and prolongation of local pain insensitivity by anesthetic liposomes. Anesth Analg. 1992;75:615–621. [DOI] [PubMed] [Google Scholar]
- [28]. Mahor S, Rawat A, Dubey PK, et al. Cationic transfersomes based topical genetic vaccine against hepatitis B. Int J Pharm. 2007;340:13–19. [DOI] [PubMed] [Google Scholar]
- [29]. Choi MJ, Maibach HI. Elastic vesicles as topical/transdermal drug delivery system. Int J Cosm Sci. 2005;27:211–221. [DOI] [PubMed] [Google Scholar]
- [30]. Guo J, Ping Q, Sun G, et al. Lecithin vesicular carriers for transdermal delivery of cyclosporin A. Int J Pharm. 2000;194:201–207. [DOI] [PubMed] [Google Scholar]
- [31]. Paul A, Cevc G, Bachhawat BK. Transdermal immunisation with an integral membrane component, gap junction protein, by means of ultradeformable drug carriers, transfersomes. Vaccine. 1998;16:188–195. [DOI] [PubMed] [Google Scholar]
- [32]. Gmm EM, Williams AC, Barry BW. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J Pharma Pharmacol. 2001;53:1069–1077. [DOI] [PubMed] [Google Scholar]
- [33]. Costa CAM, Moraes AM. Encapsulation of 5-fluorouracil in liposomes for topical administration. Acta Sci Tech. 2003;25:53–61. [Google Scholar]
- [34]. Alvi IA, Madan J, Kaushik D, et al. Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: preparation, characterization, in-vitro release, and cytotoxicity analysis. Anticancer Drugs. 2011;8:774–782. [DOI] [PubMed] [Google Scholar]
- [35]. Ita KB, Du Preez J, Lane ME, et al. Dermal delivery of selected hydrophilic drugs from elastic liposomes: effect of phospholipid formulation and surfactants. J Pharma Pharmacol. 2007;59:1215–1222. [DOI] [PubMed] [Google Scholar]
- [36]. El Zaafarany GM, Awad GAS, Holayel SM, et al. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397:164–172. [DOI] [PubMed] [Google Scholar]
- [37]. Badran M, Shalaby K, Al-Omrani A. Influence of the flexible liposomes on the skin deposition of a hydrophilic model drug, carboxyfluorescein: dependency on their composition. Sci World J. 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Cevc G, Blume G, Schatzlein A. Transferosomes-mediated transepidermal delivery improves the regiospecificity and biological activity of corticosteroids in vivo. J Control Release. 1997;45:211–226. [Google Scholar]
- [39]. Chen J, Lu WL, Gu W, et al. Skin permeation behavior of elastic liposomes: role of formulation ingredients. Expert Opin Drug Deliv. 2013;10:845–856. [DOI] [PubMed] [Google Scholar]
- [40]. Dragicevic-Curic N, Gräfe S, Gitter B, et al. Surface charged temoporfin-loaded flexible vesicles: in vitro skin penetration studies and stability. Int J Pharm. 2010;384:100–108. [DOI] [PubMed] [Google Scholar]
- [41]. Dubey V, Mishra D, Asthana A, et al. Transdermal delivery of a pineal hormone: melatonin via elastic liposomes. Biomaterials. 2006;27:3491–3496. [DOI] [PubMed] [Google Scholar]
- [42]. Myschik J, Rades T, Hook S. Advances in lipid based subunit vaccine formulations. Curr Immunol Rev. 2009;5:42–48. [Google Scholar]
- [43]. Kumar R, Philip A. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Tropic J Pharm Res. 2007;6:633–644. [Google Scholar]
- [44]. Owens DR. New horizons – alternative routes for insulin therapy. Nat Rev. 2002;1:529–540. [DOI] [PubMed] [Google Scholar]
- [45]. Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141:277–299. [DOI] [PubMed] [Google Scholar]
- [46]. Cevc G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin Pharmacokinet. 2003;42:461–474. [DOI] [PubMed] [Google Scholar]
- [47]. Kumar R, Philip A. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Tropic J Pharm Res. 2007;6:633–644. [Google Scholar]
- [48]. Maurya SD. Enhanced transdermal delivery of Indinavir sulphate via transfersomes. Int J Comp Pharm. 2010;1:1–7. [Google Scholar]
- [49]. Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. [DOI] [PubMed] [Google Scholar]
- [50]. Singh H, Utreja P, Tiwary A, et al. Elastic liposomal formulation for sustained delivery of colchicine: in vitro characterization and in vivo evaluation of anti-gout activity. Aaps J. 2009;11:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Abdallah M. Transferosomes as a transdermal drug delivery system for enhancement the antifungal activity of nyastatin. Int J Pharm Pharm Sci. 2013;5:560–567. [Google Scholar]
- [52]. Sazoka F, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci. 1978;75:4194–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Rai K. Transfersomes: self-optimizing carriers for bioactives. PDA J Pharm Sci Technol. 2008;62:362–379. [PubMed] [Google Scholar]
- [54]. Kumar A, Adde S, Kamble R, et al. Development and characterization of liposomal drug delivery system for nimesulide. Int J Pharm Pharm Sci. 2010;2:87–89. [Google Scholar]
- [55]. Charcosset C, Juban A, Valour J, et al. Preperation of liposomes at large scale using ethanol injection method: effect of scale up and injection devices. Chem Eng Res Des. 2015;94:508–515. [Google Scholar]
- [56]. Maestrelli F, Rodriguez M, Rabasco A, et al. Effect of preparation techniques on the properties of liposomes encapsulating ketoprofen- cyclodextrine complexes aimed for transdermal delivery. Int J Pharm. 2006;312:53–60. [DOI] [PubMed] [Google Scholar]
- [57]. Zhang Y, Ng W, Feng X, et al. Lipid vesicular nanocarrier: quick encapsulation efficiency determination and transcutaneous application. Int J Pharm. 2017. 10;516:225–230. [DOI] [PubMed] [Google Scholar]
- [58]. Schlich M, Lai F, Murgia S, et al. Needle-free jet injection of intact phospholipid vesicles across the skin: a feasibility study. Biomed Microdevices. 2016;18:67. [DOI] [PubMed] [Google Scholar]
- [59]. Meng S, Zhang C, Shi W, et al. Preparation of osthole-loaded nano-vesicles for skin delivery: characterization, in vitro skin permeation and preliminary in vivo pharmacokinetic studies. Eur J Pharm Sci. 2016;92:49–54. [DOI] [PubMed] [Google Scholar]
- [60]. Hassanpour AM, Ghanbarzadeh S, Javadzadeh Y, et al. Aggregated nanotransfersomal dry powder inhalation of itraconazole for pulmonary drug delivery. Adv Pharm Bull. 2016;6:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. González-Rodríguez ML, Arroyo CM, Cózar-Bernal MJ, et al. Deformability properties of timolol-loaded transfersomes based on the extrusion mechanism, statistical optimization of the process. Drug Dev Ind Pharm. 2016;42:1683–1694. [DOI] [PubMed] [Google Scholar]
- [62]. Garg V, Singh H, Bhatia A, et al. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS Pharmscitech. 2017;18:58–71. [DOI] [PubMed] [Google Scholar]
- [63]. Shreya AB, Managuli RS, Menon J, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: in vitro and in vivo performance evaluations. J Liposome Res. 2016;26:221–232. [DOI] [PubMed] [Google Scholar]
- [64]. Seidel EJ, Rother M, Regenspurger K, et al. A randomised trial comparing the efficacy and safety of topical ketoprofen in Transfersome(®) gel (IDEA-033) with oral ketoprofen and drug-free ultra-deformable Sequessome™ vesicles (TDT 064) for the treatment of muscle soreness following exercise. J Sports Sci. 2016;34:88–95. [DOI] [PubMed] [Google Scholar]
- [65]. Lu K, Xie S, Han S, et al. Preparation of a nano emodin transfersome and study on its anti-obesity mechanism in adipose tissue of diet-induced obese rats. J Transl Med. 2014;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Sarwa KK, Mazumder B, Rudrapal M, et al. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015;22:638–646. [DOI] [PubMed] [Google Scholar]
- [67]. Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed Res Int. 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Ghannoum M, Isham N, Henry W, et al. Evaluation of the morphological effects of TDT 067 (terbinafine in Transfersome) and conventional terbinafine on dermatophyte hyphae in vitro and in vivo. Antimicrob Agents Chemother. 2012;56:2530–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Zhang YT, Xu YM, Zhang SJ, et al. In vivo microdialysis for the evaluation of transfersomes as a novel transdermal delivery vehicle for cinnamic acid. Drug. 2014;40:301–307. [DOI] [PubMed] [Google Scholar]
- [70]. Sigurgeirsson B, Ghannoum M. Therapeutic potential of TDT 067 (terbinafine in Transfersome): a carrier-based dosage form of terbinafine for onychomycosis. Expert Opin Investig Drugs. 2012;21:1549–1562. [DOI] [PubMed] [Google Scholar]
- [71]. Rajan R, Vasudevan DT. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res. 2012;3:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Kaur CD, Saraf S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation-damaged skin. J Cosmet Dermatol. 2011;10:260–265. [DOI] [PubMed] [Google Scholar]
- [73]. Duangjit S, Opanasopit P, Rojanarata T, et al. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. J Drug Deliv. 2011;2011:418316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Rother M, Seidel EJ, Clarkson PM, et al. Efficacy of epicutaneous Diractin (ketoprofen in Transfersome gel) for the treatment of pain related to eccentric muscle contractions. Drug Des Devel Ther. 2009;3:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Cevc G, Mazgareanu S, Rother M. Preclinical characterisation of NSAIDs in ultradeformable carriers or conventional topical gels. Int J Pharm. 2008;360:29–39. [DOI] [PubMed] [Google Scholar]
- [76]. Zheng Y, Hou SX, Chen T, et al. Preparation and characterization of transfersomes of three drugs in vitro. Zhongguo Zhong Yao Za Zhi. 2006;31:728–731. [PubMed] [Google Scholar]
- [77]. Li S, Qiu Y, Zhang S, et al. Enhanced transdermal delivery of 18β-glycyrrhetic acid via elastic vesicles: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2012;38:855–865. [DOI] [PubMed] [Google Scholar]
- [78]. Chen G, Li D, Jin Y, et al. Deformable liposomes by reverse-phase evaporation method for an enhanced skin delivery of (+)-catechin. Drug Dev Ind Pharm. 2014;40:260–265. [DOI] [PubMed] [Google Scholar]
- [79]. Trotta M, Peira E, Debernardi F, et al. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int J Pharm. 2002;241:319–327. [DOI] [PubMed] [Google Scholar]
- [80]. Elsayed MM, Abdallah OY, Naggar VF, et al. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322:60–66. [DOI] [PubMed] [Google Scholar]
- [81]. Cadena PG, Pereira MA, Cordeiro RBS, et al. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim Biophys Acta. 2013;1828:309–316. [DOI] [PubMed] [Google Scholar]
- [82]. Gupta PN, Mishra V, Rawat A, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm. 2005;293:73–82. [DOI] [PubMed] [Google Scholar]
- [83]. Vanic Z, Hafner A, Bego M, et al. Characterization of various deformable liposomes with metronidazole. Drug Dev Ind Pharm. 2013;39:481–488. [DOI] [PubMed] [Google Scholar]
- [84]. Schmitt JV, Miot HA. Actinic keratosis: a clinical and epidemiological revision. An Bras Dermatol. 2012;87:425–434. [DOI] [PubMed] [Google Scholar]
- [85]. Memon AA, Tomenson JA, Bothwell J, et al. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol. 2000;142:1154–1159. [DOI] [PubMed] [Google Scholar]
- [86]. Gupta AK, Paquet M, Villanueva E, et al. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Alvi IA, Madan J, Kaushik D, et al. Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: preparation, characterization, in-vitro release, and cytotoxicity analysis. Anticancer Drugs. 2011;22:774–782. [DOI] [PubMed] [Google Scholar]
- [88]. Khan MA, Pandit J, Sultana Y, et al. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: in vitro characterization and in vivo study. Drug Deliv. 2015;22:795–802. [DOI] [PubMed] [Google Scholar]
- [89]. Holmes SA, Malinovszky K, Roberts DL. Changing trends in non-melanoma skin cancer in South Wales, 1988-1998. Br J Dermatol. 2000;143:1224–1229. [DOI] [PubMed] [Google Scholar]
- [90]. Marks R, Staples M, Giles G. Trends in non-melanocytic skin cancer treated in Australia: the second national survey. Int J Cancer. 1993;53:585–590. [DOI] [PubMed] [Google Scholar]
- [91]. Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. [DOI] [PubMed] [Google Scholar]
- [92]. Lear JT, Tan BB, Smith AP, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997;90:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Corona R, Dogliotti E, Errico M, et al. Risk factors for basal cell carcinoma in a Mediterranean population. Arch Dermatol. 2002;137:1162–1168. [DOI] [PubMed] [Google Scholar]
- [94]. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. Arch Dermatol. 1995;131:157–163. [PubMed] [Google Scholar]
- [95]. Yamada M, Kodama K, Fujita S, et al. Prevalence of skin neoplasms among the atomic bomb survivors. Radiat Res. 1996;146:223–226. [PubMed] [Google Scholar]
- [96]. Maloney ME. Arsenic in dermatology. Dermatol Surg. 1996;22:301–304. [DOI] [PubMed] [Google Scholar]
- [97]. Hartevelt MM, Bavinck JN, Kootte AM, et al. Incidence of skin cancer after renal transplantation in the Netherlands. Transplantation. 1990;49:506–509. [DOI] [PubMed] [Google Scholar]
- [98]. Fadel M, Samy N, Nasr M, et al. Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm Dev Technol. 2017;22(4): 545–550. [DOI] [PubMed] [Google Scholar]
- [99]. Friedman-Kien AE, Saltzman BR, Cao YZ, et al. Kaposi’s sarcoma in HIV-negative homosexual men. Lancet. 1990;335(8682):168–169. [DOI] [PubMed] [Google Scholar]
- [100]. Penn I. Kaposi’s sarcoma in organ transplant recipients: report of 20 cases. Transplantation. 1979;27:8–11. [DOI] [PubMed] [Google Scholar]
- [101]. Pathak K, Sharma V, Sharma M. Optimization, in vitro cytotoxicity and penetration capability of deformable nanovesicles of paclitaxel for dermal chemotherapy in Kaposi sarcoma. Artif Cells Nanomed Biotechnol. 2016;44:1671–1683. [DOI] [PubMed] [Google Scholar]
- [102]. Urba WJ, Washington CV, Nadiminti H. Cancer of the skin In: Longo DL, Fauci AS, Kasper DL, editors. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill Medical; 2012. [Google Scholar]
- [103]. American Cancer Society What are the key statistics about melanoma? [cited 2017. January 01]. Available from: www.cancer.org/Cancer/SkinCancer-Melanoma/Detailed-Guide/melanoma-skin-cancer-key-statistics [Google Scholar]
- [104]. O’Bryant CL, Poust JC. Melanoma In: DiPiro JT, Talbert RL, Yee GC, editors. Pharmacotherapy: a pathophysiologic approach. 8th ed. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- [105]. Lin M-W, Huang Y-B, Chen C-L, et al. A formulation study of 5-aminolevulinic encapsulated in DPPC liposomes in melanoma treatment. Int J of Med Sci. 2016;13:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Dorrani M, Olga BG, Tamara M, et al. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J Control Release. 2016;228:150–158. [DOI] [PubMed] [Google Scholar]
- [107]. Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. [DOI] [PubMed] [Google Scholar]
- [108]. Lee DA, Miller SJ. Nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2009;17:309–324. [DOI] [PubMed] [Google Scholar]
- [109]. Akgul B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol. 2006;208:165–175. [DOI] [PubMed] [Google Scholar]
- [110]. Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154:498–504. [DOI] [PubMed] [Google Scholar]
- [111]. Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48:169–175. [DOI] [PubMed] [Google Scholar]
- [112]. Gupta V, Karthikeyan C, Trivedi P. Localized delivery of cisplatin for the effective management of squamous cell carcinoma from protransfersome formulation. Arch Pharm Res. 2012;35:51–859. [DOI] [PubMed] [Google Scholar]
- [113]. Ming K, Lin H, Juan W, et al. Enhanced transdermal lymphatic drug delivery of hyaluronic acid modified transfersomes for tumor metastasis therapy. J Royal Soc Chem. 2014;51(8):1453–1456. [DOI] [PubMed] [Google Scholar]
- [114]. Paolino D, Licciardi M, Celia C, et al. Folate- targeted supramolecular vesicular aggregates as a new frontier for effective anticancer treatment in in vivo model. Euro J Pharm and Biopharm. 2012;82:94–102. [DOI] [PubMed] [Google Scholar]
- [115]. Alvi IA, Madan J, Kaushik D, et al. Comparative study of transfersomes, liposomes, and niosomes for topical delivery of 5-fluorouracil to skin cancer cells: preparation, characterization, in-vitro release, and cytotoxicity analysis. Anticancer Drug. 2011;22:774–782. [DOI] [PubMed] [Google Scholar]
- [116]. Mahmood S, Taher M, Mandal UK. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int J Nanomedicine. 2014. 12;9:4331–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Bragagni M, Mennini N, Maestrelli F, et al. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012;19:354–361. [DOI] [PubMed] [Google Scholar]
- [118]. Maswadeh H, Demetzos C, Dimas K, et al. Accumulation of vinblastine into transfersomes and liposomes in response to a transmembrane ammonium sulfate gradient and their cytotoxic/cytostatic activity in vitro. Anticancer Res. 2001;21:2577–2583. [PubMed] [Google Scholar]
- [119]. Cevc G. Preparation for the application of agents in mini-droplets. United States patent US 6165500 A. 2000.
- [120]. Cevc G. Preparation for the transport of an active substance across barriers. United States patent US 20020048596 A1. 2002.
- [121]. Perricone N. Topical drug delivery using phosphatidylcholine. United States patent US 20060105955 A1. 2006.
- [122]. Cevc G. Formulation for topical non-invasive application in vivo. United States patent US 7175850 B2. 2007.
- [123]. Cevc G. Preparation for the application of agents in mini-droplets. United States patent US 20070042030 A1. 2007.
- [124]. Cevc G, Richardsen H, Weiland-Waibe A. Method for the improvement of transport across adaptable semi-permeable barriers. United States patent US 7591949 B2. 2009.
- [125]. William H, Henk-Andre K, Linda S. Methods of reducing the proliferation and viability of microbial agents. PCT WO2010/090654 A1. 2010.
- [126]. Cevc G, Chopra A. Non-invasive vaccination through the skin. United States patent US 7867480 B1. 2011.
- [127]. Richard WG, William H. Vesicles .PCT/European Patent. CA 2919971 A1. 2015.
- [128]. Modi P. Stabilized and solubilized drug formulation for topical application and transdermal efficacy for cosmetic improvement and methods of formulation. United States patent US 20150157728 A1. 2015.


