Abstract
While reactive ductules (RDs) have been observed in viral hepatitis, biliary atresia, nonalcoholic fatty liver disease, and adult hepatocellular carcinoma (HCC), RDs in pediatric liver cancer remain uncharacterized. This study investigated the relationship of RDs with angiogenic paracrine factors, the extent of angiogenesis, and tumor cell proliferation in pediatric hepatoblastoma (HBL)/HCC livers. We quantified the extent of RDs and their expression of paracrine factors that include vascular endothelial growth factor (VEGF), vascular endothelial growth factor D (VEGFD), platelet‐derived growth factor C, and angiopoietin 1 (ANGPT1). In addition, we performed immunohistochemical detection of the endothelial marker clusters of differentiation (CD)34 and the proliferation marker Ki67 followed by correlation analyses. In HBL, we found the percentage of RDs with Ki67 expression (% Ki67+ RDs) significantly correlated with intratumoral Ki67+ areas (r = 0.5138, P = 0.0349) and % ANGPT1+ RDs positively correlated with % Ki67+ RDs (r = 0.5851, P = 0.0136). In HCC, the high ANGPT1+ RDs group (i.e., cases with % ANGPT1+ RDs ≥50) exhibited high intratumoral Ki67+ areas compared to the low ANGPT1+ RDs group. In the combined HBL and HCC liver tumor group, there was a positive association between % platelet‐derived growth factor C+ RDs and intratumoral Ki67+ areas (r = 0.4712, P = 0.0099) and the high VEGFD+ RDs group (≥50%) exhibited a high number of peritumoral CD34+ vessels compared to the low VEGFD+ RDs group. Conclusion: Paracrine factor‐expressing RDs are associated with angiogenesis and proliferation of pediatric liver tumors.
Abbreviations
- ANGPT1
angiopoietin 1
- CD
clusters of differentiation
- EpCAM
epithelial cell adhesion molecule
- HBL
hepatoblastoma
- HCC
hepatocellular carcinoma
- HPC
hepatic progenitor cell
- NAFLD
nonalcoholic fatty liver disease
- PDGFC
platelet‐derived growth factor C
- RD
reactive ductule
- VEGF
vascular endothelial growth factor
- VEGFD
vascular endothelial growth factor D
The annual incidence of primary malignant liver neoplasm in children is approximately 1.5 per million in the United States, and liver cancer accounts for 1.1% of pediatric malignancies.1 Hepatoblastoma (HBL) and hepatocellular carcinoma (HCC) are the first and second most common liver malignancies in children, respectively.2 Approximately 100‐150 new cases of liver tumors are diagnosed in pediatric patients in the United States annually.3 Although liver tumors in young patients are relatively rare, they have gained significant attention in the last few decades due to the sharp increase of incidence.1 For HBL, the overall survival rates at 5 years remain around 60%‐70%.4 It has also been reported that in 218 pediatric HCC cases, the overall 5‐ and 20‐year survival rates of the entire cohort were 24% and 8%, respectively,5 underscoring the urgent need to identify a novel therapeutic target.
Reactive ductules (RDs) consist of cells with a ductular phenotype that accumulate in response to several human liver diseases, such as chronic biliary obstruction, massive hepatic necrosis from acetaminophen toxicity, nonalcoholic fatty liver disease (NAFLD), primary biliary cirrhosis, and others.6, 7 It has been widely accepted that RDs mainly arise from the biliary compartment, although there is some evidence suggesting that hepatocytes also contribute.7, 8, 9 Epithelial cells constituting RDs, also referred to as hepatic progenitor cells (HPCs) or oval cells, express biliary markers, such as cytokeratin, epithelial cell adhesion molecule (EpCAM), clusters of differentiation (CD)133 (encoded by prominin 1), and sex determining region Y box 9. Recent evidence suggests potential involvement of ductular reactions in progressive fibrosis and hepatocarcinogenesis.9, 10 Expression of progenitor markers by HCC is likely to be associated with poor prognosis and recurrence after surgical resection and liver transplantation,11, 12, 13 and it has been proposed that HPCs are the cellular origin of liver cancer based on correlative data.14, 15, 16, 17, 18 However, using two mouse models of liver cancer induced by hepatotoxin treatment, we demonstrated that mature hepatocytes, not HPCs, are the cell of origin for tumors.19
An increasing number of reports imply a paracrine role of RDs in pathogenesis. For example, RDs in pediatric NAFLD livers express multiple adipokines, including resistin, glucagon‐like peptide 1, and adiponectin.20 Also, vascular endothelial growth factor (VEGF)‐expressing RDs are associated with the extent of angiogenesis in patients with chronic viral hepatitis and primary biliary cirrhosis.21 These findings suggest a potential involvement of ductular reactions in disease progression in an autocrine/paracrine manner. However, whether RDs accumulate in pediatric HBL/HCC livers, their relationship with paracrine factors, angiogenesis, and tumor cell proliferation remain largely unknown.
The goal of our study is to address whether RDs accumulate in the liver from pediatric patients with HBL and HCC and correlate the percentage of paracrine factor+ RDs, proliferation, and angiogenesis in peritumoral and intratumoral areas in a comprehensive histopathologic analysis of 32 livers. For correlation analyses, we selected four angiogenic factors: VEGF, vascular endothelial growth factor D (VEGFD), platelet‐derived growth factor C (PDGFC), and angiopoietin 1 (ANGPT1). These were selected based on their roles in animal models and association with other types of liver disease, such as adult HCC and cholangiocarcinoma.22, 23, 24, 25, 26, 27
Materials and Methods
Clinical Samples and Histologic Grading
Formalin‐fixed paraffin‐embedded serial sections of liver specimens from patients with HBL and HCC were obtained from the Cincinnati Biobank at Cincinnati Children’s Hospital Medical Center. This was a retrospective study using archived specimens from the Cincinnati Biobank (collected from 2005 to 2016). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board. HBL and HCC livers from 20 and 12 patients were analyzed, respectively. Tumor nodules and surrounding areas were identified by a pathologist based on histomorphology of hematoxylin and eosin‐stained sections. HBL histology classification was based on the international pediatric liver tumor consensus classification: Proceedings of the Los Angeles Children Oncology Group liver tumors symposium.28 Tumor grades for HCC were assigned using the four‐scale Edmondson–Steiner grading system.29 In the case of multifocal tumors, a section containing at least one tumor nodule and the adjacent nontumorous area was selected and subjected to evaluation of tumor grades.
Immunohistochemical Staining
Serial 5‐μm‐thick sections of paraffin‐embedded tissues were deparaffinized, rehydrated, and subjected to antigen retrieval in R‐buffer A (62606‐10; Electron Microscopy Sciences, Hatfield, PA) for 1 hour. After quenching with 3% hydrogen peroxide for 15 minutes, sections were treated with avidin/biotin blocking solution (SP‐2001; Vector Laboratories, Burlingame, CA) for 15 minutes, 3% normal donkey or horse serum and 2.5% Triton X‐100 in phosphate‐buffered saline for 1 hour, and then primary antibodies overnight at 4°C. After washing steps, sections were incubated with appropriate secondary antibodies diluted in phosphate‐buffered saline with 3% serum and 2.5% Triton X‐100 at 37°C for 30 minutes. Sections were treated with peroxidase–avidin complexes (PK‐6106; Vector Laboratories) for 30 minutes at 37°C followed by the substrate 3,3'‐diaminobenzidine tetrahydrochloride. Hematoxylin was used for counterstaining. Antibodies and antigen‐retrieval conditions are summarized in Supporting Table S1.
Quantification of Immunohistochemical Markers
Liver sections were photographed with an Aperio AT2 Digital Slide Scanner (Leica Biosystems Inc., Buffalo Grove, IL). Total EpCAM+ areas within nontumoral tissues were measured using Image J software.30 For quantification of peritumoral RDs, total number of EpCAM+ ductules in 10 randomly selected peritumoral areas within 300 μm of tumor edges were counted and divided by total surface area of captured fields (mm2). RDs were identified based on the following definition by Roskams and Colleagues: reactive ductules with a biliary/HPC phenotype arranged in an irregularly shaped structure.9 For measurement of % paracrine factor+ RDs, % Ki67+ RDs, and % CD34+ area in peritumoral areas, at least four random pictures centered on the EpCAM‐positive RDs were captured. Serial sections of the same area were stained with antibodies for paracrine factors, CD34, and Ki67. The extent of paracrine factor and Ki67 expression by RDs in a given area (0.2025 mm2) was calculated as the ratio of the number of stained ductules to the total number of ductules expressed as a percentage. The area occupied by CD34 and Ki67‐stained cells in intratumoral areas was calculated using Image J software.
Statistical Analysis
Statistical analysis was performed using SAS version 9.3 for Windows (SAS Institute Inc., Cary, NC). Pearson’s correlation coefficient was used to determine correlations between continuous normally distributed variables. To compare the means between two groups, the Student t test was performed. P < 0.05 was considered statistically significant.
Results
Patient and Immunohistochemical Characteristics
Liver sections from 32 pediatric patients (aged 18 years or younger) with cancer were used for the analysis (n = 20 and n = 12 for HBL and HCC, respectively) (Table 1). The HBL group included both epithelial (n = 15) and mixed epithelial and mesenchymal tumors (n = 5). The background of the HCC cases included both cirrhotic (n = 5) and noncirrhotic cases (n = 7), and the tumor was graded 1 (well differentiated), 2 (moderately differentiated), 3 (poorly differentiated), and 4 (undifferentiated). Fourteen postchemotherapy and six prechemotherapy cases, and three postchemotherapy and nine prechemotherapy cases were analyzed for HBL and HCC, respectively. In this study, the term RD refers to reactive ductule with a biliary/HPC phenotype arranged in an irregularly shaped structure and residing in the peritumoral area.9 Representative images of peritumoral RDs in HBL and HCC livers are shown in Fig. 1. The expression of the human Ki67 protein is strictly associated with cell proliferation, and the fraction of Ki67+ tumor cells often correlates with the clinical course of the disease.31 Therefore, the extent of proliferation of RDs and tumor cells was determined by Ki67 staining. CD34 is a transmembrane glycoprotein expressed by endothelial cells, lymphohematopoietic stem cells, and leukemic cells and is widely used as a marker of neovascularization.32, 33 Hence, we investigated the number of CD34+ vessels and CD34+ areas to analyze the extent of angiogenesis in peritumoral and intratumoral areas. We detected numerous RDs positive for paracrine factors (Figs. 2A, 3A; Supporting Figs. S1 and S2) and Ki67 (Fig. 3B) in peritumoral areas. For quantification purposes, we defined peritumoral areas as areas within 300 μm of the tumor border in which RDs are detected, and we measured the area stained with CD34 in the same region of interest in subsequent serial sections (Fig. 2B). Intratumoral Ki67+ areas and CD34+ areas were quantified and correlations with % paracrine factor+ RDs, % Ki67+ RDs, and peritumoral CD34+ areas have been investigated. The percentage of EpCAM+ area in nontumoral tissues that detects all biliary cells, including peritumoral RDs, the total number of peritumoral RDs per mm2, and the percentages of RDs positive for VEGF, VEGFD, ANGPT1, PDGFC, and Ki67 in the livers with HBL and HCC is summarized in Table 2. HBL livers exhibited high % VEGF+ RDs (P = 0.0193) and intratumoral Ki67+ areas (P = 0.0039) compared to HCC livers, while there were no statistically significant differences between the two groups in terms of EpCAM+ areas, the number of peritumoral RDs, % VEGFD+ RDs, % ANGPT1+ RDs, % PDGFC+ RDs, and peritumoral and intratumoral CD34+ areas and vessel numbers. In summary, our data indicate that proliferating RDs positive for multiple angiogenic factors are detected in peritumoral areas of both HBL and HCC livers, indicating that the presence of RDs is a common feature of pediatric liver cancer, while expression levels of ductular VEGF and intratumoral Ki67 are different between the two groups.
Table 1.
Patient Characteristics
| Parameters | Liver Tumor, Combined (HBL and HCC, n = 32) | HBL (n = 20) | HCC (n = 12) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 6.5 (6.0) | 3.1 (3.4) | 12.3 (4.8) |
| Sex | |||
| Female | 12 | 7 | 5 |
| Male | 20 | 13 | 7 |
| Chemotherapy | |||
| After | 17 | 14 | 3 |
| Before | 15 | 6 | 9 |
| Cirrhosis | |||
| Cirrhotic | ‐ | ‐ | 5 |
| Noncirrhotic | ‐ | ‐ | 7 |
| Tumor Grade | |||
| 1 | ‐ | ‐ | 4 |
| 1‐2 | ‐ | ‐ | 2 |
| 2 | ‐ | ‐ | 5 |
| 2‐3 | ‐ | ‐ | 1 |
| Histologic classification | |||
| Epithelial | ‐ | 15 | ‐ |
| Mixed epithelial and mesenchymal | ‐ | 5 | ‐ |
Figure 1.
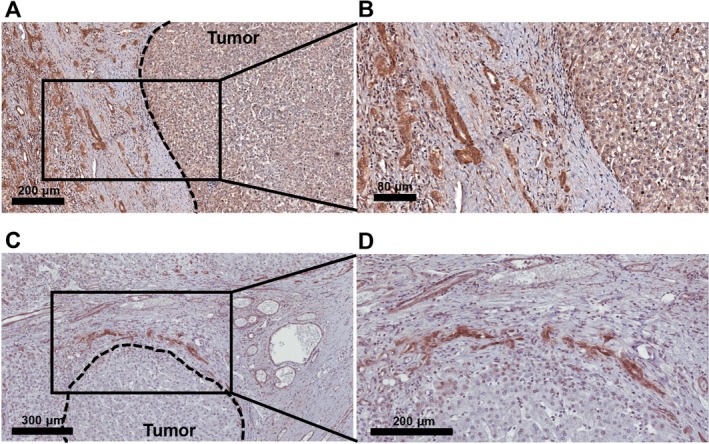
Representative immunostaining of EpCAM+ RDs in HBL and HCC. RDs are detected in peritumoral areas in (A,B) HBL and (C,D) HCC livers. Tumor borders are marked with dashed lines.
Figure 2.
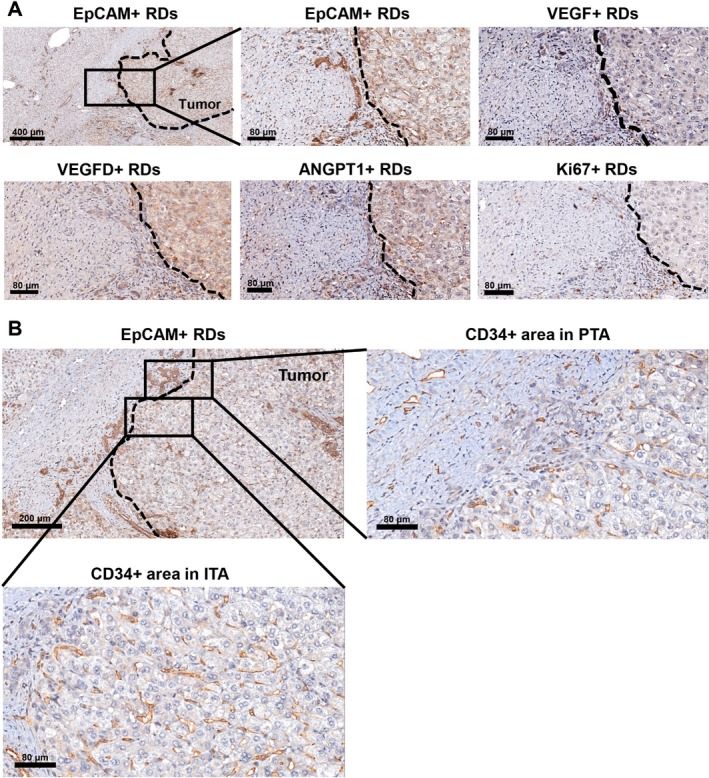
RDs positive for VEGF, VEGFD, and ANGPT1 are detected in the peritumoral area (HBL). (A) RDs in the peritumoral area are identified by EpCAM staining. Shown are subsequent serial sections of the same peritumoral area for VEGF+ RDs, VEGFD+ RDs, ANGPT1+ RDs, and Ki67+ RDs. (B) CD34+ cells in peritumoral and intratumoral areas are identified in a subsequent serial section of the same area. Tumor borders are marked with dashed lines. Abbreviations: ITA, intratumoral area; PTA, peritumoral area.
Figure 3.
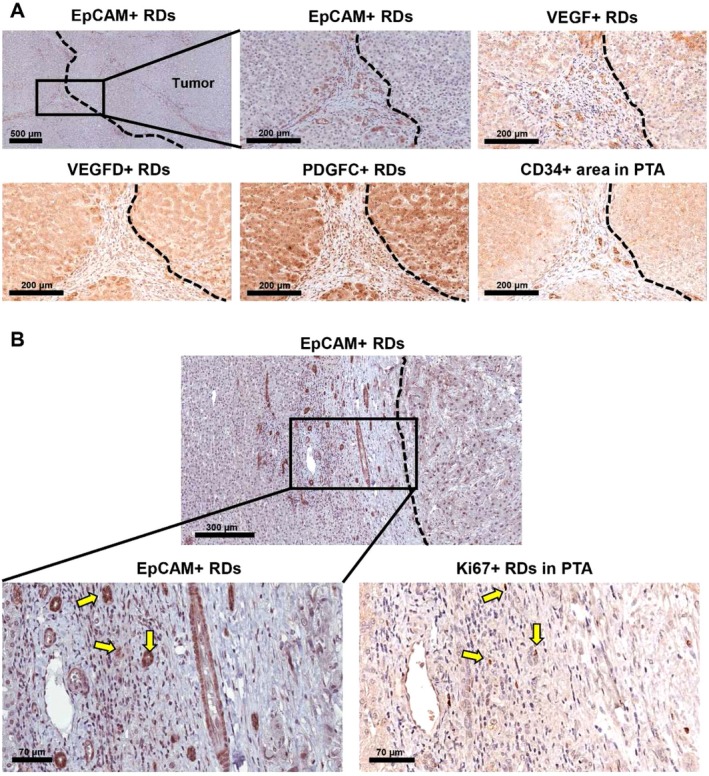
RDs positive for VEGF, VEGFD, and PDGFC are detected in the peritumoral area (HCC). (A) RDs in the peritumoral area are identified by EpCAM+ staining. Shown are subsequent serial sections of the same peritumoral area for VEGF+ RDs, VEGFD+ RDs, PDGFC+ RDs, and CD34+ vessels. (B) Ki67+ cells in the peritumoral area are identified. Arrows indicate EpCAM+Ki67+ RDs in serial sections of the same area. Tumor borders are marked with dashed lines. Abbreviation: PTA, peritumoral area.
Table 2.
Percentage of Paracrine Factor+ RDs, Angiogenesis, and Proliferation
| Liver Tumor, Combined (HBL and HCC, n = 32) | HBL (n = 20) | HCC (n = 12) | P value (HBL vs. HCC) | |
|---|---|---|---|---|
| EpCAM+ area in NTA (%)* | 1.47 ± 1.7 | 1.45 ± 1.7 | 1.50 ± 1.9 | 0.9362 |
| Number of peritumoral RDs (per mm2)† | 99.5 ± 54.2 | 109.1 ± 59.3 | 83.4 ± 41.6 | 0.1978 |
| VEGF+ RDs (%)‡ | 65.3 ± 19.4 | 71.4 ± 18.5 | 55.1 ± 16.2 | 0.0193 |
| VEGFD+ RDs (%)‡ | 79.2 ± 21.7 | 82.2 ± 15.6 | 74.2 ± 28.9 | 0.3196 |
| ANGPT1+ RDs (%)‡ | 56.8 ± 25.1 | 51.2 ± 22.3 | 66.1 ± 26.9 | 0.1072 |
| PDGFC+ RDs (%)‡ | 74.0 ± 27.1 | 80.9 ± 20.3 | 63.1 ± 32.9 | 0.0740 |
| Ki67+ RDs (%)§ | 56.1 ± 24.3 | 53.2 ± 22.3 | 60.4 ± 26.5 | 0.4417 |
| Ki67+ area in ITA (%)|| | 8.9 ± 4.4 | 10.8 ± 4.1 | 6.2 ± 3.3 | 0.0039 |
| Number of CD34+ vessels in PTA¶ | 11.7 ± 7.0 | 12.7 ± 7.4 | 10.1 ± 5.8 | 0.3020 |
| CD34+ area in PTA (%)|| | 8.3 ± 2.8 | 8.3 ± 3.0 | 8.3 ± 2.2 | 0.9956 |
| Number of CD34+ vessels in ITA¶ | 32.3 ± 20.3 | 34.2 ± 22.2 | 29.1 ± 15.6 | 0.5073 |
| CD34+ area in ITA (%)|| | 13.1 ± 5.4 | 14.2 ± 5.5 | 11.4 ± 4.5 | 0.1557 |
All data are expressed as mean ± SD.
*EpCAM+ area in NTA (%) = 100 × nontumoral EpCAM+ area/total NTA.
‡Number of peritumoral RDs = total number of EpCAM+ ductules in 10 randomly selected areas within 300 μm of the tumor border/area of 10 captured fields in mm2.
†Paracrine factor+ RDs (%) = 100 × number of paracrine factor+ ductules/total number of ductules.
§Ki67+ RDs (%) = 100 × number of Ki67+ ductules/total number of ductules.
||Ki67 or CD34+ area (%) = occupied staining+ area/selected field (0.2025 mm2).
¶Number of CD34+ vessels = number of CD34+ vessels in selected intratumoral areas or peritumoral areas (0.2025 mm2).
Abbreviations: ITA, intratumoral area; NTA, nontumoral area; PTA, peritumoral area.
Correlations Among the Extent of Paracrine Factor Expression, Proliferation, and CD34 Expression
As mentioned above, RDs express adipokines and multiple VEGF isoforms in the setting of pediatric NAFLD, chronic viral hepatitis, and primary biliary cirrhosis,20, 21 but the relationship among RDs, angiogenic paracrine factors, and pediatric cancer remains underinvestigated. Therefore, we analyzed correlations among % paracrine factor+ RDs, proliferation, and angiogenesis in peritumoral and intratumoral areas (Table 3). In the combined tumor group, % ANGPT1+ RDs correlated with % Ki67+ RDs (r = 0.4567, P = 0.0128). Interestingly, % ANGPT1+ RDs negatively correlated with peritumoral CD34+ areas (r = −0.3562, P = 0.0454) and intratumoral CD34+ areas (r = −0.4556, P = 0.0088). The number of RDs per mm2 and % PDGFC+ RDs showed a statistically significant correlation with the area stained with Ki67 in intratumoral areas (r = 0.4671, P = 0.0093; r = 0.4712, P = 0.0099, respectively). In HBL, % ANGPT1+ RDs correlated with % Ki67+ RDs (r = 0.5851, P = 0.0136), peritumoral CD34+ areas (r = −0.5219, P = 0.0183), and intratumoral CD34+ areas (r = −0.6356, P = 0.0026). The number of RDs per mm2 correlated with intratumoral Ki67 areas (r = 0.5046, P = 0.0327). In HCC, we did not detect statistically significant correlations among the number of RDs, paracrine factor+ RDs, proliferation, and angiogenesis. This result is likely due to the relatively low number of subjects with HCC (n = 12) analyzed in this study and diverse underlying diseases (Supporting Table S2). Interestingly, our data from the combined group (Table 3) suggest that ANGPT1 promotes the proliferation of RDs but plays a negative role in peritumoral and intratumoral angiogenesis. Our results also imply that PDGFC is involved in intratumoral proliferation but does not modulate proliferation of RDs. The number of RDs was also associated with intratumoral proliferation but not with the degree of angiogenesis. These data indicate that although RDs express both ANGPT1 and PDGFC, these factors play distinct roles in ductular proliferation and angiogenesis, highlighting the importance to investigate individual effector molecules expressed by RDs.
Table 3.
Correlations Among Paracrine Factors, Proliferation, and Angiogenesis
| Liver Tumor, Combined | HBL | HCC | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67+ RDs (%) | Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | Ki67+ RDs (%) | Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | Ki67+ RDs (%) | Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | ||
| EpCAM+ area in NTA (%) |
0.0919*
0.6353† |
0.0525 0.7830 |
–0.2260 0.2207 |
0.1136 0.5358 |
0.0934 0.6113 |
0.0285 0.8768 |
0.1823 0.4839 |
0.1558 0.5371 |
–0.1312 0.5813 |
0.0974 0.6828 |
0.1869 0.4301 |
–0.1295 0.5865 |
–0.0066 0.9838 |
–0.1292 0.6890 |
–0.4240 0.1695 |
0.1588 0.6220 |
–0.1039 0.7480 |
0.3563 0.2556 |
|
| No. of peritumoral RDs (per mm2) |
–0.1308 0.4989 |
0.4671 0.0093 |
0.2595 0.1515 |
0.1298 0.4788 |
0.1088 0.5532 |
0.1837 0.3143 |
0.0168 0.9490 |
0.5046 0.0327 |
0.1305 0.5834 |
0.3091 0.1848 |
0.1951 0.4097 |
0.1548 0.5148 |
–0.3400 0.2888 |
0.2269 0.4782 |
0.5430 0.0681 |
–0.4907 0.1053 |
–0.3183 0.3134 |
0.0600 0.8531 |
|
| VEGF+ RDs (%) |
–0.0707 0.7154 |
0.3262 0.0785 |
–0.2933 0.1033 |
–0.0789 0.6677 |
–0.1266 0.4901 |
–0.0340 0.8533 |
0.2292 0.3763 |
0.3447 0.1613 |
–0.4058 0.0758 |
0.0014 0.9953 |
–0.1651 0.4866 |
–0.0765 0.7487 |
–0.2958 0.3506 |
0.1153 0.7213 |
0.2085 0.5156 |
–0.2987 0.3456 |
–0.4139 0.1810 |
0.1128 0.7270 |
|
| VEGFD+ RDs (%) |
–0.2257 0.2391 |
0.1430 0.4509 |
0.0996 0.5876 |
–0.1576 0.3890 |
–0.1403 0.4437 |
–0.0207 0.9103 |
–0.3154 0.2175 |
–0.1543 0.5409 |
–0.0331 0.8898 |
–0.1680 0.4789 |
–0.2953 0.2062 |
–0.2084 0.3779 |
–0.1243 0.7002 |
0.2696 0.3967 |
0.2131 0.5060 |
–0.1930 0.5485 |
–0.0330 0.9190 |
0.0818 0.8004 |
|
| ANGPT1+ RDs (%) |
0.4567 0.0128 |
0.0227 0.9051 |
–0.1900 0.2975 |
–0.3562 0.0454 |
–0.2657 0.1417 |
–0.4556 0.0088 |
0.5851 0.0136 |
0.0508 0.8414 |
–0.2918 0.2119 |
–0.5219 0.0183 |
–0.2747 0.2412 |
–0.6356 0.0026 |
0.2672 0.4012 |
0.4949 0.1018 |
0.1247 0.6995 |
–0.0905 0.7797 |
–0.1978 0.5378 |
–0.0174 0.9573 |
|
| PDGFC+ RDs (%) |
0.1363 0.4809 |
0.4712 0.0099 |
–0.1271 0.4957 |
–0.1508 0.4180 |
0.0362 0.8469 |
0.1964 0.2896 |
0.3710 0.1427 |
0.3426 0.1782 |
–0.4522 0.0519 |
–0.2866 0.2341 |
–0.1046 0.6700 |
–0.1512 0.5366 |
0.0448 0.8900 |
0.4907 0.1052 |
0.0867 0.7887 |
–0.0073 0.9821 |
0.1310 0.6849 |
0.5070 0.0918 |
|
*Pearson correlation coefficient (r).
† P value.
Abbreviations: ITA, intratumoral area; NTA, nontumoral area; PTA, peritumoral area.
Correlations Among Paracrine Factors Expressed by RDs
Endothelial and epithelial cells exchange multiple paracrine signals,34 especially during morphogenesis and remodeling of the tissue.35, 36 Angiogenic growth factors and their receptors are associated with vascular growth and differentiation. In pediatric patients with liver tumors, however, the relationship among paracrine factors expressed by RDs is still unknown. To test our hypothesis that paracrine factors expressed by RDs are associated with each other, we analyzed correlations among the percentages of paracrine factor+ RDs in pediatric liver tumors (Table 4). We found that % PDGFC+ RDs significantly correlated with % VEGFD+ RDs (r = 0.4308, P = 0.0155) and % ANGPT1+ RDs (r = 0.3880, P = 0.0310) in the combined liver tumor group. In HBL, % PDGFC+ RDs also significantly correlated with % ANGPT1+ RDs (r = 0.5445, P = 0.0159), and in HCC, % PDGFC+ RDs correlated with % VEGFD+ RDs (r = 0.7058, P = 0.0103). Positive associations among PDGFC, VEGFD, and ANGPT1 expressed by RDs suggest the existence of potential regulatory loops among these factors.
Table 4.
Correlations Among Paracrine Factors Expressed by RDs
| Liver Tumor, Combined | HBL | HCC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VEGFD+ RDs (%) | ANGPT1+ RDs (%) | PDGFC+ RDs (%) | VEGFD+ RDs (%) | ANGPT1+ RDs (%) | PDGFC+ RDs (%) | VEGFD+ RDs (%) | ANGPT1+ RDs (%) | PDGFC+ RDs (%) | |
| VEGF+ RDs (%) |
0.2727*
0.1310† |
–0.1384 0.4509 |
0.1983 0.2848 |
0.0175 0.9418 |
0.0781 0.7436 |
0.1182 0.6297 |
0.4830 0.1117 |
–0.1943 0.5452 |
0.0269 0.9338 |
| VEGFD+ RDs (%) |
0.1810 0.3215 |
0.4308 0.0155 |
0.2055 0.3848 |
–0.1167 0.6343 |
0.2937 0.3542 |
0.7058 0.0103 |
|||
| ANGPT1+ RDs (%) |
0.3880 0.0310 |
0.5445 0.0159 |
0.5229 0.0811 |
||||||
*Pearson correlation coefficient (r).
† P value.
Correlations Between the Extent of Proliferation and Angiogenesis
Evidence suggests that the peritumoral region may be a favorable microenvironment for expanding tumor cells.37 Intrahepatic venous metastasis has been demonstrated to be associated with a unique immune/inflammation response signature in the peritumoral area, implying that the peritumoral environment influences disease progression.38 Therefore, we investigated correlations between the extent of angiogenesis and proliferation in peritumoral areas and intratumoral areas. The number of CD34+ vessels in intratumoral areas significantly correlated with the number of CD34+ vessels and CD34+ areas in peritumoral areas in the combined liver tumor group (r = 0.3779, P = 0.0330; r = 0.3884, P = 0.0280, respectively) (Table 5). In addition, intratumoral CD34+ areas significantly correlated with intratumoral Ki67+ areas (r = 0.4431, P = 0.0142) and peritumoral CD34+ areas (r = 0.4915, P = 0.0043) in the combined liver tumor group. In HBL, relatively strong correlations were also detected; % Ki67+ RDs correlated with intratumoral Ki67+ areas (r = 0.5138, P = 0.0349). The number of CD34+ vessels in intratumoral areas correlated with the number of CD34+ vessels in peritumoral areas (r = 0.5247, P = 0.0175). There was a significant correlation between intratumoral CD34+ areas and peritumoral CD34+ areas as well (r = 0.5195, P = 0.0189). However, we could not detect such correlations in HCC. Altogether, analyses of the combined and HBL groups revealed correlations between peritumoral and intratumoral angiogenesis, and proliferation of peritumoral RDs was associated with intratumoral proliferation in the HBL group. Although these correlations do not prove the causal role of RDs, our data imply crosstalk between peritumoral and intratumoral tissues as well as the contribution of RDs in progression of pediatric liver cancer.
Table 5.
Correlations Between Extent of Angiogenesis and Proliferation
| Liver Tumor, Combined | HBL | HCC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | Ki67+ Area in ITA (%) | No. of CD34+ Vessels in PTA | CD34+ Area in PTA (%) | No. of CD34+ Vessels in ITA | CD34+ Area in ITA (%) | ||
| Ki67+ RDs (%) |
0.0707*
0.7156† |
–0.1845 0.3381 |
–0.0562 0.7722 |
–0.0749 0.6995 |
–0.3080 0.1041 |
0.5138 0.0349 |
–0.2842 0.2690 |
–0.0429 0.8703 |
–0.2014 0.4383 |
–0.4168 0.0960 |
–0.3779 0.2259 |
0.0321 0.9210 |
–0.0998 0.7577 |
0.1711 0.5950 |
–0.0887 0.7840 |
|
| Ki67+ area in ITA (%) |
–0.0225 0.9061 |
0.3081 0.0976 |
0.0434 0.8197 |
0.4431 0.0142 |
–0.2820 0.2569 |
0.3479 0.1571 |
–0.0653 0.7968 |
0.3029 0.2218 |
0.3543 0.2586 |
0.4931 0.1033 |
0.1746 0.5873 |
0.5019 0.0964 |
||||
| No. of CD34+ vessels in PTA |
0.1751 0.3378 |
0.3779 0.0330 |
–0.0948 0.6058 |
0.2457 0.2963 |
0.5247 0.0175 |
–0.2139 0.3651 |
–0.0433 0.8937 |
–0.1638 0.6110 |
0.0415 0.8980 |
|||||||
| CD34+ area in PTA (%) |
0.3884 0.0280 |
0.4915 0.0043 |
0.3748 0.1035 |
0.5195 0.0189 |
0.4520 0.1402 |
0.4789 0.1152 |
||||||||||
| No. of CD34+ vessels in ITA |
0.1932 0.2894 |
0.0500 0.8342 |
0.5482 0.0650 |
|||||||||||||
Abbreviations: ITA, intratumoral area; PTA, peritumoral area.
*Pearson correlation coefficient (r).
† P value.
Relationships Among Age, Sex, Chemotherapy, and Paracrine Factor+ RDs
To investigate the relationship among age, sex, chemotherapy status, and paracrine factor+ RDs, these traits were categorized in the combined liver tumor, HBL, and HCC groups. Age, sex, chemotherapy, and histologic classification were not associated with significant differences in % EpCAM+ areas in nontumoral tissues and the number of peritumoral RDs (Supporting Table S3). In the combined liver tumor group (Supporting Table S4), age was categorized into two groups (>3 years versus ≤3 years). In the case of % paracrine factor+ RDs, 50% was used as the cut‐off value. Younger age was significantly associated with greater % VEGF+ RDs, % PDGFC+ RDs, and intratumoral Ki67+ areas (P = 0.0191, P = 0.0243, and P = 0.0052, respectively). There was no significant correlation between sex and % paracrine factor+ RDs. Postchemotherapy status in the combined group was significantly associated with high % VEGFD+ RDs (P = 0.0284). Although we showed correlations among % paracrine factor+ RDs, proliferation, and angiogenesis in Table 3, stronger correlations were detected by dividing RDs into two groups according to the expression levels of paracrine factors (≥50% versus <50%) in Supporting Tables S4‐S6. The high VEGFD+ RDs group (≥50%) showed high % ANGPT1+ RDs and peritumoral CD34+ vessel number compared to the low VEGFD+ RDs group (<50%) (P = 0.0410 and P = 0.0332, respectively), and the high PDGFC+ group (≥50%) exhibited high % VEGFD+ RDs compared to the low PDGFC+ group (<50%) (P = 0.0105) (Supporting Table S4). The high ANGPT1+ group (≥50%) was associated with low intratumoral CD34+ areas compared to the low ANGPT1+ group (<50%) (P = 0.0016), in line with our observations in Table 3.
In patients with HBL (Supporting Table S5), young age was significantly correlated with high % VEGF+ RDs and a low number of CD34+ vessels in peritumoral areas (P = 0.0397 and P = 0.0445, respectively). Postchemotherapy status was associated with high % VEGFD+ RDs (P = 0.0072). The high ANGPT1+ RD group (≥50%) showed high % PDGFC+ RDs (P = 0.0287) and low peritumoral and intratumoral CD34+ areas (P = 0.0111 and P = 0.0013, respectively). Mixed epithelial and mesenchymal histology was associated with a high number of CD34+ vessels in the peritumoral area (P = 0.0158). Relationships among age, sex, chemotherapy status, and paracrine factor+ RDs in HCC are shown in Supporting Table S6. The high PDGFC+ RDs group (≥50%) showed significantly high % VEGFD+ RDs compared to the low PDGFC RDs group (<50%) (P = 0.0004). The high ANGPT1+ RDs group (≥50%) exhibited high intratumoral Ki67+ areas compared to the low ANGPT1+ RDs group (<50%) (P = 0.0159). Younger age was associated with smaller peritumoral CD34+ areas (P = 0.0222). Interestingly, while the number of peritumoral RDs was inversely associated with tumor grades (P = 0.0446, Supporting Table S3), there was a positive association between tumor grades and peritumoral CD34+ areas (P = 0.0313).
Overall, expression of ANGPT1 by RDs was inversely associated with angiogenesis in the combined liver tumor and HBL groups (Supporting Tables S4 and S5), consistent with our data in Table 3. In addition, young age correlated with high % VEGF+ RDs and postchemotherapy status correlated with high % VEGFD+ RDs (Supporting Tables S4 and S5), implying that demographic and clinical parameters influence disease progression. Our analyses also revealed a positive association between ANGPT1 and intratumoral proliferation in HCC (Supporting Table S6), which was not detected in HBL, suggesting that accurate diagnosis can be critical for optimal therapeutic strategies. Furthermore, peritumoral angiogenesis was associated with histologic classification in HBL (Supporting Table S5) and tumor grades in HCC (Supporting Table S6), supporting involvement of peritumoral environments in disease progression.
Discussion
Definitive evidence supporting the role of RDs in pediatric liver cancer is largely unavailable due to the rarity of the disease. Our study revealed not only accumulation of EpCAM+ RDs that express multiple angiogenic factors in the livers of pediatric patients with HBL and HCC but also a previously unknown association between several parameters, such as a correlation between expression of ANGPT1 by RDs and proliferation of RDs, correlation between expression of PDGFC by RDs and intratumoral proliferation, correlation among several paracrine factors expressed by RDs, and association between peritumoral CD34+ areas and intratumoral CD34+ areas/vessel numbers. These results point to a potential role of peritumoral ductules in tumor angiogenesis.
During the course of chronic diseases, biliary cells exhibit increased secretory activities39, 40, 41 and proliferate to compensate for the impaired function of injured ducts.42 Moreover, a recent report suggests that VEGFs play both autocrine and paracrine functions in the expansion of HPCs and endothelial cells.21 Therefore, it is logical to assume that paracrine factor secretion by RDs is implicated in liver diseases and carcinogenesis. In addition, it has been demonstrated that proliferation of ductular reactions is positively associated with poor prognosis of adult HCC.43 Based on these reports, we hypothesized that RDs that express paracrine factors are correlated with angiogenesis and tumoral proliferation in the setting of pediatric liver cancer.
Our study based on the combined liver tumor group revealed that the percentage of PDGFC+ RDs positively correlates with intratumoral proliferation. This is in line with recent studies showing that PDGFC is involved in paracrine modulation of endothelial cells and hepatic stellate cells.23 Furthermore, although there is no significant correlation between the percentage of VEGF+ RDs and markers of proliferation/angiogenesis, we observed that the high VEGFD+ RDs group (≥50%) shows a high number of vessels in peritumoral areas compared to the low VEGFD+ RDs group. Moreover, in the setting of HCC, the high ANGPT1+ RDs group was associated with high intratumoral Ki67+ areas. It has been demonstrated that ANGPT1 cooperates with VEGF during ductal and arterial development in the liver.44 VEGFD exhibits not only angiogenic activity on endothelial cells but also mitogenic/motogenic activity on tumor‐derived cells.27, 45 In total, our results strongly support the potential role of RD‐derived angiogenic factors in disease progression.
Nevertheless, we also observed that the percentage of ANGPT1+ RDs negatively correlates with peritumoral/intratumoral CD34+ areas in the setting of HBL, while the percentage of Ki67+ RDs positively correlates with tumoral proliferation. Furthermore, our data imply that ANGPT1 functions in a disease‐dependent manner, i.e., inhibition of angiogenesis in HBL and promotion of tumoral proliferation in HCC. Although our study focused on ANGPT1 expression by peritumoral ductules, it has been proposed that the balance between ANGPT1 and ANGPT2 determines angiogenesis in the setting of adult HCC.26 Therefore, to clarify the relationships among ANGPT1+ RDs, angiogenesis, and proliferation of tumor cells, future research that will investigate the levels of both ANGPT1 and ANGPT2 is needed. Our results also indicate that while RDs express both ANGPT1 and PDGFC, functions of these factors may be different. In the combined group, as mentioned above, ANGPT1 positively correlated with RD proliferation but was inversely associated with angiogenesis. In contrast, PDGFC was associated with tumoral proliferation but did not correlate with proliferation of RDs and angiogenesis. It is worth noting that biliary cells express the ANGPT1 receptor TIE246 and PDGF receptors are expressed on stellate cells, endothelial cells, and HCC,47 implying that expression patterns of receptors on various hepatic cell types may explain distinct roles of each factors.
Mechanisms underlying gene expression in RDs in the setting of pediatric liver disease remain largely undetermined. This study reveals that while RDs in both HBL and HCC express paracrine factors, HBL livers manifest a high percentage of VEGF+ RDs compared to HCC livers. This result may be explained by the fact that patients with HBL are younger than patients with HCC and is in line with our data indicating that young age is associated with high VEGF expression. Another interesting observation is that postchemotherapy status correlates with high VEGFD expression, implying that RDs can be activated in response to chemotherapy. Furthermore, we demonstrate positive correlations among paracrine factors, suggesting that a common signaling pathway regulates expression of these factors and/or RDs regulate their own gene expression using an autocrine feedback mechanism. In addition, while proliferation of RDs often occurs in the setting of pre‐existing liver damage, pediatric liver cancer can arise in the absence of the underlying liver disease. The mechanisms underlying activation of the HPC compartment and expression of paracrine factors in pediatric livers with cancer are also subjects of further investigation.
Analyses of the extent of RDs also led to a number of intriguing observations. Total EpCAM+ areas in nontumoral tissues capturing the entire biliary populations, including peritumoral RDs, did not exhibit any correlations with clinical and immunostaining parameters, but the number of peritumoral RDs was significantly associated with intratumoral proliferation in the combined liver tumor and HBL groups, supporting the validity of our analyses focusing on peritumoral areas. Interestingly, while the number of RDs in the HCC group was inversely associated with tumor grades, the percentage of ANGPT1+ RDs positively correlated with intratumoral proliferation. These results underscore the importance to determine specific markers expressed by RDs and imply the existence of RD‐produced factors that differentially regulate dedifferentiation and proliferation of tumor cells. It is also possible that that RD expansion precedes expression of effector molecules (i.e., paracrine factors) that regulate proliferation of tumor cells. Of note, a meta‐analysis study that demonstrated a positive association between the proliferative index and HCC grades included high‐grade cases (grades 3 and 4)48 while the vast majority of cases analyzed in this study were grades 1 and 2. Therefore, inclusion of high‐grade tumors may further clarify the relationships among RDs, tumor grades, and proliferation.
The limitations of this study include the small sample size, variable underlying liver diseases and status after chemotherapy, and limited clinical information. Nevertheless, this study led to novel findings demonstrating that peritumoral RDs are present in pediatric HBL and HCC livers and express at least four paracrine factors and that clinical parameters influence expression of paracrine factors. Although this study has focused on the correlations of RDs with angiogenesis and proliferation, evidence suggests that paracrine factors, such as PDGFC and ANGPT1, also play important roles in fibrosis and inflammation,23, 49 supporting the broad clinical implications of our findings. Having confirmed that paracrine factor‐positive RDs exist in HBL and HCC livers, this study also sets the stage for future large‐scale studies. The pediatric hepatic international tumor trial (PHITT), which aims to build the world’s largest collection of pediatric liver cancer specimens, has been initiated, highlighting the importance of identifying novel cellular and molecular targets of investigation.50 In conclusion, our study demonstrates that peritumoral RDs expressing paracrine factors accumulate in the liver of young patients with HBL and HCC and reveals novel relationships among RDs, paracrine factors, angiogenesis, and tumoral proliferation. Our results open the door to future research that will investigate RDs as potential therapeutic targets for treatment of pediatric liver cancer and risk stratification.
Supporting information
View this article online at wileyonlinelibrary.com.
Funding
Supported in part by the National Institutes of Health Public Health Service Grant P30 DK078392 (Pilot and Feasibility Project and Integrative Morphology Core of the Digestive Diseases Research Core Center in Cincinnati), the Cincinnati Children’s Research Foundation and the Cincinnati Biobank, and the Better Outcomes for Children Biorepository.
Potential conflict of interest: Nothing to report.
References
- 1. Emre S, Umman V, Rodriguez‐Davalos M. Current concepts in pediatric liver tumors. Pediatr Transplant 2012;16:549‐563. [DOI] [PubMed] [Google Scholar]
- 2. Darbari A, Sabin KM, Shapiro CN. Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003;38:560‐566. [DOI] [PubMed] [Google Scholar]
- 3. Litten JB, Tomlinson GE. Liver tumors in children. Oncologist 2008;13:812‐820. [DOI] [PubMed] [Google Scholar]
- 4. Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford) 2013;15:741‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allan BJ, Wang B, Davis JS, Parikh PP, Perez EA, Neville HL, et al. A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 2014;49:166‐171. [DOI] [PubMed] [Google Scholar]
- 6. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011;458:251‐259. [DOI] [PubMed] [Google Scholar]
- 7. Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology 2011;54:1853‐1863. [DOI] [PubMed] [Google Scholar]
- 8. Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 2011;141:1432‐1438, 1438.e1431‐e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac‐Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739‐1745. [DOI] [PubMed] [Google Scholar]
- 10. Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis 2004;24:43‐48. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha‐fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res 2008;68:1451‐1461. [DOI] [PubMed] [Google Scholar]
- 12. Guo X, Xiong L, Sun T, Peng R, Zou L, Zhu H, et al. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagn Pathol 2012;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010;59:953‐962. [DOI] [PubMed] [Google Scholar]
- 14. Kung JW, Currie IS, Forbes SJ, Ross JA. Liver development, regeneration, and carcinogenesis. J Biomed Biotechnol 2010;2010:984248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee TK, Castilho A, Ma S, Ng IO. Liver cancer stem cells: implications for a new therapeutic target. Liver Int 2009;29:955‐965. [DOI] [PubMed] [Google Scholar]
- 16. Sell S. Cellular origin of hepatocellular carcinomas. Semin Cell Dev Biol 2002;13:419‐424. [DOI] [PubMed] [Google Scholar]
- 17. Song K, Wu J, Jiang C. Dysregulation of signaling pathways and putative biomarkers in liver cancer stem cells (Review). Oncol Rep 2013;29:3‐12. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest 2013;123:1911‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin S, Wangensteen KJ, Teta‐Bissett M, Wang YJ, Mosleh‐Shirazi E, Buza EL, et al. Genetic lineage tracing analysis of the cell of origin of hepatotoxin‐induced liver tumors in mice. Hepatology 2016;64:1163‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 2012;56:2142‐2153. [DOI] [PubMed] [Google Scholar]
- 21. Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, et al. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg Nutr 2013;2:68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fava G, Demorrow S, Gaudio E, Franchitto A, Onori P, Carpino G, et al. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int 2009;29:1031‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright JH, Johnson MM, Shimizu‐Albergine M, Bauer RL, Hayes BJ, Surapisitchat J, et al. Paracrine activation of hepatic stellate cells in platelet‐derived growth factor C transgenic mice: evidence for stromal induction of hepatocellular carcinoma. Int J Cancer 2014;134:778‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francis H, Onori P, Gaudio E, Franchitto A, DeMorrow S, Venter J, et al. H3 histamine receptor‐mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res 2009;7:1704‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis 2009;41:156‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, et al. Overexpression of angiopoietin‐1 and angiopoietin‐2 in hepatocellular carcinoma. J Hepatol 2004;40:799‐807. [DOI] [PubMed] [Google Scholar]
- 27. Marconcini L, Marchiò S, Morbidelli L, Cartocci E, Albini A, Ziche M, et al. c‐fos‐induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci U S A 1999;96:9671‐9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez‐Terrada D, Alaggio R, de Davila MT, Czauderna P, Hiyama E, Katzenstein H, et al. Children's Oncology Group Liver Tumor Committee. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 2014;27:472‐491. [DOI] [PubMed] [Google Scholar]
- 29. Schlageter M, Terracciano LM, D'Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955‐15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scholzen T, Gerdes J. The Ki‐67 protein: from the known and the unknown. J Cell Physiol 2000;182:311‐322. [DOI] [PubMed] [Google Scholar]
- 32. Greaves MF, Brown J, Molgaard HV, Spurr NK, Robertson D, Delia D, et al. Molecular features of CD34: a hemopoietic progenitor cell‐associated molecule. Leukemia 1992;6(Suppl. 1):31‐36. [PubMed] [Google Scholar]
- 33. Paschoal JP, Bernardo V, Canedo NH, Ribeiro OD, Caroli‐Bottino A, Pannain VL. Microvascular density of regenerative nodule to small hepatocellular carcinoma by automated analysis using CD105 and CD34 immunoexpression. BMC Cancer 2014;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS. VEGF expression in hypoxia and hyperglycemia: reciprocal effect on branching angiogenesis in epithelial‐endothelial co‐cultures. J Am Soc Nephrol 2002;13:2027‐2036. [DOI] [PubMed] [Google Scholar]
- 35. Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 1999;10:2125‐2134. [DOI] [PubMed] [Google Scholar]
- 36. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular‐specific growth factors and blood vessel formation. Nature 2000;407:242‐248. [DOI] [PubMed] [Google Scholar]
- 37. Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, et al. High expression of macrophage colony‐stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008;26:2707‐2716. [DOI] [PubMed] [Google Scholar]
- 38. Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006;10:99‐111. [DOI] [PubMed] [Google Scholar]
- 39. Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, et al. Gastrin inhibits cholangiocyte growth in bile duct‐ligated rats by interaction with cholecystokinin‐B/Gastrin receptors via D‐myo‐inositol 1,4,5‐triphosphate‐, Ca(2+)‐, and protein kinase C alpha‐dependent mechanisms. Hepatology 2000;32:17‐25. [DOI] [PubMed] [Google Scholar]
- 40. Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 2001;281:G182‐G190. [DOI] [PubMed] [Google Scholar]
- 41. Lesage G, Glaser SS, Gubba S, Robertson WE, Phinizy JL, Lasater J, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin‐induced ductal secretion. Gastroenterology 1996;111:1633‐1644. [DOI] [PubMed] [Google Scholar]
- 42. Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 2004;127:1565‐1577. [DOI] [PubMed] [Google Scholar]
- 43. Ye F, Jing YY, Guo SW, Yu GF, Fan QM, Qu FF, et al. Proliferative ductular reactions correlate with hepatic progenitor cell and predict recurrence in HCC patients after curative resection. Cell Biosci 2014;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol 2012;56:1159‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Identification of a c‐fos‐induced gene that is related to the platelet‐derived growth factor/vascular endothelial growth factor family. Proc Natl Acad Sci U S A 1996;93:11675‐11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol 2011;300:G303‐G315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kikuchi A, Monga SP. PDGFRalpha in liver pathophysiology: emerging roles in development, regeneration, fibrosis, and cancer. Gene Expr 2015;16:109‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohamed WS, Omar MM, Khayri TM, Fakhr IM. Assessment of the proliferative marker Ki‐67 and p53 protein expression in HBV‐ and HCV‐related hepatocellular carcinoma cases in Egypt. Int J Health Sci (Qassim) 2008;2:27‐34. [PMC free article] [PubMed] [Google Scholar]
- 49. Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, et al. Angiopoietin‐1 therapy enhances fibrosis and inflammation following folic acid‐induced acute renal injury. Kidney Int 2008;74:300‐309. [DOI] [PubMed] [Google Scholar]
- 50. Moroz V, Morland B, Tiao G, Hiyama E, Kearns P, Wheatley K. The paediatric hepatic international tumour trial (PHITT): clinical trial design in rare disease. Trials 2015;16(Suppl. 2):P224. Author names in bold designate shared co‐first authorship. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


