Abstract
Background:
Studies in animal models document that forced abstinence from usual consumption of alcohol changes subsequent seeking and consumption; with increases or decreases depending on the species, duration of abstinence, number of deprivations, and sex. Human laboratory-based alcohol deprivation studies are rare.
Methods:
We conducted a 2-session, within-participant, randomized-order comparison of intravenous, progressive ratio, alcohol self-administration during 2.5 hours of progressive work f+or alcohol and/or vehicle; once while the participants’ pursued their usual drinking habits and once after 2 weeks of closely monitored, voluntary outpatient abstinence from alcohol. The schedule of work for rewards and the incremental increases in breath alcohol concentration following completion of an alcohol work-set were identical across participants. Fifty young-adult (27 men), heavy-drinking participants completed both sessions. Our primary hypothesis was that motivation to work for alcohol after 2 weeks of abstinence would be greater in participants with a weekly binge pattern of drinking, compared to those who regularly drink heavily, and we intended to explore associations with biological family history of alcoholism and sex.
Results:
We detected no change in work for alcohol associated with recent drinking history. However, females, on average, increased their work for alcohol upon resumption after 2 weeks of abstinence (mean ± sem = +16.3 ± 9.6 %) while males decreased that work (−24.8 ± 13.8 %). The sex difference was substantial and significant (p <.03), with a medium effect size (Cohen’s d = 0.63).
Conclusion:
We believe a more comprehensive study of mechanisms underlying the sex-differences in the human post abstinence response is warranted.
Keywords: alcohol, sex, motivation, self-administration, short-term abstinence
The premise for this research project was that short-term abstinence from alcohol consumption is likely to increase the motivation to drink upon resumption. The transient increase in alcohol seeking and consumption in animals provided routine access to alcohol after short-term periods of abstinence has been termed the Alcohol Deprivation Effect (ADE) and is thought to inform human craving and relapse (Vengeliene et al., 2014 for a review). The phenomenon has been studied in considerable detail, is influenced by the genetic background of the animal, and is frequently used to examine potential pharmacological treatments for human alcohol use disorders (Bell et al., 2017, Reichel and Bevins, 2009). The ADE has been attributed to changes in the rewarding properties of alcohol (Rodd et al., 2005, Toalston et al., 2008, Dhaher et al., 2012). Its magitude varies with the duration of the abstinence interval (Bell et al., 2008, Sinclair et al., 1973), and is observed in studies of both alcohol seeking and consumption (Sinclair and Senter, 1968, Heyser et al., 2003, McKinzie et al., 1998). Notably, the effect of a single abstinence is transient, but repeated deprivations increase the effect and can result in the development of loss of control over drinking. Sex-related differences in the ADE have been reported (Garcia-Burgos and Manrique, 2010, Füllgrabe et al., 2007, Vengeliene et al., 2005). A similar phenomenon of increased alcohol drinking attributable to abstinence and intermittent alcohol access has also been found in primates (Weerts et al., 2006, Lindell et al., 2017), but the direct observation of such a phenomenon has been relatively unexplored in humans.
If an ADE exists in humans, studying it could illuminate how patterns of usual drinking, including the common occurrence of elective intervals of abstinence, contribute to the development of increased risk for alcohol use disorders. Our project is the first to use a laboratory paradigm to explore a human analogue of the ADE, here labeled the Post-Abstinence Response (PAR). We chose a different acronym because of underlying differences in assumptions of baseline alcohol consumption/exposure affecting the translation from animal models to human participants.
In natural settings such as college or work life, there is a weekly cadence to intervals of abstinence or substantially reduced consumption. A steady progression of drinking during college (Maggs et al., 2011) and changes in drinking patterns with short-term abstinence in that population (Burish et al., 1981) might reflect a positive PAR phenomenon. Self-reported increases in subjective craving during abstinence, in association with an increased risk of resumption of drinking (Oslin et al. 2009) may offer indirect evidence of PAR effects in humans. Craving also predicts alcohol drinking during treatment (Flannery et al., 2003), and increased post-abstinence craving for alcohol is higher among individuals with multiple past detoxifications versus single episodes (Malcolm et al., 2000a, 2000b; Hillemacher et al., 2006). Notwithstanding their utility, these findings do not control abstinence experimentally, and remain subject to a number of confounds, including differences in patient demographics between different types of self-imposed abstinence. We perceived an experimental approach to an abstinence effect would provide critically important data.
In developing the PAR paradigm, we chose a sample population of young, healthy, heavier-drinking, but non-alcohol dependent adults. We speculated that a PAR effect might be more readily identified in non-treatment-seeking alcohol use disorder participants. Unfortunately, induction of outpatient abstinence may place those individuals at risk for severe alcohol withdrawal and it could be of questionable ethics to provide those persons with access to alcohol after they have achieved abstinence. Moreover, influenced by ADE differences across the alcohol preferring and non-preferring rodent lines, we reasoned that PAR, if it exists, could reflect an underlying risk for developing an alcohol use disorder and should be tested at an earlier stage of human illness. Our choice is also consistent with the intermittent access approaches used to induce and augment alcohol drinking in laboratory animals (Weerts et al., 2006, Lindell et al., 2017, Carnicella et al., 2014).
Possible mechanisms underlying a potential PAR effect in humans are manifold. Abstinence could alter craving, subjective perceptions of intoxication, neural responses to alcohol, acute tolerance to alcohol, and/or alcohol elimination rates. Rather than optimize our experimental design around testing a specific potential underlying mechanism, we chose a sample population recruited to survey the most likely associated risk factors for developing an AUD: recent drinking style, density of biological family history of alcoholism, and sex. Our hypotheses were that participants who were binge drinkers, had higher family history density of alcoholism, or were male would seek more alcohol upon resumption from 2 weeks of abstinence, compared to regular drinkers, those with lower family history density and females, respectively.
The only abstinence-related human laboratory experiment that we are aware of examined the response to stress (McCaul et al., 2017). The authors examined alterations in alcohol-related, simple-button press, progressive-ratio responding in 30 non-treatment seeking participants with an alcohol use disorder (21 males, 9 females) after a 4-day abstinence +/− stress. Stress increased the rate of alcohol responding after 4 days, but the authors reported neither a direct examination of abstinence on motivation for alcohol nor inquiry into a sex-related difference. In preclinical studies, many of those that evaluated sex differences found that females had a greater ADE than males (Bell et al., 2008, Füllgrabe et al., 2007).
Modeled after preclinical procedures, our laboratory has developed several relevant human paradigms for relating abstinence to alcohol self-administration. All employ the Computer-assisted Alcohol Infusion System (CAIS) developed by the Indiana Alcohol Research Center (Zimmermann et al., 2008, 2009, 2011; Plawecki et al., 2011). CAIS achieves the same incremental change in the time course of end-expiratory breath (BrAC), and therefore brain, alcohol concentration for every alcohol reward in every participant in every session. Thus, CAIS avoids the confounding effects of the substantial variability in incremental BrAC that attend administering oral doses (Ramchandani et al., 2009). The CAIS “Progressive Work” paradigm is meant to assess the participant’s motivation for seeking alcohol; determined by operant responding (‘you will have to work at a difficult, boring task for longer and longer intervals to earn the successive rewards’) (Plawecki et al., 2013). CAIS Progressive Work parameters are quite similar to intensity and breakpoint, respectively, as defined in behavioral economic studies (e.g., Amlung et al., 2017), but are ascertained with actual, rather than hypothetical, drug self-administration. We employed the CAIS Progressive Work paradigm in this study because quantifying the effect of abstinence on alcohol motivation was our primary goal (Jones and Comer, 2013).
MATERIALS AND METHODS
Participants
Sixty healthy, young-adult (aged 21 to 30 years), heavy drinkers were recruited from those responding to local advertisements. We balanced enrollment by sex and included a substantial range of immoderate recent drinking styles (see Table 1).
Table 1.
Demographics and Results for the analytical sample population by Sex. DD = drinking day, TBW = total body water, U = Usual drinking habits session, R = Resumption session (terminating abstinence interval), PAR = Post Abstinence Response index; ((R−U)/(R+U)/2), expressed as percent change.
| Measure | Units | Women | Men | p value |
|---|---|---|---|---|
| Demographics | ||||
| (total) White : African-American : Latino : Asian | (23) 17 : 3 : 2 : 1 | (27) 21 : 4 : 2 : 0 | ||
| Age | years | 23.0 ± 0.4 | 23.8 ± 0.5 | 0.192 |
| TBW | liters | 32.8 ± 0.8 | 47.4 ± 1.0 | 0.000 |
| Drinking Days (DD) | # out of 35 days | 14.7 ± 0.8 | 15.6 ± 1.2 | 0.642 |
| Heavy Drinking Days | # out of 35 days | 6.3 ± 0.6 | 7.6 ± 0.9 | 0.251 |
| # Binges | # out of 35 days | 8.3 ± 0.6 | 9.6 ± 0.9 | 0.24 |
| # Extreme Binges | # out of 35 days | 0.6 ± 0.3 | 2.0 ± 0.4 | 0.01 |
| gmEtoh per week | gm/wk | 134 ± 10 | 196 ± 15 | 0.002 |
| gmEtoh per week/TBW | gm/wk per liter TBW | 4.1 ± 0.3 | 4.1 ± 0.3 | 0.889 |
| gmEtoh per DD | gm/day | 47.4 ± 3.5 | 68.6 ± 5.7 | 0.002 |
| gmEtoh per DD/TBW | gm/day per liter TBW | 1.45 ± 0.10 | 1.45 ± 0.12 | 0.998 |
| total gmEtoh in 35 D | gm Ethanol | 670 ± 50 | 980 ± 70 | 0.001 |
| total gmEtoh in 35 D/TBW | gm Ethanol per liter TBW | 20.6 ± 0.2 | 20.6 ± 0.1 | 0.899 |
| AUDIT | # | 9.4 ± 0.8 | 11.8 ± 1.0 | 0.087 |
| RESULTS | ||||
| Cumulative Work Data and PAR Calculations | ||||
| U - alcohol | # CAT trials | 248 ± 31 | 476 ± 52 | 0.001 |
| R - alcohol | # CAT trials | 285 ± 34 | 375 ± 45 | 0.121 |
| PAR - alcohol | % | 16.3 ± 9.6 | −24.8 ± 13.8 | 0.027 |
| U - water | # CAT trials | 296 ± 40 | 179 ± 41 | 0.051 |
| R - water | # CAT trials | 310 ± 48 | 221 ± 48 | 0.197 |
| PAR water | % | −0.2 ± 14.4 | 25.3 ± 23.1 | 0.36 |
| PAR calculations for baseline subjective perceptions | ||||
| PARcraving | % | +33 ± 16 | +35 ± 17 | 0.94 |
| PARneeding | % | +21 ± 16 | +30 ± 22 | 0.75 |
| PARwanting | % | +24 ± 2 | +2.5 ± 18 | 0.37 |
We screened respondents by phone for basic eligibility (self-reported age, recent drinking, and perceived health); those passing undertook an in-depth, face-to-face, enrollment interview after providing informed consent approved by the Indiana University School of Medicine Institutional Review Board. During that interview, we conducted the following procedures: collection of demographic information, assessment of most recent 35-day drinking (Time-Line Follow Back; Sobell and Sobell, 1992; Sobell et al., 1996), assessment of antecubital vein access and vital signs completed by a Nurse from the Indiana Clinical Research Center (ICRC of the Indiana Clinical and Translational Sciences Institute), drawing of 10 ml of venous blood for testing liver function and urine sample for drug testing and pregnancy. Trained technicians performed the Eyesenck Personality Assessment (Eysenck et al. 1985), Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), the Family History of Alcoholism module of the SSAGA (Rice et al., 1995), a brief nicotine use survey (Fagerstrom Survey), the Alcohol Use Disorders Identification Test (AUDIT; Bush et al.,1998), Family History Questionnaire, the Center for Epidemiologic Studies Depression Scale, UPPS Impulsive Behavior Scale (UPPS-SF), and the retrospective Self-Reported Effects of Alcohol (SRE) (Shuckit and Smith, 2004).
Exclusion criteria were current or prior history of any serious medical illness (requiring hospitalization or medication), current DSM-IV alcohol or drug dependence as defined by the SSAGA, prior treatment for any substance use disorder, use of medication known to interact with alcohol within 2 weeks of the study, and pregnancy or the intention to conceive in women.
In addition, each enrolled/tested participant undertook a brief face-to-face interview before each laboratory session. Participants described any alcohol problems since the earlier interview and provided an interim drinking history, current BrAC and urine samples. We monitored participants for Adverse Events and protocol deviations throughout participation in the project; presenting such incidents to the Indiana University School of Medicine Alcohol Studies Data Safety Monitoring Board.
Drinking Styles
We were interested in including a range of heavier recent drinking styles. We recruited participants averaging more than 14 European standard drinks (12 gm ethanol) per week for men; more than 10 for women. We operationalized the NIAAA definition of binge drinking, consistent with an episode in which women/men consumed at least 4/5 drinks (https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking). Extreme binges comprised 10 or more drinks per occasion for men and 8 or more for women. We defined regular, heavy drinking as a drinking pattern with less than 3 days between drinking days and more than 2.0 gm ethanol per liter total body water (about 5 standard drinks per drinking day for the average women and 7 per drinking day for the average man in this study).
Assessment of change in motivation to drink associated with 2 weeks of abstinence.
We employed a within-participant, multiple-session, randomized-order design (Figure 1) with identical operant alcohol self-administration procedures used in each session. At least one session was conducted during the participant’s usual drinking habits (the U session); the other terminating abstinence (the R session) and marking the resumption of drinking. Cumulative Work performed for Alcohol rewards (CWA) comprised the most sensitive measure of motivation in our previous studies (Plawecki et al., 2013, Cyders et al., 2016; VanderVeen et al., 2016), so we examined distributions of normalized change in laboratory CWA from the U to the R session;
Figure 1.
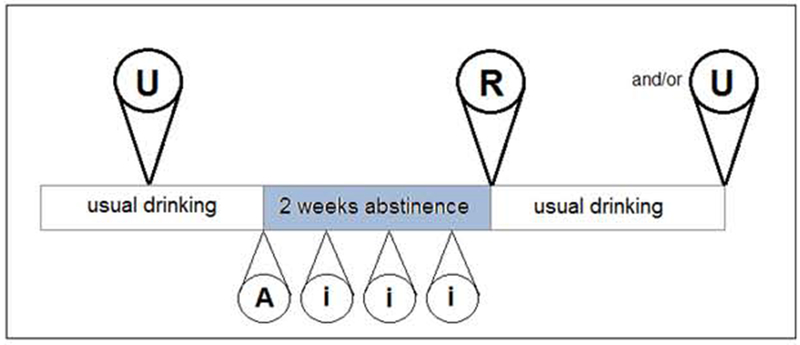
Experimental design for detecting a PAR effect. The resumption session (R) always terminated 2 weeks of monitored abstinence from alcohol. Most participants performed one usual session (U) during their usual drinking habits, either preceding the abstinence interval by at least 1 week or succeeding it by 2 weeks. We randomized the session order across participants, but the first 12 participants undertook both U sessions in order to assess test-retest reliability. Participants wore a transdermal alcohol concentration-monitoring device for all sessions and for the entire abstinence interval, beginning at time “A”. At least three additional visits to the laboratory occurred during the abstinence interval, “i”.
Maintenance of Abstinence
We considered self-report of alcohol non-use to be insufficient when abstinence from alcohol is a prerequisite of experimental design. Our participants wore a Secure Continuous Remote Alcohol Monitoring (SCRAMx®) transdermal, alcohol-monitoring device, manufactured by AMS, Highlands Ranch, CO. It provides records of transdermal alcohol concentrations obtained every 30 minutes, and documents any interval when the device was tampered with or removed. We installed the anklet device for both experimental sessions and for the entire abstinence interval. AMS uses a conservative threshold of >20 mg/dl of the peak transdermal sweat alcohol concentration reading for detection of drinking. In forensic applications, the threshold has been proven quite reliable for capturing drinking events including consumption of more than 5 drinks (Barnett et al., 2014). Application of the AMS device to abstinence-oriented research is somewhat less conclusive. Nonetheless, 100% of drinking events with 2-3 drinks (Roache et al., 2015) produce positive readings. Thus, we considered any non-zero reading as a possible drinking event.
In addition, participants visited our lab every 3rd or 4th day during their abstinence interval (marked “i” in Figure 1). During each such visit, a technician interviewed the participant directly, documenting a timeline of events since the last visit by the participant, completing the Clinical Institute Withdrawal Assessment of Alcohol Scale – Revised (Stuppaeck et al., 1994), and downloading data from the SCRAMx® device. The latter were analyzed immediately by our technicians and by the device manufacturer within 24 hours, for any possible drinking event. Any overt or suspected drinking event led to immediate dismissal of the participant from the project and any CIWA score greater than 8 would have led to dismissal after referral to treatment (no such event obtained).
Laboratory Sessions
For the U session, we instructed participants to avoid consuming alcohol after 4 PM on the day before testing and not to eat anything after midnight. For both U and R sessions, the participant was admitted to the outpatient section of the Indiana Clinical Research Center at Indiana University Hospital at 8 AM, asked to provide BrAC and urine samples precluding alcohol, drug use, and - for females - pregnancy. The Center provided a standardized 350 cal. breakfast at 9 AM, and then a nurse installed an indwelling 20-gauge IV catheter in an ante-cubital vein of the non-dominant arm. The participant rested for 15 min, then sat comfortably in a 5×7’, sound-dampened, testing chamber. The catheter was Y-connected to two sets of dual IMED infusion pumps, each set capable of delivering 4-1998 ml/hr of infusate. For alcohol rewards, we employed infusions of 6.0% (v/V) ethanol in half-normal saline (0.45% NaCl), and infused the vehicle alone for “water” rewards.
We organized each session into a sequence of work-sets, each immediately followed by intravenous delivery of the relevant reward. When ready, participants initiated a work-set by preselecting the next reward, pressing one of two available buttons, labeled “A” for alcohol or “W” for water, respectively. Participants could take as much time as they desired to initiate or complete a work-set, including the options of pausing or ceasing work altogether, but were required to finish the set and receive the reward before another work-set could be initiated. We placed no constraint upon reward selection and its sequence – participants could select alcohol only, vehicle only, any combination and sequence they desired, pause ad lib, or choose not to work. Each 2.5-hour self-administration session began without priming of any sort and required the participant to remain in the lab for the entire session.
Completion of a work-set comprised successful performance of a predetermined number of trials of the Constant Attention Task (CAT) (Plawecki et al., 2013). Briefly, each CAT trial: is initiated ad lib by the participant, cannot be performed successfully while the participant is paying attention to any other stimulus, and is continuously adapted to yield a 50% success rate independent of practice, fatigue, or intoxication. In this study, the sequence of the number of correct CAT trials required for the completion of up to 20 successive work-sets was [1, 2, 3, 4, 6, 8, 11, 15, 20, 26, 34, 45, 59, 77, 101, 132, 172, 224, 292, and 380].
As soon as an alcohol work-set was completed, CAIS exercised its embedded physiologically-based pharmacokinetic model of alcohol distribution and elimination (Plawecki et al., 2008). With parameters tailored to the individual participant, the model enables computation and delivery of the infusion pump rate profile that, starting at the participant’s current value of BrAC, raises it by 12.5 mg/dl (at +5.0 mg//min for 2.5 min), then decreases it at −0.5 mg/dl/min until the next alcohol delivery begins (Figure 2). Thus, incremental brain exposures to alcohol following completion of an alcohol work-set were identical for all participants. As soon as a work-set performed for water was finished, an infusion of vehicle alone was added to any ongoing alcohol infusion; increasing and then decreasing over 2.5 min (for a total volume of approximately 11mL). CAIS precluded initiation of the next work-set during the 2.5 min; any additional delays were at the discretion of the participant or, if the BrAC was near the safety limit, by CAIS for safety purposes. The combination of work sequence, task parameters, and alcohol reward kinetics meant that, if a participant worked as quickly as possible and worked only for alcohol, s/he could achieve a peak BrAC of 160 mg/dl.
Figure 2.
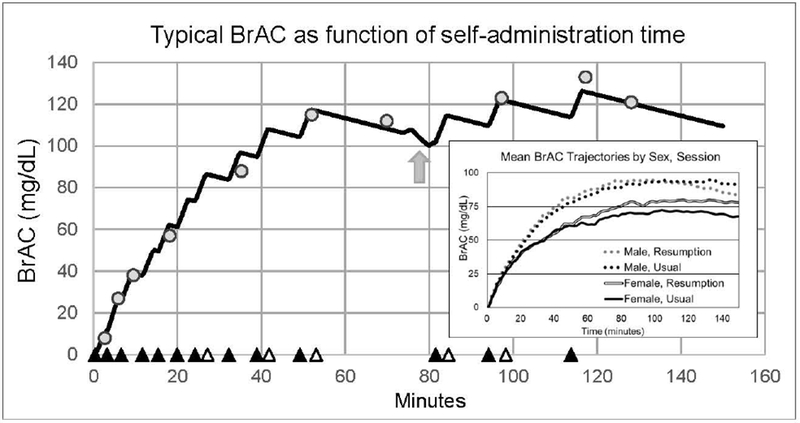
Typical trajectory of estimated BrAC during a PAR testing session (participant P0200-R). This technician’s screen of the Computer-assisted Alcohol Infusion System updates the continuous estimate of breath alcohol concentration (BrAC) as the session progresses. Circles denote BrAC measurements. Solid triangles note the onset of the delivery of alcohol rewards; open triangles: of vehicle. The arrow indicates a bathroom break in this example; during work sets, subjects could take bathroom breaks ad-lib (infusions were discontinued, but compensated with pre - and post - loading calculated to minimize variation in BrAC. Inset: Average Usual and Resumption Session BrAC trajectories by sex.
Once a session was under way, we limited interaction with the CAIS technician to measurement of BrAC approximately every 20 min. We excluded other distractions including TV, phone, or reading, and required participants to remain in the testing room for the duration of the experiment. Afterwards, we placed the participant in a private room on the ICRC until 6 PM or until their BrAC dropped below 20 mg/dl, whichever occurred later, in order to maximize safety and to discourage curtailing work for an early release. We compensated participants for their time in cash at the conclusion of each visit, employing a progressive schedule of payments that had a total value of $470 for those completing both testing sessions.
Dependent Measures
CAIS counted CWA as the total number of CAT trials (whether performed correctly or incorrectly) in service of achieving an alcohol reward, measured separately during each of the R and U sessions. As the primary outcome measure, we computed a continuous dependent variable based on an individual’s normalized change in CWA from the U to the R session, accounting for both increases and decreases among participants following abstinence. We expressed the normalized difference score as a percent change:
We normalized PAR by (R+U)/2, instead of U alone, in order to minimize the distortion that participants with small values of U would impose on the value/distribution of PAR. PARcwa is a continuous variable that exists for every participant who completed both sessions; it has a positive value if the participant worked more for alcohol upon resumption, and a negative value if the participant worked less. We considered using PAR computations for session breakpoint for alcohol and the peak BrAC achieved, but found these measures to be too highly correlated with PARcwa to employ them in analyses. We employed an identical method and calculation of a PAR effect for the alternative reinforcer.
We also collected three subjective perceptions related to the desire for alcohol, and examined them for a PAR effect attributable to 2 weeks of abstinence. We categorized desire in three ways: the subjective craving, wanting and needing of alcohol, measured at baseline in both R and U sessions. Participants used a video display of a cursor position along a 10-point scale under each of the following statements presented in a random order, consistent with our previously published work (Kosobud et al., 2015):
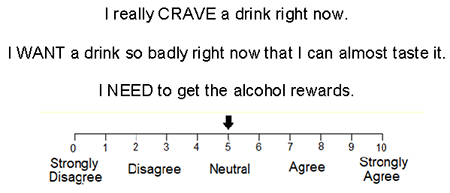
Participants moved the arrow via keypad left/right clicks to select their response of choice for each statement; our software logged and time-stamped the responses with 0.1 level accuracy for off-line analysis. We based calculations for PAR of subjective perceptions of alcohol’s effects on normalized change scores and expressed them as a ± percent change.
The first 12 participants performed U sessions both before and after abstinence in order to assess the test-retest reliability of the procedure. For PAR calculations in those cases, we set U to the average of the two U sessions. Since test-retest reliability was adequate (see Results), only one U session was performed in subsequent participants with the order of U and R sessions pseudo-randomized.
Statistical Analyses
Associations of PARcwa with known risk factors were examined within the sample of N=50 participants who completed the study. We tested the primary hypothesis, (an increase in alcohol-seeking behavior during the resumption session would correlate with current risky drinking) by calculating Pearson’s Product-Moment coefficients between PARcwa and TLFB variables. Secondary hypotheses and demographic distributions were tested as follows: 1) Sex differences were tested using multivariate General Linear Model (mGLM) analyses with variables grouped by relevance to the question being asked; 2) continuous variables were compared using Pearson’s Product-Moment correlations; and 3) t-tests were used for questions requiring a simple comparison of two groups. All data analyses employed software version 9.4 from the SAS Institute Inc., Cary, NC, or Excel statistical functions.
RESULTS
Participants
We encountered an unusually steep recruiting/retention cascade for this experiment. In order to address our goal of testing 60 enrolled participants, 2816 participants responded to advertisement, 571 passed the initial phone screen, and 118 were eligible and offered enrollment after an in-depth interview. Participants who were eligible, but unwilling to participate cited the need for 7 visits, a month-long commitment, and reluctance to wear the monitoring device, especially in the summer. Of those 74 still interested, other difficulties included scheduling around special events that could change drinking habits. Of the 60 participants enrolled for testing, five (4 female), could not resolve new conflicts with the schedule before testing began. Four participants (m:f = 2:2) were dismissed from the project for documentation or suspicion of drinking during the abstinence interval, 1 female withdrew after reporting embarrassment associated with wearing the anklet, and 1 female felt nauseated in her second session. A slight sex bias was manifest in those 50 analyzed (m:f = 27:23). Demographics of participants included in the analysis appear in Table 1.
Test/Retest reliability and order effects for PARcwa
We calculated a Pearson’s Product Moment Correlation coefficient [Pearson’s (r)] on data from 12 participants assessing motivation during usual drinking habits both before and after the abstinence interval. The coefficient for CWA from the 2 sessions was .87, indicating adequate reliability and suggested that the effect of a single abstinence period was transient, consistent with the ADE effect observed in laboratory animals. We observed no significant order effect on outcomes attributable to the sequence of U and R sessions.
Differences in PAR effects by Drinking Style:
Our original intent was to categorize recent drinking style into binge and regular heavy drinking styles, but nearly all participants reported at least one binge episode in the last 35 days, making that grouping inappropriate. The calculation of Pearson’s Product-Moment coefficients revealed no significant relationship of PARcwa to any recent drinking history variable (when normalized by total body water where appropriate).
Differences in PAR effects by Density of Family History of Alcoholism:
Similarly, we intended to examine Density of Family History of Alcoholism (adapted from Stoltenberg et al., 1998) as a potential correlate of PARcwa. However, as family history was not an emphasis of participant recruitment, the distribution of density did not support formal analysis as an independent variable.
Sex differences in Recent Drinking History
We assessed the potential for sex differences in TLFB indices of recent drinking history by using mGLM. By self-report, men drank substantially more than women (see recent drinking history data in Table 1). However, as expected, men had a significantly greater volume of distribution for alcohol than did women (total body water, liters: 47.4 ± 1.1 vs. 32.8 ± 0.8 liters, p < 0.001). Since that volume of distribution proportionally dilutes ingested alcohol, we normalized recent drinking measures connoting amounts of alcohol consumed by the individual’s total body water in order to compare likely concentrations of brain exposure to alcohol between the sexes. After this standardization, no important difference by sex for any recent drinking history variable was apparent (all p > 0.8). The number of Drinking Days, the number of NIAAA defined binges, and the number of Heavy Drinking Days did not differ significantly by sex (all p >.25), but the number of extreme binges in 35 days did: men reported 2.0 ± 0.40; women 0.60 ± 0.30 (p < 0.02).
Sex differences in Progressive Ratio Task Performance Parameters:
Across both U and R sessions, the sample reflected significantly more motivation for seeking alcohol (mean number of CAT trials =360, sem ± 30) than for vehicle (250 ± 31) (p < 0.05), although there was a sex difference in this regard. Men worked significantly more for alcohol than for vehicle in both sessions, but decreased the effort in the R, compared to U session, whereas we observed a trend for the opposite pattern in women. (Table 1, Supplemental Figure 1). The peak BrACs achieved during all 112 sessions averaged 98.6 mg/dl with a standard deviation of 29.6 mg/dl. Women achieved a peak BrAC in the U session (86.0 ± 5.2 mg/dl) that was significantly lower than for men (107.1 ± 4.8; p =.005), but the sex-difference disappeared in the R session (women: 93.5 ± 5.9, men: 105.1 ± 6.1, p =.198). Visual examination of BrAC trajectories by session suggested that, on average, women increased their BrAC in the R compared to the U session (Figure 2 Inset, Supplemental Figure 2). The peak values of these average trajectories is less than the mean peak value for individuals because the peaks occurred at different times.
Differences in PAR effects by (self-reported) Sex
Sex accounted for a significant difference in PARcwa (Figure 3). Females, on average, worked more for alcohol after 2 weeks of abstinence (PARcwa: +16.3 ± 9.6 %), compared to males who decreased their cumulative work for alcohol by (−24.8 ± 13.8 %). The difference in sex group means was substantial and significant (p <.03), and the effect size was moderate (Cohen’s d = 0.63). The sex difference in PARcwa is also apparent upon examination of its rank-ordered distribution (Figure 4). While each sex demonstrated a range of PARcwa, the distributions were skewed such that more females than males increased their alcohol seeking and more males than females decreased their alcohol seeking post abstinence. We identified no sex-difference in PAR effects for the vehicle rewards (p > 0.36).
Figure 3.
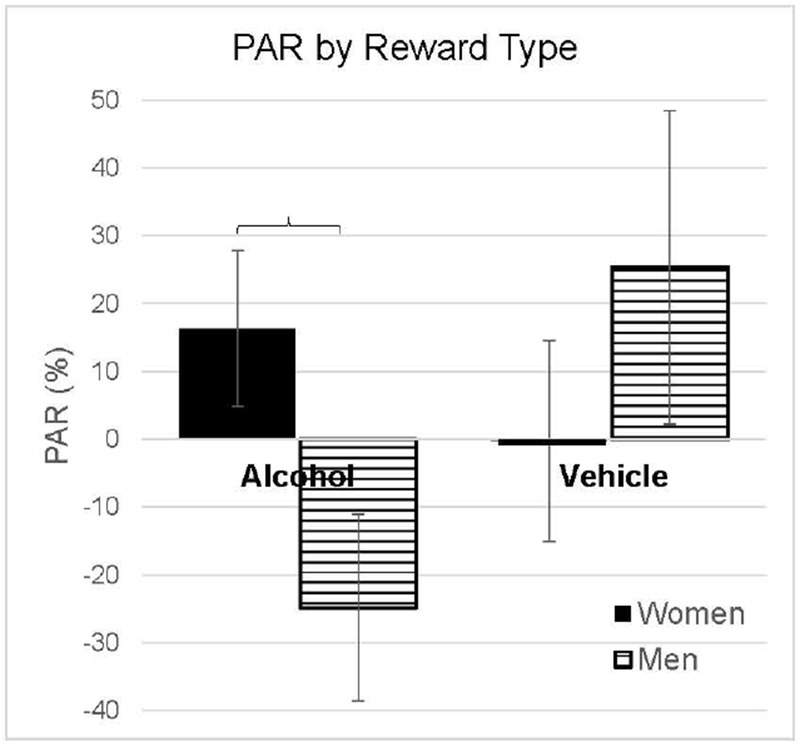
Post-Abstinence Response (PAR) by Reward Type. PAR, calculated using the cumulative work performed for each reward each individual. Men and women diverged in their motivation for seeking alcohol upon resumption from 2 weeks of abstinence.
Figure 4.
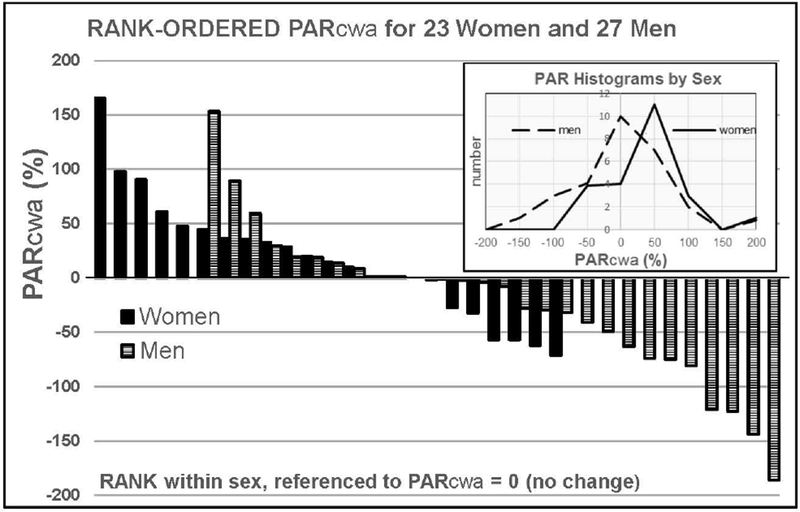
Rank-ordered distribution of PARcwa (%) for the 27 men (hashed) and 23 women (black) completing testing. The distributions have been shifted so that both distributions intersect PARcwa = zero at the same locus. Inset: Histogram of the male and female PARcwa distributions.
We used mGLM to test for sex differences in a set of measures of alcohol risk and abuse, including the AUDIT, SRE, and density of Family History of Alcoholism scores. Only SRE scores showed a significant sex difference, and these behaved as did other indices asking about number of drinks: men reported needing significantly more drinks to feel an effect during their first serious drinking episode and more drinks to achieve the same effect for the heaviest 3 months drinking interval (all p < 0.005) compared to women. However, significance vanished for scores when the number of drinks required was normalized by the individual’s current total body water volume. Age of onset of regular drinking trended towards significance (men: 18.9 ± 0.4; women: 19.9 ± 0.3; p <0.12), but not for any other demographic outcome measure, including age of first drink (all p > 0.40). We used another mGLM to test for sex differences in age, body mass index, and impulsivity scores (Cyders et al., 2014), Center for Epidemiologic Studies- Depression score, and Sensation Seeking (Karyadi et al, 2013); no significant differences were observed (all p > 0.13).
Changes in baseline subjective perceptions related to abstinence
In percentage terms, baseline subjective perception scores in the overall sample population all increased from the U to the R session; PARcraving by 34.1 ± 11.7 % (different from zero, p = 0.003); PARwanting by 26.1 ± 13.8 % (p = 0.021), but PARneeding by just 9.5 ± 14.6 % points (p > 0.26). Structured in PAR format, no change in baseline subjective perception index differed significantly by sex (see Table 1, all p > 0.38). Neither men nor women exhibited an important correlation of PARcwa with any of PARcraving, PARwanting, or PARneeding (maximum r2 =.07).
DISCUSSION
We documented a human analogue of the pharmacological alcohol deprivation effect apparent in animal studies, demonstrating that such an effect is quantifiable in humans, that the CAIS Progressive Work self-administration paradigm is sufficient to quantify it, and that the results of exploring it can inform research.
Our primary hypothesis was that the PAR phenomenon would be related to recent drinking style, but testing proved uninformative after accounting for different total body water volumes in men and women. Sex was the main mediator of the primary outcome measure, PARcwa; men worked less for alcohol upon resumption and women worked more; the observation unexplained by other available demographic and risk variables. Examination of the average male and female BrAC trajectories by Session implied a change in temporal distribution of the work performed for alcohol (Supplemental Figure 1), but more detailed trajectory analyses remain beyond the scope of this manuscript.
The decline in work for alcohol rewards after abstinence in men is consistent with epidemiological studies of treatment populations, indicating that self-imposed abstinence during treatment for AUD predicts greater odds of recovery (Kirshenbaum et al., 2009). It is also consistent with literature showing signs of neurological recovery in both sexes following abstinence (Bartsch et al., 2007; Van Eijk et al., 2013), although such studies used longer periods of abstinence than the 2 weeks reported here.
We chose to initiate this line of research in young adult heavy drinkers undertaking 2 weeks of monitored abstinence in their usual environment (Figure 1). A focus on PAR in an advanced alcohol use disorder sample population would miss the opportunity to explore its potential relevance to the development of the illness, and could be complicated by alcohol withdrawal. The effect of abstinence in an even earlier stage of drinking would also have been of interest, but alcohol may not be administered to those under 21years of age in Indiana. We chose a 2-week interval of abstinence because it was the shortest interval that interrupted both weekly binge and regular styles of drinking and because we had postulated that drinking style would be the principal determinant of PAR.
We designed this project as a translational venture, using the substantial literature on the preclinical alcohol deprivation effect to inform design of a human laboratory study of a similar phenomenon. At first glance, the decline in intake after abstinence in men seems inconsistent with preclinical data in rodents that shows increased alcohol intake following abstinence (references in first paragraph of Introduction). However, other preclinical data have shown decreases in intake following deprivation in C57Bl/6J mice, which decreased drinking following a 2-week deprivation period even though they showed an ADE after 1 week of deprivation (Melendez et al., 2006). Following 3 days of deprivation, one congenic strain of C57Bl/10SnY mice also showed decreases in alcohol intake (Salimov et al., 1995). Selectively bred crossed High Alcohol Preferring mice show a decline in drinking after one week of deprivation following shorter term pre-abstinence alcohol access (2 weeks) but not following longer-term access (5 weeks; (O’Tousa and Grahame (2016)).
We could not infer clear expectations for human outcomes from the animal literature, since differences in ADE, including those results that are sex-based, appear to depend on the line studied and the conditions of alcohol access versus deprivation. Bell et al., 2008 reported differences in the robust ADE expression across populations of alcohol-preferring and high-alcohol drinking rats. Using a short duration and repeated deprivation protocol, both lines displayed a 1-hour ADE, only the high-alcohol drinking lines developed a 24-hour ADE, and the females of the alcohol-preferring line consumed more than the males. Using a prolonged alcohol access model, however, Rodd-Hendricks et al. 2000 and 2001 reported a robust ADE in female alcohol-preferring rats that increased in magnitude and/or duration with repeated deprivations. Vengeliene et al. 2005 reported sex-dependent ADE effects in congenital learned helpless and congenital non-learned helpless rats, with the females of the former consuming more alcohol than the latter. Füllgrabe et al., 2007 reported a larger ADE in female Wistar rats as compared to their prior work, but not the expected shift in preference to higher alcohol concentrations, and with a conflicting result regarding age of onset compared to male rats (Siegmund et al. 2005). Since their environment is carefully controlled, experiments with animal lines reflect a special emphasis on the genetic component of a family history influence on development and expression of excess drinking. We were interested in a potential genetic component, beyond sex, of PAR effects, but the distribution of the density of family history of alcoholism in our sample did not provide a sensitive basis for a meaningful examination.
If the 2 weeks of monitored abstinence that was employed in this project is considered to be a stressful experience, one might consider differential response to stress as a mechanism underlying the sex differences in PAR that were observed. Sex differences in the relationship of the response to stress and drinking abound in the literature (e.g. Becker et al., 2012, Becker and Koob, 2016, Chaplin et al., 2008). Other studies relate differential craving for alcohol by sex to the response to emotional stress (e.g. Higley et al., 2011). The relationship of stress/craving/relapse (Kirshenbaum et al., 2009) appear to have a replicable basis in neuroadaptations leading to drug addictions including alcohol dependence (Breese et al., 2011, Sinha R, 2013). Some evidence supporting sex differences in the stress/craving hypothesis were apparent in this PAR project. In debriefing sessions at the end of project participation, women described substantially more conscious difficulty with the day-to-day decisions to stick with abstinence, compared to men. Both sexes reported significant increases in the subjective PAR effects for baseline craving and wanting alcohol (Table 1), but no important sex-based differences in those increases emerged. Neither the PAR effect for baseline craving nor wanting was significantly correlated with the behavioral PAR effect. Rather than a simple relationship between baseline perceptions and drinking behavior, some other phenomenon must be involved.
Hormonal influences on the motivation for consumption of alcohol under various conditions (Roberts et al., 1998) may be relevant to sex differences in preclinical ADE studies. We did not include this dimension in the PAR project because a rigorous study of the subjective effects of alcohol and volume of alcohol consumed did not vary across the menstrual cycle in healthy women at four stages of the menstrual cycle (Holdstock and de Wit, 2000).
Some research suggests a ‘telescoping’ effect in the progression of AUD severity in women (Randall et al., 1999; Hernandez-Avila et al., 2004 among others) at least in treatment samples, wherein women start drinking later than men do, but appear to progress more quickly through the stages of illness. Intermittent abstinence may influence that differential progression, and could be linked mechanistically to the PAR effect observed in this study. A tempting hypothesis is that repeated abstinence contributes to the development of drinking in women but not men, thus accounting for the apparently faster acquisition of dependence in women. However, further studies would need to be performed at different stages in the development of alcoholism to better understand these sex differences.
If craving and self-control interact with abstinence differently in men and women, they probably have different temporal trajectories in relation to the duration of abstinence. Based on preclinical literature, the duration of the abstinence interval (from days to weeks) appears to interact with the number of repetitions to influence the magnitude and/or duration of the current ADE (Sinclair and Li, 1989, Bell et al., 2004, Bell et al., 2008, Rodd et al., 2003, Rodd-Hendricks et al., 2000, Rodd-Hendriks 2001, and also reviewed in Rosenwasser et al., 2012). The present project examined only one duration and did not consider number of preceding abstinence intervals. More than one study would be required to pursue such dynamics, and a more comprehensive evaluation of PAR effects and underlying mechanisms pertinent to one abstinence interval should be undertaken first. Nonetheless, work has begun on mathematical modeling applicable to the interrelation between duration and repetition (Grasman et al., 2016).
Age is an important variable in the study of PAR. This project focused on young-adult heavy drinkers, but sex differences in the desire for and consumption of alcohol probably begin earlier (Dir et al., 2017). A project recently conducted in German adolescents also found sex-differences. Using the CAIS Free Access paradigm, Jünger et al., 2016, reported that women self-administered to lower peak BrAC compared to men. The current study found a similar sex difference in a sample about 8 years older, using a Progressive Work paradigm. The sex difference in desired peak BrAC may be a result of an intravenous route of self-administration or of the requirement for work, since all measures of recent drinking history (presumably seeking the same effect) were quite similar by sex after individual normalization by the volume of distribution of alcohol. A study on the interactive effects of affective lability, negative urgency and sensation seeking in young adults (Karyadi et al., 2013) reported significant differences by both age and sex on self-reported hazardous alcohol use in these variables.
Limitations of the current study include the relatively small size and nature of the sample population. Our decision to examine PAR as a risk factor for the development of problematic drinking versus a goal to identify its presence in those already suffering from an alcohol use disorder may have reduced or changed the impact of abstinence. Further, our study would have been strengthened by collection of abstinence-related naturalistic drinking behavior metrics. Prolonged transdermal alcohol monitoring or additional TLFB data collection could provide insight into how the abstinence influenced “real-life” drinking versus intravenous alcohol self-administration (discussed below). Finally, we made every effort to conduct the U and R sessions with identical procedures in all participants and to apply the same criteria for dropping out or dismissal from the study, but certain exclusion of systematic errors is impossible.
Our project speaks to the effect of abstinence on the pharmacological effect of ethanol self-administration. Alcohol is not administered intravenously in the community, and our protocol did not include the sensory and environmental cues participants routinely experience when ingested; such differences may limit generalizability of our results. Nonetheless, we deliberately chose a sterile lab environment because we were interested in asserting that the pharmacological effect of alcohol is the influence underlying observed differences. Most environmental cues mean different things to different individuals and likely confound the pharmacologic influence we sought; such cues include setting and social intercourse, noise or music or videos, or the taste, volume, price, alcohol concentration, odor, temperature, texture, or familiarity with the beverage ingested. We deliberately chose infusion of alcohol because (a) cortical capillary alcohol concentration is the prime determinant of the pharmacological effects we sought, (b) BrAC is a very good proxy for the contemporaneous cortical capillary alcohol concentration, (c) CAIS directly controls BrAC rather than the ingested dose of alcohol; avoiding the inter-individual differences in alcohol absorption and overcoming differences in distribution and elimination kinetics; yielding (d) virtually identical incremental pharmacologic rewards across participants. Advantages (c and d) are simply not possible with ingestion of alcohol.
We perceive the main unaddressed ecological variable in this study to be the potential influence of social setting and social interactions on the measurements. In particular, it is possible that women reacted to the social isolation of the laboratory setting differently from men, although no such effect was apparent in a study of social vs. isolation settings using ingestion (Kirkpatrick and de Wit, 2013). Some may argue that our results could reflect sex differences in response to demand characteristics (Eagly, 1978), but random session ordering and using participants as their own controls in PAR calculations probably minimized such an effect.
The utility of the PAR paradigm in future research has several dimensions. Extension to explore the effect of different durations of the abstinence interval on resumption drinking, particularly in relationship to sex differences is possible. Exploration of contributing factors such as age and drinking history would be informative, as windows of relative risk might be identified. Further, our study does not suggest a mechanism underlying the PAR phenomenon. The ADE has been attributed to metabolism and changes in rewarding properties of the drug among other causes, and specific experimental paradigms to assess those possibilities may yield insight into interventions. Finally, the results invite eventual translational studies of the effect of drugs or other interventions on shifting the distribution of PAR, particularly in women, towards more negative values.
Supplementary Material
Acknowledgements:
The cooperation and support of the IU research pharmacy in the preparation of infusate, and of the Indiana Clinical and Translational Sciences Institute Clinical Research Center personnel in the preparation of participants for testing were essential for this research project. The diligent and expert performance of testing procedures by Jim Hays, James Millward, Shreya Velamakanni and David Haines are sincerely appreciated. Recruiting for this study proved to be challenging, and the exceptional efforts of Christina Soeurt, Amanda Korsmo, Rachel Baum, Azziza Ahdoot, Claire Carron, Kathryn Snyder and Cari Tsinovoi were indispensable. The research was performed through NIAAA funding of the Indiana Alcohol Research Center, P60 AA07611-28, and with Indiana Clinical and Translational Institute Clinical Research Center, UL1TR001108, support.
The research was performed through NIAAA funding of the Indiana Alcohol Research Center, P60 AA07611-28, and with Indiana Clinical and Translational Sciences Institute Clinical Research Center, UL1TR001108, support.
Footnotes
Invitations: Investigator interest in obtaining the de-identified database from this project in order to test relevant and pre-agreed hypotheses is welcome, as is interest in adapting the capabilities of the Computer-assisted Alcohol Infusion System to their own research. In either case, please send an email to oconnor1@iu.edu.
REFERENCES
- Amlung M, MacKillop J Monti PM 3, Miranda R Jr (2017) Elevated Behavioral Economic Demand for Alcohol in a Community Sample of Heavy Drinking Smokers. J Stud Alcohol Drugs 78(4):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Meade EB, & Glynn TR (2014). Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Experimental and clinical psychopharmacology, 22(1): 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, Stefano ND, Solymosi L, Bendszus M (2007) Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130:36–47. [DOI] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB (1981) Effects of Voluntary Short-term Abstinence from Alcohol on Subsequent Drinking Patterns in College Students. J.Stud. Alcohol 42:11 1013–1020 [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 158(16):1789–95 [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 68(2):242–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C (2012) Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 7;3(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ (2004) Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin Exp Res. 28(12):1867–74. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, McBride WJ (2008) Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 42(5):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, Rodd ZA (2017) Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology. 122:201–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G, Sinha R, Heilig M (2011) Chronic Alcohol Neuroadaptation and Stress Contribute to Susceptibility for Alcohol Craving and Relapse. Pharmacol Ther. 129(2): 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, Reich T, Schmidt I, Schuckit MA (1994) A new, semistructured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55:149–158. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 48(3):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R (2008) Sex Differences in Response to Emotional Stress: An Assessment Across Subjective, Behavioral, and Physiological Domains and Relations to Alcohol Craving. Alcohol Clin Exp Res. 32(7): 1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, VanderVeen JD, Plawecki M, Millward JB, Hays J, Kareken D, O’Connor SJ (2016) Sex Specific Effects of Mood on Alcohol Seeking Behaviors: Preliminary Findings Using Intravenous Alcohol Self-Administration. Alcohol Clin Exp Res. 40(2): 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Littlefield AK, Coffey S, Karyadi K (2014) Examination of a Short Version of the UPPS-P Impulsive Behavior Scale. Addict. Behav. 39(9): 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Toalston JE, Hauser SR, Bell Richard L., McKinzie David L., McBride William J., Rodd Zachary A.. (2012) Effects of naltrexone and LY255582 on ethanol maintenance, seeking, and relapse responding by alcohol-preferring (P) rats. Alcohol. 46(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dir AL, Bell RL, Adams ZW, Hulvershorn LA (2017) Gender Differences in Risk Factors for Adolescent Binge Drinking and Implications for Intervention and Prevention. Front Psychiatry 8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagly AH (1978) Sex differences in influenceability. Psychological Bulletin, Vol 85(1), 86–116. [Google Scholar]
- Eysenck SB, Eysenck HJ, Barrett P (1985) A revised version of the psychoticism scale. Personality and individual differences, 6(1): 21–29. [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli Jr. (2003) Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 64(1):120–6. [DOI] [PubMed] [Google Scholar]
- Füllgrabe MW, Vengeliene V, Spanagel R (2007) Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 86(2):320–326. [DOI] [PubMed] [Google Scholar]
- Garcia-Burgos D, Manrique ZT, M GT, F GR. (2010) Sex differences in the alcohol deprivation effect in rats. Psicothema. 22(887–892). [PubMed] [Google Scholar]
- Grasman J, Raoul P, Grasman HL, van der Maas J (2016) The Dynamics of Addiction: Craving versus Self-Control, PLoS One, 11(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74:265–272 [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. (2003) Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 28(8):1463–1471 [DOI] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ (2011) Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl). 218(1): 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemacher T, Bayerlein K, Wilhelm J, Poleo D, Frieling H, Ziegenbein M, Sperling W, Kornhuber J, Bleich S. (2006) Volume intake and craving in alcohol withdrawal. Alcohol and Alcoholism. 41(1):61–5. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H (2000) Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology (Berl). 150(4):374–82). [DOI] [PubMed] [Google Scholar]
- Jones JD, Comer SD (2013) A review of human drug self-administration procedures. Behav Pharmacol. 24(0): 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jünger E, Gan G, Mick I, Seipt C, Markovic A, Sommer C, Plawecki MH, O’Connor S, Smolka MN, Zimmermann US (2016) Adolescent Women Induce Lower Blood Alcohol Levels Than Men in a Laboratory Alcohol Self-Administration Experiment. Alcohol Clin Exp Res. 40(8):1769–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karyadi K, Coskunpinar A, Allyson L. Dir AL, Cyders MA (2013) The Interactive Effects of Affect Lability, Negative Urgency, and Sensation Seeking on Young Adult Problematic Drinking J Addict. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, de Wit H (2013) In the company of others: Social factors alter acute alcohol effects. Psychopharmacology (Berl). 230(2)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bickel WK (2009) A quantitative review of the ubiquitous relapse curve. J Subst Abus Treat 36:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Wetherill L, Plawecki MH, Kareken DA, Liang T, Nurnberger JL, Windisch K, Xuei X, Edenberg HJ, Foroud TM, O’Connor SJ. (2015) Adaptation of Subjective Responses to Alcohol is Affected by an Interaction of GABRA2 Genotype and Recent Drinking. Alcohol Clin Exp Res. 39(7):1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell SG, Schwandt ML, Suomi S, Rice KC, Heilig M, Barr CS. (2017) Intermittent access to ethanol induces escalated alcohol consumption in primates. J Addict Behav Ther Rehabil. 6(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggs JL, Williams LR, Lee CM. (2011) Ups and downs of alcohol use among first-year college students: Number of drinks, heavy drinking, and stumble and pass out drinking days. Addict Behav. 36(3):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Herron JE, Anton RF, Roberts J, Moore J. (2000a) Recurrent detoxification may elevate alcohol craving as measured by the Obsessive Compulsive Drinking scale. Alcohol. 20(2):181–5 [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF (2000b) Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 22(3):159–64 [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Weerts EM, Xu X. (2017) A paradigm for examining stress effects on alcohol-motivated behaviors in participants with alcohol use disorder. Addiction Biology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK (1998) The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res. 22(5):1170–6. [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW (2006) Development of an Alcohol Deprivation and Escalation Effect in C57BL/6J Mice. Alcohol Clin Exp Res 30:2017–2025. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, Blow FC. (2009) Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug Alcohol Depend. 103(3):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa DS, Grahame NJ (2016). Long-term alcohol drinking reduces the efficacy of forced abstinence and conditioned taste aversion in crossed High Alcohol Preferring mice. Alcohol Clin Exp Res 40, 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Decarlo R, Ramchandani VA, O’Connor S. (2007) Improved transformation of morphometric measurements for a priori parameter estimation in a physiologically-based pharmacokinetic model of ethanol. Biomed Signal Process Control. 2(2):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O’Connor SJ. (2008) Physiologically based pharmacokinetic (PBPK) models for ethanol. Biomedical Engineering, IEEE Transactions on (12):2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Zimmermann US, Vitvitskiy V, Doerschuk PD, Crab DW, O’Connor S (2011) Alcohol Exposure Rate Control through Physiologically-Based Pharmacokinetic Modeling. Alcoholism: Clinical and Experimental Research. 36(6):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann U , and O’Connor S (2013) Voluntary Intravenous Self-administration of Alcohol Detects an Interaction between GABAergic Manipulation and GABRG1 Polymorphism Status: A Pilot Study. Alcoholism: Clinical and Experimental Research. 37(s1), E152–E160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME (1999) Telescoping of landmark events associated with drinking: a sex comparison. J Stud Alcohol. 60:252–260 [DOI] [PubMed] [Google Scholar]
- Ramchandani, O’Connor S, Neumark Y, Zimmermann U, Morzorati S, de Wit H (2006) The Alcohol Clamp: Applications, Challenges and New Directions – An RSA 2004 Symposium Summary. Alcoholism: Clinical and Experimental Research. 30:1 155–164 [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li T-K, and O’Connor S: (2009) Intravenous Ethanol Infusions Can Mimic the Time Course of Breath Alcohol Concentrations Following Oral Alcohol Administration in Healthy Volunteers. Alcoholism: Clinical and Experimental Research. 33(5):938–944. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA (2009) Forced Abstinence Model of Relapse to Study Pharmacological Treatments of Substance Use Disorder Curr Drug Abuse Rev. 2(2): 184–194). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI Jr, Schuckit MA, Begleiter H (1995) Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research 19:1018–1023. [DOI] [PubMed] [Google Scholar]
- Roache JD, Karns TE, Hill-Kapturczak N, Mullen J, Liang Y, Lamb RJ, & Dougherty DM (2015). Using Transdermal Alcohol Monitoring to Detect Low-Level Drinking. Alcoholism: Clinical and Experimental Research, 39(7): 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF (1998): Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 22(7):1564–9 [PubMed] [Google Scholar]
- Rodd-Hendricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2001) Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 25(8):1140–50. [PubMed] [Google Scholar]
- Rodd-Hendricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK. (2000) Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcoholism: Clinical and Experimental Research. 24(1):8–16. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. (2003) Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 28(9):1614–21. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. (2005) Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 315(2):648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. (2013). Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addiction Biology. 18(3):496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimov S, Salimova N., Ratkin A, Shvets L, Maisky A (1995). Genetic control of alcohol deprivation effect in congenic mice. Alcohol 12, 469–474 [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R (2005). Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 29(7):1139–45. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. (1968) Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol 29(4):863–867 [PubMed] [Google Scholar]
- Sinclair JD, Walker S, Jordan W (1973) Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. J Stud Alcohol. 34:744–757. [PubMed] [Google Scholar]
- Sinclair JD, Li TK (1989) Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 6(6):505–9. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, & Hill EM (1998). Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction, 93(10): 1511–1520. [DOI] [PubMed] [Google Scholar]
- Stuppaeck CH, Barnas C, Falk M, Guenther V, Hummer M, Oberbauer H, Pycha R, Whitworth AB, Fleischhacker WW. (1994) Assessment of the alcohol withdrawal syndrome--validity and reliability of the translated and modified Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-A). Addiction. 89(10):1287–92 [DOI] [PubMed] [Google Scholar]
- Toalston JE, Oster SM, Kuc KA, Tylene J. Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ , Rodd ZA (2008) Effects of alcohol and saccharin deprivations on concurrent ethanol and saccharin operant self-administration by alcohol-preferring (P) rats. Alcohol. 42(4):277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G (2013) Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res 37:67–74. [DOI] [PubMed] [Google Scholar]
- VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken D, O’Connor S, Cyders MA (2016) Negative Urgency, Mood Induction, and Alcohol Seeking Behaviors. Drug Alcohol Depend. 165: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Vollmayr B, Henn FA, Spanagel R. (2005) Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology (Berl). 178(2-3):125–132 [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Spanagel R. (2014) The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol. 48(3):313–20. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. (2006) Environmental cues, alcohol seeking, and consumption in baboons: Effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 30(12):2026–2036 [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S (2008) Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans Alcohol: Clin Exp Res. 32(7):1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O’Connor S. (2009) Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self- infusion of ethanol (CASE). Psychopharmacology (Berl). 202(4):689–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2011): Modeling Alcohol Self-Administration in the Human Laboratory In Behavioral Neurobiology of Alcohol Addiction (pp. 315–353). Springer; Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


