Abstract
Atrial Fibrillation (AF) affects 10 to 50% of patients with chronic heart failure (HF) and is associated with poor long-term prognosis. AF is commonly associated with atrial structural remodeling (ASR), principally characterized by atrial dilatation and fibrosis. However, the occurrence of AF in the full spectrum of ASR encountered in patients with HF is poorly defined. Experimental studies have presented evidence that extensive ASR can be accompanied with a reduced burden of AF, secondary to a prominent depression of atrial excitability. This reduction in AF burden is associated with severe atrial fibrosis rather than with dilatation. Clinical studies of patients with HF point to the possibility that advanced ASR is associated with a less frequent AF occurrence than moderate ASR. Our goal in this review is to introduce the hypothesis that AF is less likely to occur in severe vs. moderate atrial ASR in the setting of HF and that it is severe atrial fibrosis-associated depression of atrial excitability that reduces AF burden.
Keywords: Atrial fibrillation, heart failure, structural remodeling, fibrosis, cardiac arrhythmias, atrial dilatation, electrophysiology
Introduction
AF is encountered in 10 to 50% of patients with chronic HF and is associated with poor long-term prognosis.1, 2 HF is accompanied by various degrees of atrial structural remodeling (ASR).3 AF is strongly associated with ASR,3–10 but the occurrence of AF in the full spectrum of ASR is poorly defined. Recent experimental data indicate that a high degree ASR encountered in HF can be associated with a reduced burden of AF.11 The current review examines the occurrence of AF over a wide spectrum of atrial size, volume, and fibrosis (hallmarks of ASR),4 in both experimental and clinical studies. Apart from atrial dilatation and fibrosis, ASR generally consists of multiple factors, including hypertrophy, inflammatory and fatty infiltrations, apoptosis, and changes in compliance.4, 6 However, there are little to no data relative to the range of these parameters in HF and their impact on the development of AF. In this review, the term ASR is used to depict this broad interpretation of ASR.
Functional severity of HF often poorly correlates with cardiac performance
Worsening of the New York Heart Association (NYHA) functional severity is generally associated with an increased prevalence of AF.1 However, the functional NYHA classification is based on symptoms that often poorly correlate with cardiac performance and remodeling.2 The relationships between AF occurrence, ASR, and HF functional class are poorly understood.
Window of vulnerability for development of AF during the progression of experimental HF
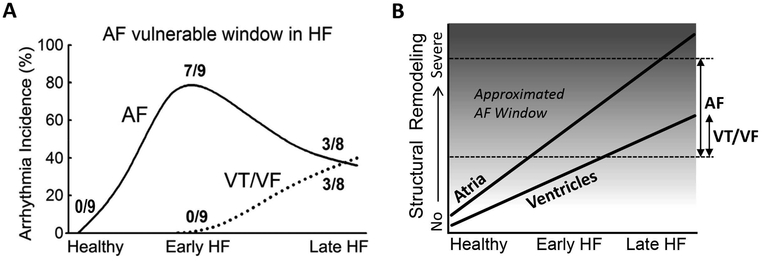
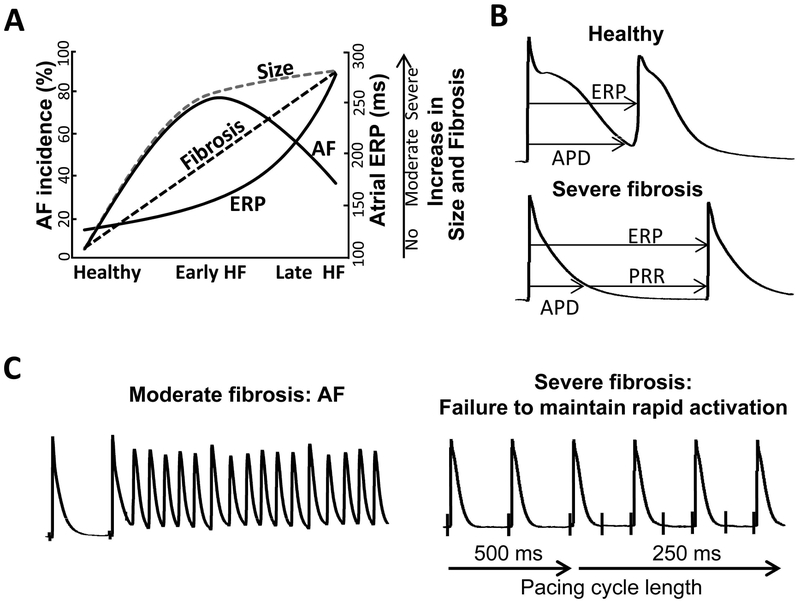
A number of groups have used ventricular tachypacing (VTP) to create a systolic non-ischemic dilated cardiomyopathy (DCM) canine models of HF characterized by progressive development of cardiac structural remodeling, which is greater in the atria than in the ventricles (Fig. 1).11–14 AF occurrence in this canine HF model has been clearly associated with the development of ASR.11, 13, 14 However, AF inducibility using a single extrastimulus has been shown to be higher at moderate vs. advanced levels of ASR, pointing to a window of vulnerability for AF inducibility during the progression of HF and associated accentuation of ASR (Figs. 1 and 2).11 A reduction of AF inducibility in advanced ASR is associated with a major prolongation of atrial effective refractory period (ERP) and rate-dependent depression of excitability (Fig. 2). This dramatic prolongation of ERP is due to the development of post-repolarization refractoriness (PRR; i.e., when ERP is longer than action potential duration at 70–90% repolarization), secondary to the depression of atrial excitability (Fig. 2B). The steep increase in ERP and reduced AF vulnerability during the transition from early to late HF are associated with a slight increase in atrial size, but a much greater increase in atrial fibrosis (Fig. 2A). During the development of VTP-induced HF, atrial size increases rapidly in the early phase followed by a much slower enlargement in the late stages (Fig. 2).11, 15, 16 In contrast, atrial fibrosis increases progressively (Fig. 2).11, 12 Thus, while AF is strongly associated with atrial fibrosis, this arrhythmia is less likely to occur in severe vs. moderate atrial fibrosis in the canine VTP-induced HF model. The reduced AF burden with advanced fibrosis is likely due to severe depression of atrial excitability (Fig. 2). At advanced stages of HF, about 50% of atrial area is either inexcitable or barely excitable, reflecting significant degradation of atrial viability and/or functionality.11
Figure 1. A temporal window of vulnerability for development of AF with advancing HF in a canine experimental model.
A: Incidence of AF and ventricular tachycardia and fibrillation (VT/VF) as a function of duration of ventricular tachypacing (2–3 weeks of pacing = “Early HF,” and 5–6 weeks of pacing = “Late HF”). The highest AF vulnerability occurs relatively early in the course of development of HF, whereas VT/VF develops later. B: Schematic representations of the temporal distinction in atrial and ventricular arrhythmia susceptibility, which is associated with more rapid and greater structural remodeling in the atria.11, 12 The dotted lines approximate the range of atrial structural remodeling in which AF is most likely to occur (i.e., “AF window”). Note that the atrium has a greater amount fibrosis under baseline conditions than the ventricle.4, 11 The scale in the Y axis is qualitative. Modified from Burashnikov et al,11 with permission.
Figure 2. A proposed mechanism for suppression of AF in the setting of advanced fibrosis in the experimental HF: severe depression of atrial excitability.
A-C: Advanced atrial fibrosis appears to be a marker of prominent electrical depression of the atrium. Atrial enlargement develops much faster during early vs. late stages of HF whereas atrial fibrosis increases progressively during both early and late HF.11, 12, 15, 16 A high AF inducibility in early HF is associated with advanced atrial dilatation but moderate fibrosis. A reduction of AF vulnerability in late HF is accompanied by both severe atrial enlargement and fibrosis. Severe atrial fibrosis is associated with a prominent depression of atrial excitability, manifesting as a dramatic prolongation of the atrial effective refractory period (ERP) due to post-repolarization refractoriness (PRR). Advanced rate-dependent depression of atrial excitability acts to reduce AF occurrence. Panels B and C depict transmembrane action potentials illustrating the end of ERP in a healthy atrium and the development of PRR in an atrium with severe fibrosis isolated from a late HF heart. The left part of panel C shows an episode of AF induced by a single extrastimulus. The right part of panel C illustrates an example showing that acceleration of stimulation rate from a pacing cycle length of 500 to 250 ms is associated with a 2:1 activation failure, due to the prolonged PRR. Panels B and C are from Burashnikov et al,11 with permission.
Greater inducibility of AF in early vs. late stages of VTP-induced HF has also been reported in sheep and goats in vivo.15, 17 In VTP-induced HF models, the duration of AF is relatively brief (commonly < 30 min).11, 14, 15
“AF window” has not been observed in non-HF experimental settings, likely due to a much less advanced ASR than in HF. In models of experimental AF not mediated by HF, persistent rapid atrial activation causes a significant ASR.5, 10, 13 Such ASR, however, is generally much less extensive than that caused by pressure and volume overload (i.e. “HF”).3, 11–13, 16
Is AF occurrence lower in HF patients with severe vs. moderate ASR?
The extent to which the observed experimental “AF window” correlates with cases of clinical HF is not clear. In the clinic the causes and progression of HF and AF are much more heterogeneous, AF is spontaneous and not electrically-induced, and the duration of AF is commonly much longer than in the VTP-induced HF model. Moreover, both ASR and AF in patients with HF can be influenced by therapeutic interventions capable of reversing ASR and/or modulating development of AF (e.g., ivabradine reverses cardiac remodeling18 but promotes AF19). Few data are available concerning AF burden in patients with HF who possess a broad spectrum of ASR.
Left ventricular ejection fraction, ASR, and AF
HF is generally divided into two basic types based on left ventricular ejection fraction (LVEF), the proportion of blood pumped out of the heart during a single contraction expressed as a percentage, with a normal range between 50 and 75%. The two types are: HF with preserved and reduced LVEF (HFpEF and HFrEF, respectively). The LVEF border line between HFpEF and HFrEF is within 40–50% in different studies.20–30 Patients having a mid-range LVEF (between 40–49%) have been increasingly recognized as a distinct subtype of HF.24, 26–28
Reduced LVEF is associated with both atrial and ventricular structural remodeling.29, 31 Mean left atrial (LA) size and maximum volume are typically greater in patients with HFrEF vs. HFpEF, irrespective of the presence of AF (Table 1).29, 31, 32 Some studies, however, report no significant difference in LA maximum volume between HFrEF and HFpEF patients (Table 1).33, 34 Patients without HF, including those with hypertension, commonly have significantly smaller average LA size/volume than HFpEF patients (Table 1).29, 31, 32, 35 LA size and fibrosis generally inversely correlate with LVEF, so that LA size and fibrosis increase progressively as LVEF declines.36–38
Table 1.
LA volume/size in non-HF, HFpEF, and HFrEF Patients.
| Parameter | Non-HF | HFpEF | HFrEF | n, patients | References | |
|---|---|---|---|---|---|---|
| LA volume (ml) | 32 | 46* (+44%) |
48* (+50%) |
193/90/84 | AF/SR | Gottdiener et al. 33 |
| Max LA volume (cm3/m2) | 43 | 52* (+21%) |
68*# (+58%) |
48/32/26 | SR | Triposkiadis et al 31 |
| Max LA volume index (ml/m2) | 21 | 36* (+71%) |
38* (+81%) |
27/25/20 | NA | Kurt et al. 34 |
| LA size (mm) |
44 | 47* (+7%) |
50*# (+14%) |
3,482/633/455 | AF | Nieuwlaat et al.32 |
| Max LA volume (ml) | 45 | 85* (+89%) |
104*# (+131%) |
40/101/97 | AF/SR | Melenovsky et al. 29 |
| 37±10 |
53±19 (+45±32%) |
62±26 (+68±26%) |
Mean values from each individual study are shown. Values in brackets in the HFpEF and HFrEF columns are % increase vs. Non-HF.
p<0.05 vs. control.
P<0.05 vs. HFpEF.
SR – patients with sinus rhythm only. AF – patients with AF only. AF/SR – mixture of patients with SR and history of AF.
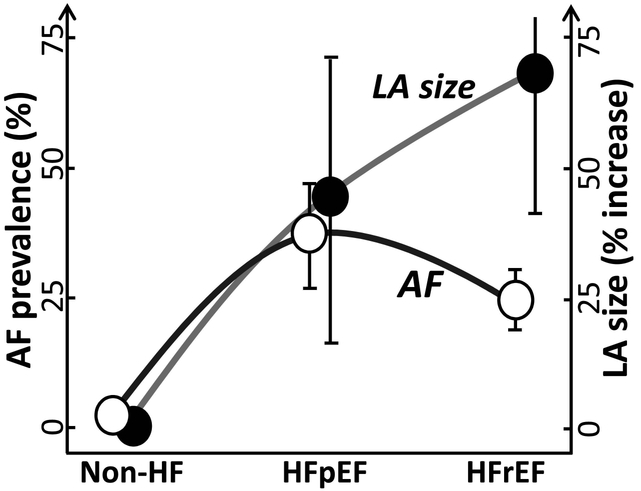
The prevalence of AF in hypertensive non-HF patients is significantly lower than in age-matched patients with HF (Fig. 3).29, 39, 40 Among those with HF, AF prevalence is reportedly higher in patients with HFpEF vs. HFrEF (Table 2; Fig. 3).20–30 In patients with LVEF of 40–49% (mid-range LVEF patients), AF prevalence is either similar or slightly lower than in patients with LVEF of ≥50 and significantly higher in those with LVEF of <40%.24, 26–28 In all but one of these studies (in which AF was chronic20), the type of AF was not specified (commonly referred to as “AF history” or “AF at presentation”)21, 24–28, 30 and there appears to be a mixture of paroxysmal, persistent, and permanent AF.
Figure 3. Relationship between AF prevalence and LVEF-associated left atrial (LA) size/volume in the clinic.
LA size/volume serves as a surrogate for atrial structural remodeling in clinical setting. In the absence of HF (hypertensive patients), LA size/volume is commonly normal or near normal and is associated with a low prevalence of AF. The mean LA size/volume is much greater in the setting of HF, but is relatively smaller in HFpEF when compared with HFrEF patients. AF prevalence increases dramatically in patients with HF, more so in patients with preserved LVEF vs. reduced LVEF (HFpEF vs. HFrEF, respectively). Please see Table 1 and 2 for the numerical data.
Table 2.
Prevalence of AF in hypertensive patients without HF and in HF patients with preserved and reduced LVEF.
| No HF (%) |
HFpEF (%) |
HFrEF (%) |
P value | n, patients | References |
|---|---|---|---|---|---|
| 33 | 28 | <0.0001 | 21,149/21,118 | Fonarow et al, 2007.24 | |
| 43 | 34 | <0.0001 | 38,056/48,950 | Kapoor et al, 2016.28 | |
| 55 | 32 | <0.001 | 539/4,474 | Toma et al, 2014.27 | |
| 27 | 18 | <0.001 | 10,347/31,625 | MAGGIC 2012.30 | |
| 32 | 24 | <0.001 | 880/1,570 | Bhatia et al, 2006.21 | |
| 41 | 29 | <0.001 | 2,167/2,429 | Owan et al, 2006.22 | |
| 35 | 24 | <0.001 | 6,210/3,914 | Gurwitz et al, 2013.26 | |
| 34 | 20 | <0.001 | 178/270 | Lee et al, 2009.25 | |
| 25 | 23 | <0.01 | 3,148/3,658 | Lenzen et al, 2004.20 | |
| 42 | 26 | <0.0001 | 101/97 | Melenovsky et al, 2015.29 | |
| 29 | 19 | <0.001 | 809/2,702 | De Ferrari et al, 2007.23 | |
| 1.1 | 39,056 | Haywood et al, 2009.40 | |||
| 1.6 | 941 | Gerdts et al, 2002.39 | |||
| 1.4±0.4 | 36±9 | 25±5 |
Interestingly, in large studies involving HFrEF or mixed HFrEF and HFpEF patients (n=3,513–99,810), those with AF had a significantly higher mean LVEF than those with SR (Table 3).23, 41–44
Table 3.
LVEF in HF-REF or mixture of HF-REF and HF-PER patients with sinus rhythm and AF in large studies.
| Sinus rhythm (%) |
AF (%) |
P value | n, patients | References |
|---|---|---|---|---|
| 34 ± 10 | 36 ± 11 | <0.001 | 809/2,702 | De Ferrari et al, 2007.23 |
| 32 ± 11 | 34 ± 12 | <0.001 | 2,657/1,391 | Tveit et al, 2011.41 |
| 39 ± 17 | 42 ± 17 | <0.001 | 68,455/31,355 | Mountantonakis et al, 2012.43 |
| 27.6 ± 8.1 | 29.0 ± 7.7 | <0.001 | 1,195/2,071 | Mentz et al, 2012.42 |
| 27 (20,35)* | 31(24,43)* | <0.001 | 4,330/2,677 | Abualnaja et al, 2015.44 |
In %. Mean±SD.
median with 25th and 75th percentile.
Thus, while average LA size/volume is progressively larger in patient populations with either no HF, HFpEF, and HFrEF, the prevalence of AF is not, displaying a bell-shaped relationship pointing to a lower AF burden in HF patients with severe vs. moderate ASR (Fig. 3, Tables 1 and 2).
AF and HF etiology
Patients with ischemic heart disease and DCM HF etiologies usually have a greater cardiac structural remodeling and a lower AF prevalence than patients with hypertensive HF prime etiology (Table 4).23, 25, 45
Table 4.
AF prevalence as a function of the primary etiology responsible for HF
| IHD (%) |
DCM (%) |
HPT (%) |
VHD (%) |
n, patients | References |
|---|---|---|---|---|---|
| 14 | 20 | 31 | 49 | 1,529/886/445/315 | De Ferrari et al, 2007.23 |
| 27 | 34 | 39 | 42 | 1,306/242/428/291 | Pecini et al, 2011.45 |
| 17 | - | 36 | 39 | 278/ - /140/42 | Lee et al, 2009.25 |
IHD – ischemic heart disease; DCM – dilated cardiomyopathy; HPT – hypertension; VHD – valvular heart disease.
A larger LA size/volume can be associated with a lower AF burden in patients with but not without HF
The clinical data presented above (Fig. 3; Tables 1, 2, and 4) suggest that severe ASR can be associated with a reduced AF burden. There are studies that directly compare AF occurrence as a function of ASR. In numerous studies a larger LA size/volume/fibrosis has been associated with a higher risk for development of AF.7, 8, 46, 47 However, these studies included few or no patients with HF.
Patients with HF commonly have a significantly greater mean atrial size/volume/fibrosis than patients without HF (Table 1 and Fig. 3). Few studies have reported AF burden as a function of atrial size/volume in patients with HF. The risk of new onset AF or AF recurrence could have either positive48, 49 or no50, 51 association with baseline LA size/volume. The prevalence of AF history was greater in 4th vs. 1st-3rd quartiles of LA volume index in mildly symptomatic systolic HF patients (16 vs. 10%, respectively, <0.001).36 Gavazzi et al. found that systolic HF patients with mild DCM had a smaller LA dimension and greater prevalence of persistent AF than patients with a “typical” form of DCM (22% vs. 3%, respectively; <0.001).52 Melenovsky et al. reported that HFpEF patients had smaller maximum LA volume and higher AF prevalence than HFrEF patients (42 vs. 26%, respectively, <0.0001).29
Thus, while the occurrence of AF over the full spectrum of ASR is poorly understood, a larger LA size/volume can be associated with a lower AF prevalence in select HF populations.29, 52 There is no such association reported in non-HF populations,7, 8, 46, 47 perhaps due to a poor representation of patients with advanced ASR.
Atrial fibrosis and enlargement in the clinical HF
While there is generally a direct relationship between atrial fibrosis and size,9 there are no clinical data that we are aware of that compare the degree of atrial enlargement to the extent of atrial fibrosis over a wide range, as in experimental models of HF (Fig. 2A).11, 12, 16 Also, we are not aware of data correlating AF occurrence over a broad range of atrial fibrosis encountered in HF.
ASR and AF
The relation between AF and ASR can be very complex. AF may cause and be caused by ASR.3–5 Patients with AF may have little ASR, and patients with no history of AF may have severe ASR.3, 4 Nevertheless, patients with AF commonly have a larger LA size/volume/fibrosis than patients with sinus rhythm4, 9, 29, 52 which is likely to be attributable to the development of AF-mediated ASR.4, 5
Atrial dilatation and fibrosis are the principal hallmarks of ASR and both are associated with AF occurrence.4, 6 AF is commonly associated with a significant atrial dilation, but this arrhythmia, including persistent AF, may occur without atrial fibrosis.4, 5, 53 The extent to which atrial fibrosis and dilatation contribute to development of AF is poorly understood and can be importantly modulated by atrial electrical remodeling principally characterized by abbreviation of the atrial ERP.4–6
How can severe fibrosis “suppress” AF?
Severe atrial fibrosis seems to be a marker of advanced electrical depression of the atrium. Prominent atrial fibrosis and dilatation are commonly associated with prolongation of atrial ERP and AF cycle length as well as a decrease in AF dominant frequency,11, 54–61 perhaps due to depression of atrial excitability (Fig. 2).11 Note that AF-induced atrial fibrosis is much less advanced than that caused by pressure and volume overload.3–5, 13, 16, 53 In experimental HF models, atrial ERP prolongs progressively as the extent of atrial dilatation and fibrosis increases.11, 15, 56 Prolongation of ERP is generally greater following an increase in fibrosis than increase in atrial size (Fig. 2A). Severe atrial fibrosis in the setting of HF has been shown to be associated with marked rate-dependent atrial electrical depression characterized by a prolonged ERP secondary to the development of PRR (Fig. 2).11
Abbreviation of the ERP is a well-known risk factor for the development of AF and prolongation of ERP is a therapeutic strategy used to terminate AF and prevent its development irrespective of the mechanism responsible for AF.5, 62 The significantly prolonged atrial ERP in the setting of severe atrial fibrosis likewise acts to prevent and terminate AF (Fig. 2).11 It is noteworthy that AF occurrence is still greater in severe when compared to little or no atrial fibrosis (Fig. 2).
Critically depressed atrial excitability in the setting of advanced ASR can be due to a number of factors, including 1) reduction of steady-state availability of the sodium channel, 2) decrease in the rate of recovery from inactivation of the sodium channel, and 3) reduction in electrotonic interactions (mediated by fibrosis and gap junctional changes). Importantly, advanced fibrotic remodeling may be associated with a major depolarization of resting membrane potential (which reduces the steady-state availability of the sodium channel and slows the recovery from inactivation of the sodium channel).11, 63
Compact, patchy, defuse, and interstitial fibrosis are all observed in HF and these diverse types of fibrosis may affect AF generation in different ways.11, 13, 14, 58, 64 However, the occurrence and distribution of various types of fibrosis in the course of HF-associated ASR development and their association with AF generation are poorly defined.
Structural remodeling: the atrium vs. ventricle in HF
Healthy atria have more fibroblasts and fibrosis than healthy ventricles and this distinction is amplified in the setting of HF.4, 11, 12 Sustained pressure and volume overload, a major cause of cardiac remodeling in HF,3 lead to greater and more rapid structural remodeling in the atria than in the ventricles (Fig. 1B) 11, 12, 17 due in large part to the fact that atria are thinner and smaller than the ventricles. AF itself produces both atrial and ventricular structural remodeling, with more extensive structural remodeling occurring in the atria.4 In the setting of sustained pressure and volume overload, the atrium appears more likely than the ventricle to achieve severe structural remodeling that is accompanied with a limited ability of the atrium to maintain rapid activation (Fig. 2).
The hypothesis
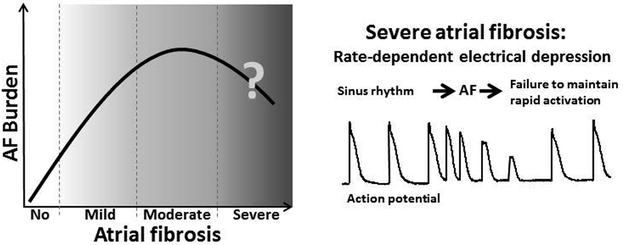
The occurrence of AF in the full spectrum of ASR is poorly defined. Our principal goal in this review is to introduce the hypothesis that AF burden is reduced under conditions of severe vs. moderate atrial fibrosis in the setting of HF (Fig. 4). Advanced atrial fibrosis is associated with rate-dependent depression of atrial excitability that acts to reduce AF burden. This hypothesis is based largely on experimental findings derived from VTP-induced experimental models of HF (Fig. 1-2). To the best of our knowledge, there are no clinical data permitting assessment of AF burden in the full spectrum of atrial fibrosis encountered in HF, so the hypothesis remains speculative. However, the available clinical data indicate either directly29, 52 or indirectly (Fig. 3; Table 1 and 2) that a larger atrial size can be associated with a lower AF burden in patients with HF. Atrial size positively correlates with atrial fibrosis.9, 38 The available data highlight the need for clinical studies aimed at providing a direct test of the hypothesis that severe fibrosis is associated with a lower burden of AF than moderate fibrosis in patients with HF.
Figure 4. The hypothesis: Aggravation of atrial fibrosis is associated with increased AF burden, but severe atrial fibrosis “reduces” AF.
Severe atrial fibrosis is accompanied with advanced rate-dependent depression of atrial excitability that acts to reduce AF burden. The “AF window” is assumed to be observed in HF but not or less likely in non-HF population, due to a greater occurrence of severe atrial fibrosis in the former. Please see text for details.
Considerations for the design of clinical studies to test the hypothesis
In contrast to the straightforward relationship between HF severity and the development of atrial fibrosis encountered in experimental models of VTP-induced HF (Fig. 1 and 2), the relationship between HF and atrial fibrosis encountered in the clinic is much more heterogeneous, being affected by HF etiology, approaches to therapy, co-morbidities and development of AF, among many other factors. Accordingly, a test of the proposed hypothesis in the clinical settings is not easily achieved.
The proposed hypothesis presumes that severe atrial fibrosis-associated electrical depression acts to suppress AF in all HF etiologies, types of AF, and stages of HF. We are not aware of any clinical studies reporting the prevalence of AF in severe vs. mild/moderate atrial fibrosis in specific HF etiologies or stages. There is indirect evidence for the manifestation of an “AF window” in DCM, i.e., systolic HF patients with mild DCM have been reported to have a smaller LA size and greater prevalence of persistent AF than patients with more severe DCM (22% vs. 3%, respectively).52 Clinical data relative to the prevalence of long- vs. short-duration AF in severe vs. mild/moderate atrial fibrosis are also lacking. The attainment of severe atrial fibrosis may be different in the various HF etiologies and should be studied.
A proper test of the hypothesis requires that AF burden be evaluated over the full spectrum of atrial fibrosis encountered in patients with HF (Fig. 4). Concomitant measurement of atrial size/volume may be useful, but the putative “AF window” may not be detected on the basis of atrial size/volume changes (Fig. 2A). The correlation between the full ranges of atrial size/volume and atrial fibrosis in the patients with HF is unknown and should be established.
Clinical quantification of atrial fibrosis is best achieved using magnetic resonance imaging (MRI).47, 65 Cardiac MRI provides a noninvasive tool for quantification of myocardial fibrosis by probing the retention of gadolinium-contrast agent in myocardial tissue. Late-gadolinium enhancement (LGE) cardiac MRI is employed in many studies for measurement of myocardial scarring in wide variety of pathologies. T1 mapping can be used to provide a direct measurement of the extracellular volume fraction of the myocardium. In contrast to LGE, T1 mapping can be used to measure diffuse myocardial fibrosis. These two techniques can be used the study the relationship between the patchy, focal or diffuse nature of fibrosis and the propensity to development of AF.
While data on AF at time of presentation may suffice for the determination of an “AF window”, data regarding AF burden would be preferable. However, quantification of AF burden is not easily achieved in the clinic. Intermittent rhythm monitoring has been shown to be unreliable in estimating true AF burden.66 Continuous monitoring devices such implantable loop recorders or pacemakers are therefore recommended for this purpose.
Evidence in support of the hypothesis that severe atrial fibrosis is attended by electrical depression can be obtained by concomitant measurement of ERP. The depression of excitability with progressive fibrosis is expected to prolong ERP, due to development of PRR, which occurs when the ERP value exceeds that of the action potential duration (APD) (Fig. 2). APD can be estimated in vivo by measurement of the monophasic action potential duration or the activation-recovery-interval using unipolar recordings from the same site. It is noteworthy that atrial ERP is commonly prolonged in patients with structural heart disease.55, 60 For these reasons, it would be helpful to determine whether atrial ERP progressively prolongs with aggravation of atrial fibrosis in patients with HF, as in experimental HF studies (Fig. 2).11, 15
The search for “AF window” can be done in a “snap-shot” fashion in studies involving different groups of HF patients having various extents of atrial fibrosis or in longitudinal studies involving the same group of patients in whom atrial fibrosis develops progressively to a severe level over a period of time.
Acknowledgments
The authors thank Dr. Peter Kowey for thoughtful discussion and valuable suggestions in the course of writing this review. The authors also wish to thank Ms. Donna Loyle for editorial assistance.
Funding Sources: Grant HL47678 from NHLBI. Martha and Wistar Morris Fund.
Funding
Supported by grant HL47678 from NHLBI and Martha and Wistar Morris Fund.
Footnotes
Disclosure
There are no conflicts of interest.
Disclosures: None
REFERENCES:
- 1.Maisel WH and Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2d–8d. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW and Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 3.Schoonderwoerd BA, Van G, I, Van Veldhuisen DJ, van Den Berg MP and Crijns HJ. Electrical and structural remodeling: role in the genesis and maintenance of atrial fibrillation. Prog Cardiovasc Dis 2005;48:153–168. [DOI] [PubMed] [Google Scholar]
- 4.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR and Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allessie MA, Ausma J and Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–246. [DOI] [PubMed] [Google Scholar]
- 6.Jalife J and Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med 2015;25:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaziri SM, Larson MG, Benjamin EJ and Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. [DOI] [PubMed] [Google Scholar]
- 8.Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, Brodsky M, Barrell P and Greene HL. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol 2005;45:2026–33. [DOI] [PubMed] [Google Scholar]
- 9.Platonov PG, Mitrofanova LB, Orshanskaya V and Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- 10.Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O’Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O and Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burashnikov A, Di Diego JM, Sicouri S, Doss MX, Sachinidis A, Barajas-Martinez H, Hu D, Minoura Y, Moise NS, Kornreich BG, Chi L, Belardinelli L and Antzelevitch C. A temporal window of vulnerability for development of atrial fibrillation with advancing heart failure. Eur J Heart Fail. 2014;16:271–280. [DOI] [PubMed] [Google Scholar]
- 12.Hanna N, Cardin S, Leung TK and Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. CardiovascRes 2004;63:236–244. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Fareh S, Leung TK and Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 14.Okuyama Y, Miyauchi Y, Park AM, Hamabe A, Zhou S, Hayashi H, Miyauchi M, Omichi C, Pak HN, Brodsky LA, Mandel WJ, Fishbein MC, Karagueuzian HS and Chen PS. High resolution mapping of the pulmonary vein and the vein of Marshall during induced atrial fibrillation and atrial tachycardia in a canine model of pacing-induced congestive heart failure. JAmCollCardiol 2003;42:348–360. [DOI] [PubMed] [Google Scholar]
- 15.Power JM, Beacom GA, Alferness CA, Raman J, Wijffels M, Farish SJ, Burrell LM and Tonkin AM. Susceptibility to atrial fibrillation: a study in an ovine model of pacing-induced early heart failure. J Cardiovasc Electrophysiol 1998;9:423–435. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Ducharme A, Li D, Gaspo R, Nattel S and Tardif JC. Remodeling of atrial dimensions and emptying function in canine models of atrial fibrillation. CardiovascRes 2001;52:217–225. [DOI] [PubMed] [Google Scholar]
- 17.Schoonderwoerd BA, Van G, I, Van Veldhuisen DJ, Tieleman RG, Grandjean JG, Bel KJ, Allessie MA and Crijns HJ. Electrical remodeling and atrial dilation during atrial tachycardia are influenced by ventricular rate: role of developing tachycardiomyopathy. J Cardiovasc Electrophysiol 2001;12:1404–1410. [DOI] [PubMed] [Google Scholar]
- 18.Tardif JC, O’Meara E, Komajda M, Bohm M, Borer JS, Ford I, Tavazzi L and Swedberg K. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox K, Ford I, Steg PG, Tardif JC, Tendera M and Ferrari R. Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: an analysis from the SIGNIFY study. Eur Heart J. 2015. [DOI] [PubMed] [Google Scholar]
- 20.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J and Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. EurHeart J. 2004;25:1214–1220. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y and Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. NEnglJ Med 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 22.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL and Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. NEnglJ Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 23.De Ferrari GM, Klersy C, Ferrero P, Fantoni C, Salerno-Uriarte D, Manca L, Devecchi P, Molon G, Revera M, Curnis A, Sarzi Braga S, Accardi F, Salerno-Uriarte JA and Group AS. Atrial fibrillation in heart failure patients: prevalence in daily practice and effect on the severity of symptoms. Data from the ALPHA study registry. Eur J Heart Fail. 2007;9:502–9. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW and Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 25.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV and Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurwitz JH, Magid DJ, Smith DH, Goldberg RJ, McManus DD, Allen LA, Saczynski JS, Thorp ML, Hsu G, Sung SH and Go AS. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med 2013;126:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toma M, Ezekowitz JA, Bakal JA, O’Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW and Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF Trial. EurJ Heart Fail. 2014;16:334–341. [DOI] [PubMed] [Google Scholar]
- 28.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW and Fonarow GC. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Heart Fail. 2016;4:464–72. [DOI] [PubMed] [Google Scholar]
- 29.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G and Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation Heart failure. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 30.The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. EurHeart J. 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 31.Triposkiadis F, Harbas C, Kelepeshis G, Sitafidis G, Skoularigis J, Demopoulos V and Tsilimingas N. Left atrial remodeling in patients younger than 70 years with diastolic and systolic heart failure. J Am Soc Echocardiogr 2007;20:177–85. [DOI] [PubMed] [Google Scholar]
- 32.Nieuwlaat R, Eurlings LW, Cleland JG, Cobbe SM, Vardas PE, Capucci A, Lopez-Sendon JL, Meeder JG, Pinto YM and Crijns HJ. Atrial fibrillation and heart failure in cardiology practice: reciprocal impact and combined management from the perspective of atrial fibrillation: results of the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol 2009;53:1690–1698. [DOI] [PubMed] [Google Scholar]
- 33.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM and Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study). Am J Cardiol 2006;97:83–89. [DOI] [PubMed] [Google Scholar]
- 34.Kurt M, Wang J, Torre-Amione G and Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. [DOI] [PubMed] [Google Scholar]
- 35.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS and Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007;49:198–207. [DOI] [PubMed] [Google Scholar]
- 36.Kuperstein R, Goldenberg I, Moss AJ, Solomon S, Bourgoun M, Shah A, McNitt S, Zareba W and Klempfner R. Left atrial volume and the benefit of cardiac resynchronization therapy in the MADIT-CRT trial. Circulation Heart failure. 2014;7:154–60. [DOI] [PubMed] [Google Scholar]
- 37.Akkaya M, Higuchi K, Koopmann M, Damal K, Burgon NS, Kholmovski E, McGann C and Marrouche N. Higher degree of left atrial structural remodeling in patients with atrial fibrillation and left ventricular systolic dysfunction. J Cardiovasc Electrophysiol 2013;24:485–497. [DOI] [PubMed] [Google Scholar]
- 38.Knackstedt C, Gramley F, Schimpf T, Mischke K, Zarse M, Plisiene J, Schmid M, Lorenzen J, Frechen D, Neef P, Hanrath P, Kelm M and Schauerte P. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2008;17:318–24. [DOI] [PubMed] [Google Scholar]
- 39.Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, Dahlof B and Devereux RB. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739–43. [DOI] [PubMed] [Google Scholar]
- 40.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT and Williard A. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). J Am Coll Cardiol 2009;54:2023–31. [DOI] [PubMed] [Google Scholar]
- 41.Tveit A, Flonaes B, Aaser E, Korneliussen K, Froland G, Gullestad L and Grundtvig M. No impact of atrial fibrillation on mortality risk in optimally treated heart failure patients. Clin Cardiol 2011;34:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mentz RJ, Chung MJ, Gheorghiade M, Pang PS, Kwasny MJ, Ambrosy AP, Vaduganathan M, O’Connor CM, Swedberg K, Zannad F, Konstam MA and Maggioni AP. Atrial fibrillation or flutter on initial electrocardiogram is associated with worse outcomes in patients admitted for worsening heart failure with reduced ejection fraction: findings from the EVEREST Trial. Am Heart J. 2012;164:884–892. [DOI] [PubMed] [Google Scholar]
- 43.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED and Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail. 2012;5:191–201. [DOI] [PubMed] [Google Scholar]
- 44.Abualnaja S, Podder M, Hernandez AF, McMurray JJ, Starling RC, O’Connor CM, Califf RM, Armstrong PW and Ezekowitz JA. Acute Heart Failure and Atrial Fibrillation: Insights From the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) Trial. J Am Heart Assoc 2015;4:e002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pecini R, Moller DV, Torp-Pedersen C, Hassager C and Kober L. Heart failure etiology impacts survival of patients with heart failure. IntJ Cardiol 2011;149:211–215. [DOI] [PubMed] [Google Scholar]
- 46.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW and Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clinic proceedings. 2001;76:467–75. [DOI] [PubMed] [Google Scholar]
- 47.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P and Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. Jama. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 48.Kato TS, Di Tullio MR, Qian M, Wu M, Thompson JL, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, Lip GY, Levin B, Mohr JP, Labovitz AJ, Estol CJ, Lok DJ, Ponikowski P, Anker SD and Homma S. Clinical and Echocardiographic Factors Associated With New-Onset Atrial Fibrillation in Heart Failure- Subanalysis of the WARCEF Trial. Circ J 2016;80:619–26. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T, Sakata Y, Miura M, Tadaki S, Ushigome R, Sato K, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Nochioka K, Takahashi J, Miyata S and Shimokawa H. Prognostic Impact of New-Onset Atrial Fibrillation in Patients With Chronic Heart Failure - A Report From the CHART-2 Study. Circ J. 2016;80:157–67. [DOI] [PubMed] [Google Scholar]
- 50.Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F and Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol 1998;32:197–204. [DOI] [PubMed] [Google Scholar]
- 51.Bonapace S, Rossi A, Cicoira M, Targher G, Marino P, Benfari G, Mugnai G, Arcaro G and Vassanelli C. Echocardiographically Derived Pulse Wave Velocity and Diastolic Dysfunction Are Associated with an Increased Incidence of Atrial Fibrillation in Patients with Systolic Heart Failure. Echocardiography. 2016;33:1024–31. [DOI] [PubMed] [Google Scholar]
- 52.Gavazzi A, De Maria R, Renosto G, Moro A, Borgia M, Caroli A, Castelli G, Ciaccheri M, Pavan D, De Vita C and et al. The spectrum of left ventricular size in dilated cardiomyopathy: clinical correlates and prognostic implications. SPIC (Italian Multicenter Cardiomyopathy Study) Group. Am Heart J. 1993;125:410–22. [DOI] [PubMed] [Google Scholar]
- 53.Remes J, van Brakel TJ, Bolotin G, Garber C, de Jong MM, van der Veen FH and Maessen JG. Persistent atrial fibrillation in a goat model of chronic left atrial overload. J Thorac Cardiovasc Surg 2008;136:1005–11. [DOI] [PubMed] [Google Scholar]
- 54.Haissaguerre M, Lim KT, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P and Virag N. Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace. 2007;9 Suppl 6:vi64–70. [DOI] [PubMed] [Google Scholar]
- 55.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB and Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. [DOI] [PubMed] [Google Scholar]
- 56.Verheule S, Wilson E, Everett T, Shanbhag S, Golden C and Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stambler BS, Fenelon G, Shepard RK, Clemo HF and Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol 2003;14:499–507. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O and Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. CircRes 2007;101:839–847. [DOI] [PubMed] [Google Scholar]
- 59.Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL, Kasprowicz K, Bhatta L, Puskas F, Kalifa J and Jalife J. Left versus right atrial difference in dominant frequency, K+ channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhm JS, Mun HS, Wi J, Shim J, Joung B, Lee MH and Pak HN. Prolonged atrial effective refractory periods in atrial fibrillation patients associated with structural heart disease or sinus node dysfunction compared with lone atrial fibrillation. Pacing Clin Electrophysiol 2013;36:163–71. [DOI] [PubMed] [Google Scholar]
- 61.Platonov PG, Corino VD, Seifert M, Holmqvist F and Sornmo L. Atrial fibrillatory rate in the clinical context: natural course and prediction of intervention outcome. Europace. 2014;16 Suppl 4:iv110–iv119. [DOI] [PubMed] [Google Scholar]
- 62.Burashnikov A and Antzelevitch C. New development in atrial antiarrhythmic drug therapy. Nat RevCardiol 2010;7:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miragoli M, Gaudesius G and Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 2006;98:801–10. [DOI] [PubMed] [Google Scholar]
- 64.Hansen BJ, Zhao J and Fedorov VV. Fibrosis and Atrial Fibrillation: Computerized and Optical Mapping; A View into the Human Atria at Submillimeter Resolution. JACC Clin Electrophysiol 2017;3:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambale-Venkatesh B and Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nature reviews Cardiology. 2015;12:18–29. [DOI] [PubMed] [Google Scholar]
- 66.Charitos EI, Ziegler PD, Stierle U, Robinson DR, Graf B, Sievers HH and Hanke T. Atrial fibrillation burden estimates derived from intermittent rhythm monitoring are unreliable estimates of the true atrial fibrillation burden. Pacing Clin Electrophysiol 2014;37:1210–8. [DOI] [PubMed] [Google Scholar]