Abstract
Dehydroalanine (DHA) and dehydrobutyrine (DHB) intermediates, formed through β-elimination, induce protein irreversible glutathionylation and protein-protein crosslinking in human lens fiber cells. In total, irreversible glutathionylation was detected on 52 sites including cysteine, serine and threonine residues in 18 proteins in human lenses. In this study, the levels of GSH modification on three serine residues and four cysteine residues located in seven different lens proteins isolated from different regions and different aged lenses were quantified. The relative levels of modification (modified/nonmodified) were site-specific and age-related, ranging from less than 0.05% to about 500%. The levels of modification on all of the sites quantified in the lens cortex increased with age and GSH modification also increased from cortex to outer nucleus region suggesting an age-related increase of modification. The levels of modification on sites located in stable regions of the proteins such as Cys117 of βA3, Cys80 of βB1 and C27 of γS, continued increasing in inner nucleus, but modification on sites located in regions undergoing degradation with age decreased in the inner nucleus suggesting GSH modified proteins were more susceptible to further modification. Irreversible GSH modification in cataract lenses was typically higher than in age-matched normal lenses, but the difference did not reach statistical significance for a majority sites, with the exception Cys117 of βA3 crystallin in WSF. Except for S59 of αA and αB crystallins, GSH modification did not induce protein insolubility suggesting a possible role for this modification in protection from protein-protein crosslinking.
Keywords: glutathione, proteomics, posttranslational modification, crystallins
1. Introduction
The formation of protein mixed disulfides where, for example, glutathione (GSH) modifies cysteine residues through a disulfide linkage, is generally a reversible process of posttranslational modification. This process increases globally during oxidative stress, protects protein thiols from irreversible oxidation and prevents pro-aggregatory protein-protein crosslinking through disulfide bonds (Gallogly and Mieyal, 2007, Leichert and Jakob, 2004). In the ocular lens, protein-thiol mixed disulfide levels increase with age (Lou and Dickerson, 1992), oxidative stress (Lou et al., 1990) and correlate with lens nuclear color and opalescence among nuclear cataract lenses (Lou et al., 1990). In addition to reversible disulfide modifications, GSH can also irreversibly modify proteins, in an age-dependent manner, through the formation of a thioether bond by reacting with dehydroamino acids such as dehydroalanine (DHA) and dehydrobutyrine (DHB) (Finley and Friedman, 1977, Sen et al., 1977, Townsend et al., 2014). The formation of DHA and DHB residues in proteins results from a well-known base-catalyzed β-elimination (Finley and Friedman, 1977, Sen et al., 1977). This β-elimination reaction has been confirmed to occur under physiological conditions in long-lived proteins as evidenced by detection of DHA in tissues such as human lens (Srivastava et al., 2004) and dentin (Cloos and Jensen, 2000, Masters, 1985). Both enzyme-catalyzed and non-enzymatic mechanisms have been reported for formation of DHA in proteins (Brennan and Barford, 2009, Finley and Friedman, 1977, Sen et al., 1977, Townsend et al., 2014). The residues that undergo β-elimination usually include cysteine (Bar-Or et al., 2008), serine and phosphoserine (Cloos and Jensen, 2000, Sen et al., 1977) and threonine and phosphothreonine (Brennan and Barford, 2009, Sen et al., 1977). Once β-elimination products are formed, GSH can react with DHA and DHB residues in proteins resulting in irreversible glutathionylation. DHA and DHB can also react with other nucleophiles such as cysteine, histidine and lysine to form lanthionine (LAN), histidinoalanine (HAL) and lysinoalanine (LAL) (Kanayama et al., 1987, Linetsky et al., 2004, Linetsky and LeGrand, 2005) resulting in protein modification and protein-protein crosslinking (Bessems et al., 1987, Cloos and Jensen, 2000, Linetsky et al., 2004).
The presence of LAN, HAL and LAL residues has been detected in aged proteins, for example in dentin (Cloos and Jensen, 2000), and in both normal and cataractous lenses with the level of these crosslinks significantly higher in cataractous lenses (Bessems et al., 1987, Linetsky et al., 2004). Direct ELISA of reduced lens proteins showed a concentration-dependent immunoreactivity suggesting protein glutathionylation through nonreducible thioether bonds (Linetsky and LeGrand, 2005). Recently, using mass spectrometry techniques, we directly detected a number of lens proteins that were modified by glutathione, cysteine, glycine-cysteine dipeptide, as well as homocysteine through a thioether bond (Wang et al., 2014). This result unambiguously confirmed the previous report of irreversible protein glutathionylation based on a predicted mechanism of thioether formation (Linetsky and LeGrand, 2005). In addition, in vitro experiments indicate that both DHA formation and nucleophilic addition can occur spontaneously at physiological pH and temperature (Wang et al., 2014). DHA formed from β-elimination also induces protein-protein crosslinking and crosslinks resulting from this chemistry between lens crystallins have been detected in human lenses (Wang et al., 2014). These results provide direct evidence for a mechanism involving spontaneous formation of DHA and DHB intermediates leading to protein modification and protein-protein crosslinking.
The ocular lens is a unique tissue that contains fiber cells of varying ages from newly differentiated cells in the outer cortex to those as old as the organism in the central lens core. A continuous series of biochemical and biophysical changes occur throughout lens growth and aging (Michael and Bron, 2011). Importantly, there is no significant protein turnover in the differentiated lens fiber cells. With age, lens proteins are highly modified and degraded; therefore, continuous formation of DHA and DHB is predicted with fiber cell age resulting in higher levels in older, nuclear fiber cells. On the contrary, GSH levels are relatively high in the cortex of normal lenses and are substantially lower in the lens nucleus (Kamei, 1993, Lou, 2003, Pau et al., 1990). Furthermore, GSH levels decrease with lens age (Kamei, 1993). Thus, a balance exists between predicted increasing DHA/DHB formation and decreasing GSH levels with fiber cell age. In addition, once formed, GSH thioether adducts can further degrade to form CysGly or Cys adducts (Wang et al., 2014).
Previously, the levels of LAN, HAL and LAL residues in normal and cataract lenses were quantified by analyzing acid hydrolyzes samples (Bessems et al., 1987, Linetsky et al., 2004); however, after acid hydrolysis, knowledge of whether LAN, HAL and LAL come from protein GSH modification or protein-protein crosslinking is lost. In this study, we quantified the level of GSH modification at specific sites on several lens proteins isolated from different lenses. The level of modification in normal lenses and cataract lenses was also compared. Our results indicate that rate of formation and degradation of GSH modification in human lens highly depends on the modification sites. Statistically significant differences for some modification sites can be detected with lens region, lens age or cataract. Our results provide important information toward understanding the reaction mechanism and the consequence of this irreversible, age-related modification.
2. Materials and Methods
2.1 Isolation of different regions of the lens
Human lenses were obtained from NDRI (Philadelphia, PA). All lenses were isolated from the donor no later than 8 hours post mortem and shipped on dry ice. All lenses received were stored at −80°C until use. The lenses were attached to a cryostat chuck with optimal cutting temperature (OCT) medium (Tissue-Tek®, Sakura Finetek, Torrance, CA). Lenses were sectioned at 30 μm thickness equatorially in a cryostat (LEICA CM 3050S, Leica Microsystems Inc., Bannockburn, IL) at −21°C. Only secPons from the equatorial region were collected. SecPons were picked up on a piece of parafilm and saved on dry ice. The section, on parafilm, was first air-dried at room temperature and different regions of the lens were separated by punching through the parafilm using AcuPunch trephines (Acuderm Inc, Ft. Lauderdale, FL) of different diameters. The inner nucleus region was obtained by punching the middle of the section using a 4.5 mm diameter trephine. Further punching the remaining tissue with a 6 mm diameter trephine gave the outer nucleus region. The remaining portions of the section were collected as cortex and separated from the bulk parafilm using an 8 mm diameter punch (In most cases, the section is slightly larger than 8 mm in diameter and all the tissue was included).
2.2 Preparation of different fractions of lens fiber cell proteins
Isolated lens tissue regions were separated from parafilm by five sequential washes with 100 μL of 25 mM Tris, 150 mM NaCl, 5 mM EDTA, pH 7.4. The samples were passed through a 25 G needle 5 times and centrifuged at 20,000 g for 30 min. The supernatant was collected as the water soluble fraction (WSF). The pellets were vortexed in 100 μL of 8 M urea in 25 mM Tris, 150 mM NaCl, 5 mM EDTA, pH 7.4 and centrifuged at 20,000 g for 30 min. This 8 M urea extraction was repeated and both urea extracts were pooled as the urea soluble fraction (USF). The remaining pellets were collected as the urea insoluble fraction (UIF). The UIF pellets were suspended in 100 μL of water and 5 μL was mixed with 5 μL of 5% SDS to solubilize the protein for protein assay. The protein concentration in each fraction was measured by BCA assay (Thermo Scientific, Rockford, IL).
The WSF and USF corresponding to 200 μg of total protein and the UIF suspended in water corresponding to 50 μg of total protein were reduced by adding 1 M DTT to a final concentration of 10 mM in the sample. The samples were incubated at 56 °C for one hour. Iodoacetamide was then added to a final concentration of 55 mM and the samples were incubated in the dark at room temperature for 45 min. WSF and USF proteins were precipitated by chloroform/methanol as previously described (Wessel and Flügge, 1984) and both solvents and samples were kept on ice during experiments. The UIF proteins were centrifuged at 20,000g and the supernatant was discarded. Chloroform and methanol precipitated WSF and USF proteins and the UIF pellets were digested by trypsin in 10%ACN with an enzyme-to-protein ratio of 1:100. The digestion was done overnight at 37°C. After digestion, all samples were dried in a speedvac and proteins in each sample were reconstituted in 0.1% formic acid to make a final total protein concentration of 0.25 μg/μL for subsequent analysis.
2.3 LC-MS/MS
A standard peptide mixture (Catalog Number 88320, Thermo Scientific, Rockford, IL) was spiked-in as an internal standard. The final concentration of the standard peptides was 2.38 fmol/μL. Tryptic peptides corresponding to 0.5 μg of total protein were separated on a one-dimensional fused silica capillary column (200 mm × 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size) coupled with an Easy-nLC system (Thermo Scientific, San Jose, CA). A 70-minute gradient was performed, consisting of the following: 0–60 min, 2–45% ACN (0.1% formic acid); 60–70 min, 45–95% ACN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into a Q Exactive instrument (Thermo Scientific, San Jose, CA) equipped with a nanoelectrospray ionization source. The data-dependent instrument method consisted of MS1 acquisition (R=70,000), using an MS AGC target value of 1e6, followed by up to 15 MS/MS scans (R=17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 2e5, with a maximum ion time of 200 ms, and a 5% underfill ratio, and intensity threshold of 5e4. HCD collision energy was set to 27, dynamic exclusion was set to 5 s, and peptide match and isotope exclusion were enabled.
2.4 Data Analysis
For identification of GSH modification sites, the raw data were processed and searched against a concatenated forward and reversed (decoy) Uniprot human database (Oct, 2016) as previously reported (Wang et al., 2014). Briefly, the data were searched with differential modifications of carbamidomethylation of cysteine and oxidation of methionine as well as GSH modification on Cysteine (delta mass= ), Serine ( ) and Threonine ( ). All the modifications reported in this paper were manually verified by inspection of their tandem mass spectra and measured masses were below 5 ppm mass accuracy for parent masses and below 10 ppm for product ions.
The selected ion chromatograms of modified and unnmodified peptides were extracted with a +/−10 ppm mass tolerance. The peak areas of GSH modified peptides and corresponding non-modified peptides were calculated within Xcalibur software. The relative level of modification was defined as the ratio of the peak area of modified peptide to the peak area of the nonmodified peptide. Each lens was separated into three regions and three samples were generated from each region (WSF, USF and UIF). The results are presented as mean ± standard deviation (SD) of 4 independent experiments from four different lenses in each group. Data were analyzed by Student’s t test (two-tailed). Results were considered statistically significant whenp < 0.05.
3. Results
3.1 Characterizing GSH modification on lens proteins
Previously we reported that GSH can irreversibly modify proteins in human lenses through dehydroalanine (DHA) or dehydrobutyrine (DHB) intermediates (Wang et al., 2014). We detected 26 GSH modification sites on either serine or threonine residues and a majority of these sites are known phosphorylation sites suggesting DHA and DHB are derived primarily from phosphorylated serine and threonine, respectively (Wang et al., 2014). DHA can also be derived from cysteine residues in human lenses and Cys5 of βA4 crystallin was found to be highly modified by GSH in our previous report (Wang et al., 2014). In the current study, we used a highly sensitive, high-resolution Q Exactive mass spectrometer for relative quantification of the modification and included cysteine residues as potential sites for modification in our database searching parameters to achieve a more in depth analysis of GSH-modified lens proteins. Table 1 lists additional sites identified as glutathionylation sites through thiol-ether linkage in human lenses other than those sites reported previously (Wang et al., 2014). Twenty five new GSH modification sites were identified including 18 sites on cysteines suggesting cysteine residues represent another site that is prone to β-elimination. With the exception of Ser142 on phakinin, all modification sites on serine and threonine residues are present on previously reported phosphorylation sites. In the current analysis, GSH irreversible glutathionylation was detected not only on highly abundant crystallins, but also on some relatively low abundance lens proteins such as AQP5 and peroxiredoxin-6. Together with previously reported results (Wang et al., 2014), we have now identified 52 GSH modification sites in human lenses; however, the relative abundance of modifications in the different regions of the human lenses and in different aged lenses, including cataract lenses, has not been measured.
Table 1.
New sites identified in human lens proteins that are irreversibly modified by GSH.
| Proteins | Modification Sites |
|---|---|
| β-crystallin A3 | C117, C185 |
| β-crystallin A4 | C166 |
| β-crystallin B2 | C67 |
| β-crystallin B1 | C80 |
| α-crystallin A | C142, T153 |
| α-crystallin B | S35, S153 |
| γ-crystallin C | C23, C79, C80, C130 |
| γ-crystallin D | C79 |
| γ-crystallin S | C27 |
| Phakinin | S31, S142, C65, C255, C396 |
| Filensin | C259, |
| Gap junction α-3 protein | S119 |
| Peroxiredoxin-6 | C47, C91 |
| AQP5 | T259 |
3.2 Quantification of GSH modification in different lens regions
We developed a lens dissection method and a quantitative mass spectrometry method to measure the extent of modification in different lens regions of lenses of varying age. In this study, the extent of modification on seven sites (four cysteines and three serines) was measured in both normal and cataractous lenses. These seven sites were chosen because these sites are present in highly abundant lens proteins and tryptic peptides containing these sites have good lengths for detection by mass spectrometry. With the exception of serine S235 of AQP0 (seen only in the UIF), the peptides containing these sites gave strong signals across different regions of the lenses and different fractions of samples. Thus, we could the relative modification levels amongst all samples and fractions. Peptides containing easily modified residues such as methionine were excluded from our analysis. In total 12 different lenses were used and they were divided into three groups: young normal lenses (18 y, 19 y, 21 y and 22 y), middle aged normal lenses (48 y, 56 y and two 53 y), and middle aged cataract lenses (50 y, 57 y, 58 y and 64 y). The detailed information for the lenses used in this experiment can be found in Supplemental Table 1. For each lens, three different regions were dissected (cortex, outer nucleus and inner nucleus) and WSF, USF and UIF were obtained from each sample. Results are presented for crystallin protein modification in WSF and USF fractions, and for aquaporin-0 (AQP0) in the UIF fraction. Due to reduced signal from crystallins in UIF, GSH modified crystallin signals in the UIF were below the limit of quantification; therefore, quantification of GSH modified crystallins in the UIF was not done.
3.3 GSH modification on C117 of βA3, Cys80 of βB1 crystallin, and Cys27 of γS crystallin crystallin
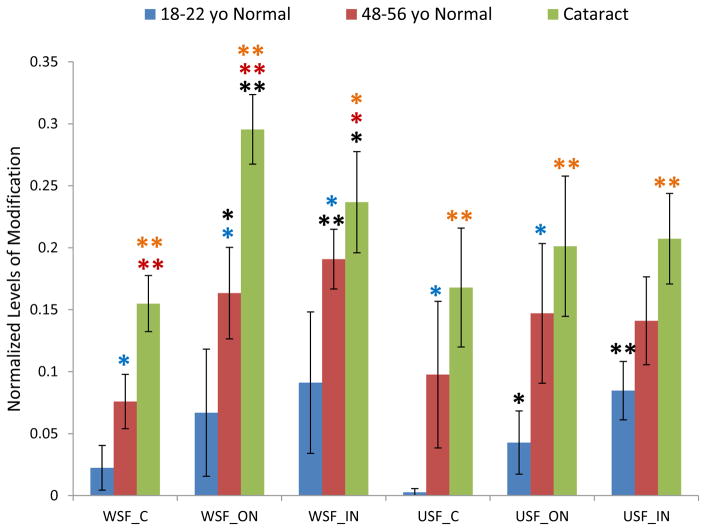
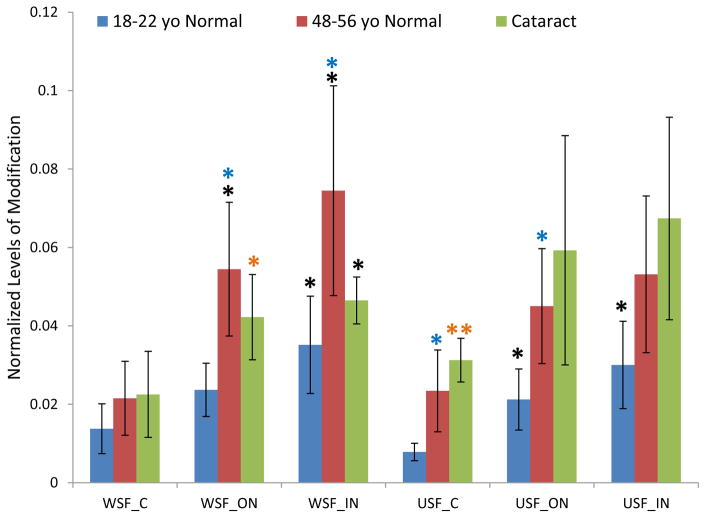
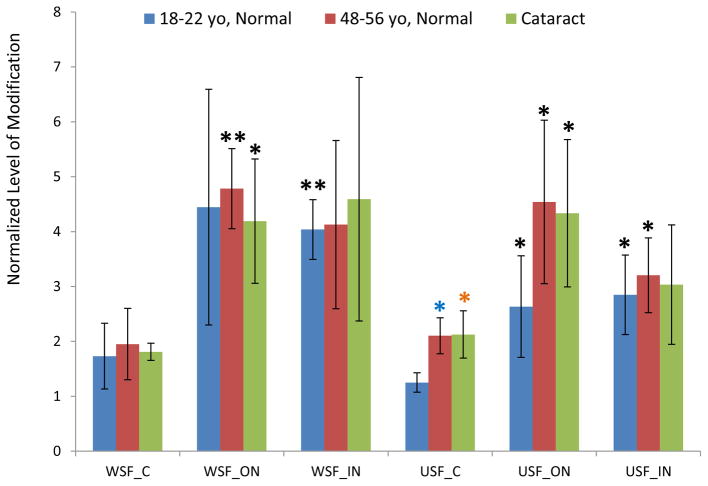
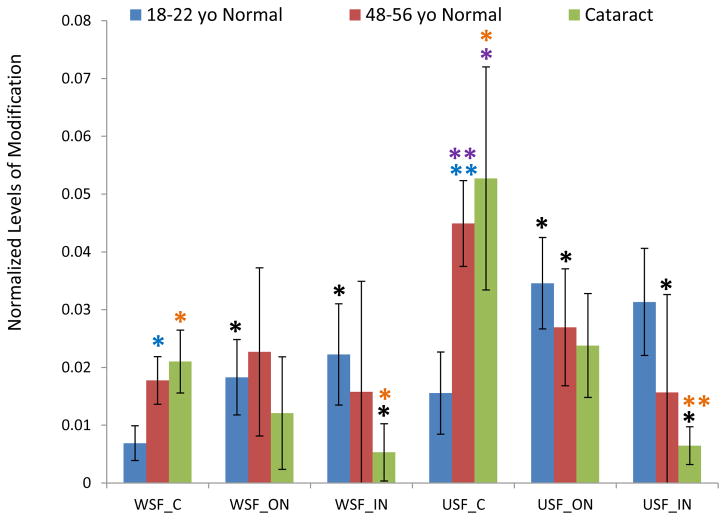
The age-related patterns of GSH modification for these three cysteine sites are similar (Figure 1–3). The relative amount of modification at these sites ranges from 0.1% in βB1 to 30% in βA3 and shows a clear lens age and lens region dependence. Modification levels in the young lenses were significantly lower than in the older lenses. Statistical analysis of samples from various regions of the lenses in both WSF and USF demonstrated that modification on Cys80 of βB1 crystallin and Cys117 of βA3 crystallin were significantly higher in middle-aged lenses than in young lenses for most of the samples analyzed. Age-related accumulation of modification can also be seen when the extents of modification in the different regions of the lenses were compared. The average levels of modification on all three sites increased from cortex to inner nucleus with fiber cell aging. For all three sites, the level of modification in USF from the young lenses in inner nucleus region was significant higher than in cortex region. Comparing the extents of modification in cataract lenses with age-matched normal lenses, modification on Cys80 of βB1 crystallin and Cys117 of βA3 crystallin showed consistent higher level in cataract lenses. The difference reached statistical significance for modification on Cys117 of βA3 crystallin in WSF of both cortex and outer nucleus. Modification on both Cys80 of βB1 crystallin and Cys117 of βA3 crystallin significantly increased in cataract lenses compared with young lens (p <0.05 or p <0.005) and both age and cataract could contribute to this increased level of modification. The extents of modification in WSF and USF did not show a statistical difference suggesting GSH modification does not induce changes of protein solubility. Modification on Cys27 of γS crystallin showed a pattern of age-related increases similar to modification on Cys80 of βB1 crystallin and Cys117 of βA3 crystallin.
Figure 1.
GSH modification on Cys117 of βA3 crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF). * Indicates p< 0.05, ** indicates p< 0.005; Black asterisks show significant increasing modification compared with cortex in the same group of lenses. Red asterisks show significant increasing of GSH modification in cataract lenses compared with middle-aged normal lenses; Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. Orange asterisks indicate significant increase in cataract lenses compared with young lenses.
Figure 3.
GSH modification on Cys27 of γS crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF). * Indicates p< 0.05; Black asterisks show significant increasing modification compared with cortex in the same group of lenses. Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. The difference of modification in cataract lenses compared with middle-aged normal lenses did not reach statistical significance.
3.4 GSH modification on Cys5 of βA4 crystallin
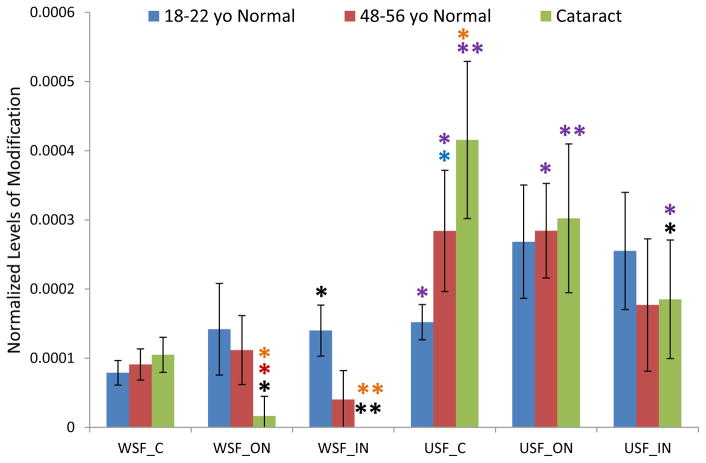
Cys5 on βA4 crystallin is highly susceptible to modification (Friedrich et al., 2017). This modification can be detected in lenses as young as 2 month old in a whole human lens sample (data not shown). At approximately 20 years old, the signal of the modified βA4 crystallin peptide exceeded that of the unmodified peptide in lens cortex. The levels of modification in different lens regions were quantified and the average results for three groups are shown in Figure 4. Since Gln 4 on βA4 crystallin undergoes significant deamination, signals for both deaminated and non-deaminated forms were summed for results shown in Figure 4. The extent of modification showed variability among lenses from different donors; however, for all the lenses analyzed, the signal of GSH modified peptide relative to unmodified peptide was greater and increased from the cortex to outer nucleus region in both WSF and USF indicating accumulation of more modified protein in older cells. Except for the WSF, 18–22y, young normal lenses, the difference of modification levels between cortex and outer nucleus regions for both WSF and USF reached statistical significance (p< 0.05). Interestingly, the modification levels remain relatively constant between outer and inner nucleus regions, i.e. they do not increase in the inner nuclear region as described above for βB1, βA3, and γS-crystallins. This pattern as well as the decrease of modified peptide abundance in the inner nucleus of the USF is likely due to further modification of the peptide; either to other amino acids in this peptide or by further degradation of adducted GSH to cysteine or Cys-Gly dipeptide. Both enzymatic (Orlowski and Meister, 1970) and nonenzymatic (Deshmukh et al., 2009) degradation of GSH to Cysteine and Cys-Gly dipeptide has been reported. Additionally, we have observed such degradation of GSH-modified peptides (Friedrich et al., 2018).
Figure 4.
GSH modification on Cys5 of βA4 crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF). * Indicates p< 0.05, ** indicates p< 0.005; Black asterisks show significant increasing modification compared with cortex in the same group of lenses. Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. Orange asterisks indicate significant increase in cataract lenses compared with young lenses. The level of modification in cataract lenses and in middle-aged normal lenses was not statistically significant.
The extent of modification on Cys5 of βA4 crystallin in cataract lenses and age-matched normal lenses did not show significant differences in any lens region. Despite the very high abundance of the modified βA4 crystallin peptide, these results suggest that the GSH modification does not induce solubility changes of βA4 crystallin.
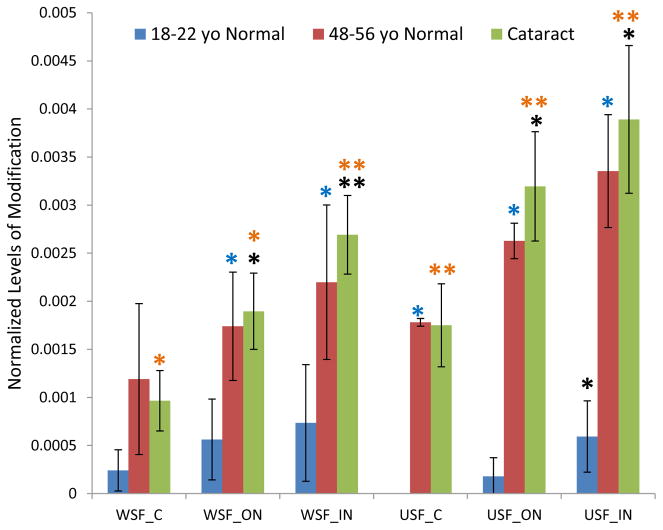
3.5 GSH modification on Ser59 on αA crystallin and αB crystallin
The lens region, solubility, and age-related changes of GSH modification on Ser59 of αA crystallin and αB crystallin show similar patterns to each other (Figure 5 and Figure 6), but different than the patterns seen for Cys modifications described above. The modification level increased from cortex to inner nucleus regions in young lenses, but decreased in the inner nucleus regions in older lenses. This trend is similar to the result obtained using a targeted method reported earlier (Wang et al., 2014). The most striking observation is the decrease of modified Ser59 in the WSF in cataract lenses and the corresponding increase in the USF in each lens region. In general, there is a higher level of modified αA Ser59 in the USF suggesting an effect on αA-crystallin solubility. A similar effect is seen for modified Ser59 in αB-crystallin. The observed decrease in modified peptide levels in the cataract lenses in different lens regions is likely due to increased additional modification or, alternatively, due to insolubilization that would shift the signals to the UIF fraction.
Figure 5.
GSH modification on Ser59 of αA crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF).* Indicates p< 0.05, ** indicates p< 0.005; Black asterisks show significant increasing or decreasing modification compared with cortex in the same group of lenses. Red asterisks show significant decreasing of GSH modification in cataract lenses compared with middle-aged normal lenses; Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. Purple asterisks indicate significant increasing in USF than in WSF.
Figure 6.
GSH modification on Ser59 of αB crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF). * Indicates p< 0.05, ** indicates p< 0.005; Black asterisks show significant increasing or decreasing level of modification compared with cortex in the same group of lenses. Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. Purple asterisks indicate significant increasing level of modification in USF than in WSF.
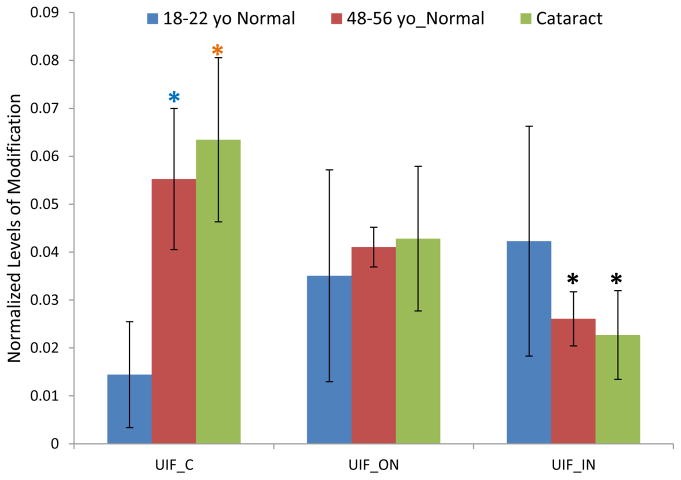
3.6 GSH modification on Ser235 on AQP0
AQP0 appears in the UIF and a GSH modification is detected on Ser235; the major site of phosphorylation (Ball et al., 2004, Schey et al., 2000). When modified by phosphorylation, trypsin also mis-cleaves R233 and a similar mis-cleavage was observed when S235 is modified by GSH. Therefore, the signal of the modified peptide 229–238 was normalized by the signal of the expected tryptic peptide 234–238 since mis-cleavage does not occur for the unmodified peptide. The extent GSH modification on AQP0 Ser235 within each lens region is shown in Figure 7 and the observed age-related changes in GSH modification are similar to those seen for Ser59 of αA and αB crystallin. Modification in the young lenses increased from cortex to nucleus, but the difference was not statistically significant. The level of modification was significantly higher in middle-aged normal lenses than young lenses in the lens cortex. Similar to Ser59 of αA and αB crystallin, the level of modification significantly decreased in the inner nucleus region compared with the cortex region for both middle-aged normal lenses and cataract lenses; again, likely due to further modification of the GSH modified peptide. No significant different level of modification was detected in cataract lenses compared with age-matched normal lenses.
Figure 7.
GSH modification on Ser235 of AQP0 in urea insoluble fraction (UIF) of dissected lens regions. * Indicates p< 0.05; Black asterisks show significant decreasing modification compared with cortex in the same group of lenses. Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. The level of modification in cataract lenses compared with middle-aged normal lenses was not statistically significant.
4. Discussion
In this paper, we quantified the level of DHA or DHB mediated glutathionylation in different regions of human lenses of varying age and in WSF, USF, and UIF fractions. Unlike glutathionylation through disulfide bonds, i.e. protein mixed disulfides, GSH modification through a thioether linkage is expected to be irreversible and to accumulate in the lens with age. It is important to note, however, that GSH can degrade to form Cys and CysGly adducts (Wang et al., 2014). The extents of modification to cysteine residues of βB1 crystallin, γS crystallin and βA3 crystallin support the age-related accumulation in the lenses. For other modification sites, the increased levels of modification from cortex to outer nucleus region also support age-related accumulation of GSH modification. Instead of increased level of modification in the oldest fiber cells of the inner nucleus, the extents of modification on Cys5 of βA4, Ser59 of αA, Ser59 of αB crystallin and Ser235 of AQP0 decrease in a majority of lenses from the outer nucleus to inner nucleus regions. We attribute this to additional modification to these peptides in oldest fiber cells, including degradation of the GSH to Cys and CysGly (Orlowski and Meister, 1970, Deshmukh et al., 2009, Wang et al., 2014, Friedrich et al., 2018). In this study, the level of modified protein was normalized to the non-modified protein. If both GSH modified and unmodified protein undergo the same level of degradation, the normalized level of modification would not change with protein degradation. Our results demonstrate that the normalized level of GSH modification decreases in the inner nucleus region for some modification sites which suggests that GSH modified proteins are more easily degraded. This is consistent with previous reports that indicate that glutathionylation enhances protein degradation (Liang and Pelletier, 1988, Zetterberg et al., 2006). Similarly, Kamei (1993) reported that reversible glutathionylation levels in both soluble and insoluble lens proteins dropped noticeably in the 50-year and older age groups. Previous reports suggest more protein-protein crosslinking in cataract lenses due to increased levels of lanthionine (LAN), histidinoalanine (HAL) and lysinoalanine (LAL) measured after acid hydrolysis (Bessems et al., 1987, Linetsky et al., 2004). Our results suggest that irreversible glutathionylation partially contributes to the signal detected in acid hydrolysis samples. Comparing the level of modification in normal and cataract lenses, the extents of thioether-linked GSH modification on some sites such as Cys80 of βB1 and Cys117 of βA3 are higher in cataract lenses than normal lenses. Both Cys80 of βB1 and Cys117 of βA3 were reported to form higher level of disulfide in cataract lenses (Fan et al., 2015) and it is noteworthy that disulfide formation is a potential intermediate in DHA formation that can lead to GSH thioether formation (Friedrich et al., 2017, Friedrich et al., 2018). Considering the lower levels of GSH reported in cataract lenses, we expected to observe lower levels of irreversible glutathionylation; however, this modification requires two-steps, DHA/DHB formation and nucleophilic attack by GSH. The formation of DHA and DHB could be higher in cataract lenses and this step could be the rate-limiting step. Like reversible glutathionylation, accessibility is another factor that may affect modification. Clearly DHA containing proteins can be detected in the lens samples, and these proteins are at risk for forming protein-protein crosslinks (Wang et al., 2014).
Since protein glutathionylation adds additional mass and charge to the modified proteins, this modification could change protein three-dimensional structure and affect protein function (Grek et al., 2013). Modification on serine and threonine that replace known phosphate groups at known phosphorylation sites, e.g. in α-crystallins or AQP0, could affect protein function. Increased levels of modification in the USF on Ser59 of both αA and αB crystallin suggests GSH modified proteins could cause misfolding and aggregation. Alternatively, protein conformation changes of insoluble αA and αB crystallin could result in modification sites becoming more accessible to GSH. Although GSH modification could induce solubility changes, our results indicate that modification on majority sites does not induce protein insolubility. Irreversible glutathionylation may play important role in blocking reactive DHA and DHB formation in the proteins and serves to prevent protein-protein crosslinking (Wang et al., 2014). Potential limitations of the current approach are that low abundance modified peptides have intensities below our limit of quantitation. Furthermore, extraction of urea insoluble peptides may be limited and produce an underestimation of GSH modification levels in this fraction.
In conclusion, irreversible glutathionylation occurs on many lens proteins and susceptibility to modification on different sites is different, but age-related accumulation of modification can be detected for all sites quantified. Unlike expected decreased level of modification due to low GSH concentration in cataract lenses, the level of modification showed a trend of increasing in cataract lenses for majority of sites monitored indicating DHA formation is the rate-limiting step for irreversible glutathionylation. Modified proteins likely undergo further degradation and the degree of degradation is also site-specific. Therefore, the accumulation of modified proteins in the lens results from the combinatorial effect of formation and degradation with age. Irreversible glutathionylation may affect protein structure and result in solubility changes, but results from quantification suggest that, with the exception of the α-crystallins, GSH modification does not induce protein insolubility. For these sites, a protective role from protein-protein crosslinking is hypothesized.
Supplementary Material
Figure 2.
GSH modification on Cys80 of βB1 crystallin in dissected lens regions separated into water soluble fraction (WSF) and urea soluble fraction (USF).* Indicates p< 0.05, ** indicates p< 0.005; Black asterisks show significant increasing modification compared with cortex in the same group of lenses. Blue asterisks show significant increasing of modification in middle-aged normal lenses compared with young lenses. Orange asterisks indicate significant increase in cataract lenses compared with young lenses. The difference of modification in cataract lenses compared with middle-aged normal lenses did not reach statistical significance.
Highlights.
52 sites of irreversible thioether-linked glutathionylation on 18 lens proteins are reported
Modified residues include serine, threonine, and cysteine
Quantification of modified lens crystallins indicated increased modification with lens age, fiber cell age, and cataract status
Acknowledgments
This work was supported by the National Institutes of Health grants: EY024258 and EY008126.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 2004;43:9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- Bar-Or R, Rael LT, Bar-Or D. Dehydroalanine derived from cysteine is a common post-translational modification in human serum albumin. Rapid Commun Mass Spectrom. 2008;22:711–716. doi: 10.1002/rcm.3421. [DOI] [PubMed] [Google Scholar]
- Bessems GJ, Rennen HJ, Hoenders HJ. Lanthionine, a protein cross-link in cataractous human lenses. Exp Eye Res. 1987;44:691–695. doi: 10.1016/s0014-4835(87)80139-2. [DOI] [PubMed] [Google Scholar]
- Brennan DF, Barford D. Eliminylation: a post-translational modification catalyzed by phosphothreonine lyases. Trends Biochem Sci. 2009;34:108–114. doi: 10.1016/j.tibs.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Cloos PA, Jensen AL. Age-related de-phosphorylation of proteins in dentin: a biological tool for assessment of protein age. Biogerontology. 2000;1:341–356. doi: 10.1023/a:1026534400435. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Kutscher H, Stein S, Sinko P. Nonenzymatic, self-elimination degradation mechanism of glutathione. Chem Biodivers. 2009;6:527–539. doi: 10.1002/cbdv.200800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Zhou S, Wang B, Hom G, Guo M, Li B, Yang J, Vaysburg D, Monnier VM. Evidence of Highly Conserved β-Crystallin Disulfidome that Can be Mimicked by In Vitro Oxidation in Age-related Human Cataract and Glutathione Depleted Mouse Lens. Mol Cell Proteomics. 2015;14:3211–3223. doi: 10.1074/mcp.M115.050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW, Friedman M. New amino acid derivatives formed by alkaline treatment of proteins. Adv Exp Med Biol. 1977;86B:123–130. doi: 10.1007/978-1-4757-9113-6_8. [DOI] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Oakley AJ, Schey KL, Truscott RJW. Hotspots of Age-Related Protein Degradation. The Importance of Neighbouring Residues for the Formation of Non-Disulfide Crosslinks derived from Cysteine. Biochem J. 2017;474:2475–2487. doi: 10.1042/BCJ20170268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL, Truscott RJW. DehydroalanylGly, a new post translational modification resulting from the breakdown of glutathione. Biochim Biophys Acta. 2018;1862:907–913. doi: 10.1016/j.bbagen.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei A. Glutathione level of human crystalline lens in aging and antioxidant effect against the oxidation of lens protein. Biol Pharm Bull. 1993;16:870–875. doi: 10.1248/bpb.16.870. [DOI] [PubMed] [Google Scholar]
- Kanayama T, Miyanaga Y, Horiuchi K, Fujimoto D. Detection of the cross-linking amino acid, histidinoalanine, in human brown cataractous lens protein. Exp Eye Res. 1987;44:165–169. doi: 10.1016/s0014-4835(87)80001-5. [DOI] [PubMed] [Google Scholar]
- Leichert LI, Jakob U. Protein thiol modifications visualized in vivo. PLoS Biol. 2004;2:e333. doi: 10.1371/journal.pbio.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JN, Pelletier MR. Destabilization of lens protein conformation by glutathione mixed disulfide. Exp Eye Res. 1988;47:17–25. doi: 10.1016/0014-4835(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Linetsky M, Hill JM, LeGrand RD, Hu F. Dehydroalanine crosslinks in human lens. Exp Eye Res. 2004;79:499–512. doi: 10.1016/j.exer.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Linetsky M, LeGrand RD. Glutathionylation of lens proteins through the formation of thioether bond. Mol Cell Biochem. 2005;272:133–144. doi: 10.1007/s11010-005-6908-1. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE, Jr, Garadi R. The role of protein thiol mixed disulfides in cataratogenesis. Exp Eye Res. 1990;50:819–826. doi: 10.1016/0014-4835(90)90133-f. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992;55:889–896. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- Lou MF, Dickerson JE, Jr, Tung WH, Wolfe JK, Chylack LT., Jr Correlation of nuclear color and opalescence with protein S-thiolation in human lenses. Exp Eye Res. 1999;68:547–552. doi: 10.1006/exer.1998.0638. [DOI] [PubMed] [Google Scholar]
- Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Masters PM. In vivo decomposition of phosphoserine and serine in noncollagenous protein from human dentin. Calcif Tissue Int. 1985;37:236–241. doi: 10.1007/BF02554869. [DOI] [PubMed] [Google Scholar]
- Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:1278–1292. doi: 10.1098/rstb.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970;67:1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau H, Graf P, Sies H. Glutathione levels in human lens: regional distribution in different forms of cataract. Exp Eye Res. 1990;50:17–20. doi: 10.1016/0014-4835(90)90005-f. [DOI] [PubMed] [Google Scholar]
- Schey KL, Little M, Fowler JG, Crouch RK. Characterization of human lens major intrinsic protein structure. Invest Ophthalmol Vis Sci. 2000;41:175–182. [PubMed] [Google Scholar]
- Sen LC, Gonzalez-Flores E, Feeney RE, Whitaker JR. Reactions of phosphoproteins in alkaline solutions. J Agric Food Chem. 1977;25:632–638. doi: 10.1021/jf60211a057. [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Kirk MC, Srivastava K. Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem. 2004;279:10901–10909. doi: 10.1074/jbc.M308884200. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Lushchak VI, Cooper AJ. A comparison of reversible versus irreversible protein glutathionylation. Adv Cancer Res. 2014;122:177–198. doi: 10.1016/B978-0-12-420117-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lyons B, Truscott RJ, Schey KL. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 2014;13:226–234. doi: 10.1111/acel.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Zetterberg M, Zhang X, Taylor A, Liu B, Liang JJ, Shang F. Glutathiolation enhances the degradation of gammaC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 2006;47:3467–3473. doi: 10.1167/iovs.05-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.