Abstract
Objectives
While exercise significantly reduces craving for cigarettes, the effect of exercise on self-initiation of quit attempts is less known. Therefore, this randomized pilot study explored the effect of starting an exercise program on self-initiated quit attempts, as well as the feasibility and acceptability of a novel exercise intervention, High Intensity Interval Training (HIIT), as compared to a more traditional continuous aerobic (CA) exercise intervention.
Methods
Participants smoked (≥ 5 cigarettes/day), were aged 18-40 years and wanted to increase their exercise. Participants were randomized into one of three groups: HIIT, CA, and delayed-control. All participants attended follow-up visits at Weeks 4, 8 and 12. Outcomes included measures of feasibility (e.g., visit attendance) and acceptability (e.g., satisfaction), as well as changes in smoking behavior (e.g., quit attempts during follow-up) and proxies to quit attempts (e.g., positive affect).
Results
Overall, there were no differences in terms of feasibility and acceptability between the HITT (n=12) and CA (n=9) groups. Based on both self-report and objective measurement, the exercise groups (HIIT and CA) increased their physical activity as compared to the delayed treatment group (n=11). Compared to HIIT and delayed-control, CA (n=9) had significant favorable changes in positive affect (e.g., at Week 8, HIIT: +0.25±2.21, delayed-control: −5.11±2.23, CA: +5.50±2.23; p=0.0153).
Conclusions
These observations suggest that HIIT is as feasible and acceptable as CA, though CA may have a more favorable effect on proxies to quit attempts (e.g., positive affect). Fully-powered studies are needed to examine the effect of HIIT versus CA on quit attempts.
Keywords: Feasibility, Exercise, Smoking Cessation
INTRODUCTION
While exercise significantly reduces craving for cigarettes, as well as a favorably alter number of other barriers to smoking cessation (e.g., mood management, stress relief, limit post-cessation weight gain) (Bock et al., 1999; Marcus et al., 1999; Ussher et al., 2014), the effect of exercise as a smoking cessation intervention is inconclusive (Ussher et al., 2014). The limited existing evidence, though somewhat mixed, suggests that exercise interventions are most effective when they are initiated prior to a pre-set quit date (Patten et al., 2001; Ussher et al., 2014). However, the effect of exercise on the decision to self-initiate a quit attempt is relatively unexplored. This is a significant gap in the existing literature as an estimated 52% of quit attempts are self-initiated (Larabie, 2005). Two published studies are available on the effect of exercise on self-initiated quit attempts. The first, a prospective study (n=24) that implemented a workplace walking program, observed favorable effects on a number of proxies to smoking cessation (e.g., negative mood), and more than 20% of participants reported being smoking abstinence at one-year follow-up post-enrollment (Mantoani et al., 2014). The second, a randomized study (n=99), compared a walking intervention to a usual care control in a sample of disadvantaged individuals who smoke. Those in the exercise condition had a higher prevalence of biochemically-verified smoking abstinence at four weeks compared to those in the control group (23% versus 6%) (Thompson et al., 2016). While the generalizability of these findings are limited due to the small sample sizes, they suggest that initiating exercise may prompt self-initiated quit attempts.
Unfortunately, smoking cessation exercise interventions have been plagued by low adherence rates (Ussher et al., 2014), perhaps due to a lack of time and/or boredom. Therefore, High-Intensity Interval Training (HIIT) may provide a solution given the total amount of time spent per weeks is substantially less than more traditional forms of aerobic exercise. HIIT, which alternates short periods of intense activity with less intense recovery periods of light exercise, is less time intensive, more engaging and more enjoyable than more traditional continuous aerobic (CA) exercise interventions, and may have similar physiological benefits (Dunham, Harms, 2012; Kessler et al., 2012; Shiraev, Barclay, 2012). Further, like other forms of exercise, HIIT can easily be adapted for a variety of physical fitness levels (Shiraev, Barclay, 2012). Although protocols for examining HIIT in adults who smoke have been recently published (Pavey et al., 2015), the feasibility and acceptability of HIIT in this group remains unknown.
The primary goal of this pilot unblinded randomized trial was to assess the feasibility and acceptability of a HIIT exercise intervention versus a more traditional CA exercise intervention in a sample of adults who smoke. We also sought to make a preliminary comparison of those exercise interventions (HIIT/CA) to those randomized to a delayed-treatment control condition in terms of proxies for smoking cessation (e.g., craving, quit intentions) and changes in smoking behavior (e.g., quit attempts). In this manuscript, we describe these results and share some of our lessons learned while conducting this study (e.g., recruitment, working with local gyms, contracted personal trainers, and Fitbit® data).
METHODS
Study Participants
A convenience sample of young adults (i.e., ages of 18 and 40) were recruited in Minneapolis, Minnesota with social/mass media advertisements, as well as by flyers posted in local businesses. Eligible participants currently smoked (i.e., ≥ 5 cigarettes/day for ≥ last 6 months), and were minimally active (i.e., ≤ 2 planned exercise sessions per week), ambulatory, interested in increasing physical activity (i.e., ≥ 7 on a 10-point Likert-type scale), interested in quitting smoking within the next six months, in stable physical/mental health, willing and able to complete the study protocol, and able to provide informed consent. Exclusion criteria included recent (<6 months) pregnancy, planning to become pregnant within the next three months, or contraindications to increasing physical activity (e.g., high blood pressure, abnormal electrocardiogram or VO2 peak test results, recent [<6 months] heart attack or stroke).
Study Protocol
All procedures were approved by the University of Minnesota’s Human Subjects Research Protection Program. Eligibility was initially assessed with a telephone interview followed by an in-person clinic visit, at which informed consent was obtained. During the informed consent process, participants were told that the goal of the study was to examine changes in physical activity in individuals who smoke when an exercise program was initiated. We opted to not inform participants of the true purpose of the study to limit the effect of the study on smoking behavior rather than the exercise interventions. The in-person screening visit included a brief physical/mental examination by a nurse practitioner, followed by a 12-lead electrocardiogram (Quinton Q-Stress) and indirect calorimetry (Beck’s Physiological Systems) to measure maximal oxygen consumption (VO2 peak) on a Precor 842i cycle ergometer. Participants also completed validated measures to capture demographics, baseline measures of smoking characteristics (including expired carbon monoxide as an acute indicator of smoking status) and physical activity.
During a baseline visit, conducted within two weeks of the screening visit, participants were stratified based on their readiness to quit smoking (intending to quit < 30 days versus intending to quit ≥ 30 days). After stratification, participants were randomized (1:1:1 ratio) into one of three study groups: HIIT, CA, or delayed-treatment control. Participants were informed of their randomization assignment at their baseline visit. All participants, regardless of randomization, were given a Fitbit Flex® and instructed to wear it daily to monitor physical activity. Participants in the exercise conditions (HIIT/CA) were provided with a voucher for a free three-month membership at a local gym franchise, which offered six local locations. They were also paired with one of five contracted personal trainers. Participants in the exercise conditions met with personal trainers once per week for 12 weeks at the local gym. Personal trainers were instructed not to discuss smoking behavior with participants. The control condition participants were instructed to maintain their current activity level until the end of the study. Upon completion of the 12-week follow-up, control participants were provided with a three-month gym membership, and paired with one of the personal trainers, if desired.
All participants attended three follow-up clinic visits at Weeks 4, 8 and 12. At each follow-up visit, Fitbit Flex® data were downloaded, vital signs were measured, and validated questionnaires were completed via REDCap (Harris et al., 2009). Timeline FollowBack (Sobell et al., 1996) interviews were performed to retrospectively capture cigarettes smoked per day and minutes of exercise per day. In addition to the three-month gym membership (valued at $140) and Fitbit Flex® (valued at $100), all participants were compensated up to $165 in Visa gift cards.
Exercise Interventions
The HIIT intervention consisted of a single 20-minute session of exercise per week, modeled after the Wingate Protocol, one of the most commonly used HIIT programs (Shiraev, Barclay, 2012). This program was completed on a stationary bike at the local gym with the personal trainer. Participants started with a five-minute warm-up followed by a two-minute low intensity interval at approximately 40-50 revolutions per minute (RPM) and 35-45% of heart rate reserve (HRR). Next, they increased 80-90 RPM for 30 seconds attempting to reach their target heart rate (THR). Since participants were minimally active individuals who also smoke, we used a progressive program to determine their THR, targeting 75-80% of their HRR during the first two weeks, 80-90% HRR during the next three weeks, and then targeting >90% HRR for the remaining weeks. The personal trainer adjusted the wheel resistance or RPM in order to keep the participants within their THR. They repeated this pattern three times for a total of four bouts (each bout lasting 2.5 minutes; work-to-rest ratio of 1:4), then performed a three-minute cool down and some light stretching to complete the 20-minute session.
The CA exercise intervention included three 30-minute sessions per week, one of which was with the personal trainer at the local gym. This was modeled after the popular “Couch to 5k” (http://www.nhs.uk/LiveWell/c25k/Pages/couch-to-5k.aspx) program, which is designed to train an inactive individual to be able to run a 5k (3.1 mile) race in 12 weeks. This program starts with mostly walking (i.e., the first session includes five minutes of warm-up walking followed by 60 seconds of jogging then 90 seconds of walking repeated for 20 minutes, concluding with five minutes of walking to cool down) and gradually increases to mostly jogging (i.e., the last session includes five minutes of warm-up walking followed by 30 minutes of jogging).
Study Measures
To address our first aim (i.e., feasibility/acceptability), we explored group differences for a number of measures including attendance to the exercise sessions, data collection clinic visits and adverse events. Next, as an indicator of the adoption of the exercise program, we evaluated the change in physical activity from baseline to follow-up via the International Physical Activity Questionnaire (IPAQ) (Craig et al., 2003), self-reported minutes of exercise per day via the TimeLine FollowBack (TLFB) (Sobell et al., 1996), and, for a subset of participants, minutes of activity and number of steps via the Fitbit Flex®. On the IPAQ, participants indicate the number of times they engaged in mild, moderate, and strenuous activity for more than 15 minutes over the past week. A composite exercise score is calculated using the weighted sum of each exercise intensity: (mild × 3) + (moderate × 5) + (strenuous × 9). The composite score is a weekly MET value (units of metabolic equivalence). Finally, to determine acceptability, we compared satisfaction with the interventions via a brief four-question survey developed for this protocol. Each item was assessed using a 10-point Likert-type scale (with higher numbers indicating greater satisfaction).
For our second aim (i.e., effect on proxies to cessation), we used several validated questionnaires. Participants completed the following validated scales: Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes, Hatsukami, 1986), Questionnaire of Smoking Urges – Brief (QSU) (Cox et al., 2001), Centers for Epidemiologic Study – Depression (CESD) (Radloff, 1977), Positive and Negative Affect Scale (PANAS) (Watson et al., 1988), and Perceived Stress Scale (PSS) (Cohen et al., 1983), Decisional Balance Scale (DBS) (Velicer et al., 1985), Risk Perception Scale (RPS) (Williams et al., 2011), Transtheoretical Stages of Change (SOC) (Prochaska et al., 2001; Prochaska et al., 1993), and the Contemplation Ladder (Biener, Abrams, 1991).
Our last aim (i.e., effect on smoking behavior) was assessed by the following information collected via TLFB(Sobell et al., 1996): (1) reduction in the number of cigarettes smoked per day from baseline to follow-up, (2) the prevalence of a self-initiated quit attempt, defined as the self-report of >24 hours of abstinence (Starr et al., 2005) during the follow-up period, and (3) a percentage of days abstinent during the follow-up period.
Statistical Analysis
Descriptive statistics were used to summarize the demographics and baseline characteristics of the study sample by randomization group. Given the small sample size of this pilot study, nonparametric statistical tests were used for analyses. Baseline differences by randomization status were assessed with Kruskal Wallis tests and Fisher’s Exact tests. Within-person change of continuous variables (physical activity measures and proxy measurements for smoking cessation) from baseline to follow-up were tested using Wilcoxon signed rank sum tests. Between-group comparisons in change of continuous variables between HIIT, CA, and Control groups were tested first overall using Kruskal Wallis tests, and where significant, post-hoc pairwise comparisons were completed with Wilcoxon-Mann-Whitney tests (t-approximation). Differences in satisfaction with the exercise programs between the HIIT and CA groups at follow-up were assessed using Wilcoxon-Mann-Whitney tests (t-approximation). All changes from baseline in physical activity and proxy measures for smoking cessation were examined at each follow-up time point separately (e.g. Baseline to Week 4, Baseline to Week 8, and Baseline to Week 12). SAS Software v. 9.4 was used for analysis (SAS Institute Inc., Cary, NC), and p-values <0.05 were considered statistically significant.
RESULTS
Study Participants
A total of 473 individuals contacted the study expressing an interest in participating. Of these, 176 (37%) were ineligible for participation and 265 (56%) were deemed “not interested” due to not completing the eligibility survey or stated conflicts with the protocol (Figure 1). Therefore, our final sample size was 32 participants (Table 1). Fewer females were randomized to the HIIT group (33%) versus the CA group (78%) or control group (82%; p=0.0368). There were no other statistically significant group differences at baseline. Prior to randomization, nearly all participants indicated they wanted to be randomized to an exercise condition, with a similar percentage for HIIT (44%) and CA (50%).
Figure 1.
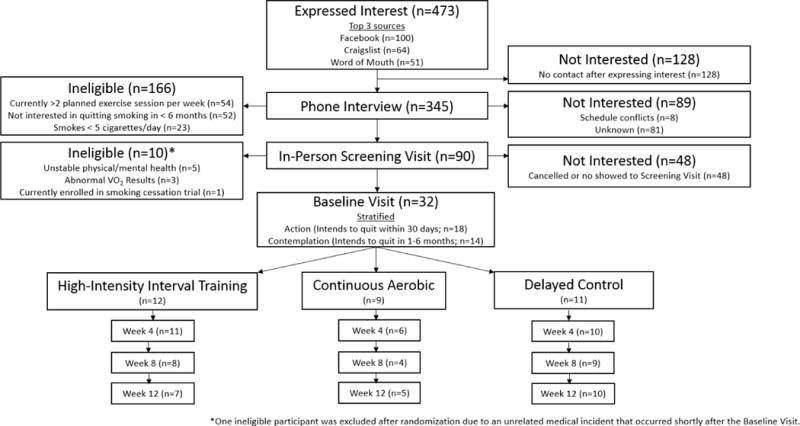
CONSORT Diagram
Table 1.
Baseline Participant Characteristics by Randomization Group (n=32)1
| All (n=32) |
HIIT (n=12) |
CA (n=9) |
Control (n=11) |
p-value2 | |
|---|---|---|---|---|---|
| Age | 30.3±1.0 | 30.4±1.5 | 29.2±1.8 | 31.0±2.2 | 0.6859 |
| Gender (n, % female) | 20 (63%) | 4 (33%) | 7 (78%) | 9 (82%) | 0.0368 |
| Cigarettes/Day | 13.1±0.9 | 14.1±1.4 | 10.8±1.5 | 14.0±1.5 | 0.2155 |
| Body Mass Index (BMI) | 27.1±1.1 | 25.1±1.6 | 27.3±2.2 | 29.1±2.0 | 0.3568 |
| Race (n, %) | |||||
| African American/Black | 3 (9%) | 1 (8%) | 1 (11%) | 1 (9%) | 0.9351 |
| American Indian, Alaskan Native | 3 (9%) | 2 (17%) | 1 (11%) | 0 (0%) | |
| Other/Mixed Race | 3 (9%) | 1 (8%) | 1 (11%) | 1 (9%) | |
| White | 23 (72%) | 8 (67%) | 6 (67%) | 9 (82%) | |
| Education Level (n, %) | |||||
| High school graduate | 9 (28%) | 5 (42%) | 0 (0%) | 4 (36%) | 0.1975 |
| Some college/2yr degree | 19 (59%) | 6 (50%) | 7 (78%) | 6 (55%) | |
| College graduate/4yr degree | 4 (13%) | 1 (8%) | 2 (22%) | 1 (9%) | |
| Readiness to Quit within 30 days (n, % at screening) | 18 (56%) | 7 (58%) | 5 (56%) | 6 (55%) | 1.0000 |
| Baseline exercise level (IPAQ), median (IQR) METs, missing n=6 | 1,073 (2,574) | 2,013 (6,270) | 528 (1,013) | 1,510 (4,232) | 0.4405 |
| Baseline exercise level (IPAQ), n (%) | |||||
| 1st Quartile (99-264) | 6 (23%) | 3 (30%) | 2 (25%) | 1 (13%) | |
| 2nd Quartile (396-759) | 7 (27%) | 1 (10%) | 4 (50%) | 2 (25%) | |
| 3rd Quartile (1,386-2,772) | 6 (23%) | 2 (20%) | 1 (13%) | 3 (28%) | |
| 4th Quartile (2,970-15,048) | 7 (27%) | 4 (40%) | 1 (13%) | 2 (25%) |
Unless otherwise stated values are Mean±SE
p-values from Kruskal Wallis tests or Fisher’s Exact tests
Feasibility and Acceptability
Approximately half of the personal trainer sessions were attended in both the HIIT (mean ± standard error: 52±9%) and CA (49±13%) groups (Table 2). Though not statistically different, the clinic visit attendance was lower in the CA group (56±15%) compared to HIIT (79±10%) or controls (86±10%). A total of 20 adverse events were reported during the follow-up period. Most (19 incidents) were unrelated to the study (e.g., cold/flu), with one deemed possibly related (low back pain in a CA participant). Compared to the control group, which reported a 78% decline in physical activity per the score on the IPAQ, the HIIT group reported a significant (125%) increase in physical activity (p=0.0127) (Table 2). Overall, trends indicate that the HIIT and CA groups reported an increase in physical activity whereas the control group reported either no change or a decrease in physical activity. Participants in the HIIT and CA groups reported relatively high satisfaction with the interventions, with no statistically significant difference in responses by exercise groups (Table 3).
Table 2.
Indicators of Exercise Intervention Feasibility and Adherence1
| HIIT (n=12) |
CA (n=9) |
Control (n=11) |
p-value2 | |
|---|---|---|---|---|
| Attendance | ||||
| Personal Trainer Sessions | 52±9% | 49±13% | ---- | 0.8021 |
| Clinic Visit | 79±10% | 56±15% | 86±10% | 0.1897 |
| Self-Report Physical Activity | ||||
| IPAQ – Baseline (METs) | 3,605 ± 1,379 | 955 ± 373 | 3,543 ± 1,799 | 0.4405 |
| IPAQ – Change to W4 (METs) | +1,569 ± 2,509 | +3,006 ± 1,059 | +321 ± 3,355 | 0.1641 |
| IPAQ – Change to W8 (METs) | +936 ± 2,024 | +1,716 ± 504 | +402 ± 572 | 0.3563 |
| IPAQ – Change to W12 (METs) | +4,533 ± 2,167†a | +903 ± 731ab | −2,767 ± 2,073†b | 0.0187 |
| TLFB – Baseline | 14.9 ± 7.3 | 30.0 ± 23.6 | 11.1 ± 8.0 | 0.6517 |
| TLFB – Change to W4 | +10.9 ± 6.7 | −5.1 ± 13.8 | +1.3 ± 4.8 | 0.1392 |
| TLFB – Change to W8 | +22.2 ± 12.2 | −8.9 ± 31.6 | −2.4 ± 2.2 | 0.1152 |
| TLFB – Change to W12 | +8.8 ± 9.1 | +22.6 ± 15.5 | +0.0 ± 3.9 | 0.3446 |
| Fitbit Steps3 | ||||
| Baseline | 7,524 ± 1,110 | 8,235 ± 802 | 9,176 ± 1,452 | 0.6805 |
| Change to W4 | +1,379 ± 1,268 | −1,602 (n=1) | −2,769 ± 1,484 | 0.1328 |
| Change to W8 | −538 ± 3,546 | +2,614 ± 645 | −2,342 ± 1,400 | 0.2883 |
| Change to W12 | +986 ± 4,548 | +1,213 ± 1,294 | −4,222 ± 1,793 | 0.2034 |
Values are Mean ± SE or Mean ∆ ± SE ∆
p-value for comparison between HIIT, CA, and Control per Kruskal Wallis test or Fisher’s exact test.
Fitbit data from a subset of participants including HIIT (n=7), CA (n=3), and Control (n=6)
p<0.05 significant within-person change from baseline per Wilcoxon signed rank sum tests.
Pairwise comparisons are within rows per Wilcoxon-Mann-Whitney tests. Values with different superscript letters are significantly different (p<0.05); HIIT vs. Control: p=0.01, CA vs. Control: p=0.06, HIIT vs. CA: p=0.19.
IPAQ: International Physical Activity Questionnaire(Craig et al., 2003)
TLFB: TimeLine FollowBack as reported in minutes of activity per day(Sobell et al., 1996)
Table 3.
Satisfaction Survey by Randomization Group and Time Point 1
| HIIT (n=11) |
CA (n=6) |
p-value2 | |
|---|---|---|---|
| How satisfied are you with this exercise program? | |||
| Week 4 | 7.73±0.62 | 7.00±0.45 | 0.2895 |
| Week 8 | 7.88±0.61 | 7.25±0.85 | 0.4918 |
| Week 12 | 7.75±0.88 | 8.40±0.81 | 0.7147 |
| How much did you enjoy the exercise program? | |||
| Week 4 | 7.64±0.73 | 7.17±0.60 | 0.5463 |
| Week 8 | 7.25±0.82 | 6.50±1.04 | 0.5465 |
| Week 12 | 7.50±0.63 | 8.00±0.81 | 0.7155 |
| Would you recommend this exercise program to a friend? | |||
| Week 4 | 8.00±0.82 | 7.67±0.71 | 0.5039 |
| Week 8 | 8.00±0.82 | 7.25±1.38 | 0.7337 |
| Week 12 | 6.75±0.88 | 8.60±0.75 | 0.2069 |
| Do you think you will continue this exercise program on your own? | |||
| Week 4 | 7.36±0.75 | 7.83±0.48 | 1.0000 |
| Week 8 | 8.00±0.80 | 7.00±1.47 | 0.5444 |
| Week 12 | 6.00±1.02 | 8.60±0.75 | 0.1289 |
Values are Mean ± SE on a 10-point Likert-type scale with 10 as the highest
p-value for comparison between HIIT and CA per Wilcoxon-Mann-Whitney tests test (t approximation)
Proxies to Attempts at Smoking Cessation
Three significant between-group differences were noted (Table 4), indicating favorable changes in positive affect among the CA group at Weeks 8 and 12 compared to HIIT and Control, as well as favorable changes in risk perception among the control group at Week 12 compared to HIIT and CA. Finally, though not statistically significant, the CA group had the highest prevalence of action stage participants at each time point and also had the most consistent favorable changes on the following subscales: craving, withdrawal, intention to smoke, positive affect, and contemplation ladder.
Table 4.
Proxies to Smoking Cessation by Randomization Group and Time Point 1
| HIIT (n=12) | CA (n=9) | Control (n=11) | p-value2 | |
|---|---|---|---|---|
| Cessation-Related Symptoms | ||||
| Craving (MNWS) | ||||
| Baseline | 1.58 ± 0.36 | 1.78 ± 0.32 | 2.27 ± 0.33 | ---- |
| Change to W4 | +0.09 ± 0.16 | −0.17 ± 0.79 | −0.20 ± 0.25 | 0.6984 |
| Change to W8 | +0.00 ± 0.19 | −1.00 ± 0.71 | −0.56 ± 0.29 | 0.2263 |
| Change to W12 | +0.25 ± 0.31 | −0.60 ± 0.51 | −0.50 ± 0.27 | 0.1894 |
| Withdrawal (MNWS) | ||||
| Baseline | 0.62 ± 0.15 | 1.25 ± 0.38 | 1.01 ± 0.22 | ---- |
| Change to W4 | −0.01 ± 0.10 | −0.50 ± 0.38 | +0.39 ± 0.26 | 0.1364 |
| Change to W8 | +0.23 ± 0.13 | −0.68 ± 0.39 | +0.33 ± 0.22 | 0.0560 |
| Change to W12 | +0.07 ± 0.11 | −0.09 ± 0.24 | +0.14 ± 0.18 | 0.7826 |
| Intention to Smoke (QSU) | ||||
| Baseline | 2.45 ± 0.56 | 2.78 ± 0.43 | 3.04 ± 0.48 | ---- |
| Change to W4 | +0.09 ± 0.55 | −0.53 ± 0.71 | −0.38 ± 0.47 | 0.8011 |
| Change to W8 | −0.40 ± 0.30 | −0.75 ± 0.67 | −0.78 ± 0.45 | 0.4667 |
| Change to W12 | −0.98 ± 0.60 | −1.40 ± 0.48 | −0.06 ± 0.57 | 0.3136 |
| Anticipated Relief from Negative Affect (QSU) | ||||
| Baseline | 0.48 ± 0.27 | 0.78 ± 0.26 | 0.60 ± 0.27 | ---- |
| Change to W4 | +0.25 ± 0.21 | +0.20 ± 0.56 | +0.48 ± 0.27 | 0.8507 |
| Change to W8 | −0.10 ± 0.15 | +0.05 ± 0.50 | +0.00 ± 0.12 | 0.8986 |
| Change to W12 | −0.13 ± 1.22 | −0.16 ± 0.07 | +0.14 ± 0.18 | 0.3764 |
| Depressive Symptoms (CESD) | ||||
| Baseline | 5.42 ± 1.23 | 6.44 ± 1.19 | 5.45 ± 0.92 | ---- |
| Change to W4 | +0.64 ± 1.05 | −0.17 ± 2.04 | +3.30 ± 1.34† | 0.3373 |
| Change to W8 | +1.13 ± 0.67 | +0.25 ± 1.93 | +3.11 ± 1.49 | 0.5377 |
| Change to W12 | +0.50 ± 1.09 | +4.00 ± 3.05 | +1.50 ± 0.93 | 0.6307 |
| Negative Affect (PANAS) | ||||
| Baseline | 12.67 ± 1.02 | 16.11 ± 1.93 | 16.64 ± 1.87 | ---- |
| Change to W4 | +0.36 ± 1.27 | −1.67 ± 3.47 | −0.40 ± 1.37 | 0.8144 |
| Change to W8 | +1.00 ± 1.12 | −2.50 ± 4.79 | −1.22 ± 1.61 | 0.6260 |
| Change to W12 | +0.63 ± 1.08 | +1.60 ± 1.78 | −0.10 ± 1.68 | 0.7160 |
| Positive Affect (PANAS) | ||||
| Baseline | 26.33 ± 2.66 | 25.89 ± 2.33 | 30.55 ± 1.60 | ---- |
| Change to W4 | +1.55 ± 2.39 | +3.50 ± 1.67 | −2.10 ± 1.92 | 0.1979 |
| Change to W8 | +0.25 ± 2.21ab | +5.50 ± 1.94a | −5.11 ± 2.23b† | 0.0153 |
| Change to W12 | +2.88 ± 1.39a | +4.40 ± 2.06a | −2.70 ± 1.56b | 0.0197 |
| Perceived Stress (PSS) | ||||
| Baseline | 11.42 ± 1.68 | 13.67 ± 1.76 | 12.45 ± 2.16 | ---- |
| Change to W4 | +0.45 ± 0.88 | +0.17 ± 2.20 | +1.70 ± 2.30 | 0.7311 |
| Change to W8 | +1.38 ± 1.87 | −2.50 ± 2.06 | +1.89 ± 1.98 | 0.4249 |
| Change to W12 | +2.75 ± 1.16 | +1.60 ± 2.94 | +2.90 ± 2.10 | 0.5889 |
| Risk Perceptions | ||||
| Cons of Smoking (DBS) | ||||
| Baseline | 27.00 ± 5.59 | 35.75 ± 1.70 | 34.71 ± 2.58 | ---- |
| Change to W12 | −2.86 ± 2.02 | −5.00 ± 2.86 | −1.57 ± 2.21 | 0.5152 |
| Pros of Smoking (DBS) | ||||
| Baseline | 20.57 ± 3.05 | 24.5 ± 4.43 | 25.71 ± 2.52 | ---- |
| Change to W12 | −1.43 ± 1.39 | −1.00 ± 1.08 | −1.43 ± 1.17 | 0.9906 |
| Absolute Risk (RPS) | ||||
| Baseline | 3.29 ± 0.13 | 3.75 ± 0.35 | 4.00 ± 0.21 | ---- |
| Change to W12 | −0.36 ± 0.33a | 0.00 ± 0.10ab | +0.38 ± 0.16b | 0.0261 |
| Relative Risk (RPS) | ||||
| Baseline | 2.40 ± 0.26 | 3.29 ± 0.10 | 3.24 ± 0.18 | ---- |
| Change to W12 | +0.24 ± 0.26 | −0.21 ± 0.21 | +0.26 ± 0.21 | 0.3279 |
| Motivation to Quit | ||||
| Action Stage (TSOC)3 | ||||
| Baseline | 10 (83%) | 5 (56%) | 4 (36%) | 0.0716 |
| W4 | 5 (45%) | 5 (83%) | 3 (30%) | ---- |
| W8 | 5 (63%) | 4 (100%) | 4 (44%) | ---- |
| W12 | 6 (75%) | 4 (80%) | 4 (40%) | ---- |
| Contemplation Ladder | ||||
| Baseline | 7.42± 0.65 | 6.56± 0.56 | 7.09 ± 0.64 | ---- |
| Change to W4 | −0.09 ± 0.62 | +1.67 ± 0.92 | −0.10 ± 0.74 | 0.2135 |
| Change to W8 | −0.13 ± 0.35 | +2.25 ± 1.31 | +0.56 ± 0.75 | 0.1375 |
| Change to W12 | +1.38 ± 0.84 | +2.20 ± 1.07 | +1.10 ± 0.75 | 0.7914 |
Values are Mean ± SE, Mean ∆ ± SE ∆, or n (%)
p-value for comparison between HIIT, CA, and Control per Kruskal Wallis test
Defined as tried to quit in past 30 days or willing to quit within next 30 days
p<0.05 significant within-person change from baseline: Wilcoxon signed rank sum tests
Pairwise comparisons are within rows per Wilcoxon-Mann-Whitney tests; Values with different superscript letters are significantly different (p<0.05).
MNWS: Minnesota Nicotine Withdrawal Scale (Hugheset al., 1986)
QSU: Questionnaire of Smoking Urges – Brief (Cox et al., 2001)
CESD: Center for Epidemiological Study – Depression (Radloff, 1977)
PANAS: Positive and Negative Affect Scale (Watson et al., 1988)
PSS: Perceived Stress Scale (Cohen et al., 1983)
DBS: Decisional Balance Scale (Velicer et al., 1985)
RPS: Risk Perception Scale (Williams et al., 2011)
TSOC: Transtheoretical Stages of Change (Prochaska et al., 2001, 1993)
Self-Initiated Attempts at Smoking Cessation
All participants significantly reduced the number of cigarettes smoked per day by 2.4±0.7 at Week 12; this did not vary by randomization group (data not shown). Though not statistically different, more participants in the HIIT and CA groups made a quit attempt during follow-up (42% and 44%, respectively) compared to the control group (27%; p=0.7265). Also, the percentage of days abstinent during follow-up was higher in the CA group (14.8±7.7%) versus HIIT (2.3±1.1%) and control (7.7±6.7%; p=0.6450).
DISCUSSION
The results from this study suggest that both this study design, as well as the HIIT exercise intervention are both indeed acceptable and feasible in young adults who smoke. Specifically, compared to the CA group, the HIIT group had similar or better attendance rates and changes in physical activity. This is important given the significantly shorter total time per week dedicated to planned exercise (20 minutes/week versus 90 minutes/week). Further, there were no related adverse events among the HIIT participants. In terms of the acceptability of the intervention, at enrollment there were similar levels of interest in the two programs, indicating good acceptability for both interventions. While both exercise interventions received relatively high satisfaction scores from study participants, the HIIT participants ranked the intervention highest at Week 8 whereas the CA participants rated the intervention highest at Week 12. This may be related to the fact that the CA program incorporates goal setting. That is, CA participants were learning to run a 5k over the course of 12 weeks. Therefore, satisfaction might have increased later in the program when participants were closer to achieving that goal. In contrast, the HIIT program did not contain goal setting, and participants completed the same exercise protocol weekly, though with increasing workloads. Thus, while the HIIT intervention was successful in increasing initial motivation, perhaps enthusiasm may have waned in subsequent weeks. While these ideas need to be tested in future work, these data suggest that goal setting might help sustain satisfaction with longer exercise protocols. A recent meta-analysis examining 52 physical activity interventions observed a medium positive effect of goal setting on subsequent physical activity behavior (Cohen’s d = 0.55, 95% confidence interval = 0.43-0.67) (McEwan et al., 2016). Further, in a small study of 11 adults who smoke, exercise that also include goal setting, self-talk and relaxation breathing reduced craving for cigarettes more than exercise alone (Hatzigeorgiadis et al., 2016). Therefore, incorporating goal setting in exercise smoking cessation interventions may improve the adherence rates, as well as the effect of exercise on reducing smoking-related symptoms.
The exercise condition participants appeared to have more favorable changes in the smoking-related outcomes than the control group. For example, while not statistically significant, nearly half of the participants in the exercise groups (42-44%) made a quit attempt compared to 27% in control. Further, the CA group had more favorable changes in a number of proxies to smoking cessation (e.g., craving, motivation to quit, stage of change), and also had the highest prevalence of participants in the action stage of change at each follow-up visit. While many of these differences were not statistically significant, the results concur with prior observations that exercise may indeed prompt self-initiated quit attempts (Mantoani et al., 2014; Thompson et al., 2016). Unfortunately, relatively little is known about how to prompt self-initiated quit attempts. Future, fully-powered studies are needed to examine whether an exercise program prompts self-initiated quit attempts, and which types of exercise programs are most effective.
Lessons Learned
This pilot study provides insight into several useful methodological considerations for future studies.
(1) Recruitment and enrollment
Although we experienced a high volume of phone calls and emails from interested individuals, relatively few enrolled into the study (9%). One major barrier to enrolling was the high participant burden during the four-hour screening visit. For safety purposes, participants had to complete a fitness test (VO2 peak), as well as a physical examination and electrocardiogram prior to enrollment. This resulted in a lengthy visit that had to match the schedules of five different individuals (study participant, study coordinator, nurse practitioner, study physician, and technicians for the VO2 peak). As a result, we were only able to schedule about two screening visits per week, more than half of which were “no shows.” To address this problem, we would recommend the use of an orientation visit. This half-hour visit is an opportunity for potential study participants to learn more about the study prior to committing to the four-hour screening visit. We added an orientation visit (offered weekly) in the final 1.5 months of our recruitment; this resulted in a striking change in the screening visit attendance rate from 38% (27/71) to 71% (17/24).
(2) Exercise intervention delivery
Working with a local gym proved challenging. We observed a high rate of management turnover during the study. We expected to have access to gym attendance records based on discussions with the initial manager. However, subsequent managers told us this was not possible. To avoid this loss of data, we would recommend future studies use study-owned technology to directly capture gym attendance. For example, the PiLR system (www.pilrhealth.com) allows for the collection of sensor data and location-triggered data collection. To use this type of approach, participants would add an application to their smartphones, and researchers would either place a sensor or use GPS data to note a location of interest (e.g., local gym). Then, the smartphone application would be triggered when the phone is near the location of interest. This approach was recently successfully implemented in a study examining changes in physical activity and the redesign of city parks (Huang et al., 2016).
We initially started the study with two contracted personal trainers, but quickly discovered this was insufficient. We utilized experienced, certified personal trainers who independently contracted their services to the study. Although this was advantageous to the study in terms of budget (i.e., we only paid for services rendered), participants experienced some barriers when scheduling with personal trainers due to lack of availability as they were not full-time employees of the study. When we expanded our pool to five personal trainers, this barrier was reduced. Therefore, we would encourage future projects to employ a large pool of independent contractors with varying availability.
(3) Activity tracking
We also experienced multiple technical difficulties with the Fitbit® data. We originally intended to have Fitbit® data for all participants for the entire 12-week follow-up period. However, we only ended up with data for approximately half of the study sample. The missing data were the result of a variety of problems including loss of Fitbits® by study participants (n=4), and missing data for unknown reasons (e.g., no data stored on the device though participant reported use of the Fitbit®). Further, we downloaded data on a monthly basis (at each clinic visit), which may have resulted in additional loss of data. The Fitbit® website states that the Fitbit Flex® has memory to track daily totals for the past 30 days (https://www.fitbit.com/en-ca/flex). We also confirmed this directly with Fitbit® (personal communication, 12/14/2015). However, we found that the number of days of data the devices held was much lower and very inconsistent (approximately 14-21 days). Future studies could avoid missing data by use of real time uploading of data via EMA mobile technology or with a system like Fitabase (https://www.fitabase.com/), which is designed for research data collection purposes.
Strengths and Limitations
While this novel pilot study was strengthened by the use of a randomized study design and collection of data via validated measures, there are limitations. First, we had a small, highly educated, homogenous sample. This limits the generalizability of our results, and the power to detect statistically significant differences. Second, the randomization groups were not exchangeable (e.g., fewer females in HIIT) and, therefore, our observations may be confounded. To address these limitations, future studies should be conducted with a larger and more diverse study sample. Third, we did not conduct a psychometric analysis of the satisfaction survey and, thus, the validity and reliability were not determined.
CONCLUSIONS
This research advances the current literature by demonstrating that HIIT is a feasible and acceptable exercise intervention in young adults who smoke. This work also suggests that exercise may favorably effect proxies to quit attempts, and that the CA program may have more promise for doing so. This formative work lays a foundation to support future work to examine how exercise may encourage self-initiated quit attempt.
Acknowledgments
We extend our thanks to Kimberly Nagel, Himal Purani, Emily Lekah, Angela Tipp, and Jane Schulz for their participation in recruitment and data collection, as well as Dr. Otto Sanchez, Mary Whipple, Erika Tipp and Dereck Salisbury for their assistance with implementation of the exercise test. Additional thanks is given to Denise Bradley, Brenda Steger, Mary Hass, Ulice Payne III, and Sarah Mork for delivering the exercise interventions. Finally, thank you to Evelyn Rens for her editorial assistance.
Sources of Support: Funding for this project was provided by ClearWay Minnesota (RC-2015-0004), the University of Minnesota’s Building Interdisciplinary Research Careers in Women’s Health Grant (BIRCWH K12HD055887; Allen) from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), the Office of Research on Women’s Health, and the National Institute on Aging, National Institutes of Health, administered by the University of Minnesota Deborah E. Powell Center for Women’s Health. Support was also provided by Research Services in the Department of Family Medicine and Community Health, Medical School, University of Minnesota (S. Carlson).
Contributor Information
Alicia Allen, Family & Community Medicine Department, College of Medicine, University of Arizona.
Samantha C. Carlson, Department of Family Medicine & Community Health, Medical School, University of Minnesota.
Tyler A. Bosch, College of Education and Human Development, University of Minnesota.
Lynn E. Eberly, Division of Biostatistics, School of Public Health, University of Minnesota.
Kola Okuyemi, Department of Family & Preventive Medicine, Medical School, University of Utah.
Uma Nair, Health Promotion Sciences Department, Mel & Enid Zuckerman College of Public Health, University of Arizona.
Judith S. Gordon, Community and Systems Health Science Division, College of Nursing, University of Arizona.
References
- Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 1991;10(5):360–5. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 24(3):399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Key Outcome Indicators for Evaluating Comprehensive Tobacco Control Programs. DEPARTMENT OF HEALTH AND HUMAN SERVICES; 2005. Retrieved from https://www.cdc.gov/tobacco/tobacco_control_programs/surveillance_evaluation/key_outcome/pdfs/frontmaterial.pdf. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Craig CL, MARSHALL AL, Sjöström M, BAUMAN AE, BOOTH ML, AINSWORTH BE, OJA P. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Medicine & Science in Sports & Exercise. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Dunham C, Harms CA. Effects of high-intensity interval training on pulmonary function. European Journal of Applied Physiology. 2012;112(8):3061–8. doi: 10.1007/s00421-011-2285-5. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzigeorgiadis A, Pappa V, Tsiami A, Tzatzaki T, Georgakouli K, Zourbanos N, Theodorakis Y. Self-regulation strategies may enhance the acute effect of exercise on smoking delay. Addictive Behaviors. 2016;57:35–37. doi: 10.1016/j.addbeh.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Huang TTK, Wyka KE, Ferris EB, Gardner J, Evenson KR, Tripathi D, Thorpe LE. The Physical Activity and Redesigned Community Spaces (PARCS) Study: Protocol of a natural experiment to investigate the impact of citywide park redesign and renovation. BMC Public Health. 2016;16(1):1160. doi: 10.1186/s12889-016-3822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Medicine (Auckland, NZ) 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Larabie LC. To what extent do smokers plan quit attempts? Tobacco Control. 2005;14(6):425–428. doi: 10.1136/tc.2005.013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantoani LC, Furlanetto KC, Kovelis D, Proença M, Zabatiero J, Bisca G, Pitta F. Long-term Effects of a Program to Increase Physical Activity in Smokers. Chest. 2014;146(6):1627–32. doi: 10.1378/chest.14-0459. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, Abrams DB. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Internal Medicine. 1999;159(11):1229–34. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- McEwan D, Harden SM, Zumbo BD, Sylvester BD, Kaulius M, Ruissen GR, Beauchamp MR. The effectiveness of multi-component goal setting interventions for changing physical activity behaviour: a systematic review and meta-analysis. Health Psychology Review. 2016;10(1):67–88. doi: 10.1080/17437199.2015.1104258. [DOI] [PubMed] [Google Scholar]
- Pavey TG, Gartner CE, Coombes JS, Brown WJ. Assessing the effectiveness of High Intensity Interval Training (HIIT) for smoking cessation in women: HIIT to quit study protocol. 2015 doi: 10.1186/s12889-015-2631-3. [DOI] [PMC free article] [PubMed]
- Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 1993;12(5):399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, Fava JL, Ruggiero L, Laforge RG, Rossi JS, Lee PA. Counselor and stimulus control enhancements of a stage-matched expert system intervention for smokers in a managed care setting. Preventive Medicine. 2001;32(1):23–32. doi: 10.1006/pmed.2000.0767. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Shiraev T, Barclay G. Evidence based exercise - clinical benefits of high intensity interval training. Australian Family Physician. 2012;41(12):960–2. [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Thompson TP, Greaves CJ, Ayres R, Aveyard P, Warren FC, Byng R, Taylor A. An Exploratory Analysis of the Smoking and Physical Activity Outcomes From a Pilot Randomized Controlled Trial of an Exercise Assisted Reduction to Stop Smoking Intervention in Disadvantaged Groups. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco. 2016;18(3):289–97. doi: 10.1093/ntr/ntv099. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, Faulkner GEJ. Exercise interventions for smoking cessation. The Cochrane Database of Systematic Reviews. 2014;8:CD002295. doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. Journal of Personality and Social Psychology. 1985;48(5):1279–1289. doi: 10.1037/0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97(3):346–353. doi: 10.1037/0021-843X.97.3.346. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Herzog TA, Simmons VN. Risk perception and motivation to quit smoking: a partial test of the Health Action Process Approach. Addictive Behaviors. 2011;36(7):789–91. doi: 10.1016/j.addbeh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


