Abstract
The C57BL/6J (B6) is the most common inbred mouse strain used in biomedical research in the United States. Yet, this strain is notoriously known for being deficient in the biosynthesis of melatonin, an important effector of circadian clocks in the brain and in the retina. Melatonin deficiency in this strain results from non-functional alleles of the genes coding two key enzymes of the melatonin synthesis pathway: arylalkylamine-N-acetyltransferase (Aanat) and N-acetylserotonin-O-methyltransferase (Asmt). By introducing functional alleles of the Aanat and Asmt genes from the melatonin-proficient CBA/CaJ (CBA) mouse strain to B6, we have generated a B6 congenic line that has acquired the capacity of rhythmic melatonin synthesis. In addition, the melatonin-dependent rhythm of dopamine release in the retina is restored in the B6 congenic line. Finally, we have partially characterized the Aanat and Asmt genes of the CBA strain and have identified multiple differences between CBA and B6 alleles, including single nucleotide polymorphism and deletion/insertion of DNA segments of various sizes. As an improved model organism with functional components of the melatonin synthesis pathway and melatonin-dependent circadian regulations, the new line will be useful to researchers studying melatonin physiological functions in a variety of fields including, but not limited to, circadian biology and neuroscience. In particular, the congenic line will be useful to speed up introduction of melatonin production capacity into genetically-modified mouse lines of interest such as knockout lines, many of which are on B6 or mixed B6 backgrounds. The melatonin-proficient B6 congenic line will be widely distributed.
Keywords: Melatonin, AANAT, ASMT, C57BL/6J, CBA/CaJ, C3Heb/FeJ, congenic, retina, circadian rhythms
Introduction
Melatonin is a universal time-keeping neurohormone whose synthesis and release are under the control of circadian clocks [1, 2]. With few exceptions in the animal kingdom, plasma melatonin levels are low during the daytime and high at night. Thereby, melatonin relays important information to the body about both the time of day—through the position of the nocturnal peak– and the time of the year—through the duration of the nocturnal peak [1, 2]. In mammals, melatonin is primarily produced in the pineal gland and, at a much lower level, in the neural retina [1–4]. Melatonin is synthesized from serotonin via the successive actions of the enzymes arylalkylamine-N-acetyltransferase (AANAT; E.C. 2.1.3.87) and N-acetylserotonin-O-methyltransferase (ASMT; E.C. 2.1.1.4; also known as hydroxyindole-O-methyltransferase or HIOMT) [1–4].
Most domestic mouse strains used in biomedical research are deficient in melatonin production, displaying constitutive low levels of plasma melatonin and/or a nocturnal peak of small amplitude or absent [5–12]. Melatonin-deficient mouse strains include the most common C57BL/6J (B6) strain [5–12]. It has been proposed that melatonin-deficiency has a favorable impact on domestic mice in breeding colonies by eliminating the antigonadal action of melatonin [5, 12]. Although both melatonin-proficient and melatonin-deficient strains have normal circadian rhythms of locomotor activity and quite similar circadian clock function [12], subtle differences between the two groups have been reported, so that the full repertoire of circadian rhythms is incomplete or their amplitude altered, in melatonin-deficient strains [see for instance 13-16].
In the retina, melatonin is a critical effector of a circadian clock located in the photoreceptors [3–4]. The presence of a circadian rhythm of dopamine (DA) release has been observed only in melatonin-proficient mice and not in melatonin-deficient mice, suggesting that rhythmic DA release in the retina depends directly on rhythmic melatonin release [4, 17]. Because melatonin inhibits DA release [18] and melatonin levels peak at night, melatonin generates an antiphase rhythm of DA release characterized by high levels during the day and low levels at night [3, 4]. In agreement with this, timed extra-ocular injections of melatonin in melatonin-deficient mice restore rhythms of DA release in the retina [17]. The absence of melatonin in the B6 strain and transgenic mouse lines with B6 background have long imposed limitations to the study of DA-dependent rhythmic processes in the retina, such as the strength of electrical coupling between photoreceptors [19, 20] or gene expression [3, 4]. One of the major goals of this project was to create a melatonin-proficient B6 congenic mouse line and test whether restoring a melatonin rhythm would restore a circadian rhythm of DA release in the retina.
Previous studies have provided valuable information on the chromosome location and genomic DNA sequences of the Aanat and Asmt genes in the B6 strain as well as in some melatonin-deficient strains [7, 12]. Using polymerase chain reaction (PCR) and Sanger sequencing based methods, we first confirmed the differences between functional CBA type alleles and the non-functional B6 ones. Using identified sequence differences, we then developed PCR methods to genotype these different alleles. We have been using this method to genotype offspring at each generation. Generating the congenic line will require at least 10 generations of backcrossing to the B6 recipient line to complete. Here, we report that a rhythm of melatonin production and function is restored in N3 animals. We also report that a circadian rhythm of DA release is present in the N3 animals. Finally, we partially characterized genomic DNA sequences of the Aanat and Asmt genes in the CBA strain and identified long pieces of insertion compared to B6, which have not been reported in previous studies.
Materials and methods
Animals and backcrossing strategy
C57BL/6J (B6) (JAX stock# 000664) and CBA/CaJ (CBA)(JAX stock# 000654) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed on a 12 h light/12 h dark cycle. Animal manipulations and procedures followed all federal, state and local guidelines and regulations and were fully approved by the Animal Welfare Committee (Institutional Animal Care and Use Committee) of the University of Texas Health Science Center at Houston.
To introduce the functional Aanat and Asmt genes into B6 background, we crossed B6 mice with CBA mice. Specifically, a female CBA mouse was used as donor animal to preserve the B6 Y chromosome in the offspring [12]. Backcross was continued afterward with B6 animals. Selection of the donor (i.e. CBA type) allele was performed at each generation. To generate animals homozygous for CBA-type alleles, crosses were setup between siblings heterozygous for both loci.
Tissue from a C3H/He strain (C3Heb/FeJ strain) was generously provided by Dr. Shen-An Hwang (McGovern Medical School, Houston, TX).
Identification and characterization of different alleles of the Aanat and Asmt genes
PCR was performed to genotype the different alleles of the Aanat and Asmt genes. Most PCR primers were designed in such a way that agarose gel electrophoresis of the PCR product was sufficient to tell apart differences, that is either the primers were specific to only one allele or the PCR products were of different sizes. When gel electrophoresis was not able to distinguish alleles, direct sequencing of the PCR product was performed (GeneWiz LLC., South Plainfield, NJ) and single nucleotide polymorphism (SNP) was used as a marker for strain-specific alleles. PCR enzymes were either Taq enzyme (New England Biolabs, Ipswich, MA) or Takara LA Taq (Clontech Laboratories, Mountain View, CA). In more details, we first used a PCR method [12] to amplify the genomic DNA and then directly sequence the PCR product to establish the genomic DNA sequence of the CBA alleles since this information was not readily available. Our results confirmed the polymorphism of both genes. Based on the sequence information we obtained, we then designed sets of allele-specific PCR primers differing from each other by their 3′ ends. Using these allele-specific PCR primers allowed us to preferentially amplify only one specific allele. To further increase the specificity of the genotyping PCR, we sometimes introduced another mutation at the third nucleotide position from the 3′ end. The method was validated by using the parental strains as positive and negative controls; besides, CBA x B6 F1 offspring were used as a positive control since it contained both possible alleles. All primer sequences and purposes of the PCRs are listed in Table 1.
Table 1.
Sequence of PCR primers
| Purpose | Name | Sequence 5′-3′ |
|---|---|---|
| Genotyping Aanat CBA allele | AANAT-GT-CBA-F | atc att ttc att gct act ccg |
| AANAT-GT-R | gct aca cct gtt tct cca aac c | |
| Genotyping Aanat B6 allele | AANAT-GT-B6-F | atc att ttc att gct act cca |
| AANAT-GT-R | gct aca cct gtt tct cca aac c | |
| Genotyping Asmt CBA allele | ASMT-GT-F | caa gcc ctc agg gtt cag gaa |
| ASMT-GT-CBA-R | gcg ccc acc tga cag gaa acg | |
| Genotyping Asmt B6 allele | ASMT-GT-F | caa gcc ctc agg gtt cag gaa |
| ASMT-GT-B6-R | gcg ccc acc tga cag gaa aca | |
| PCR of 5′ end of Aanat gene | AANAT-5′-F | ctt gca gtc agg agt ctc ag |
| AANAT-5′-R | caa tgt gtc tgt cct atc agc | |
| PCR of 3′ end of Aanat gene | AANAT-3′-F | aca aag gca aga cca gga tg |
| AANAT-3′-R | cac gtg ttt gct cca gtc ag | |
| PCR of 5′ end of Asmt gene | ASMT-5′-F | agg cts agt akc tcg cgt ccc acg atg (*) |
| ASMT-5′-R | acc tgt aga tgg cgg tga agg (*) | |
| PCR of 3′ end of Asmt gene | ASMT-3′-F | ggt gct gct ggt gga gag |
| ASMT-3′-R | cct gtc tgt gac gtc gcg |
from [12].
Tissue collection and processing
Mice were transferred to a dark room at the end of the day or light phase and kept dark-adapted for up to 36 h when required for the circadian experiments. Before tissue collection, animals were anesthetized with ketamine/xylazine (100/10 mg/kg body weight, i.p.) and decapitated. When required, eyeballs were enucleated in the dark with the help of infrared goggles. The pineal gland was dissected out of the brain after removal of eyeballs. The dissected tissues were immediately sonicated in ice cold homogenizing solution composed of 0.4 M perchloric acid, 0.1 mM disodium EDTA and 0.1 mM sodium metabisulfite. The resulting homogenate was centrifuged at 12,000 g for 30 minutes at 4˚C and the supernatant was collected for DA or melatonin measurements.
Measurements of melatonin, DA and 3,4-Dihydroxyphenylacetic acid (DOPAC)
A high performance liquid chromatography (HPLC) coupled with electrochemical detection (ECD) method was used to detect melatonin in the pineal gland. The same method with slightly modified parameters (e.g. methanol concentration in the mobile phase) was used to measure DA and its metabolite DOPAC in the eyeball. The liquid chromatograph and detection system consisted of a Prominence HPLC system (Shimadzu Scientific Instruments, Columbia, MD) with a Hypersil ODS C18 column (250 × 4.6 mm, 5-μm particle size, Thermo Scientific, Grand Island, NY) and an amperometric detector (Model LC-4C; BioAnalytical System, West Lafayette, IN). An isocratic mobile phase was used and was composed of 50 mM KH2PO4, 0.01-0.02% octyl sodium sulphate (OSS, ACROS Organics, NJ), and 10-30% methanol, depending upon the target of detection. The pH was adjusted to 3.1 before methanol was added. The mobile phase flow rate was set to 1 mL/min, and the column temperature to 30°C. The concentration of OSS and methanol were adjusted within the ranges abovementioned to achieve optimal peak separation. The potential of the working carbon electrode was set to +0.600 V for DA and DOPAC, and +0.825 V for melatonin detection. The substances of interest were identified by their peak retention times compared to those of standards and were quantified based on peak height. Depending on the chemical subjected to measure, 5 to 20 μl of sample was injected. See full details of the technique in previous publications [21, 22]. Although we were able to measure melatonin in the pineal gland of CBA and congenic mice at any time of the day, we were not able to consistently detect it in the eye or in any other tissue.
Measurement of wheel-running activity
Mice aged 2–6 months from CBA and B6 strains and congenic (N3 generation) line were individually housed in wheel-running cages (Columbus Instruments, Columbus, Ohio). To control the lighting conditions, the cages were enclosed in a light-tight cubicle within a room illuminated by dim red light. When required, light was provided by fluorescent white light bulbs that delivered an intensity of ∼100 lux at the level of the cages. Mice were kept in a 12-h light/12-h dark cycle (12L/12D) or in constant dark conditions (D/D) for at least 9 days. Wheel-running activity was continuously recorded and binned in 6 minute duration as described previously [23]. Data analysis was performed using NIH ImageJ software with the ActogramJ plug-in [24].
Data analysis
Results are given as individual values or mean ± SEM. Statistical analyses were performed with OriginPro 8.6-64 bit (OriginLab Corporation, Northampton, MA). Additional details are included in the figure legends.
Results
Characterization and identification of Aanat and Asmt alleles in B6 and CBA strains
Based on the information provided in a previous publication [12], PCR of the genomic DNA was used to amplify segments of both B6 and CBA alleles of the Aanat and Asmt genes. The PCR products were sequenced and comparison of the sequences was made among CBA, B6 and another melatonin-proficient mouse strain, C3H/He (C3H) [12]. A comparison of a portion of the sequence of the PCR products is shown in figure 1A. In this area, the CBA sequence differs from the B6 by multiple single nucleotide polymorphism (SNP) sites, for both the Aanat and Asmt genes. The CBA sequence greatly resembles that of C3H (Fig. 1A), which indicates that the SNPs are important for normal gene function.
Figure 1.
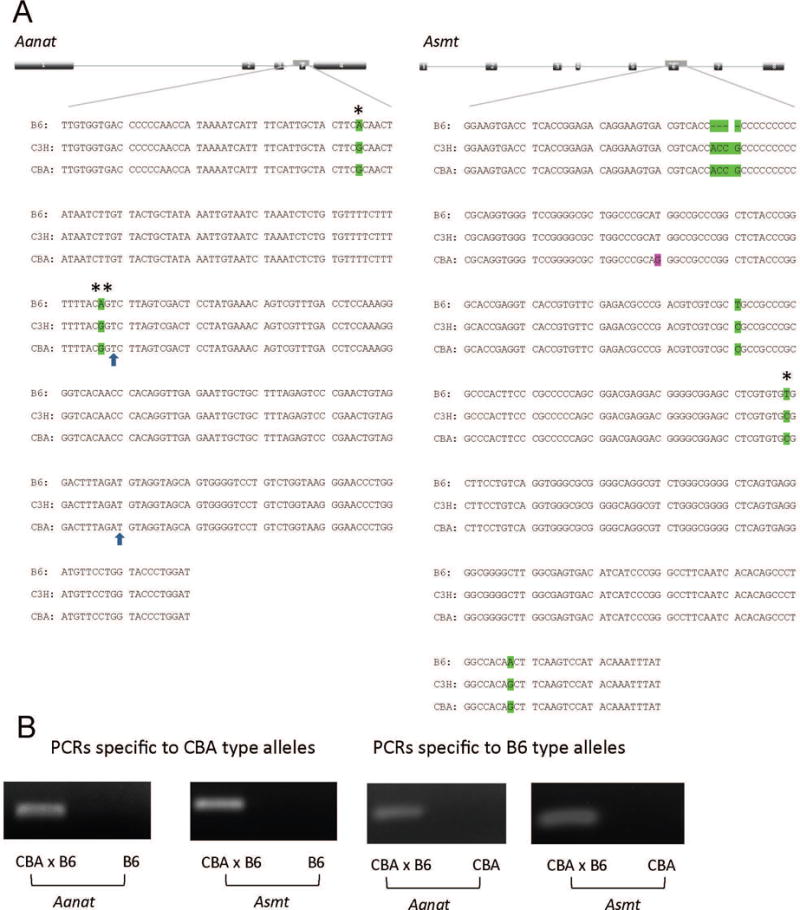
Identification and genotyping of alleles of the Aanat and Asmt genes in B6, C3H and CBA strains. A: Comparison of the sequences of Aanat and Asmt alleles. Locations of the sequences compared are indicated by the gene diagrams, where the exons are numbered. P: Pseudo-exon of the B6 type Aanat. Green: CBA sequence is identical to that of C3H but different from that of B6. Magenta: CBA sequence is different from those of C3H and B6. *: SNP used to design PCR primers. The two SNPs in the Aanat sequence (*, **) have been reported in B6 mice and the second one (**) shown to be responsible for the decreased AANAT enzymatic activity in this strain [7]. The boundaries of the pseudo-exon are exposed by the 2 arrows. B: Allele-specific PCR on the basis of SNPs was used to distinguish the different alleles. Visible PCR products represent the existence of a specific allele. The size of the PCR product is 284 bp for Aanat and 311 bp for Asmt.
We used SNPs to design PCR primers specific to the different alleles (Table 1). The PCR product was visualized by agarose gel electrophoresis as shown in figure 1B. PCRs were specific enough to distinguish the different alleles of a same gene. PCRs specific to CBA alleles were performed as a genotyping method at each backcross generation to select the animals with the correct genotype. However, these PCRs usually were not quantitative enough to distinguish homozygotes (2 copies of the same allele) from heterozygotes (1 copy of each allele). To solve this problem, an additional PCR specific to the B6 allele was performed. A heterozygote was tested positive by both PCRs while a homozygote only by one allele-specific PCR.
CBA-type alleles rescued melatonin synthesis in the B6 congenic line
To test if introduction of the functional CBA-type Aanat and Asmt alleles could be sufficient to restore the ability of melatonin synthesis in the congenic line, we assayed nocturnal melatonin content in the pineal glands of animals of various genotypes along the process of backcrossing. Measurements of the pineal melatonin content at circadian times (CT) CT19-CT20 was about 50 pg per gland in CBA animals (Fig. 2, CBA). In contrast, B6 pineal melatonin content was < 5 pg per gland that is, below detection threshold, at that time (Fig. 2, B6). We next examined the F1 offsprings of the CBA and B6 cross. These animals are double heterozygous for both Aanat and Asmt, i.e. carry one functional allele from the CBA strain for each gene. We were able to detect melatonin in the pineal gland of some of these animals, although the levels were on average lower than in CBA mice and not significantly different than in B6 (Fig. 2, F1). We next examined animals backcrossed to B6 at N2. Mice that were heterozygous for both genes (i.e. one copy of CBA functional allele in each gene) had low levels of pineal melatonin (Fig. 2, N2**). Low levels of pineal melatonin were also observed when only one of the two genes had one functional allele (Fig. 2, N2*). All together the results indicate that a single functional allele of either Aanat and/or Asmt is not sufficient to fully restore melatonin synthesis, suggesting haplo-insufficiency of one or both genes. To confirm this, we crossed mice heterozygous for both genes to generate animals homozygous for CBA type alleles at N3 generation. When these animals were tested, we found that their pineal melatonin levels were comparable to those of the CBA animals (Fig. 2, N3). The results thus establish that two functional alleles of both Aanat and Asmt genes are necessary for full restoration of melatonin biosynthesis in the B6 congenic line. We did not try to further clarify which gene, out of Aanat and Asmt, was more affected by haplo-insufficiency. The results also indicate that the B6 genome does not contain other elements significantly impeding melatonin production.
Figure 2.
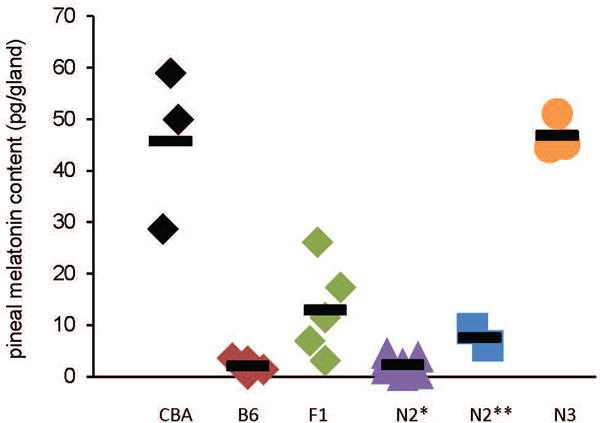
Functional Aanat and Asmt alleles rescued nocturnal pineal melatonin biosynthesis in B6 congenic mice. Each dot represents an individual animal; horizontal short lines represent group means. Pineal glands were collected at CT19-CT20 in the dark. F1: double heterozygous for CBA alleles; N2*: maximum 1 CBA-type allele in either gene; N2**: double heterozygous for CBA-type alleles; N3: double homozygous for CBA-type alleles. The population variances are not significantly different [Brown-Forsythe test; F(5,15) = 2.402 (P = 0.0778)] but the population means are significantly different [one-way ANOVA: F(5,15) = 32.470 (P = 2.105E-8)]. Tukey post-hoc analysis indicates that CBA and N3 groups are not statistically different to one another (P > 0.5) but each is different from the other 3 groups (i.e. F1, N2*, and N2**) (P < 0.001); none of the F1, N2*, and N2** groups is significantly different from the 2 others (P > 0.05). n = 2-7.
We analyzed the locomotor activity rhythms of CBA, B6 and N3 congenic mice under various lighting conditions (Fig. 3). Mice from all genotypes were able to entrain to a 12L/12D cycle and were able to run freely under D/D conditions with a period slightly shorter than 24 h (Figs. 3A-C). When submitted to a 6-h phase advance, mice from all genotypes entrained to the new lighting regimen within a few days (Fig. 3A), although at slightly different speeds (Fig. 3D). These results indicate that the congenic animals have normal organization and regulation of their daily rhythms of locomotor activity.
Figure 3.
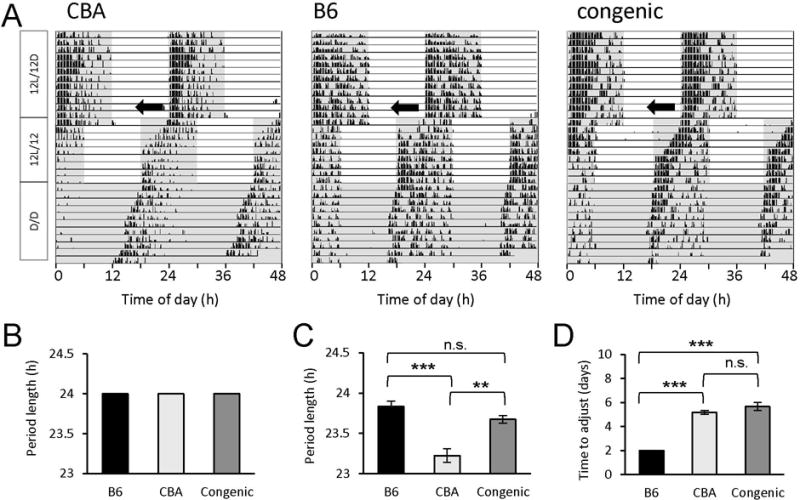
Locomotor activity rhythms of CBA, B6 and congenic mice under a 12-h light/12-h dark cycle (12L/12D) or in constant dark conditions (D/D). A: Representative double-plotted profiles of wheel-running activity of a CBA mouse (left panel), a B6 mouse (middle panel), and a double-homozygous congenic mouse (right panel), obtained (from top to bottom) under a 12L/12D cycle, including after a 6-h phase shift (jetlag paradigm, black arrows), and D/D. B and C: Average period lengths of the wheel activity rhythm under 12L/12D (B) and D/D conditions (C). Mice from all genotypes had a period length of exactly 24.0 h under 12L/12D (B) and were able to run freely under D/D conditions with a period slightly shorter than 24 h (C). A two-way ANOVA of the data illustrated in (B) and (C) revealed significant differences between the lighting regimens (F(1,18) = 129, P = 1.23e-9) as well as between genotypes (F(2,18) = 25.6, P = 5.42e-6) and interaction lighting cycles x genotypes (F(2,18) = 25.6, P = 5.42e-6). Tukey’s post hoc test revealed a significant difference between CBA and B6 mice (***, P = 2.92e-4) and CBA and congenic mice (**, P = 0.00679) but not between B6 and congenic animals (not significant [n.s.], P = 0.256). D: Time to adjust the onset of activity after a 6-h phase advance. A one-way ANOVA revealed significant differences between genotypes in the speed to which mice adjusted to the new phase (F(2,9) = 76.5, P = 2.25e-6). Specifically, B6 mice adjusted much faster (within 2 days) to the new phase compared to CBA (***, P = 4.37e-6) or congenic mice (***, P = 4.27e-6), which did so in ~ 5 to 6 days. No difference was found between CBA and congenic mice (n.s., P = 0.246). Data were collected from 3 B6, 6 CBA, and 4 congenic mice. Error bars represent SEM. Shaded areas in A indicate periods of darkness.
CBA-type alleles of Aanat and Asmt rescue circadian rhythms in melatonin synthesis and melatonin-dependent retinal DA release in B6 congenic mice
To test if circadian rhythms in melatonin production are restored in the congenic animals double homozygous for CBA-type, functional alleles of Aanat and Asmt (N3 generation), we collected pineal samples during the subjective day (CT6) and subjective night (CT20). Our results demonstrate that the subjective day/subjective night difference in pineal melatonin content is present in these animals (Fig. 4A). Pineal melatonin levels were under detection threshold (< 5 pg) in B6 mice at both circadian times (data not illustrated). We noted however that the nighttime pineal melatonin content of the congenic line as well as that of the CBA strain we measured was lower than previously reported [8–10, 12]. We speculated that we might have collected pineal tissue in the ascending portion of the nocturnal peak rather than at the peak. Indeed, previous studies have reported that melatonin production peaks somewhat late at night in CBA mice that is around CT21-22 [8, 10]. To directly address this possibility, we collected pineal glands at CT21-22. At that time point, we observed much higher values for melatonin pineal content in both CBA and congenic mice: 315 ± 14 (n=3) and 220 ± 51 (n = 2) pg/pineal gland, respectively (mean ± S.E.M.) (Fig. 4A). These values are in agreement with the peak levels of melatonin reported previously in CBA pineal glands (~ 276 pg/gland [8] and ~ 460 pg/gland [10]). Thus, the values of peak melatonin production at night measured in the B6 congenic mice are comparable to those obtained in the CBA strain, indicating that the congenic line has the same capability to synthesize melatonin as the CBA strain.
Figure 4.
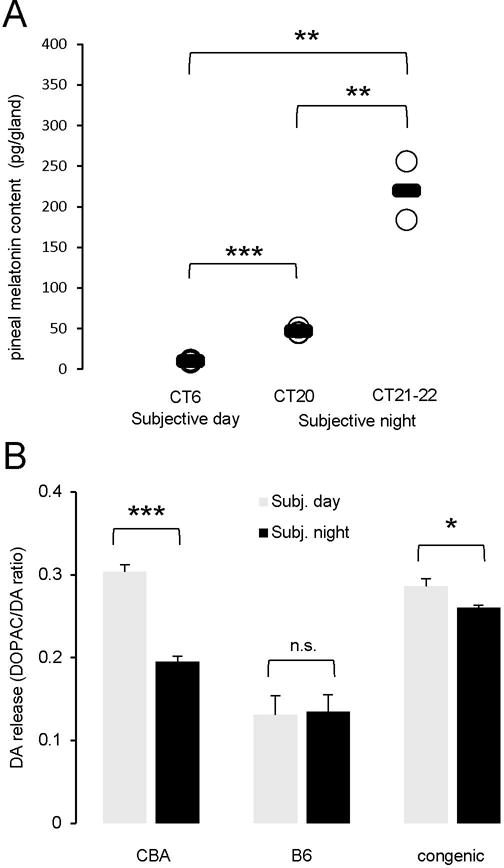
Rhythmicity of melatonin biosynthesis and melatonin-dependent processes was restored in congenic animals. A: Subjective day/subjective night difference of pineal gland melatonin content in the N3 animals. Pineal glands were collected at CT6 (subjective day) and CT20 or CT21-22 (subjective night) and melatonin assayed by HPLC. Each dot represents an individual animal; group means are represented by short lines. *** P = 8.40 × 10−5, ** P = 0.004 (day vs CT21-22) or P = 0.008 (CT20 vs CT21-22), 2-tailed unpaired Student’s t-test. B: Subjective day/subjective night difference of retinal dopamine release in CBA, B6 and B6 congenic animals. Eyeballs were collected at CT6 (subjective day) and CT18-20 (subjective night) in the dark. Contents of DA and DOPAC were measured with HPLC-ECD. DA release was represented by DOPAC/DA ratio. Data are mean ± S.E.M. * P = 0.0175, *** P = 6.95 × 10−5, n.s. not significant, 2-tailed unpaired Student’s t-test. n = 4 for CBA and B6, at each time point and 6 for B6 congenic animals, at each time point.
To determine whether the circadian rhythm of DA release is restored in the retina of the B6 congenic animals, we measured DA and DOPAC contents in the eyeballs and used the DOPAC/DA ratio as an index of dopamine release (see Methods for details). As in CBA animals, we found a significant subjective day/subjective night difference in DA release in the congenic animals, although with a somewhat reduced amplitude (Fig. 4B). In contrast, no subjective day/subjective night difference was found in B6 animals (Fig. 4B). The daytime increase in the DOPAC/DA ratio observed in CBA and congenic animals is essentially the result of a daytime increase in DOPAC content, and not of a daytime decrease in DA content, which to the contrary increases during the daytime (Table 2). These results indicate that melatonin synthesis is restored in the congenic line, not only in the pineal gland but also in other melatonin-producing tissues, such as the retina. Furthermore, these observations strengthen the view that the circadian rhythm of dopamine release in the mouse retina depends on melatonin [3, 4].
Table 2.
DOPAC, DA and DOPAC/DA ratio in B6, CBA and congenic mice
| Strain/line | Time (n) | DOPAC (pg/eye) | Dopamine (pg/eye) | DOPAC/DA ratio |
|---|---|---|---|---|
| B6 | CT06 (4) | 243.5 ± 44.7 | 2,041.0 ± 66.4 | 0.122 ± 0.0253 |
| CT18-20 (4) | 282.7 ± 43.7 | 1,719.4 ± 80.1 (*) | 0.165 ± 0.0256 | |
| CBA | CT06 (5) | 790.5 ± 20.2 (†††) |
2,091.6 ± 40.1 | 0.379 ± 0.0121 (†††) |
| CT18-20 (5) | 484.1 ± 18.7 (***, †††) |
1,855.1 ± 32.1 (**) |
0.261 ± 0.00996 (***, †††) |
|
| B6 congenic | CT06 (6) | 352.6 ± 8.55 (†, ###) |
1,236.6 ± 32.6 (†††, ###) |
0.286 ± 0.00883 (†††, ##) |
| CT18-20 (6) | 311.8 ± 3.92 (***, ###) |
1,200.9 ± 17.4 (*, †††, ###) |
0.269 ± 0.00259 (*, †††) |
|
| ANOVA | CT06 |
F(2,12)=140.2 (P=4.8e-9) |
F(2,12)=128.4 (P=7.9e-9) | F(2,12)=68.5 (P=2.7e-7) |
| CT18-20 |
F(2,12)=22.7 (P=8.4e-5) |
F(2,12)=73.7 (P=1.8e-7) |
F(2,12)=16.1 (P=4.0e-4) |
Data are mean ± SEM from n animals. Subjective day/subjective night difference was tested within each genotype using 2-tailed unpaired Student’s t-test:
P<0.05,
P<0.01,
P<0.001.
Differences between daytime values or between nighttime values were tested between genotypes using one-way ANOVA followed by Tukey post-hoc analysis.
P<0.05,
P<0.001 (compared to B6),
P<0.01,
P<0.001 (compared to CBA).
Characterization of additional sequences of the Aanat and Asmt genes
In the practice of backcrossing, the donor sequence (in our case, the CBA type) is incorporated into the recipient’s genome through homologous recombination. While very unlikely, there is a possibility that recombination may occur within the gene [25]. That is, backcrossing may result in the generation of a new allele composed of parts of the two parental ones. This mosaic allele may be difficult to detect by our genotyping methods as it may be undistinguishable from one of the parental alleles as long as it contains the right SNPs. While a hybrid allele can be functional or partially functional, we avoided such a situation by ensuring the congenic animals contain the whole CBA-type alleles for both Aanat and Asmt. To this purpose, we genotyped the breeding animals not only for the small regions inside of the genes but also the 5′ and 3′ ends of them. We rationalized that if an animal carries both 5′ and 3′ ends and a sequence in between of a given allele, there is high likelihood that it does carry such an allele completely. Only animals that carried all sequences of a functional allele were selected for breeding.
Whereas full characterization of the genes was not a main objective of this research project, we did find interesting differences between alleles, especially in the Asmt gene (detailed below). Our PCR primers were designed based on the available B6 genomic sequences (Ensembl genome browser and [7, 12]). For Aanat, the 5′ primer covered the 1st exon and the 3′ primer the 3′ end to the last exon. For Asmt, the 5′ primer covered the first 3 exons completely and the 3′ primer targeted a sequence inside the last (8th) exon. For the Aanat gene 5′ and 3′ ends, PCRs with such primers readily yielded amplicons of expected sizes (Fig. 5A). Partially sequencing these PCR products revealed that the difference between alleles were essentially SNPs. This finding was consistent with the near-identical size of PCR products. These SNPs are sufficient to provide information to genotype an animal (Fig. 5B). In contrast to the Aanat gene, a similar PCR approach yielded very different results for the Asmt gene. Using B6 genomic DNA as a template, we could reliably amplify the target sequences at both 5′ and 3′ ends; and the sizes of the PCR products were as expected. However, PCR products were very different when CBA genomic DNA was used as a template. Specifically, for the 5′ end of the gene, we visualized a PCR product about 1 kb longer than expected based on the available B6 sequence (Fig. 5C). We directly sequenced the PCR product and obtained information on a small part of the sequence. The PCR product contains exon 1 and part of the intron 1 of the Asmt gene, therefore ruling out non-specific amplification. The homology to intron 1 of B6 sequence stops at around 86 nt after the start of the first intron. For the rest of the sequence we obtained, we could not find any meaningful homolog in the mouse and human genomic databases (Basic Local Alignment Search Tool (BLAST) -NCBI - NIH). However, practically, the 1-kb size difference provides an efficient way to genotype the 5′ end of the gene (Fig. 5C). For the 3′ end, we could not amplify the target sequences when the primers spanned the 7th intron, suggesting significant differences in this region between the B6 and CBA alleles (Fig. 5D). To identify the alleles, we resorted to a PCR whose target sequence resided completely inside exon 8. A SNP inside this region was used to distinguish the two alleles (Figs. 5E, 5F). Along the process of continuous backcrossing, we tested our breeders for the whole genes every several generations. Such test will also be performed before the congenic line is eventually released.
Figure 5.
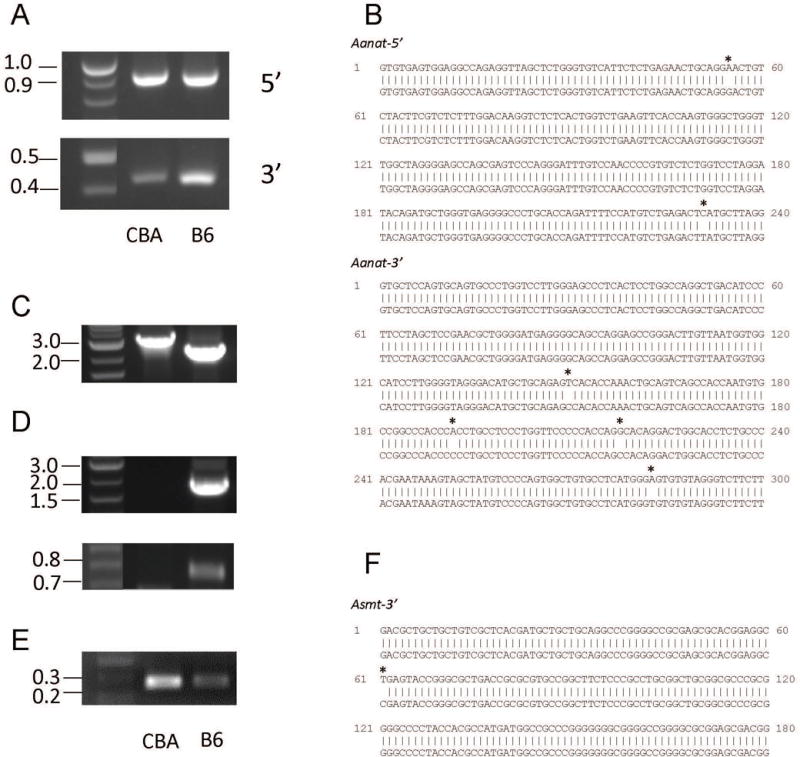
Characterization of the 5′ and 3′ ends of the Aanat and Asmt genes. A. Visualization of PCR products of Aanat 5′ and 3′ ends amplified from CBA and B6 genomic DNA. Note the similar size of the PCR products of CBA and B6. B. comparison of CBA and B6 sequences for the Aanat 5′ and 3′ ends. SNPs are marked by asterisks. C. PCR product of 5′ end of CBA and B6 Asmt gene. Note the size difference of about 1 kb. D. Visualization of products of 2 PCR targeting 3′ end of Asmt gene using CBA and B6 genomic DNA. The PCR primers covered intron 7 for these 2 reactions. E. Visualization of products of PCR targeting 3′ end of Asmt gene using CBA and B6 genomic DNA. The PCR primers are both located inside Exon 8. F. Comparison of CBA and B6 sequences of 3′ end of the Asmt gene. A single SNP, marked with an asterisk, distinguish different alleles. For panels B and F, upper sequences are CBA and lower are B6.
Discussion
As one of the most common mouse models used in biomedical research, the C57Bl/6J strain has been characterized in great details (more than 17,000 entries returned to “C57Bl/6J” in Pubmed as of 10/2017). The wealth of information accumulated over decades on this specific mouse strain is highly valuable. Therefore, B6 has become the recipient strain of choice when backcrossing animals with mutant genes of interest. However, the use of B6 has been limited when normal melatonin function is required for the experimentation.
In this project we aimed to improve the B6 strain by generating a congenic B6 mouse line that is melatonin-proficient. The data up to date demonstrate that this can be done by continual backcrossing to B6 and selecting the functional Aanat and Asmt genes. The congenic line has the ability to synthesize melatonin in the pineal gland with a normal circadian rhythm. Furthermore, we were able to analyze the Aanat and Asmt genomic sequences to some details. This approach revealed an extensive polymorphism in the Asmt sequence between the CBA and B6 strains (Fig. 5C). Previous research reported that the Asmt cDNA sequences across many domestic mouse strains differ by SNPs [12]. Because Kasahara et al. [12] did not find mutations other than SNPs in the CBA Asmt cDNA, it is therefore likely that the long insertion(s) we found in the CBA Asmt gene does not affect the splicing of pre-mRNA. Nonetheless, it remains to determine if this sequence has any impact on the regulation of Asmt gene expression. Interestingly, we found this long insertion in the C3H gene as well (Z.Z., E.S., N.J. and C.P.R., unpublished data), suggesting that it might be a feature of melatonin proficiency in mice.
It is well acknowledged that melatonin controls or modulates important physiological functions, such as seasonal reproduction, sleep, and the synchronization of peripheral oscillators [1–2, 26, 27]. In addition, melatonin possess diverse biological activities such as antioxidant properties and anti-tumor action [26, 27]. The melatonin-proficient mouse model we have developed will be useful to scientists in these fields as it will serve as a replacement of the widely used B6 strain to study relevant questions about melatonin actions. This congenic animal line, when fully established, will be distributed through the Jackson Laboratories and made available to all investigators.
Acknowledgments
This work was supported by the National Institutes of Health, grants EY018640 (CPR) and EY028102 (vision core grant), the Hermann Eye Fund (CPR) and an Unrestricted Challenge Grant from Research to Prevent Blindness to the Ruiz Department of Ophthalmology and Visual Science. The authors are grateful to Dr. Takaoki Kasahara (RIKEN Brain Science Institute, Japan) for providing helpful suggestions on PCR methods, to Drs. Chai-An Mao (McGovern Medical School, Houston, TX) and Marie-Paule Felder-Schmittbuhl (University of Strasbourg, France) for constructive comments on the manuscript, to Dr. Shen-An Hwang (McGovern Medical School, Houston, TX) for sharing mouse tissue and to Dr. Kimberly Mankiewicz (McGovern Medical School, Houston, TX) for editing the manuscript.
Footnotes
DR CHRISTOPHE P RIBELAYGA (Orcid ID : 0000-0001-5889-2070)
Author Contributions
Conception and design of the experiments: Z.Z. and C.P.R. Collection, analysis and interpretation of the data: Z.Z., E.S., N.G. and C.P.R. Drafting of the article and revising it critically for important intellectual content: Z.Z. and C.P.R. All authors have read and approved the final submission.
References
- 1.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 2.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105:170–82. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Iuvone PM, Tosini G, Pozdeyev N, et al. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–56. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 6.Conti A, Maestroni GJ. HPLC validation of a circadian melatonin rhythm in the pineal gland of inbred mice. J Pineal Res. 1996;20:138–144. doi: 10.1111/j.1600-079x.1996.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 7.Roseboom PH, Namboodiri MA, Zimonjic DB, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 8.Vivien-Roels B, Malan A, Rettori MC, et al. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms. 1998;13:403–409. doi: 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- 9.von Gall C, Lewy A, Schomerus C, et al. Transcription factor dynamics and neuroendocrine signaling in the mouse pineal gland: a comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur J Neurosci. 2000;12:964–972. doi: 10.1046/j.1460-9568.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 10.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. Melatonin in mice: rhythm, response to light, adrenergic stimulation, and metabolism. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R358–R365. doi: 10.1152/ajpregu.00360.2001. [DOI] [PubMed] [Google Scholar]
- 11.Khaldy H, Leon J, Escames G, et al. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinol. 2002;75:201–208. doi: 10.1159/000048238. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara T, Abe K, Mekada K, et al. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107:6412–6417. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storch KF, Paz C, Signorovitch J, et al. Intrisic circadian clock of the mammalian retina: importance of retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimomura K, Lowrey PL, Vitaterna MH, et al. Genetic suppression of the circadian clock mutation by the melatonin biosynthesis pathway. Proc Natl Acad Sci U S A. 2010;107:8399–8403. doi: 10.1073/pnas.1004368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiragaki S, Baba K, Coulson E, et al. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeffer M, Korf HW, Wicht H. The role of the melatoninergic system in light-entrained behavior of mice. Int J Mol Sci. 2017;18:e530. doi: 10.3390/ijms18030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 18.Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- 19.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin N, Ribelayga CP. Direct evidence for daily plasticity of electrical coupling between rod photoreceptors in the mammalian retina. J Neurosci. 2016;36:178–184. doi: 10.1523/JNEUROSCI.3301-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Zhang Z, Blackburn MR, et al. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao SN, Zhang Z, Ribelayga CP, et al. Multiple cone pathways are involved in photic regulation of retinal dopamine. Sci Rep. 2016;6:28916. doi: 10.1038/srep28916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao C-A, Li H, Zhang Z, et al. T-box transcription regulator Tbr2 is essential for the formation and maintenance of Opn4/melanopsin-expressing intrinsically photosensitive retinal ganglion cells. J Neurosci. 2014;34:13083–13095. doi: 10.1523/JNEUROSCI.1027-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid B, Helfrich-Forster C, Yoshii T. A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J Biol Rhythms. 2011;26:464–467. doi: 10.1177/0748730411414264. [DOI] [PubMed] [Google Scholar]
- 25.Markel P, Shu P, Ebeling C, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 26.Tosini G, Owino S, Guillaume JL, Jockers R. Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays. 2014;36:778–787. doi: 10.1002/bies.201400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tordjman S, Chokron S, Delorme R, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]


