Abstract
Study Design
case-control
Objective
To understand the role of high resolution MR in identifying regional cord volume loss in cervical spondylotic myelopathy (CSM).
Summary of Background Data
Preliminary studies suggest that compression of the ventral region of the cord may contribute disproportionately to CSM symptomology, however, tract-specific data is lacking in the CSM population. The current study is the first to use 3T MRI images of CSM patients to determine specific volume loss at the level of detail of individual descending white matter tracts.
Methods
Twelve patients with CSM and 14 age- matched were enrolled prospectively and underwent 3 Tesla MR imaging of the cervical spine. Using the high-resolution images of the spinal cord, straightening and alignment with a template was performed and specific spinal cord tract volumes were measured using Spinal Cord Tool-box version 3.0.7. Modified Japanese orthopedic association (mJOA) and Nurick disability scores were collected in a prospective manner and were analyzed in relation to descending spinal tract volumes.
Results
Having CSM was predicted by anterior/posterior diameter, eccentricity of the cord (OR 0.000000621, p=0.004), ventral reticulospinal tract volume (OR 1.167, p=0.063), lateral corticospinal tract volume (OR 1.034, p=0.046), rubrospinal tract volume (OR 1.072, p=0.011), and ventrolateral reticulospinal tract volume (OR 1.474, p=0.005) on single variable logistic regression. Single variable linear regression showed decreases in anterior/posterior spinal cord diameter (p=0.022), ventral reticulospinal tract volumes (p=0.007) and ventrolateral reticulospinal tract volumes (p=0.017) to significantly predict worsening mJOA scores. Similarly, decreases in ventral reticulospinal tract volumes significantly predicted increasing Nurick scores (p=0.039).
Conclusion
High resolution 3T MRI can detect tract-specific volume loss in descending spinal cord tracts in CSM patients. Anterior/posterior spinal cord diameter, ventral reticulospinal tract, ventrolateral reticulospinal tract, lateral corticospinal tract and rubrospinal tract volume loss are associated with CSM symptoms.
Keywords: Cervical Spondylotic Myelopathy, 3T MRI, Spinal Cord Toolbox, White Matter Tract Volume, Reticulospinal Tract, CSM, Rubrospinal Tract, Corticospinal Tract, Myelopathy, Spinal Cord Distortion, Spinal Cord Volume Loss
Cervical Spondylotic Myelopathy (CSM), a degenerative disease characterized by incremental compression and injury to the cervical spine, is a common cause of morbidity in elderly patients.1–4 The exact pathophysiology of CSM is largely not understood.1,3 While cord compression is generally accepted to be associated with worse CSM symptoms, it however is not always predictive of CSM.1,5,6 It is not uncommon for patients with seemingly high-grade cord compression on MRI to present asymptomatically.1,3,6 Similarly, patients with seemingly no compression evidenced on MRI may present with severe and debilitating symptoms.1,5,6 As such, it has been hypothesized that symptoms may instead result from compression of specific descending white matter regions, contributing to the large variability in symptom severity amongst patients with compression.1,7 Preliminary studies suggest that compression of the ventral region of the cord may contribute disproportionately to CSM symptomology, however, tract-specific data is lacking in the CSM population.7,8
High resolution 3T MRI has been increasingly evaluated as a neuroimaging tool that may allow physicians to reliably predict clinical symptomology in cervical myelopathy.9,10 This technology provides increasingly detailed imaging capabilities for visualizing individual white and gray matter tracts within the spinal cord.11 As such, high resolution 3T MRI may have a future in helping to guide clinician decision making by assisting with the preoperative evaluation of patients with CSM.
The current study is the first to use 3T MRI images of CSM patients, paired with age matched control patients to determine specific volume loss at the level of detail of individual descending white matter tracts. Tract specific volume loss was additionally evaluated in relation to clinical symptoms. It is our hypothesis that reduction of volume in individual descending white matter tracts of the ventral and lateral spinal cord would prove predictive of worsening clinical symptoms.
Methods
We prospectively enrolled a cohort of 12 CSM patients and 14 aged-matched controls (matching coefficient 0.64) to undergo MRI imaging of the cervical spine. All CSM patients included were diagnosed at a single large academic institution based upon a combination of both clinical and radiographic findings. Inclusion criteria for entry included the following in all patients diagnosed with CSM: classic CSM symptoms, including exam findings of weakness, hyperreflexia, or change in coordination; radiographic signs of spinal compression; Nurick grade I-IV12; and modified Japanese Orthopedic Association (mJOA) scores of <1813. Exclusion criteria included the following: age <21 or >80, comorbid neural disease (e.g., multiple sclerosis), pregnant or nursing, active systemic rheumatological disease, active peripheral or vascular neuropathy, urgent need for surgery. The study was conducted with the approval of the university’s Institutional Review Board (IRB).
Image Acquisition and Analysis
All imaging data were collected with a 3.0 Tesla Siemens Prisma magnetic resonance scanner (Siemens, Erlangen, Germany) equipped with a 64-channel head/neck coil. Participants were placed supine on the scanner bed, and a localizer scan was obtained to identify the location of the intervertebral discs of the cervical spine (C2–3, C3–4, C4–5, C5–6, C6–7, and C7- T1). Six high-resolution transverse slices with high white matter to gray matter contrast were acquired within the plane of each cervical intervertebral disc using a multi-echo gradient-echo sequence (TR=300 ms, TE=18 ms, Flip angle=30°, FOV=180×180, Matrix size=384×384, In-plane resolution=0.47×0.7 mm2, Slice thickness=4 mm, number of averages=2). Image processing and analysis were performed separately for each transverse slice using the Spinal Cord Toolbox (version 3.0.7) and the PAM50 spinal cord template.11,14 To permit registration of the spinal cord template to the two-dimensional slices, each transverse slice was concatenated along superior-inferior axis to form a three-dimensional volume three slices thick. The spinal cord was then manually segmented from the volume, and vertebral landmarks masks were generated such that the superior and inferior slices corresponded to the vertebral bodies immediately superior and inferior to the respective intervertebral disc level. The spinal cord template was then initially registered to the volume using the spinal cord segmentation mask to first co-register the images followed by column wise deformation in the transverse plane to account for any cord compression. The spinal cord gray matter was then segmented from the images, and the registration of the template was finely-tuned using the internal structure of the spinal cord. The spinal cord template white matter atlas was then warped to the volume, and cross-sectional morphometric measures (i.e., anterior-posterior spinal cord diameter and eccentricity) and white matter tract volumes were extracted. For the white-matter volumes, the maximum a posteriori (map) method was used to account for partial volume effect.15 A representative example of the registration and masking process is shown in Figure 1A–E.
Figure 1.
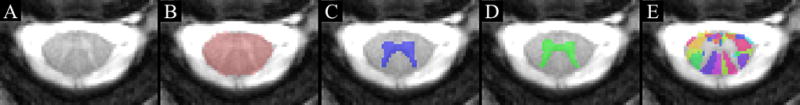
High resolution transverse slices with high white matter to gray matter contrast were acquired at the plane of each cervical intervertebral disc level (A). Image processing and analysis was performed using the Spinal Cord Toolbox and the PAM50 spinal cord template. The spinal cord template was initially registered to the volume using a spinal cord segmentation mask (B) to first co-register the images. The initial registration of the gray matter is shown in C. The spinal cord gray matter was then segmented from the images, and the registration of the template was finely-tuned using the internal structure of the spinal cord (D). The spinal cord template and white matter atlas (E) were then warped to the volume, and cross-sectional morphometric measures (i.e., anterior-posterior spinal cord diameter and eccentricity) and white matter tract volumes were extracted. The example images are from the C2–3 disc level.
Ad-hoc analysis of specific descending spinal cord white matter tract volumes included the ventral corticospinal tract, ventral reticulospinal tract, medial reticulospinal tract, lateral corticospinal tract, rubrospinal tract, lateral reticulospinal tract, and the ventrolateral reticulospinal tract. Tract measurements for each unique slice were considered each their own unique data point for ease of analysis. The cross-sectional morphometric measures and white matter volumes were compared between CSM patients and controls. Differences in tract volumes were further analyzed for correlation to clinical symptoms.
Statistical Methods
Statistical analysis was performed using and Stata 12.0 (StataCorp, College Station, TX, USA). Skewness and kurtosis tests for normality were applied to all ratings data. Parametric data was given as mean ± standard deviation and compared using a t-test. Non-parametric data was compared using Wilcoxon rank-sum test (Mann-Whitney U test), Chi-square test, or Fisher’s exact test, as appropriate. Regression analysis was performed using stepwise, multivariable logistic regression, with an inclusion threshold for the multivariable model of p<0.10 for candidate variables on single-variable logistic regression. A value of p<0.05 was considered statistically significant. Figures were generated using Prism 6.0b (GraphPad Software, Inc., La Jolla, CA, USA).
Results
The patient group consisted of 13 adults (8 males, 5 females) with a mean age of 62 ± 11 years and mean BMI of 28.23±5.8 kg/m2. Mean mJOA score was 14.3± 1.8 (ranging from 12–17) and Nurick grade ranged from 1–4. The control group consisted of six females and seven males with a mean age of 51±11 years and mean BMI of 25.6±2.5 kg/m2.
Figure 2AB shows a representative comparison of a patient with CSM (63 year old female, reported mJOA=15) and an age and sex matched control (63 year old, Female, mJOA= 18) at the level of compression observed in the patient (C2-C3). A decrease in anterior/posterior diameter in the patient was observed (6.1mm vs. 7.5mm). Cord distortion was reflected in increase in eccentricity (0.88 in the patient vs. 0.80 in control). White matter tracts show reduction in area as highlighted- rubrospinal (red), lateral reticulospinal (green), ventrolateral reticulospinal (yellow), ventral reticulospinal (blue).
Figure 2.
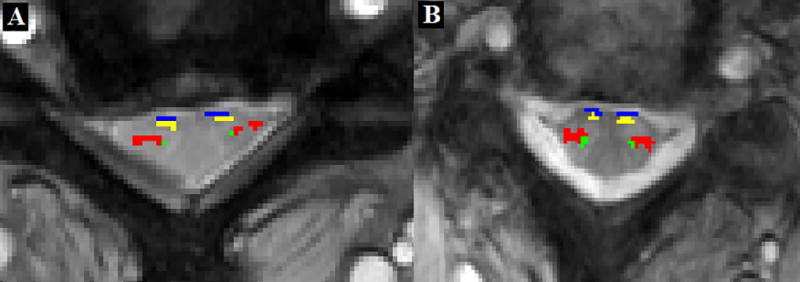
Morphological and tract specific changes in a patient (A) with CSM as compared to an age and sex matched control (B). White matter tracts highlighted- rubrospinal (red), lateral reticulospinal (green), ventrolateral reticulospinal (yellow), ventral reticulospinal (blue).
Morphological parameters of the spinal cord and specific white matter tract volumes were significantly different among patients as compared to controls (Table 1). Anterior-posterior cord diameter was lower in patients, while eccentricity was significantly higher. Patients exhibited a loss of white matter tract volumes, specifically ventral reticulospinal tract, ventrolateral reticulospinal tract, lateral corticospinal tract and rubrospinal tract volumes were significantly lower in patients than in controls.
Table 1.
Morphological parameters and white matter tract volumes among patients and controls.
| A/P diameter (mm) |
eccentricity | reticulospinal tract Volume (mm3) |
lateral corticospinal tract volume (mm3) |
rubrospinal tract volume (mm3) |
ventrolateral reticulospinal tract volume (mm3) |
|
|---|---|---|---|---|---|---|
| Controls | 8.31± 0.9 | 0.799± 0.6 | 9.25± 1.8 | 48.51± 12.0 | 19.79± 8.1 | 8.78± 2.0 |
| Patients | 7.72± 0.7 | 0.831± 0.4 | 8.21± 1.6 | 43.43 ± 13.0 | 15.76± 7.3 | 7.77± 1.3 |
| P value | p=0.0004 | p=0.0114 | p=0.0026 | p=0.0417 | p=0.0088 | p=0.0024 |
Mean ± SD, and p-values are reported.
Having CSM was predicted by anterior/posterior diameter (OR 2.497 [1.450, 4.302], p=0.001), eccentricity of the cord (OR 0.000000621 [0.000, 0.009], p=0.004), ventral reticulospinal tract volume (OR 1.167 [0.992, 1.373], p=0.063), lateral corticospinal tract volume (OR 1.034 [1.001, 1.069], p=0.046), rubrospinal tract volume (OR 1.072 [1.016, 1.131], p=0.011), and ventrolateral reticulospinal tract volume (OR 1.474 [1.127, 1.927], p=0.005) on single variable logistic regression. No variables remained significant predictors on stepwise multivariate regression.
Single variable linear regression showed decreases in anterior/posterior spinal cord diameter (b=0.589, p=0.022), ventral reticulospinal tract volumes (b=0.334, p=0.007) and ventrolateral reticulospinal tract volumes (b=0.307, p=0.017) to significantly predict worsening mJOA scores. On stepwise multivariable linear regression, however, only ventral reticulospinal tract volume remained a significant predictor of worsening scores (p=0.007).
Single variable linear regression showed decreases in ventral reticulospinal tract volumes to significantly predict increasing Nurick scores (p=0.039). There were no significant predictors of worsening Nurick scores on multivariable analysis.
Discussion
Cervical Spondylotic Myelopathy is a common cause of morbidity in elderly patients.1 The exact pathophysiology of CSM is largely not understood. While cord compression is generally known to be associated with worse CSM symptoms, it should be noted that compression alone is not always predictive of CSM.1,5,6 As such, it has been hypothesized that symptoms may instead result from compression of specific descending white matter regions, contributing to the large variability in symptom severity and surgical response not associated with presentation on clinical MRI.1,7 The current study provides insight into the tract-specific pathophysiologic processes contributing to symptoms associated with CSM.
In the current study, we found anterior posterior diameter and eccentricity to be significantly different between CSM patients and controls, consistent with previous literature suggesting global compression plays a role in CSM development.2–4,16 Similarly, our findings that tracts of the ventral cord (ventral reticulospinal tract, ventrolateral reticulospinal tract and rubrospinal tract respectively) differed significantly between CSM patients and controls is consistent with current literature suggesting anterior cord compression to be a main pathophysiologic driver of the disease.1,7 CSM patients classically present with hand weakness and hyperreflexia of the upper extremities17–20, consistent with known anatomy suggesting that the descending white matter tracts innervating such regions and muscles all pass through the anterior region of the cord at the cervical level.7 We similarly found ventral reticulospinal tract volume and ventrolateral reticulospinal tract volume to predict worsening mJOA scores and ventral reticulospinal tract volume to predict worsening Nurick scores.
To our knowledge, our findings are the first in the literature to find an association between descending reticulospinal white matter tract volume loss with increasing severity of CSM symptoms.7 Research on the function of the reticulospinal tract in humans is scarce, with the majority of the literature reported in non-human primate studies.21 While primarily thought to play a role in recovery after corticospinal injury in humans, the reticulospinal tract has also been shown to be associated with locomotion and postural adjustment as shown in a recent literature review.21–26 Furthermore, in studies on non-human primates the reticulospinal tract has been hypothesized to play a role in coordination of activity primarily performed by corticospinal tract such as reaching movements in the upper extremities and possibly finger movements.21,22,27–29 Exact function of such tracts in humans and their role in CSM symptoms remains under-investigated and further research on the topic is needed to better understand CSM pathophysiology.
Of particular note, our study found that anterior corticospinal tract volume loss was not significantly different between CSM and controls, nor did it significantly predict CSM, mJOA scores or Nurick scores. This differs from current literature suggesting pathophysiologic processes contributing to CSM symptoms are hypothesized to be most heavily driven by descending corticospinal tract damage.7,30 It is unclear to the authors why the current study is not consistent with such findings. These findings, while possibly a result of sampling bias, could also suggest a more prominent role played by the reticulospinal tracts than previously understood. Further study is indicated to determine the true relative contributions of each individual descending white matter tracts and their unique relationship to CSM symptoms.
The current study is one of the first to use high resolution 3T MRI to look at specific white matter tracts within the spinal cord in a clinical setting. Currently used as standard of care in functional cranial neurosurgery,31,32 white matter tract mapping has yet to make a significant clinical contribution in spinal cord patients.33 Currently, much of the white matter spinal tract specific literature stems from the development of diffusion tensor imaging (DTI) and magnetic transfer imaging (MTI).33–35 The current study acts as a proof of concept for yet another potential resource capable of assessing descending white matter tracts, made possible by the new creation of Spinal Cord Toolbox software. Spinal Cord Toolbox provides software tools necessary to analyze any high-resolution MRI sequence. While the future of spinal cord white matter tract mapping still remains unclear, studies such as the current one prove promising as a prominent step forward towards identifying adoptable a clinically useful application of tract mapping for spinal surgeons. Further investigation, validation and trials are needed to better understand the usefulness of this new method.
Our study is not without limitations. With only a small cohort, our data is subject to sample bias. Similarly, validation data on methods used by Spinal Cord Toolbox is limited and as such, findings must be interpreted within context.15,36 As the quantitative spinal cord MRI is new and growing field much remains to be optimized in the areas of spinal cord image acquisition (i.e., sequence and imaging parameters) and the analysis of spinal cord images. Several multi-site projects are currently underway to improve and standardize the imaging and analysis methods for quantitative spinal cord MRI.33,37,38 Standardization and streamlining of the methods will facilitate multi-site, multi-vendor studies and increase the clinical feasibility of quantitative spinal cord MRI applications. Furthermore, the current method looking at individual tract volume relies heavily on high-resolution, distinct imaging and leading to limitations the measuring of volume loss in patients with very severe cord compression. This limitation is less concerning to the authors as often such severe clinical presentations seldom requires a further need for more pre-operative diagnostic imaging. Lastly, while the current study measures tract specific volume loss, it need be noted that volume loss may not be a perfect surrogate for tract dysfunction. As such, further study is indicated to understand the true relevance of tract specific volume loss and its subsequent correlation with tract dysfunction and myelin loss.
The current preliminary study shows promise in the use of high resolution 3T MRI for the characterization and further study of pathophysiologic processes of the spinal cord disease. As imaging technology advances and becomes more clinically available, individual tract measurements may play an important role in prediction and determination of responsiveness to surgery and further contribute to diagnostic clinical utility in equivocal patients. Next steps include larger, prospective trials to better elucidate which tracts and regions are most predictive of CSM symptoms.
Conclusion
High resolution 3T MRI can detect tract-specific volume loss in descending spinal cord tracts in CSM patients. Anterior/posterior spinal cord diameter, ventral reticulospinal tract, ventrolateral reticulospinal tract, lateral corticospinal tract and rubrospinal tract volume loss are associated with CSM symptoms. Identifying tract specific injuries provides the necessary first steps needed to identify more robust thresholds for treatment in the CSM patient population.
Supplementary Material
Acknowledgments
The National Institute of Drug Abuse funds were received in support of this work.
Footnotes
No relevant financial activities outside the submitted work.
References
- 1.Ichihara K, Taguchi T, Sakuramoto I, et al. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg. 2003;99:278–85. doi: 10.3171/spi.2003.99.3.0278. [DOI] [PubMed] [Google Scholar]
- 2.Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976) 2013;38:S149–60. doi: 10.1097/BRS.0b013e3182a7f449. [DOI] [PubMed] [Google Scholar]
- 3.Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am. 2010;41:193–202. doi: 10.1016/j.ocl.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Matz PG, Holly LT, Mummaneni PV, et al. Anterior cervical surgery for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:170–3. doi: 10.3171/2009.3.SPINE08724. [DOI] [PubMed] [Google Scholar]
- 5.Grabher P, Mohammadi S, Trachsler A, et al. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci Rep. 2016;6:24636. doi: 10.1038/srep24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen CY, Cui JL, Mak KC, et al. Diffusion tensor imaging of somatosensory tract in cervical spondylotic myelopathy and its link with electrophysiological evaluation. Spine J. 2014;14:1493–500. doi: 10.1016/j.spinee.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Cloney MB, Smith ZA, Weber KA, 2nd, et al. Quantitative Magnetization Transfer MRI Measurements of the Anterior Spinal cord Region are Associated with Clinical Outcomes in Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2017 doi: 10.1097/BRS.0000000000002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanty C, Massicotte EM, Fehlings MG, et al. Association of preoperative cervical spine alignment with spinal cord magnetic resonance imaging hyperintensity and myelopathy severity: analysis of a series of 124 cases. Spine (Phila Pa 1976) 2015;40:11–6. doi: 10.1097/BRS.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 9.Cui JL, Li X, Chan TY, et al. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. 2015;24:41–7. doi: 10.1007/s00586-014-3522-5. [DOI] [PubMed] [Google Scholar]
- 10.Gao SJ, Yuan X, Jiang XY, et al. Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur J Radiol. 2013;82:1940–5. doi: 10.1016/j.ejrad.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 11.De Leener B, Levy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. NeuroImage. 2017;145:24–43. doi: 10.1016/j.neuroimage.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Benzel EC, Lancon J, Kesterson L, et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–95. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 14.De Leener B, Fonov VS, Collins DL, et al. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2017;165:170–9. doi: 10.1016/j.neuroimage.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Levy S, Benhamou M, Naaman C, et al. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage. 2015;119:262–71. doi: 10.1016/j.neuroimage.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JT, Wang LF, Wang S, et al. Risk factors for poor outcome of surgery for cervical spondylotic myelopathy. Spinal Cord. 2016;54:1127–31. doi: 10.1038/sc.2016.64. [DOI] [PubMed] [Google Scholar]
- 17.Iyer A, Azad TD, Tharin S. Cervical Spondylotic Myelopathy. Clin Spine Surg. 2016;29:408–14. doi: 10.1097/BSD.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 18.Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013;31:287–305. doi: 10.1016/j.ncl.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16:176–87. doi: 10.1097/NRL.0b013e3181da3a29. [DOI] [PubMed] [Google Scholar]
- 20.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064–70. 73. [PubMed] [Google Scholar]
- 21.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–12. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol. 2000;84:2237–56. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- 25.Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85:679–98. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- 26.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–38. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- 27.Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- 28.Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–9. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 29.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, MacMillian EL, Jutzeler CR, et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: Evidence of demyelination. Neurology. 2017;89:602–10. doi: 10.1212/WNL.0000000000004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pujol S, Wells W, Pierpaoli C, et al. The DTI Challenge: Toward Standardized Evaluation of Diffusion Tensor Imaging Tractography for Neurosurgery. J Neuroimaging. 2015;25:875–82. doi: 10.1111/jon.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potgieser AR, Wagemakers M, van Hulzen AL, et al. The role of diffusion tensor imaging in brain tumor surgery: a review of the literature. Clin Neurol Neurosurg. 2014;124:51–8. doi: 10.1016/j.clineuro.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2016;10:192–238. doi: 10.1016/j.nicl.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banaszek A, Bladowska J, Podgorski P, et al. Role of Diffusion Tensor MR Imaging in Degenerative Cervical Spine Disease: a Review of the Literature. Clin Neuroradiol. 2016;26:265–76. doi: 10.1007/s00062-015-0467-y. [DOI] [PubMed] [Google Scholar]
- 35.Nouri A, Martin AR, Mikulis D, et al. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40:E5. doi: 10.3171/2016.3.FOCUS1667. [DOI] [PubMed] [Google Scholar]
- 36.Prados F, Ashburner J, Blaiotta C, et al. Spinal cord grey matter segmentation challenge. Neuroimage. 2017;152:312–29. doi: 10.1016/j.neuroimage.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samson RS, Levy S, Schneider T, et al. ZOOM or Non-ZOOM? Assessing Spinal Cord Diffusion Tensor Imaging Protocols for Multi-Centre Studies. PLoS One. 2016;11:e0155557. doi: 10.1371/journal.pone.0155557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin AR, De Leener B, Cohen-Adad J, et al. Clinically Feasible Microstructural MRI to Quantify Cervical Spinal Cord Tissue Injury Using DTI, MT, T2*-Weighted Imaging: Assessment of Normative Data and Reliability. AJNR Am J Neuroradiol. 2017;38:1257–65. doi: 10.3174/ajnr.A5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


