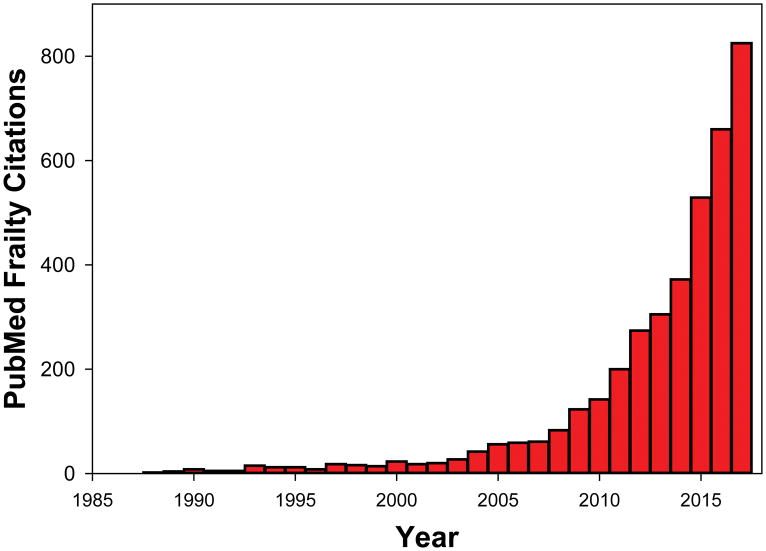
Frailty is fundamental to the science and practice of geriatric medicine and this overarching concept resonates with all involved in the care of older patients. “Frailty” citations in PubMed have quickly risen from the rare and exotic to nearly thousand “hits” in 2017 (Figure 1). This growth includes proliferation of frailty tools with a 2016 review identifying 67 different frailty instruments in the literature1. Reviews2, opinion pieces3 and consensus conference reports 4 continue to provide additional perspectives.
Figure 1.
PubMed citations of the term “frailty” 1985–2017.
While we can take pride in these achievements, lingering concerns suggest that the concept of frailty is still “work in progress”. Geriatricians profess to recognizing frailty “when they see it”, yet the precise meaning of the term, its conceptualization and even its use to describe either a state or a process can vary between equally thoughtful and well-informed individuals. Indeed, these issues generate confusion even among experienced clinicians. Also, in my role as Deputy Editor for this journal I have observed that authors often fail to provide a rationale for choosing a frailty tool or offer a conceptual framework for developing yet another frailty instrument. Finally, the conclusion of a recent editorial that “frailty is not ready for prime time as a full-fledged outcome measure in geriatrics research”5 is the most worrisome. The time lag from discovery to its translation into products, policy and practice is typically measured in decades6. Therefore, failure to test the impact of interventions on frailty could jeopardize significant progress as regards clinical frailty prevention or modification within our lifetimes.
Are there solutions to these challenges and do we need to complicate an already confusing issue further through another concept – resilience – as articulated in the contributions from Whitson et al7 and Varadhan et al8 in this issue? The answer to both questions is “yes”, but with important caveats.
Resilience is neither the converse of frailty, nor does it simply reflect its absence7. However, as articulated by Varadhan et al.8 frailty and physical resilience are closely linked to each other. This can be understood by studying underlying physiological principles contributing to normal homeostatic balance in the face of intrinsic and extrinsic stressors9, 10. When confronting complex multifactorial issues, a “swiss army knife” solution whereby one seeks to identify one single unifying idea or screening tool to answer all relevant questions under all conditions and in everyone is not realistic11. Similarly, silo-based approaches grounded within one discipline or one conceptual framework will also not work. Growing evidence indicates that in tackling frailty, resilience and related issues we must consider the human body as a complex system8, 12, 13 and that ultimate solutions will require input from different disciplines and research perspectives. Thus, it is not surprising to see that in addition to geriatricians and gerontologists, thought leaders in frailty have also included mathematicians13, biostatisticians8 and engineers8.
Engineers are trained as experts in the design, construction, maintenance and function of complex systems14. Such skills can provide insights into geriatricians’ efforts to help older adults maintain normal function and independence even when confronted with the varied stressors that can arise upon a variable and complex background of clinical, physiologic, biologic, behavioral and social changes seen with aging8.
In fully appreciating the remarkable complexity of the human body, it may be possible to identify some key overarching principals from the design of a complex system that is less complex – the Golden Gate Bridge. This graceful all-suspension bridge was designed in 1921 by an engineering team led by Joseph B Strauss. This was accomplished without computers using slide rules, pencils and paper, yet fundamental concepts remain relevant.
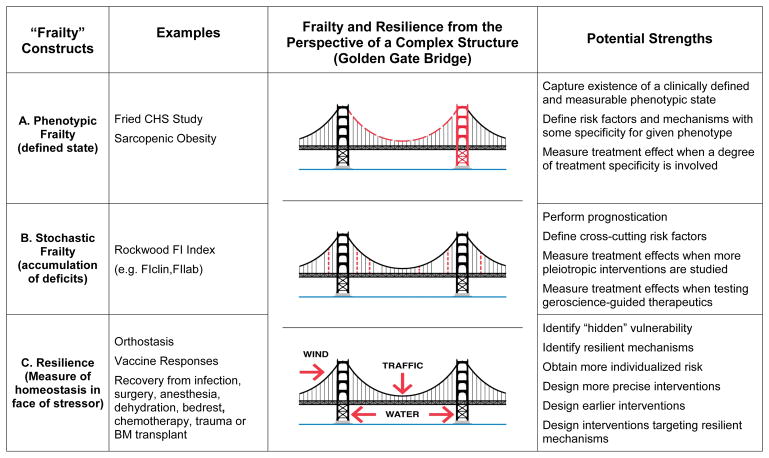
Even viewed by a non-engineer, some bridge components are critical. For example, failure of either tower or of the horizontal structure supporting the roadway would have catastrophic consequences. Failure of either main horizontal cable carrying the full weight of the bridge would render the bridge highly vulnerable while failure of both would cause its collapse. In contrast, the 250 pairs of vertical suspender cables spaced 50 feet apart across both sides of the bridge are equally important in their aggregate, yet they are individually less critical demonstrating considerable redundancy. One could envision the loss of multiple vertical cables without endangerment of the bridge’s integrity while there is no traffic or stress from prevailing winds or water currents. However, rush hour traffic compounded by a major storm could overcome the ability of this bridge, already compromised through the loss of multiple vertical cables, to remain resilient and intact. Threats to more critical structures could jeopardize the identity or phenotype of a bridge as a bridge (Figure 2A). As illustrated by Varadhan et al.8, a robust system (Figure 1b8) could be transformed into one that is less fit but still able to maintain a specific but now different phenotypic identity (Figure 1c8) with further progression resulting in even lower fitness with loss of any phenotypic identity and resilience (Figure 1d8). Such threats to more critical structures lead to a bridge that is still a bridge yet has the obvious appearance of vulnerability, thus reflecting phenotypic frailty. In contrast to such highly focused threats, more random or stochastic threats do not reflect any particular state or phenotype, but rather provide key insights into the accumulation of such damage and deficits taking place across the entire system (Figure 2B). Obviously, none of these issues can really be considered in isolation from the specific external threats in the form of traffic, wind or water currents challenging the system and thus reflected in resilience (Figure 2C).
Figure 2.
The Golden Gate Bridge is presented as a visual metaphor for the complementary contributions made by different conceptual constructs to the integrity and function of a complex system. A. Phenotypic frailty involves the existence of a specific state or phenotype that is defined by measurable changes involving structures such as a major tower (red) or horizontal cable (red long dash) that perform unique functions and are critical to the existence of the overall system. B. Stochastic frailty is defined as a process whereby the accumulation of deficits involving more redundant structures such as vertical cables (absent or red stippled) raises system vulnerability. C. Resilience evaluates the ability of the system to withstand expected stressors such as bridge traffic, wind and water currents (red arrows). All of these complementary perspectives must be considered when designing a bridge or providing care for older adults. Each approach has its strengths as regards specific questions and further research is needed. However, phenotypic frailty is most helpful when focusing on a specific clinical state associated with increased vulnerability and for measuring the effects of an intervention targeting risk factors or mechanisms that are relatively specific to that condition. Stochastic frailty may be most helpful for individual prognostication and for evaluating interventions that are more pleiotropic or that target shared risk factors or biological mechanisms.
In keeping with these concepts, the term “frailty” has been and continues to be widely used as a reflection of a specific phenotype or state of increased vulnerability associated with unintentional weight loss, sense of exhaustion, hand grip weakness, slow walking speed and low physical activity15. Other variants of this phenotype or state likely exist, with sarcopenic obesity involving declines in muscle mass and muscle performance in individuals who are obese as potentially the most distinct16. In contrast to the phenotypic approach, frailty has also been conceptualized as a process involving the accumulation of stochastic or random deficits17. Not only do these approaches not compete with each other, they provide essential and complementary insights. Moreover, frailty can have a negative impact on resilience regardless of whether it is present as a state or a process.
Finally, how should these considerations influence investigators’ decision to select a particular tool to measure frailty and/or resilience in the context of a clinical trial? Also, when and how does one justify the development of yet another frailty instrument?
Surprisingly few published clinical trials have included frailty as an outcome measure and to date none appear to have included measures of physical resilience. The LIFE study, a trial of a structured moderate-intensity physical activity program failed to improve frailty as measured using the SOF index, a simplified phenotypic index derived from inability to rise from chair 5 times without use of arms, a self-reported reduced energy level and weight loss18. In contrast, a combination of exercise and dietary restriction has been shown to result in major improvements in physical performance, weight loss and alterations in body composition used to define sarcopenic obesity19. Small trials testing a combination of testosterone and high calorie nutritional supplement20 or a goal-oriented multidisciplinary primary care plan21 have failed to demonstrate clear improvements in various versions of the Frailty Index, while showing encouraging trends.
In designing future trials, it may be necessary to consider the complementary strengths of different approaches to evaluating frailty and to select those that best match the nature of the proposed intervention and its goals (Figure 2). Tools designed to capture the existence of a specific frailty phenotype may be most appropriate outcome measures when expecting to see the kinds of detectable changes involving the criteria used to define that phenotype in the first place as seen following combined exercise and dietary restriction in older obese individuals with sarcopenic obesity19. Fried frailty phenotype and functional performance measures may also be especially helpful when evaluating interventions targeting mechanisms that are somewhat more specific for this particular state22. In contrast, benefits of more pleiotropic interventions such exercise or geroscience-guided therapeutics targeting multiple biological hallmarks of aging23 may be better captured using the Frailty Index. More research is needed, but performance characteristics of tools involving continuous as opposed to categorical measurements may need to be considered. It may also be advantageous to routinely include more than one frailty measurement in order to capture these complementary facets of frailty.
Few studies have combined measures of frailty and resilience in the same individuals. Therefore, it remains to be seen whether provocative tests will be able to uncover hidden deficits in resilience and frailty detectable only when confronted with a stressor9. An intriguing cross-sectional examination of 4,334 community-dwelling older adults recruited as part of The Irish Longitudinal Study of Ageing (TILDA) by O’Connel et al[24] in this issue, has shown an association between the frailty phenotype and decreased physiologic resilience involving reduced blood pressure and heart recovery upon standing from the lying position. In a previous analysis, these declines in resilience were shown to increase 2-year risk of injurious falls[25]. There is a great need to include both frailty and resilience measures in future longitudinal studies and clinical trials. The setting of elective surgery, vaccination, chemotherapy and bone marrow transplantation offer especially promising clinical situations for the study and implementation of such approaches9. Finally, there is no doubt that existing frailty instruments may need to be refined and new ones designed. However, all such efforts will require careful thought leading to clear goals and justification.
Acknowledgments
Financial Disclosure: This work was supported by National Institute on Aging (NIA) Grants UH2AG056925, R01AG048023, P01AG021600 and R01AG052608.
Footnotes
Conflict of Interest: Dr. Kuchel is Deputy Editor of the Journal of the American Geriatrics Society.
Author Contributions: Dr. Kuchel was responsible for manuscript preparation and revision.
Sponsor’s Role: Although NIA partners have been instrumental in framing the discussion on the emerging construct of physical resilience, the sponsor had no role in the design or preparation of the paper.
References
- 1.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Research Reviews. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
- 4.Walston J, Robinson TN, Zieman S, et al. Integrating Frailty Research into the Medical Specialties-Report from a U13 Conference. J Am Geriatr Soc. 2017;65:2134–2139. doi: 10.1111/jgs.14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RT, Covinsky KE. Frailty as an Outcome in Geriatrics Research: Not Ready for Prime Time? Ann Intern Med. 2018;168:361–362. doi: 10.7326/M17-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanney SR, Castle-Clarke S, Grant J, et al. How long does biomedical research take? Studying the time taken between biomedical and health research and its translation into products, policy, and practice. Health Res Policy Syst. 2015;13:1. doi: 10.1186/1478-4505-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitson HE, Cohen HJ, Schmader KE, Morey MC, Kuchel G, Colon-Emeric CS. Physical Resilience: Not Simply the Opposite of Frailty. J Am Geriatr Soc. 2018 doi: 10.1111/jgs.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varadhan R, Walston J, Bandeen-Roche K. Can Physical Resilience and Frailty in Older Adults be Linked by the Study of Dynamical Systems? Journal of the American Geriatrics Society. 2018 doi: 10.1111/jgs.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadley EC, Kuchel GA, Newman AB Workshop S Participants. Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. doi: 10.1093/gerona/glx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchel GA. Aging and Homeostatic Regulation. In: halter JB, Ouslander JG, Studenski S, et al., editors. Hazzard’s Geriatric Medicine and Gerontology. 7. McGraw-Hill; 2017. pp. 681–690. [Google Scholar]
- 11.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol. 2017 doi: 10.1016/j.exger.2017.08.027. [DOI] [PubMed]
- 13.Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K. Aging, frailty and complex networks. Biogerontology. 2017;18:433–446. doi: 10.1007/s10522-017-9684-x. [DOI] [PubMed] [Google Scholar]
- 14.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obesity Research. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 17.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 18.Trombetti A, Hars M, Hsu FC, et al. Effect of Physical Activity on Frailty: Secondary Analysis of a Randomized Controlled Trial. Ann Intern Med. 2018;168:309–316. doi: 10.7326/M16-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. The New England journal of medicine. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theou O, Chapman I, Wijeyaratne L, et al. Can an Intervention with Testosterone and Nutritional Supplement Improve the Frailty Level of Under-Nourished Older People? J Frailty Aging. 2016;5:247–252. doi: 10.14283/jfa.2016.108. [DOI] [PubMed] [Google Scholar]
- 21.Theou O, Park GH, Garm A, Song X, Clarke B, Rockwood K. Reversing Frailty Levels in Primary Care Using the CARES Model. Can Geriatr J. 2017;20:105–111. doi: 10.5770/cgj.20.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manini TM, Anton SD, Beavers DP, et al. ENabling Reduction of Low-grade Inflammation in SEniors Pilot Study: Concept, Rationale, and Design. J Am Geriatr Soc. 2017;65:1961–1968. doi: 10.1111/jgs.14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metabolism. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell MDL, Savva GM, Finucane C, Romero-Ortuno R, Fan CW, Kenny RA. Impairments in Hemodynamic Responses to Orthostasis Associated with Frailty: Results from TILDA. J Am Geriatr Soc. 2018 doi: 10.1111/jgs.15327. [DOI] [PubMed] [Google Scholar]
- 25.Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired Orthostatic Blood Pressure Recovery Is Associated with Unexplained and Injurious Falls. J Am Geriatr Soc. 2017;65:474–482. doi: 10.1111/jgs.14563. [DOI] [PubMed] [Google Scholar]