Abstract
Glucose tolerance is lower at night and higher in the morning. Shift workers, who often eat at night and experience circadian misalignment (i.e., misalignment between the central circadian pacemaker and the environmental/behavioral cycles), have an increased risk of type 2 diabetes. To determine the separate and relative impacts of the circadian system, behavioral/environmental cycles, and their interaction (i.e., circadian misalignment) on insulin sensitivity and β-cell function, we used the oral minimal model to quantitatively assess the major determinants of glucose control in 14 healthy adults, using a randomized, cross-over design with two 8-day laboratory protocols. Both protocols involved 3 baseline inpatient days with habitual sleep/wake cycle, followed by 4 inpatient days with same nocturnal bedtime (circadian alignment) or with 12-h inverted behavioral/environmental cycles (circadian misalignment). Our data showed that circadian phase and circadian misalignment affect glucose tolerance through different mechanisms. While the circadian system reduces glucose tolerance in the biological evening compared to the biological morning mainly by decreasing both dynamic and static β-cell responsivity, circadian misalignment reduced glucose tolerance mainly by lowering insulin sensitivity, not by affecting β-cell function.
1. Introduction
Glucose tolerance varies greatly across the day in healthy humans, peaking in the morning and with low levels in the evening/night. Diminished insulin sensitivity and β-cell function, two major determinants of type-2 diabetes (T2D) risk, are responsible for decreased glucose tolerance in the evening[1, 2]. Both the endogenous circadian system and behavioral/environmental cycles (e.g., fasting/feeding, sleep/wake, physical activity, and dark/light cycles) contribute to the diurnal variations in glucose control[3]. However, since these two factors typically cycle in synchrony in diurnally-active individuals, it is impossible to assess their separate contribution under normally entrained conditions (i.e., sleep at night, eating during the day). Furthermore, night shift workers chronically experience recurrent circadian misalignment, a condition where environmental/behavioral cycles are out-of-sync with the endogenous circadian system. This circadian misalignment may explain, in part, why shift work increases T2D risk[4]. Thus, understanding the separate and relative contribution of the endogenous circadian system and circadian misalignment—after accounting for behavioral cycle effects, on different components of glucose control is important for the general population and for shift workers.
We are not aware of any studies that could systematically address the independent effects of behavioral cycles, the endogenous circadian system, and circadian misalignment on insulin sensitivity and β-cell function in humans. This is partly because the gold-standard methods to quantify insulin sensitivity and insulin secretion (e.g., intravenous glucose tolerance tests, hyperglycemic clamp, euglycemic-hyperinsulinemic clamp) require long fasting durations and artificial manipulations of glucose levels. Thus, implementing such tests in a circadian protocol disrupts the fasting-feeding cycle and physiology, thus making it very difficult to design a balanced study to mathematically separate behavioral, circadian phase and circadian misalignment effects. The oral minimal model method that quantifies insulin sensitivity and β-cell responsivity from a mixed-meal test[5], allows us to circumvent above limitations and perform in-depth assessments of glucose control in two separate 8-day in-laboratory protocols with randomized, cross-over design.
2. Method
Other aspects of this study—which was designed to test separate hypotheses—have previously been published[6, 21, 22, 23, 24].
2.1 Participants and Experimental Design
Fourteen healthy nonsmoking, drug- and medication-free (excepting oral contraceptives) adults completed this study [mean age±SD, 28±9 y; BMI, 25.4±2.6 kg/m2; HbA1C, 5.38±0.26%; eight men]. Identical mixed meals (33.3% of calculated daily calorie intake) given 1-h and 13-h following scheduled wake time. Fasting blood was drawn 7 min before the mixed meal, and postprandial blood was drawn every 10 min for 90 min, starting 10 min after the participant began eating the test meal, and subsequently every 30 min for the next 90 min, totaling 3 h. Details of methods, subject recruitment, screening, pre-inpatient study conditions, diet, and mixed-meal test can be found elsewhere[6].
2.2 Data Analysis and Statistics
Oral minimal model method
Insulin sensitivity (SI) was estimated from plasma glucose and insulin concentrations measured during 3-hr mixed-meal tests using the oral glucose minimal model[7], which measures the overall effect of insulin on stimulating glucose disposal and inhibiting glucose production and has been successfully validated against model-independent measurements using multiple-tracer meal protocols and euglycemic-hyperinsulemic clamps.
β-cell function was quantified from C-peptide and glucose data using the C-peptide minimal model[5]. Basal β-cell function (Φb) and static β-cell function (Φs) measure insulin secretion in response to the basal glucose concentration or a given increment in glucose above basal glucose concentrations, respectively. Dynamic β-cell function responsivity (Φd) measures the stimulatory effect exerted by the rate of increase in glucose concentration on insulin secretion. Φd is likely to represent secretion of promptly releasable insulin, Φs reflects the provision of new insulin into a releasable pool. Total β-cell responsivity to glucose (Φtot), a measure of overall insulin secretion, can be calculated from Φs and Φd[8]. Finally, the disposition index (DI), assessing the appropriateness of insulin secretion for the prevailing level of insulin resistance, was calculated by multiplying Φtot by SI.
Statistics
Analyses for mixed-meal tests were performed on natural log-transformed data. Results for transformed parameters were back-transformed (exponentiated) and reported on the original scale. Estimates for log-normally distributed data were reported as geometric means (95% CI). Linear mixed models with participant as random factor, tested the independent effects of the behavioral cycle [breakfast vs. dinner (1h or 13h after wake time, respectively)], circadian phase [8AM (biological morning) vs. 8PM (biological evening)], alignment condition (circadian alignment vs. circadian misalignment), and their interaction with test day (first vs. third) on SI, Φ indices, and DI. Statistical significance was accepted as P<0.05.
3. Results
We did not find any significant interaction effects between duration of exposure (i.e., test day) and the main effects (i.e., circadian phase, circadian misalignment, and behavioral cycle; all P≥0.088). Therefore, all the percentage changes and geometric means were calculated with test day 1 and 3 combined.
Circadian phase effects, independent of behavioral cycle effects: β-cell function was higher in the biological morning than in the biological evening (Fig.1 and 2, left panels)
Figure 1.
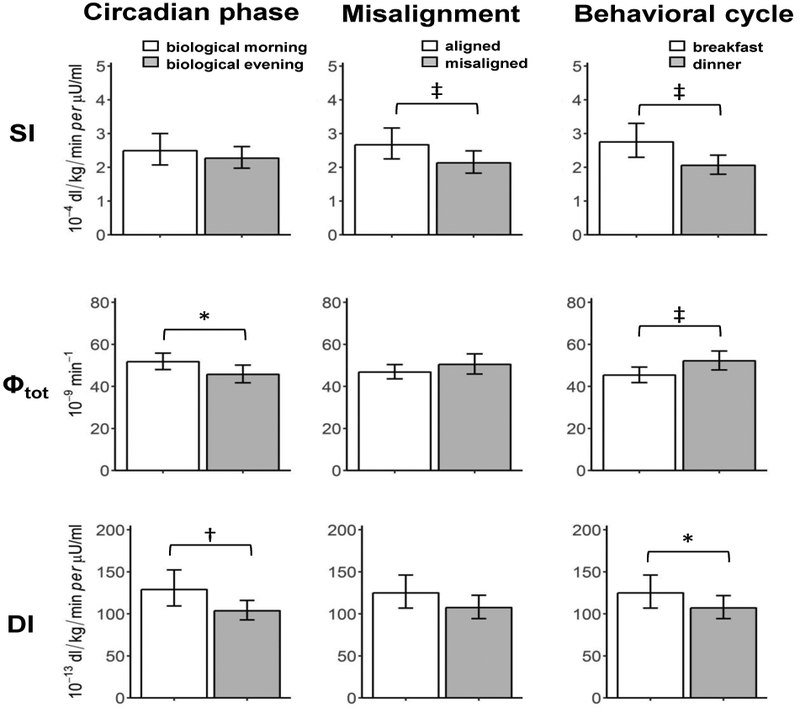
Effects of the circadian phase (left), circadian misalignment (central), and behavioral cycle (right) on indices of insulin sensitivity (SI, top), total β-cell responsivity (Φtot, middle), and Disposition index (DI, bottom). Data are reported as geometric means (95% CI). *P<0.05, †P<0.01, ‡P<0.001.
Figure 2.
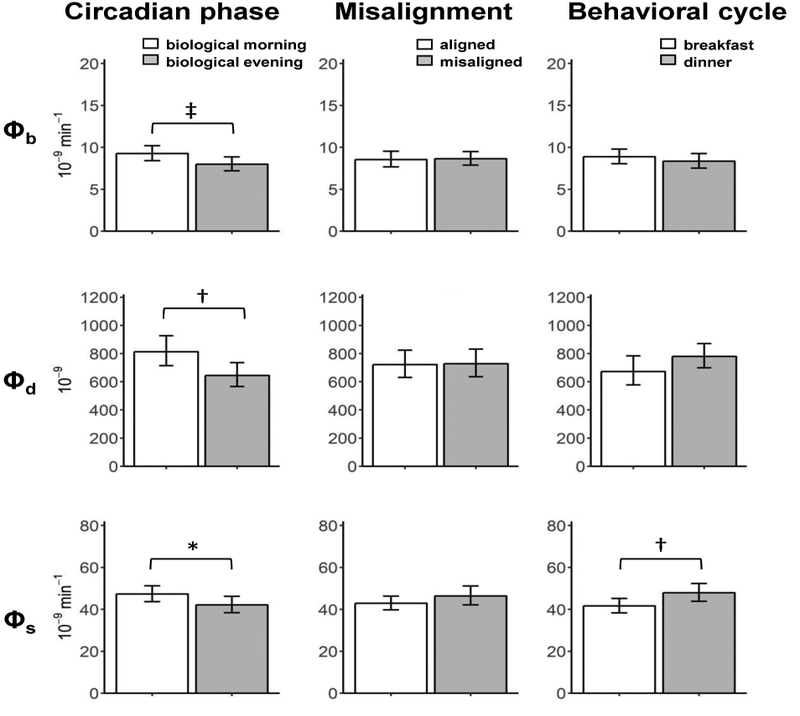
Effects of the circadian phase (left), circadian misalignment (central), and behavioral cycle (right) on indices of basal β-cell function (Φb, top), dynamic β-cell responsivity (Φd, middle), and static β-cell responsivity (Φs, bottom). Data are reported as geometric means (95% CI). *P<0.05, †P<0.01, ‡P<0.001.
All measures of β-cell function were lower in the biological evening than biological morning, with Φb by 13.7% (95% CI 6.6, 20.4 ; P<0.0001), Φd by 21.6% (95% CI 4.7, 35.6; P=0.0086), Φs by 11.3% (95% CI 1.2, 20.3,; P = 0.034), and Φtot by 14.7% (95% CI 2.0, 21.0; P=0.022). Similar results were found for DI, with a 19.1% reduction in the biological evening (95% CI 4.7, 31.4; P = .0.0034). There was no significant circadian effect on SI (95% CI −4.2, 19.0; P=0.06).
Circadian misalignment reduced insulin sensitivity independent of circadian phase or behavioral cycle effects (Fig.1 and 2, middle panels)
SI was 16.5% lower in the circadian misalignment than alignment condition (95% CI 7.9, 24.3; P=0.0007). There were no significant effects of circadian misalignment on β-cell responsivity, as all Φ indices and DI did not significantly differ between circadian alignment and misalignment conditions (all P>0.099).
Behavioral cycle effects, independent of circadian phase effects: Insulin sensitivity was lower at dinner than at breakfast, while β-cell function in response to meal was higher at dinner than at breakfast (Fig.1 and 2, right panels)
SI was 25.4% lower (95% CI 13.2, 35.9; P<0.0001) at dinner than at breakfast. While no significant difference in Φb (95% CI −2.0, 12.3; P=0.054), Φtot was 14.7% higher at dinner than at breakfast (95% CI 2.3, 28.5; P=0.0046) due to increased Φs by 15.0% (95% CI 3.0, 28.4; P=0.0046) without significant difference in Φd (95% CI −3.1, 39.0; P=0.0579). DI was 14.4% lower at dinner than at breakfast (95% CI −3.3, 29.2; P=0.038).
4. Discussion
Our results revealed that the endogenous circadian system and circadian misalignment, after controlling for behavioral cycle influences, have independent and differential impacts on insulin sensitivity and β-cell function in healthy adults. First, the endogenous circadian system strongly regulated all aspects of β-cell function, without significant effect on insulin sensitivity. Φb, representing fasting β-cell responsivity, was lower in the biological evening. Furthermore, because Φd assesses insulin secretion in response to the maximum glucose increase after meal ingestion, the lower Φd in the biological evening reveals that the circadian system governs multiple immediate steps in the insulin secretory pathway (e.g., glucose sensing, rate of granule docking, priming, and exocytosis)[5]. Circadian regulation also likely influenced the distal steps in the insulin secretory pathway (e.g., incretin stimulation, synthesis, processing, granule maturation) because Φs was also lower in the biological evening[5]. The 14.7% decrease of Φtot in the biological evening is of particular clinical relevance, since the magnitude of change is similar to the difference between elderly and young individuals[9]. Our results are consistent with the study by Sharma et al. in which rotating shift workers had impaired β-cell function in the evening of their night shift as compared to in the morning of their day shift[10]. However, our design further allows us to distinguish whether such decline of β-cell function during night shifts is due to circadian phase and/or circadian misalignment, indicating a clear effect of the circadian phase, but not misalignment, on β-cell function. The circadian regulation of β-cell function may provide an explanation for the increased risk of poor glycemic control in late eaters and shift workers who often eat in the biological evening/night[11, 12]. Our results also support the findings that avoiding large meals with high glycemic index in the late evening or nighttime may prevent postprandial hyperglycemia, thus reduce the risk of T2D in the long run[13].
Furthermore, our finding that circadian misalignment specifically reduced insulin sensitivity, without significantly changing any β-cell responsivity indices or DI, is consistent with Leproult and colleagues’ findings[14], showing that prior circadian misalignment in men, independent of sleep loss, decreased insulin sensitivity without significant reduction in DI. Our results also agree with the study by Bescos et al reporting reduced insulin sensitivity after four days of simulated night shift using hyperinsulinaemic-euglycaemic clamp [25]. However, our participants consumed highly-controlled diet throughout the two protocols, which minimized the potential influences caused by variations in caloric intake. The 16.5% decrease in SI upon exposure to circadian misalignment is notable, since this is about one-third of the difference in SI between elderly and young individuals[9]. The decrease in SI during circadian misalignment may be, in part, due to the increased growth hormone and fasting free fatty acid levels during nighttime wakefulness that we reported[6] because both decrease insulin sensitivity[15, 16]. In our short-term circadian misalignment exposure (3 days), we found no evidence of altered β-cell function due to circadian misalignment. However, the insulin resistance induced by circadian misalignment increases insulin demand, which—upon chronic exposure—may impair β-cell function, thus resulting in T2D. Indeed, other studies using longer term circadian disruption including clock gene knock-out and constant light exposure in rodents and prolonged misalignment together with sleep deprivation in humans did show β-cell dysfunction[17-19].
As for the independent effects of the behavioral cycle, SI was lower at dinner than at breakfast, while β-cell function was higher at dinner. Previous studies have reported that under normally-entrained conditions (i.e., sleep at night, evening dinner), insulin sensitivity and β-cell responsivity peak in the morning, and deteriorate as the day progresses[1, 2]. Here, we show that such decrease in insulin sensitivity is mostly caused by the behavioral cycle, while the circadian system dominates the deterioration in β-cell responsivity. Interestingly, the behavioral cycle itself significantly improved total β-cell responsivity, specifically static response (Φs), at dinner. This may be partially explained by a phenomenon called “second meal effect”[20], in which the magnitude of insulin release is enhanced by previous glucose exposure.
In conclusion, the results show separate effects of the endogenous circadian system, the behavioral cycle, and circadian misalignment on insulin sensitivity and β-cell responsivity with relevance for daily glucose regulation in diurnally active people as well as night shift workers.
Acknowledgements
We thank the research volunteers and Brigham and Women’s Hospital’s Center for Clinical Investigation nursing and technical staff.
Funding
This study was supported by NIH Grant R01HL094806 (F.A.J.L.S.) and UL1RR025758 (to Harvard University and Brigham and Women’s Hospital). J.Q. was supported in part by American Diabetes Association grant #1-17-PDF-103 (J.Q.). F.A.J.L.S. was supported in part by NIH grants R01HL094806, R01HL118601, R01DK099512, R01DK102696, and R01DK105072 (F.A.J.L.S.).
Disclosure statement
C.J.M. reports receiving salary from Grünenthal Ltd, UK., which relationship is not related to the present article. F.A.J.L.S. received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, and Vanda Pharmaceuticals.
Author contributions
J.Q., C.J.M., and F.A.J.L.S. conceived the idea and designed the study. C.J.M. and F.A.J.L.S., performed research; J.Q., C.D.M., and C.C. analyzed data; J.Q. and F.A.J.L.S. wrote the manuscript; J.Q., C.D.M., C.J.M., C.C., and F.A.J.L.S. interpreted the data and edited the manuscript.
References
- 1.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, et al. (2012). Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61, 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cauter E, Polonsky KS, and Scheen AJ (1997). Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 18, 716–738. [DOI] [PubMed] [Google Scholar]
- 3.Qian J, and Scheer FA (2016). Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol Metab 27, 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheer FA, Hilton MF, Mantzoros CS, and Shea SA (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106, 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, and Rizza R (2014). The oral minimal model method. Diabetes 63, 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FA (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A 112, E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalla Man C, Caumo A, and Cobelli C (2002). The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49, 419–429. [DOI] [PubMed] [Google Scholar]
- 8.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, and Cobelli C (2001). Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 50, 150–158. [DOI] [PubMed] [Google Scholar]
- 9.Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, and Rizza R (2007). Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293, E1–E15. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Laurenti MC, Dalla Man C, Varghese RT, Cobelli C, Rizza RA, Matveyenko A, and Vella A (2017). Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia 60, 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse SA, Ciechanowski PS, Katon WJ, and Hirsch IB (2006). Isn't this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care 29, 1800–1804. [DOI] [PubMed] [Google Scholar]
- 12.Pan A, Schernhammer ES, Sun Q, and Hu FB (2011). Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8, e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan LM, Shi JW, Hampton SM, and Frost G (2012). Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr 108, 1286–1291. [DOI] [PubMed] [Google Scholar]
- 14.Leproult R, Holmback U, and Van Cauter E (2014). Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boden G, Lebed B, Schatz M, Homko C, and Lemieux S (2001). Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50, 1612–1617. [DOI] [PubMed] [Google Scholar]
- 16.Rizza RA, Mandarino LJ, and Gerich JE (1982). Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31, 663–669. [DOI] [PubMed] [Google Scholar]
- 17.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, et al. (2015). Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian J, Block GD, Colwell CS, and Matveyenko AV (2013). Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 62, 3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, and Shea SA (2012). Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4, 129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakubowicz D, Wainstein J, Ahren B, Bar-Dayan Y, Landau Z, Rabinovitz HR, and Froy O (2015). High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia 58, 912–919. [DOI] [PubMed] [Google Scholar]
- 21.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, and Scheer FA (2015). The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 23, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris CJ, Purvis TE, Hu K, and Scheer FA (2016). Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker MA, Morris CJ, Morgan A, Yang J, Myers S, Pierce JG, Stickgold R, & Scheer FA (2017). The relative impact of sleep and circadian drive on motor skill acquisition and memory consolidation. Sleep, 40(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chellappa SL, Morris CJ, & Scheer FA (2018). Daily circadian misalignment impairs human cognitive performance task-dependently. Scientific reports, 8(1), 3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bescos R, Boden MJ, Jackson ML, Trewin AJ, Marin EC, Levinger I, … & Owens JA (2018). Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiologica, 223(2), e13039. [DOI] [PubMed] [Google Scholar]


