Abstract
Objectives
The noradrenergic system plays an important role in the pathophysiology of alcohol use disorder (AUD). Medications in this class may reduce drinking. Our aims were to investigate this in a unique sample of individuals with AUD.
Methods
36 individuals with AUD were randomized to treatment with prazosin, an alpha-1 noradrenergic antagonist, or placebo for 6 weeks (target daily dose 16 mg). Hierarchical linear modeling was used to examine the effect of treatment group on rate of change in primary [drinks per week (DPW)] and several secondary outcome measures..
Results
Prazosin did not significantly affect rate of reduction in alcohol use in the intent to treat sample (n=36) compared to placebo, but did significantly increase the rate of reduction in DPW in an optimal treatment exposure subgroup (beta=−0.3; p=0.01; event rate ratio=0.74; CI=0.59,0.93; n=27). Poor adherence and tolerability may have contributed to null effects. Diastolic blood pressure (DBP) moderated the effects of treatment group on rate of reduction in drinks per drinking day, supporting previous work in doxazosin, another alpha-1 antagonist. Specifically, prazosin was associated with greater rates of reduction in drinking compared to placebo in individuals with high but not low DBP.
Conclusions
Our findings do not support the clinical utility of prazosin for all treatment-seeking AUD, but post-hoc analyses indicate that it might have some efficacy in individuals who can tolerate it. Further work exploring the clinical utility of DBP as a treatment matching variable, and defining optimal values using sensitivity and specificity analyses, is warranted.
Keywords: prazosin, alcohol use disorder, adherence, adrenergic, blood pressure
Introduction
The norepinephrine system plays an important role in AUD development and maintenance (Kenna et al. 2016; Koob 2008; Simpson et al. 2015), with hyper-activation of this system related to greater alcohol reward (Mitrano et al. 2012), withdrawal (Kovacs et al. 2002; Patkar et al. 2003), and stress-induced relapse (Kenna et al. 2016; Koob 2009; Simpson et al. 2015). This knowledge informs theories that reductions in noradrenergic tone could enhance abstinence, and has motivated several groups to study alpha-1 noradrenergic antagonists for AUD treatment (Le et al. 2011; O’Neil et al. 2013; Rasmussen et al. 2009; Simpson et al. 2015; Simpson et al. 2009).
Recent animal (Froehlich et al. 2015; Rasmussen et al. 2009) and human studies demonstrate that noradrenergic antagonists such as prazosin may have efficacy for the treatment of AUD, producing reductions in drinking days per week and drinks per week (DPW) from 3–6 weeks (Simpson et al. 2009) in individuals with AUD alone, and in percent days drinking per week and percent days heavy drinking per week in individuals with co-occurring AUD and post-traumatic stress disorder (PTSD) (Simpson et al. 2015) relative to placebo. In a human laboratory study, prazosin reduced craving, anxiety and negative emotion induced by an alcohol cue and/or stressor relative to placebo (Fox et al. 2012). Doxazosin, another alpha-1 noradrenergic receptor antagonist, was not effective in reducing drinking overall in an AUD sample relative to placebo (Kenna et al. 2016), but did reduce DPW and heavy drinking days in individuals with a positive family history of alcoholism (Kenna et al. 2016). In another analysis of these data, doxazosin was also found to reduce DPDD in those with elevated diastolic blood pressure (DBP) (Haass-Koffler et al. 2017).
As is evident from the nuanced findings from the aforementioned doxazosin trial, AUD medication effects may be affected by heterogeneity in patient samples and by specific sub-types within the AUD spectrum (Bogenschutz et al. 2009; Hutchison 2010; Kampman et al. 2007; Mann et al. 2009; Mann et al. 2014; Roache et al. 2008; Seneviratne and Johnson 2015; Wilcox and Bogenschutz 2013). An NIH-wide initiative, Precision Medicine, highlights the importance of searching for variables that allow patients to be matched to particular treatments so as to identify treatment moderators in the context of clinical trials. For prazosin, there are several important potential moderators to consider. As mentioned previously, a positive family history and elevated DBP have been found to predict a better response to treatment with doxazosin in AUD (Haass-Koffler et al. 2017; Kenna et al. 2016). Given prazosin’s mechanism of effect (reduction of noradrenergic tone) and its efficacy in ameliorating some of the anxiety-related symptoms in PTSD (Germain et al. 2012; Raskind et al. 2007; Raskind et al. 2003; Raskind et al. 2013; Simpson et al. 2015; Taylor et al. 2008) level of anxiety is another promising potential moderator, which, to our knowledge has not yet been explored.
This study was a small clinical trial of prazosin for the treatment of AUD. Our primary aim was to measure the effects of prazosin on drinking [primary measure: DPW], and to provide further information on safety and tolerability of this medication in individuals with AUD. Our second aim was to examine several potential moderators, hypothesizing that high baseline DBP and anxiety would predict a more robust treatment response.
Materials and Methods
Participants
Thirty-six individuals (63.9% male) between the ages of 18 and 65 (39.6 ± 11.4) with AUD interested in cutting back or quitting drinking were recruited through newspapers, online postings like craigslist, and flyers. To be enrolled, individuals were required to be English-speaking, want to cut back or quit drinking (treatment seeking), have at least four heavy drinking days (> 4 standard drinks for women, > 5 for men) in the past month, and meet criteria for alcohol dependence in the past three months1. Participants were excluded if they were currently undergoing alcohol treatment, taking anti-depressant, anti-craving, anxiolytic, anti-psychotic, mood stabilizing, or anti-convulsant medications, had any severe neurologic, cardiac, hepatic, or renal medical issues or other serious medical conditions, had co-morbid diagnoses of PTSD, schizophrenia, schizoaffective disorder, bipolar I, or dependence on another drug other than nicotine or cannabis, had suicidal thoughts during the past month, or were pregnant.
Trial Procedures
A 45-day randomized double-blind placebo-controlled trial of prazosin was conducted at the University of New Mexico Center for Psychiatric Research (clinicaltrials.gov identifier NCT01916941). The study protocol was approved by the University of New Mexico Health Sciences Center Human Resources and Review Committee2. Once it was confirmed participants qualified for the study, they were randomized to a medication group by the pharmacist. While on medication, participants were treated using the COMBINE Medical Management Protocol (MM) (Anton et al. 2006) over eight visits (six in person, two over the phone). Further details on trial procedures and other methods are available in supplemental materials and Figure S1.
Study drug
Prazosin was given thrice daily, titrating over the first 2 weeks with a target dose of 16 mg, mirroring that of previous work using this medication in AUD (Simpson et al. 2009) (Table 1), and participants were treated for six weeks. The provider had discretion to reduce the participants’ dose of medication or stop the medication if they reported clinically significant side effects or experienced orthostatic hypotension by vital signs.
Table 1.
Medication Schedule
| Day(s) | Total Daily Dosage (mg) | Dose Timing (mg) (9am/3pm/9pm)* |
|---|---|---|
| 1–2 | 1 | 0/0/1 |
| 3–4 | 3 | 1/1/1 |
| 5–7 | 6 | 2/2/2 |
| 8–10 | 10 | 2/2/6 |
| 11–14 | 14 | 4/4/6 |
| 15–45 | 16 | 4/4/8 |
These are approximate times, but participants were able to adjust this as needed, with instructions to take the doses at least 4 hours apart
Randomization and Blinding
Participants were randomized to prazosin or placebo by the study pharmacist who never had contact with the patients. The randomization was stratified based on whether they had an anxiety disorder diagnosis [Structured Clinical Interview for DSM Disorders, (Kranzler et al. 1996)], given the known beneficial effects of prazosin in PTSD, and the possibility that individuals who had greater anxiety might respond better to prazosin than those with lower anxiety, and allocation was determined based on a randomization table provided to the study pharmacist at study onset. After the participants had finished the medication schedule (usually a few days after the tenth visit), participants were asked what group they thought they were assigned to (supplementary results), the blind was broken, and patients were informed of their random assignment.
Measures
Our primary drinking outcome measure was DPW, and we obtained measures at two-week intervals (90 days from baseline, 0–2 weeks after medication initiation, 2–4 weeks after medication initiation, and 4–6 weeks after medication initiation) from the Timeline Followback calendar method drinking assessment tool. We also obtained information within similar time frames on drinks per drinking day (DPDD), percent days abstinent (PDA) and percent heavy (>5 standard drinks for men, >4 for women) drinking days (PHDD).
Past week anxiety was assessed with the Patient-Reported Outcomes Measurement Information System Short Form v1.0 Anxiety 8a (PROMIS) at the baseline visit (Schalet et al. 2016).
Participant blood pressure was measured at all visits while sitting (sit) and after standing for at least a minute (std). Screening DBP was utilized in moderator analyses (instead of systolic), given the results from previous work (Haass-Koffler et al. 2017) showing that only DBP moderated response to doxazosin for the treatment of AUD.
Adverse Events
A modified version of the Systematic Assessment for Treatment Emergent Effects (SAFTEE) (Levine and Schooler 1986) was obtained at all MM visits by research staff from subjects who were still taking the medication and, in those who had discontinued, to verify side effects had subsided.
Medication Adherence
We measured adherence both by pill counts and self-report. Each of these approaches produced two total adherence percentages: full and adjusted. The full total was number of pills taken divided by the full, ideal, prescription (equivalent to 304 2mg pills). The adjusted total was number of pills taken divided by number of pills each participant was expected to take based on any changes in prescription (due to side effects) by the medical provider.
Statistical Analyses
For Aim 1, we used hierarchical linear modeling and, in particular, we used a lagged growth model. Analyses were performed with HLM 7 (Raudenbush et al. 2004), and used restricted maximum likelihood estimation and robust standard errors. Outcome data were count, and the models assumed a Poisson distribution with constant exposure. DPW was the primary outcome measure and DPDD, PDA, and PHDD were secondary outcome measures. HLM is an ideal method for handling missing data as it does not generate listwise deletion of cases and uses all available data. For drinking measures, time was coded as 0, 1, 2 for 0–2, 2–4, and 4–6 weeks following medication initiation, respectively, and entered as a fixed factor. Baseline values of the outcome measure and medication condition were entered as predictors in all models and the slope, or rate of change in the outcome over time, was our dependent variable of interest. Intercept and slope were both predicted in level 2, and both were allowed to vary. P values of <0.05 for tests of the coefficient associated with medication condition in the models predicting slope indicated that the rate of change in drinking over time differed between the two treatment groups (as opposed to tests in the models predicting intercept which would be a mean difference test).
In addition to our intent to treat analysis (n=36), we also performed analogous exploratory post-hoc analyses in the subgroup (n=27) that had adequate medication exposure [excluding those who dropped out immediately after medication initiation and who discontinued medications] and in a smaller subgroup (n=26) after excluding an outlier.
For Aim 2, several moderator analyses were performed with HLM in the intent to treat sample after eliminating an extreme outlier (n=35), using a similar set of models. We removed this outlier for all moderator analyses, as moderator results changed depending on whether this outlier was included or excluded, and graphs of drinking quantities over time showed a non-standard trajectory when the outlier was included. For these analyses, an interaction term, the original continuous or binary moderator, the baseline value of the outcome, and condition were added as predictors, and the rate of change over time in the outcome were the outcomes of interest. We limited moderator analyses to DPW and DPDD [DPW was our primary outcome and the moderator effect of DBP was measured with DPDD in previous work (Haass-Koffler et al. 2017)]. The PROMIS anxiety variable was binarized using a median split. The DBP variables (sit and std) were binarized based on values > or <90. These binary moderators were multiplied by medication condition to create the interaction term. However, diverging from previous work (Haass-Koffler et al. 2017), we used 90 as a cutoff for high versus low DBP grouping instead of 80, as only 25% of our sample had a DBP<80, whereas approximately 50% had a DBP<90. Only results from moderator analyses with a significant interaction term are reported, and statistical tests by moderator subgroup were performed in a similar fashion as the main effects tests (effect of condition on rate of change in DPW and DPDD within relevant subgroups).
Results
Participant Flow and Characteristics
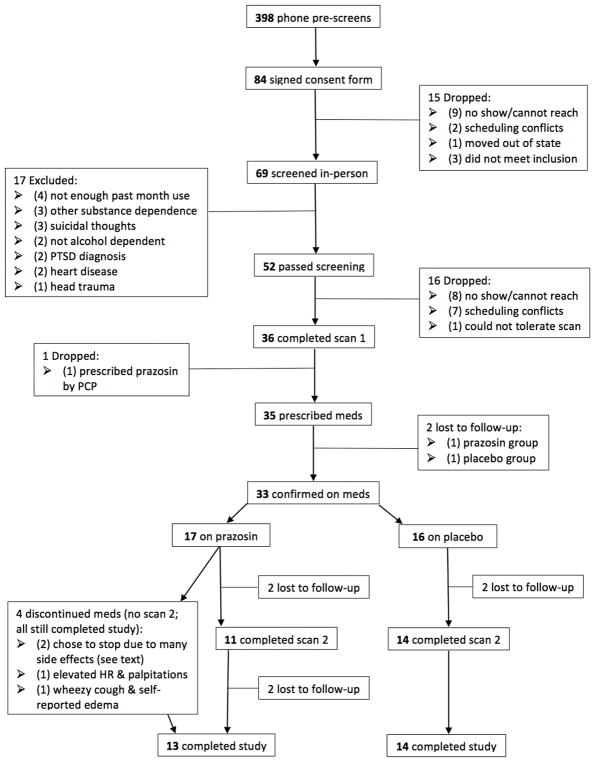
Three hundred ninety-eight individuals were screened by phone (9/13–5/16). Figure 1 summarizes the study flow. Thirty-six individuals were initiated on medications, with 33 confirmed as starting on medication (17 prazosin, 16 placebo) and 31 attending at least one follow-up visit. Three participants dropped out of the study immediately after medication initiation (placebo = 2, prazosin =1), 2 dropped out between baseline and week 2 (placebo=0, prazosin = 2), 2 dropped out of the study between week 2 and week 3 (placebo = 2, prazosin = 0), 1 (prazosin) dropped out after week 3, and 1 dropped out (between visit 10 and 11, prazosin) after completing medication. 27 completed all study visits (13 prazosin).
Figure 1.
Participant flow (CONSORT diagram).
Fifteen participants were Hispanic, 17 were Caucasian, 10 were Native American, 2 were Asian. Twenty-three were male. Participants averaged 14.03 years of education (SD = 1.91) and 5 were unemployed. Three participants had current (past month) marijuana dependence. Fourteen participants met criteria for lifetime marijuana dependence, 8 for lifetime cocaine dependence, 1 for lifetime sedative dependence, and 1 for lifetime opiate dependence. One participant met criteria for current major depression, 2 for current substance-induced mood disorder, and none for bipolar disorder. Seven participants had current co-morbid anxiety diagnoses but none met criteria for PTSD. Participants did not significantly differ on any tested variables between prazosin and placebo groups (Table 2).
Table 2.
Baseline Characteristics
| Prazosin Group (n=18) | Placebo Group (n=18) | p-value | |
|---|---|---|---|
|
| |||
| Age [mean (SD)] | 38.56 (12.35) | 40.67 (10.69) | 0.59 |
|
| |||
| Gender [% male (n)] | 66.7 (12) | 61.1 (11) | 0.73 |
|
| |||
| Yrs Educ [mean (SD)] | 13.79 (1.69) | 14.25 (2.12) | 0.49 |
|
| |||
| Ethnicity [% non-Hispanic (n)] | 55.6 (10) | 61.1 (11) | 0.99 |
|
| |||
| Race [% (n)] | NA 27.8 (5) | NA 27.8 (5) | |
| Cauc 44.4 (8) | Cauc 50 (9) | ||
| Asian 5.6 (1) | Asian 5.6 (1) | ||
| Other 22.2 (4) | Other 16.7 (3) | ||
|
| |||
| DiaSit Visit 1 [mean (SD)] | 90.22 (13.27) | 86.06 (10.43) | 0.30 |
|
| |||
| DiaStd Visit [mean (SD)] | 90.61(13.94) | 89.94 (14.50) | 0.89 |
|
| |||
| DPW [mean (SD)] | 37.41 (30.74) | 30.57 (18.36) | 0.42 |
|
| |||
| DPDD [mean (SD)] | 8.38 (4.46) | 8.63 (5.14) | 0.88 |
|
| |||
| PDA [mean (SD)] | 34.20 (31.47) | 38.39 (34.32) | 0.71 |
|
| |||
| PHDD [mean (SD)] | 40.19 (35.77) | 46.05 (30.78) | 0.60 |
|
| |||
| PromisAnx [mean (SD)] | 52.22 (10.21) | 53.85 (8.29) | 0.60 |
|
| |||
| PromisDep [mean (SD)] | 48.59 (7.57) | 51.23 (9.58) | 0.37 |
|
| |||
| SCID Current Cannabis Dependence [% (n)] | 11.1 (2) | 5.6 (1) | 0.54 |
|
| |||
| SCID Current Anxiety Disorder [% (n)] | 22.2 (4) | 16.7 (3) | 0.67 |
|
| |||
| SCID Current MDD [% (n)] | 0 (0) | 5.6 (1) | 0.31 |
|
| |||
| SCID Current Subst Ind Mood Disorder [% (n)] | 0 (0) | 11.1 (2) | 0.15 |
|
| |||
| SCID past other SUD including Cannabis [% (n)] | 66.7 (12) | 44.4 (8) | 0.18 |
|
| |||
| SCID past other SUD excluding Cannabis [% (n)] | 22.2 (4) | 27.8 (5) | 0.70 |
Key: NA = Native American, Cauc = Caucasian; DiaSit = sitting diastolic blood pressure; DiaStd = standing diastolic blood pressure; DPW = drinks per week; DPDD = drinks per drinking day; PDA = percent days abstinent; PHDD = percent heavy drinking days; SCID = Structured Clinical Interview for DSM-IV; PromisAnx = PROMIS Anxiety T-score; PromisDep = PROMIS Depression T-score; MDD = major depressive disorder; Subst Ind Mood = substance-induced mood; SUD = substance use disorder
Effect of prazosin on drinking outcomes
When analyses were performed in an intent to treat fashion (n=36), no significant differences between the active and placebo condition in rate of change from 0–2 weeks to 4–6 weeks for our primary (DPW) and secondary (DPDD, PDA, PHDD) drinking variables (Table 3; Figure 2a) were observed. However when only individuals in the optimal treatment exposure subgroup were analyzed post-hoc, there was a significant effect of active treatment indicating that the slope was more negative for those assigned to prazosin compared to placebo, or that prazosin was associated with a greater rate of reduction in DPW over time (beta =−0.304, p=0.012). Upon visual inspection of the graph for DPW for the optimal treatment exposure subgroup, we observed an “uptick” in drinking in the prazosin group from 2–4 weeks to 4–6 weeks (observable to a lesser extent in Figure 2a) that was driven by an extreme outlier in the prazosin group (>1.5*IQR). Removing this outlier, the effect of prazosin on DPW was even more robust in the optimal treatment exposure subgroup (beta=−0.427, p=0.002) and the trajectory of the two groups was more standard (Figure 2b).
Table 3.
Effects of prazosin versus placebo on rate of change (HLM) in drinking in intent to treat (n=36) and optimal treatment exposure (n=27) sample
| Coeff | p-value | Event Rate Ratio | CI | |
|---|---|---|---|---|
| DPW n=36 | 0.14 | 0.15 | 1.15 | 0.95,1.39 |
| DPW n=27 | −0.30 | 0.01 | 0.74 | 0.59,0.93 |
| DPDD n=36 | −0.06 | 0.43 | 0.95 | 0.82,1.09 |
| DPDD n=27 | −0.14 | 0.10 | 0.87 | 0.74,1.03 |
| PDA n=36 | −0.17 | 0.08 | 0.84 | 0.70,1.02 |
| PDA n=27 | −0.14 | 0.11 | 0.87 | 0.73,1.04 |
| PHDD n=36 | 0.04 | 0.81 | 1.11 | 0.69,1.77 |
| PHDD n=27 | <0.01 | >0.99 | 1.26 | 0.74,2.14 |
DPW = drinks per week; DPDD = drinks per drinking day; PDA = percent days abstinent; PHDD = percent heavy drinking days; HLM = hierarchical linear modeling; Coeff = coefficient/unstandardized beta associated with medication condition/treatment group in HLM models predicting rate of change in drinking; Event Rate Ratio = event rate ratio associated with medication condition/treatment group in HLM models predicting rate of change in drinking; CI = confidence interval
Figure 2.
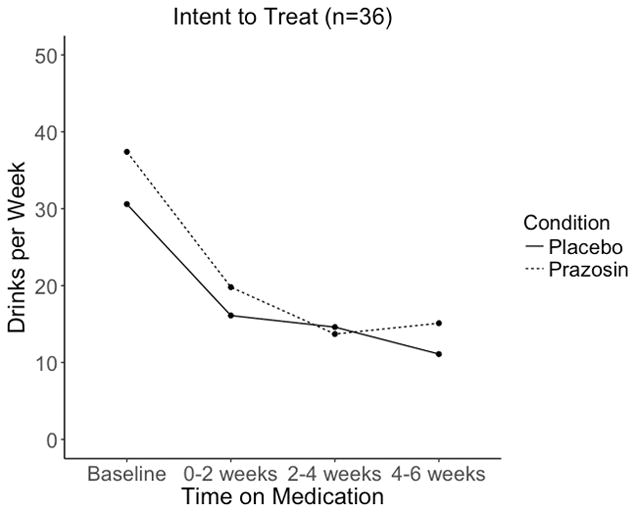
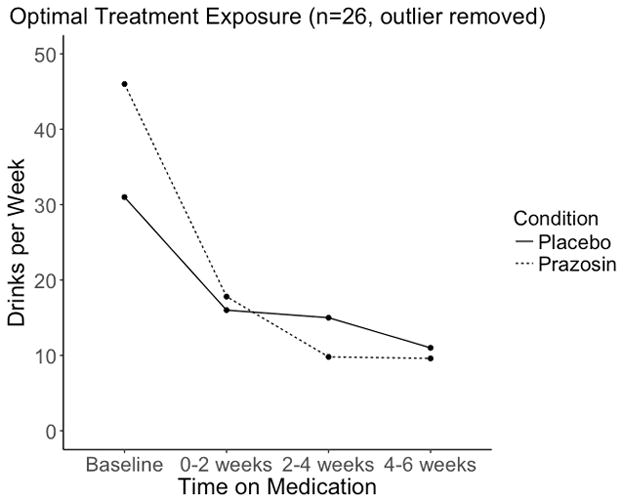
Figure 2a. This figure depicts the means, including values from all subjects present at each time point, for drinks per week over the course of the trial, divided by medication group in an intent to treat sample (n=36).
Figure 2b. This figure depicts the means, including values from all subjects present at each time point, for drinks per week over the course of the trial, divided by medication group for the subgroup of participants who did not immediately drop out of the trial and who did not stop medications for tolerability reasons, excluding an outlier (n=26).
Safety, Tolerability, Adverse events
Four participants had their medications discontinued by the study physician and/or at the patient’s request for various reasons and all were on active treatment (details in supplemental materials). Three of these individuals were in the low sitting DBP group. Dose adjustments were made for 3 participants for tolerability of which two were on active and one on placebo. Dizziness on standing was more frequent in the prazosin group compared to placebo (χ2 = 3.86, p = 0.049).
Adherence
Prazosin was associated with similar rates of adherence as placebo (p’s>0.69 on independent sample t-tests) for the adjusted total adherence variables, but a lower frequency of bringing in pill counts (chi square p’s for visit 8 were 0.032 and visits 9 and 10 were 0.070 and 0.081 respectively). Prazosin was also associated with lower overall doses [study-doctor adjusted doses were more prevalent in active (decreases n=2, discontinuations n=4) compared to placebo (decreases n=1, discontinuations n=0)], which resulted in lower full total adherences (p’s 0.041–0.103) (Table S5). For individuals with low sitting DBP, prazosin was associated with lower full total self-reported adherence (mean placebo 86.69+/−27.60, mean active 61.00 +/−43.50, t=1.55, p=0.15) and lower full total adherence by pill counts (mean placebo 82.47 +/−28.47, mean active 45.99+/− 44.04 t=2.17, p=0.045), but similar adjusted adherence levels for self-report and pill counts (p’s .94 and .56, t’s 0.08 and .59) (n=19). For individuals with high DBP, prazosin and placebo did not differ on total adherence (ps > 0.47, t’s <0.75) (n=12). These findings could indicate that individuals with low DBP are less able to tolerate the full 16 mg dose.
Moderator Analyses
Sitting DBP (DBPsit) (interaction term p=0.038) and standing DBP (DBPstd) (interaction term p=0.024) significantly moderated the effect of medication condition on rate of change in DPDD. Graphing this effect for DBPsit (Figure S2) indicated that for those with a high DBPsit, individuals on placebo improved less quickly than those on active treatment. Analyses by subgroup demonstrated that individuals with high DBPsit (beta=-0.330, p=0.036) and high DBPstd (beta=−0.271, p=0.013) had more negative betas for the effect of condition on rate of change in DPDD (indicating a faster rate of improvement on prazosin compared to placebo) than those with low DBPsit (beta=−0.027, p=0.600) and low DBPstd (beta=−0.041, p=0.516). In summary, in those with high DBP, there was a significant difference between active and placebo treatment, but, in those with low DBP, active medication was inert. The effect of condition on rate of change in DPW was not moderated by DBP nor were either DPDD or DPW moderated by anxiety.
Discussion
In summary, in the intent to treat sample, alcohol use outcomes in the prazosin group were not significantly different from placebo for our primary measure (DPW) or secondary outcome measures. However, in support of previous published studies of prazosin in AUD (Simpson et al. 2015; Simpson et al. 2009), in a post-hoc analysis, a main effect of prazosin on the rate of drinking reduction was observed in an optimal treatment exposure subgroup. Although it appeared that this faster rate of change could have been partly due to regression to the mean since individuals on prazosin had higher mean DPW at baseline than placebo (Figure 2b), these baseline differences were not statistically significant.
One possible reason for the lack of efficacy in the intent to treat sample is that medication tolerability was a problem. Four of the individuals on prazosin, but none on placebo, chose to discontinue the medication, and there was a higher rate of dizziness on standing in the prazosin group. Poor adherence appeared especially problematic in individuals with low DBP. Furthermore, at later time points, individuals on prazosin were less likely to bring in their pills for counts than those on placebo, which could indicate avoidance behavior, and not wanting to be discovered being non-adherent. That the overall effects of treatment in the expected direction were seen at weeks 2–4 but not 4–6 (Table S4, exploratory generalized linear model analysis) for DPW could indicate that the medication was helping until participants stopped taking it. Another possibility is that all subjects experienced large reductions of drinking regardless of condition, perhaps due to the efficacy of the MM intervention (Anton et al. 2006), which may have washed out medication effects.
Furthermore, similar to a study of doxazosin (Haass-Koffler et al. 2017), we observed a larger effect of prazosin compared to placebo on DPDD in individuals with high DBP compared to individuals with low DPB. This should be interpreted cautiously given the small sample size. However, other work has also found high blood pressure to successfully identify individuals with PTSD who more likely to respond to prazosin (Raskind et al. 2016). That blood pressure predicts response to treatments with alpha-1 noradrenergic antagonists in several independent samples indicates this marker has potential for clinical utility, especially given that it can be obtained in any clinical setting.
One limitation of this study which could have contributed to the null effects for the intent to treat analysis was the sample size; this small-scale NIH-funded study did not have sufficient power to detect small effects but was powered to detect moderate to large effects (we were 80% powered to detect an effect of 0.35 for the main analysis and an effect of 0.43 for the moderator analyses). However, the initial pilot study of prazosin in AUD (Simpson et al. 2009) showed robust effects of medication on drinking outcome (Cohen’s d = 2.8 for medication effect on DPW at 6 weeks in study completers n=17). Second, six-weeks is a relatively short treatment period. If the trial was longer, the superiority of prazosin over placebo in the optimal responders group and in individuals with high DBP could be lost or could be accentuated. Third, individuals with current cannabis use disorder and past other substance use disorder were not excluded. However, this could also be considered a strength, given that our findings have greater generalizability. Fourth, for the moderator analyses, we acknowledge that heterogeneity of response in the sample may have either masked or contributed to false positive results, and the small sample precluded more rigorous outlier removal; larger samples are necessary to definitively establish DBP as a moderator and to determine the sensitivity and specificity of various thresholds for decision-making during choice of treatment. Another major limitation was that we excluded individuals with PTSD in order to maintain some homogeneity, which may have limited the sample variability to test anxiety-related moderator effects. However, 10 of the subjects did meet criteria on the SCID for current or past anxiety disorder. Finally, that individuals were able to guess their treatment assignment (supplemental results, chi square p <0.002) indicates that the trial was biased in the direction of finding a treatment effect.
In summary, in the intent to treat sample, there was not a significant effect of prazosin, relative to placebo, on the rate of reduction in drinking. Post-hoc exploratory analyses in a subgroup of individuals who followed through and were able to tolerate medications indicated that prazosin increased the rate of reduction in DPW, in support of previous work (Simpson et al. 2015; Simpson et al. 2009). Poor tolerability and adherence may have contributed to the lack of medication effect when the whole sample was examined. Unlike doxazosin, a once-daily medication, prazosin is a three times a day medication, which may further challenge adherence. Also important, we replicated the findings in previous work suggesting that DBP is a moderator of treatment response to alpha-1 antagonists in AUD (Haass-Koffler et al. 2017). Future work should explore whether these findings are replicated in a larger sample and explore the diagnostic utility (sensitivity and specificity) of DBP for predicting response to treatment with this medication class.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant number K23-AA021156 awarded to Claire Wilcox. We, the authors, have no financial conflicts of interest to report.
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Scott Tonigan J, Pettinati HM. Effects of alcoholism typology on response to naltrexone in the COMBINE study. Alcohol Clin Exp Res. 2009;33:10–18. doi: 10.1111/j.1530-0277.2008.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Fischer S, Wise B, Rasmussen DD. Prazosin Reduces Alcohol Intake in an Animal Model of Alcohol Relapse. Alcohol Clin Exp Res. 2015;39:1538–1546. doi: 10.1111/acer.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Richardson R, Moul DE, Mammen O, Haas G, Forman SD, Rode N, Begley A, Nofzinger EA. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72:89–96. doi: 10.1016/j.jpsychores.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Goodyear K, Zywiak WH, Magill M, Eltinge SE, Wallace PM, Long VM, Jayaram-Lindstrom N, Swift RM, Kenna GA, Leggio L. Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend. 2017;177:23–28. doi: 10.1016/j.drugalcdep.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: realizing the promise of pharmacogenomics and personalized medicine. Annu Rev Clin Psychol. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, Tirado C, Oslin DW, Sparkman T, O’Brien CP. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27:344–351. doi: 10.1097/JCP.0b013e3180ca86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L. Role of the alpha1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2016;21:904–914. doi: 10.1111/adb.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GL, Soroncz M, Tegyei I. Plasma catecholamines in ethanol tolerance and withdrawal in mice. Eur J Pharmacol. 2002;448:151–156. doi: 10.1016/s0014-2999(02)01939-8. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Kadden RM, Babor TF, Tennen H, Rounsaville BJ. Validity of the SCID in substance abuse patients. Addiction. 1996;91:859–868. [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res. 2009;33:674–683. doi: 10.1111/j.1530-0277.2008.00884.x. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38:2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, Weinshenker D. alpha-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology. 2012;37:2161–2172. doi: 10.1038/npp.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD. The alpha1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcohol Clin Exp Res. 2013;37:202–212. doi: 10.1111/j.1530-0277.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Naik PC, Murray HW, Vergare MJ, Marsden CA. Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal. Alcohol Alcohol. 2003;38:224–231. doi: 10.1093/alcalc/agg055. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Millard SP, Petrie EC, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Hill J, Daniels C, Hendrickson R, Peskind ER. Higher Pretreatment Blood Pressure Is Associated With Greater Posttraumatic Stress Disorder Symptom Reduction in Soldiers Treated With Prazosin. Biol Psychiatry. 2016;80:736–742. doi: 10.1016/j.biopsych.2016.03.2108. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O’Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP, Rohde K, O’Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Scientific Software International Inc; Skokie, IL: 2004. [Google Scholar]
- Roache JD, Wang Y, Ait-Daoud N, Johnson BA. Prediction of serotonergic treatment efficacy using age of onset and Type A/B typologies of alcoholism. Alcohol Clin Exp Res. 2008;32:1502–1512. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, Riley W, Cella D. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–127. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne C, Johnson BA. Advances in Medications and Tailoring Treatment for Alcohol Use Disorder. Alcohol Res. 2015;37:15–28. [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, Varon D, Raskind M, Saxon AJ. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39:808–817. doi: 10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, Peskind ER, Raskind MA. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–632. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Bogenschutz MB. Psychopharmacologies for Alcohol and Drug Use Disorders. In: McCrady BS, Epstein EE, editors. Addictions: A Comprehensive Guidebook. Oxford University Press; New York, NY: 2013. pp. 526–550. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.