Abstract
Objective
Ibudilast (IBUD) is a neuroimmune modulator that inhibits phosphodiesterase-4 and -10 and macrophage migration inhibitory factor. A randomized, placebo-control, crossover human laboratory trial advanced IBUD development for alcohol use disorder and found that IBUD reduced tonic levels of alcohol craving. Given the importance of considering medication effects on other appetitive behavior, the present study tested the effect of IBUD (50 mg. bid) on food craving.
Method
The present study is a secondary data analysis of the trial of IBUD in non-treatment seekers with alcohol use disorder (N = 19). High-fat/high-sugar food craving was measured daily. Moreover, because substantial literatures show that small alcohol doses and psychological stress increase eating of high-fat/high-sugar food, craving for high-fat/high-sugar food was measured after alcohol infusion and stress reactivity.
Results
Results indicated that IBUD did not alter tonic high-fat/high-sugar food craving. Alcohol infusion did not generally increase high-fat/high-sugar food craving but psychological stress did. Likewise, IBUD did not affect high-fat/high-sugar food craving after alcohol infusion but IBUD did increase high-fat/high-sugar food craving after psychological stress. Follow-up analyses revealed that, among individuals with lower depressive symptomatology, IBUD compared to placebo heightened the effect of psychological stress on high-fat/high-sugar food craving.
Conclusions
These results advance the development of IBUD for addiction indications by demonstrating that IBUD compared to placebo does not suppress other appetitive responses, namely craving for high-fat/high-sugar food among individuals with alcohol use disorder.
Keywords: depression, eating, human laboratory, ibudilast, NCT02025998
Introduction
Ibudilast (IBUD) is a neuroimmune modulator that inhibits phosphodiesterase-4 and -10 and macrophage migration inhibitory factor. It is theorized that IBUD promotes neurotrophin expression via inhibition of negative regulation and reduces neuroinflammation via inhibition of proinflammatory signaling (Johnson, Matsuda, & Iwaki, 2014). Increases in neurotrophins may restore mesolimbic dopamine function and reduction in proinflammatory signaling may decrease drug-seeking behavior (Blednov et al., 2012; Barak et al., 2015). A randomized, placebo-control, human laboratory trial advanced IBUD development for alcohol use disorder (AUD) and found that IBUD reduced tonic levels of alcohol craving—and for individuals reporting higher levels of depressive symptoms—reduced the stimulant and rewarding effects of alcohol (Ray et al., 2017a). IBUD has similarly been studied for its safety and initial efficacy among individuals with methamphetamine use disorder (DeYoung et al., 2016; Worley et al., 2016) and opioid use disorder (Cooper et al., 2016; Metz et al., 2017). In sum, IBUD may be a promising pharmacotherapy for AUD and other substance use disorders.
Critically, pharmacotherapies for addiction can alter other appetitive responses, particularly food craving. In detail, pharmacotherapies for addiction have had broad effects on eating frequency and amounts (de Zwaan & Mitchell, 1992). However, studies with more precise measurement have revealed that these medications change cravings particularly for foods that are high in fat, sugar, or both fat and sugar (de Zwaan & Mitchell, 1992; Langleben et al., 2012); these food types (which we will label “high-fat/high-sugar food”) strongly engage the reward system (Volkow et al., 2011). Thus, studying IBUD effects on food craving—particularly high-fat/high-sugar food craving—would further medication development.
Moreover, IBUD may uniquely impact high-fat/high-sugar food craving because proinflammatory signaling may influence eating behavior. Chronic, elevated peripheral levels of proinflammatory cytokines are associated with depression, and both decreased and increased motivation to eat (Dowlati et al., 2010). Some individuals with depression engage in more comfort eating (Konttinen et al., 2010), which is defined as eating high-fat/high-sugar food to reduce negative emotions like stress (Tomiyama, Finch, & Cummings, 2015). Modulating proinflammatory signaling may consequently decrease or increase motivation to eat, especially in the context of comfort eating. Thus, testing IBUD effects on high-fat/high-sugar food craving may provide insight into the nuances between proinflammatory signaling and eating behavior.
The present study had two objectives: (1) to test the effects of IBUD versus placebo (PLAC) on tonic levels of high-fat/high-sugar food craving and (2) to test the effects of IBUD versus PLAC on high-fat/high-sugar food craving following alcohol infusion and stress. Certainly, a sizeable number of research studies indicate that small alcohol doses stimulate eating, particularly eating of high-fat/high-sugar food (Hofmann, 2008; Yeomans, 2010; Eiler et al., 2015; Schrieks et al., 2015; Christiansen et al., 2016). This effect is not consistent and seems to nullify with increases in alcohol dose (Mattes, 1996; Poppitt et al., 1996; Yeomans & Phillips, 2002; Caton et al., 2004; Rose, Hardman, & Christiansen, 2015). Moreover, a large body of research demonstrates that psychological stress increases eating of high-fat/high-sugar food; although for a subgroup (~30%), stress decreases eating (see Adam & Epel, 2007 for a review). In light of this literature, the present study tests the main effects of alcohol administration and stress-induction on high-fat/high-sugar food craving. Given that other pharmacotherapies for addiction have reduced eating and high-fat/high-sugar food craving in samples with substance use disorder (de Zwaan & Mitchell, 1992; Langleben et al., 2012), we hypothesized that IBUD compared to PLAC would reduce tonic levels of high-fat/high-sugar food craving as well as reduce high-fat/high-sugar food craving following alcohol infusion and stress.
Lastly, prior work demonstrated that IBUD interacted with depressive symptomatology to attenuate alcohol’s effect (Ray et al., 2017a). Depressive symptomology may be especially relevant to examining the effects of IBUD on high-fat/high-sugar craving following psychological stress due to associations among chronic inflammation, depression, and comfort eating. Therefore, exploratory analyses tested the moderating role of depressive symptomatology in any effect of IBUD compared to PLAC on high-fat/high-sugar food craving following psychological stress. Additional exploratory analyses testing the moderating role of participant characteristics including biological sex, age, and Body Mass Index (BMI) in any effect are provided in Supplemental Digital Content.
Method
Participants
The objectives of the present study were tested using secondary data from the first randomized, placebo-control, human laboratory trial developing IBUD for AUD. See Ray et al. (2017a) for a full description of the methods for the trial, which was registered in clinicaltrials.gov (NCT02025998). IBUD is still investigational for treatment of addictions. The University of California, Los Angeles Institutional Review Board approved all research activities. In order to test the present study aims, measures of food craving were amended into the study in July 2014. A total of 24 non-treatment seeking participants with current DSM-5 AUD completed the trial, of which 19 completed measures of food craving. Table 1 presents demographics, which were similar to those present in the whole sample.
Table 1.
Final Sample Characteristics (N = 19)
| Mean | SD | |
|---|---|---|
| % | ||
| Age | 31.37 | 9.20 |
| Sex (% Male) | 63.20% | |
| Ethnicity | ||
| Caucasian | 21.10% | |
| African American | 31.60% | |
| Asian American | 5.30% | |
| Native American | 15.80% | |
| Latino/Hispanic | 26.30% | |
| % Reporting Occasional or Daily Cigarette Smoking1 | 21.05% | |
| % Reporting Marijuana Use in Past 30 Days2,3 | 20.83% | |
| Drinking Days per Month | 21.68 | 5.54 |
| Drinks per Drinking Day | 7.42 | 4.89 |
| AUD Symptom Count | 4.47 | 2.57 |
| Mild AUD | 42.10% | |
| Moderate AUD | 36.85% | |
| Severe AUD5 | 21.05% | |
| Body Mass Index | 25.79 | 4.49 |
| Underweight | 5.3% | |
| Normal | 47.4% | |
| Overweight | 31.6% | |
| Obese I | 10.5% | |
| Obese II | 5.3% | |
| RED | ||
| BDI-II4 | 7.68 | 9.62 |
Notes:
Smoking status was defined by the entry item to the Fagerstrom Test for Nicotine Dependence with 4 participants identifying as smokers (2 of whom reported occasional smoking and 2 who reported daily smoking).
Marijuana use was obtained from the 30-day Timeline Follow Back indicating that 5 participants reported using marijuana in the past 30 days. These 5 participants reported marijuana use on 1, 3, 5, 23, and 29 days.
A total of 5 participants tested positive for cannabis in the IBUD condition and 3 tested positive for cannabis in the PLAC condition.
The observed range for the BDI-II was 0 to 25.
Procedure
All participants provided informed consent and the visit procedure followed all the guidelines for experimental investigation with human participants required by the Universty of California, Los Angeles and California. Participants were deemed eligible for the study after a screening and physical exam by the study physician. The screening included a urine toxicology test (CLIA Waived, 10-panel drug test; Medimpex United) negative for all drugs (excluding marijuana). The toxicology panel comprised cocaine, marijuana, opiate (heroin, morphine, and codeine), amphetamine, methamphetamine, PCP, benzodiazepine, barbiturate, methadone, and oxycodone (Ray et al., 2017a). The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and the Reward-Based Eating Drive Scale (Epel et al., 2014) was administered during the screening.
Each participant completed two separate 7-day intensive outpatient protocols, during which medication adherence was observed in the AM by a nurse and in the PM by a riboflavin (50 mg) tracer. At each AM visit, the nurse confirmed participant sobriety with a daily BrAC and toxicology test negative for all drugs (excluding marijuana). IBUD administration was as follows: 20 mg (i.e., 2 10mg capsules) bid during days 1–2 and 50 mg (i.e., 5 10mg capsules) bid during days 3–6. After reaching a stable target dose of the study medication (or placebo), participants completed a stress reactivity paradigm (on day 5; PM) and an intravenous (IV) alcohol administration (day 6; PM), which started at 1PM and was followed by an overnight visit and discharge (day 7). The protocol required a minimum 7-day washout period (Mean = 16.58 days, SD = 10.44, Range = 7 – 40) (Ray et al., 2017a).
High-Fat/High-Sugar Food Craving Measurement
Tonic
At each AM assessment, participants reported cravings for high-fat/high-sugar foods via the item: “How strong is your urge to eat high-fat/high-sugar food right now?” Responses were recorded on a 10-point Likert scale wherein higher scores indicate greater urge. Single-item Likert scale measures of food craving are typically used in medication trials (Langleben et al., 2012; Mason et al., 2015). In the present study, we sought to validate this item against scores on the Reward-Based Eating Drive Scale (RED; Epel et al., 2014) measured during the screening. The RED is a 9-item self-report that assesses factors that drive overeating and may stem from reward-related neural circuitry (e.g., lack of control, lack of satiation, preoccupation with food). On day 1 for both study conditions, urge for high-fat/high-sugar food positively correlated with RED scores (IBUD day 1: r(17) = .52, p < .05, PLAC day 1: r(17) = .59, p < .05), adding validity to the present study’s food craving measurement.
IV Alcohol Administration
Based on prior research, we anticipated that IV alcohol infusion would increase high-fat/high-sugar food craving; we sought to test IBUD effects on this potential alcohol-induced high-fat/high-sugar food craving. The alcohol infusion was performed using a nomogram developed and validated in our previous work (.166-ml/minute × weight in kilogram for males/ .126-ml/minute × weight in kg for females; Ray & Hutchison, 2004). Target BrACs included .02, .04, .06, and .08 g/dl. See Ray et al. (2017a) for full details.
On the day of IV alcohol administration, participants ate a standardized lunch before the IV alcohol administration during both IBUD [M(SD) = 43.00(12.85) minutes before, Range = 30–80] and PLAC conditions [M(SD) = 40.42(11.19) minutes before, Range = 25–68]. The lunch consisted of a sandwich, chips, drink, and a fruit/dessert. Table 2 provides caloric information for the lunch along with percent of fat, carbohydrates, and protein. Participants chose one item from each section of the lunch. Participants were not forced to finish the lunch.
Table 2.
Caloric Information for Standardized Lunch
| Calories | %Fat | %Carb | %Protein | |
|---|---|---|---|---|
|
| ||||
| Sandwich | ||||
| Turkey sandwich (3 oz. turkey breast, 1 oz. cheese, 2 slices wheat bread, lettuce tomato, 1 package light mayonnaise, 1 package mustard) | 375 | 21% | 39% | 40% |
| Ham sandwich (3 oz. ham, 1 oz. cheese, 2 slices wheat bread, lettuce tomato, 1 package light mayonnaise, 1 package mustard) | 375 | 27% | 37% | 36% |
| Chips | ||||
| Doritos | 140 | 50% | 44% | 6% |
| Potato chips | 160 | 57% | 38% | 5% |
| Drink | ||||
| Cranberry juice | 130 | -- | 100% | -- |
| Apple juice | 110 | -- | 100% | -- |
| Lemon lime soda | 120 | -- | 100% | -- |
| Fruit/Dessert | ||||
| Orange | 50 | -- | 93% | 7% |
| Apple | 50 | 2% | 94% | 3% |
| Mixed fruit cocktail | 60 | -- | 95% | 5% |
| Cheesecake | 90 | 62% | 31% | 7% |
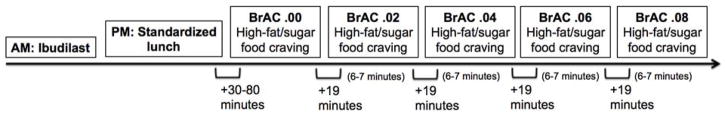
High-fat/high-sugar food craving was measured at baseline and following each target BrAC via the item: “How strong is your urge to eat high-fat/high-sugar food right now?” Additionally, high-fat/high-sugar food craving was measured following each target BrAC via the item: “What was the highest urge to eat high-fat/high-sugar food that you felt during the time that the alcohol was present?” All responses were recorded on a 10-point Likert Scale wherein higher scores indicate greater urge. We have used similarly worded items to assess task-related alcohol and cigarette craving in prior medication trials (Ray et al., 2007). Timing of the high-fat/high-sugar food craving measurements and the experimental procedure are graphically displayed in Figure 1.
Figure 1.
Graphical display of the timing of the high-fat/high-sugar food craving measurements and the experimental procedure. Baseline high-fat/high-sugar food craving was measured at BrAC .00 after a standardized lunch. There was approximately 19 minutes between each BrAC level and 6–7 minutes held stable at each BrAC level.
Stress Reactivity
Based on prior research, we anticipated psychological stress would increase high-fat/high-sugar food craving; we sought to test IBUD effects on this potential stress-induced high-fat/high-sugar food craving. Participants were exposed to 5-minute tape-recorded scripts recounting current and unresolved stressful events in the participants’ lives following standardized procedures (Sinha, 2009). This procedure significantly increased negative mood and decreased positive mood in the study sample (Ray et al., 2017a).
High-fat/high-sugar food craving was measured at baseline and post-stress via the item: “How strong is your urge to eat high-fat/high-sugar food right now?” High-fat/high-sugar food craving was additionally measured post-stress via the item: “What was the highest urge to eat high-fat/high-sugar food that you felt during the time that the imagery was presented?” All responses were recorded on a 10-point Likert Scale wherein higher scores indicate greater urge.
Statistical Analyses
To test our first objective, we conducted a repeated measures analysis of variance (ANOVA) to test the effects of IBUD on tonic high-fat/high-sugar food craving. We simultaneously tested the main effect of Time (Day 1–Day 7), the main effect of Medication (IBUD vs. PLAC), and the Medication x Time interaction. To test our second objective, we conducted repeated measures ANOVAs to test the effects of IBUD on the potential alcohol- and stress-induced high-fat/high-sugar food craving. We simultaneously tested the main effect of Trial (baseline vs. target BrAC levels and pre- vs. post-stress exposure), the main effect of Medication (IBUD vs. PLAC), and the Medication x Trial interaction. In exploratory analyses, we tested the moderating role of depressive symptomatology in any effect of IBUD on high-fat/high-sugar food craving following psychological stress by adding BDI-II scores as a between-subjects factor in the repeated measures ANOVA. We used the continuous BDI-II scores and log-transformed them to account for data non-normality. Across analyses, our sample size provided between .82 and .93 power for a large expected effect size based on power analyses conducted in G*Power Version 3.1.7.
Results
Tonic Food Craving
Urge for high-fat/high-sugar food did not change over time (Day 1–7: F(6,108) = 1.59, p = .16). IBUD versus PLAC did not alter urge for high-fat/high-sugar food (IBUD: F(1,18) = 0.04, p = .85, IBUD x Day 1–7: F(6,108) = 1.07, p = .39).
Food Craving during IV Alcohol Administration
Table 3 presents correlations between current urge for high-fat/high-sugar food following each target BrAC and highest urge for high-fat/high-sugar food during infusion. The items were positively correlated following all target BrACs. However, the strength of the correlations weakened with increasing BrAC. This variability between items suggested that each item would uniquely relate to increasing BrAC. Indeed, there was no main effect of alcohol infusion on current urge for high-fat/high-sugar food (BrAC: F(4,72) = 0.86, p = .49). However, there was a significant main effect of alcohol infusion on highest urge for high-fat/high-sugar food during infusion (BrAC: F(4,72) = 3.37, p = .01). Post-hoc tests indicated the effect was non-linear: highest urge for high-fat/high-sugar food significantly increased from baseline to BrAC .02 (p = .01), marginally decreased from BrAC .02 to .04 (p = .08), and did not significantly change from BrAC .04 to .06 (p = .19) or BrAC .06 to .08 (p = .53).
Table 3.
Correlations between urge and highest urge for high-fat/high-sugar food at each time point
| .02 BrAC | .04 BrAC | .06 BrAC | .08 BrAC | Post-Stress 1 | Post-Stress 2 | Post-Stress 3 | Post-Stress 4 | Post-Stress 5 | |
|---|---|---|---|---|---|---|---|---|---|
| IBUD | .93*** | .91*** | .89*** | .69** | .97*** | .90*** | .82*** | .84*** | .52* |
| PLAC | .96*** | .98*** | .99*** | .97*** | .97*** | .83*** | .75*** | .80*** | .66** |
Note:
p < .05,
p < .01,
p < .001
IBUD versus PLAC did not alter urge for high-fat/high-sugar food following alcohol infusion (IBUD: F(1,18) = 1.10, p = .31, IBUD x BrAC: F(4,72) = 0.44, p = .78) or highest urge for high-fat/high-sugar food during alcohol infusion (IBUD: F(1,18) = 2.83, p = .11, IBUD x BrAC: F(4, 72) = 0.45, p = .77).
Food Craving during Stress Reactivity
Table 3 presents correlations between urge for high-fat/high-sugar food following each post-stress time point and highest urge for high-fat/high-sugar food during stress. The items were positively correlated at all post-stress time points but the strength of the correlation weakened with increasing psychological stress. Regardless, the psychological stress protocol significantly increased both urge for high-fat/high-sugar food post-stress (Trial: F(5,90) = 2.49, p = .04) and highest urge for high-fat/high-sugar food during stress (Trial: F(5,90) = 2.96, p = .02). Post-hoc tests confirmed these effects were linear.
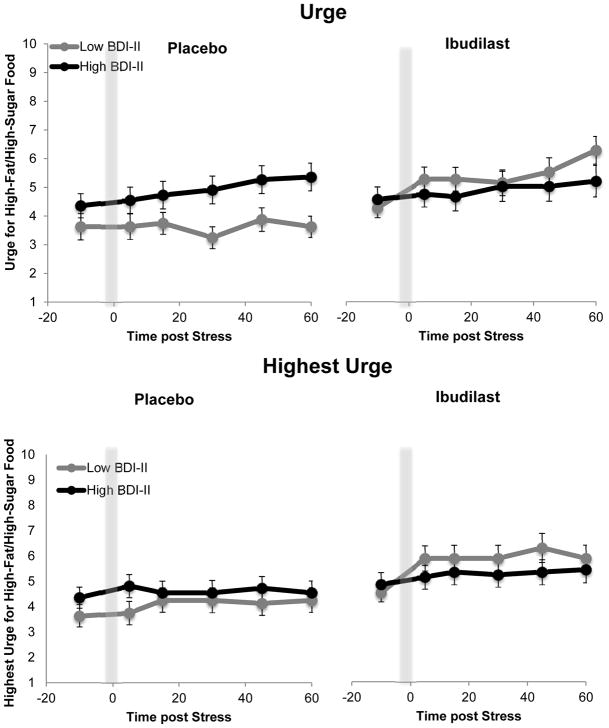
IBUD versus PLAC marginally increased urge for high-fat/high-sugar food post-stress (IBUD: F(1,18) = 3.51, p = .08) and significantly increased highest urge for high-fat/high-sugar food during stress (IBUD: F(1,18) = 7.09, p = .02); these effects did not change across trials (IBUD x Trial: F(5,90) = 0.45, p = .81 and IBUD x Trial: F(5,90) = 1.30, p = .27, respectively). Figure 2 presents these IBUD versus PLAC effects.
Figure 2.
High-fat/high-sugar craving during the stress paradigm. Urge and highest urge for high-fat/high-sugar food during stress was greater while on IBUD, relative to placebo (Urge: p = .08, Highest urge: p = .01)
BDI-II moderation
BDI-II scores did not predict urge for high-fat/high-sugar food post-stress (BDI-II: F(1,17) = 0.15, p = .71) or highest urge for high-fat/high-sugar food during stress (BDI-II: F(1,17) = 0.06, p = .80). However, BDI-II scores significantly moderated the effect of IBUD versus PLAC on urge for high-fat/high-sugar food post-stress (IBUD x BDI-II: F(1,17) = 5.58, p = .03) and highest urge for high-fat/high-sugar food during stress (IBUD x BDI-II: F(1,17) = 8.15, p = .01). As presented in Figure 3, these interactive effects were such that IBUD versus PLAC increased urge and highest urge for high-fat/high-sugar food to a greater extent among participants with lower BDI-II scores. BDI-II scores were entered as a continuous covariate into the repeated measures ANOVAs. High [M(SD) = 14.10(9.35)] and low [M(SD) = 0.56(1.13)] groups were only created (based on a median split) for the purpose of visually plotting the results.1
Figure 3.
High-fat/high-sugar food craving during stress is affected by the administration of IBUD and levels of depressive symptomatology. For ease of presentation, means and standard errors are presented based on a median split of depressive symptomatology, however, the analyses examined depressive symptomatology (i.e., log-transformed BDI-II scores) as a continuous predictor.
Discussion
The objectives of the present study were to test if the neuroimmune modulator IBUD altered tonic high-fat/high-sugar food craving as well as high-fat/high-sugar food craving following alcohol administration and psychological stress induction in a sample with AUD. Given that pharmacotherapies for addiction can alter other appetitive responses, we hypothesized that IBUD would reduce tonic levels of high-fat/high-sugar food craving as well as reduce high-fat/high-sugar food craving following alcohol infusion and stress. Understanding general- and domain-specific effects of IBUD will further medication development. A neuroimmune modulator such as IBUD may uniquely impact high-fat/high-sugar food craving because proinflammatory signaling may be associated with increased or decreased motivations to eat (Dowlati et al., 2010).
Indeed, contrary to our hypothesis, IBUD increased high-fat/high-sugar food craving during psychological stress. It is uncertain why this effect emerged, although one might speculate that because IBUD inhibits proinflammatory signaling it reduces “sickness behavior” thereby increasing motivation to eat (Kelley et al., 2003, p. S112). Indeed, IBUD effects on high-fat/high-sugar food craving only emerged during a psychological stress task and psychological stressors acutely stimulate eating of high-fat/high-sugar food (Adam & Epel, 2007) and production of proinflammatory cytokines (Maes et al., 1998), which could make modulation by IBUD more salient. Nevertheless, chronic peripheral levels of proinflammatory cytokines are associated with depression and increased comfort eating (Dowlati et al., 2010; Konttinen et al., 2010). In accordance with this, depressive symptomatology moderated the effects of IBUD versus PLAC on high-fat/high-sugar food craving during psychological stress reactivity: for those scoring higher in depressive symptoms, high-fat/high-sugar food craving remained higher regardless of IBUD. For those scoring lower in depressive symptoms, IBUD increased high-fat/high-sugar food craving to a level comparable to the level experienced by those scoring higher in depressive symptoms. These findings should be interpreted while considering that the overall mean of depressive symptoms in this study sample [M(SD) = 7.68(9.62)] was below the clinical depression cut-off. Thus, effects may reflect differences in subclinical mood dysphoria. The associations among mood dysphoria, depression, acute and chronic neuroinflammation, and comfort eating are complex and future research is needed to tease apart inflammation mechanisms that drive changes in eating.
On the other hand, IBUD did not alter high-fat/high-sugar food craving following alcohol infusion. This could be because alcohol did not generally increase high-fat/high-sugar food craving and had a non-linear effect on craving across target BrAC levels. In detail, a small amount of alcohol increased highest urge for high-fat/high-sugar food (BrAC .02), yet more alcohol decreased this urge (BrAC .04), and even more alcohol diminished effects (BrAC .06 & .08). Although there is a substantial literature documenting that small alcohol doses stimulate eating (Hofmann, 2008; Yeomans, 2010; Eiler et al., 2015; Schrieks et al., 2015; Christiansen et al., 2016), this is observed less in studies where individuals drink larger doses (Mattes, 1996; Poppitt et al., 1996; Yeomans & Phillips, 2002; Caton et al., 2004; Rose et al., 2015). The present study is the first to use a within-subjects design wherein food craving was measured at target BrAC levels. Findings add to this literature by specifying a boundary dose for alcohol’s stimulatory effect on eating (BrAC .04). Alcohol was intravenously administered; this rules out expectancy effects and suggests that the observed nonlinear effect was mediated by pharmacological or physiological mechanisms. Intravenously administering alcohol comes with the drawback of reduced real-world validity but prior research indicates that participants who drink placebos eat similarly to those in control conditions rather than those who drink alcohol (Poppitt et al., 1996; Hetherington et al., 2001), again suggesting that the stimulatory eating effect of small alcohol doses is pharmacological or physiological.
It is important to note that there was some variability across the two craving items in the results for high-fat/high-sugar food craving following psychological stress and alcohol administration. The reason for the different findings between items is uncertain. It could be that asking about highest urge felt during the tasks rather than urge felt right after the task increased response variance and improved statistical precision.
Finally—and critical to the development of IBUD for addiction indications—IBUD did not alter daily urge for high-fat/high-sugar food. This effect remained across all days of each condition in the trial. Although not what we hypothesized, in terms of medication development, this suggests that the effects of IBUD are domain-specific. The trial showed that IBUD reduced tonic levels of alcohol craving (Ray et al., 2017a). Other research on IBUD has demonstrated its safety and initial efficacy for methamphetamine use disorder (DeYoung et al., 2016; Worley et al., 2016) and opioid use disorder (Cooper et al., 2016; Metz et al., 2017). IBUD, however, did not reduce tonic levels of high-fat/high-sugar food craving. IBUD seems to be influencing drug-specific mechanisms rather than (or at a greater extant than) general appetitive mechanisms. This is in contrast to other addiction medications such as naltrexone and naloxone, which have been shown to have a notable effect on food cravings and eating (de Zwaan & Mitchell, 1992; Langleben et al., 2012). The results alternatively highlight that multiple pathways regulate eating including inflammation, satiety, and reward signaling (Ahima & Osei, 2001). Although some of these pathways may overlap with drug use, others may not.
The present study findings should be interpreted while considering limitations. The sample was moderately sized and only powered to detect large effect sizes (and at best medium effect sizes). Also, the sample comprised individuals with AUD. This sampling is typical when studies test if pharmacotherapies for addiction alter eating (Langleben et al., 2012), yet a trial examining IBUD effects in a sample with obesity or eating disorders may yield different results. In addition, the items measuring high-fat/high-sugar food craving may have been too vague. Providing a clear definition of high-fat/high-sugar food with examples may have improved measurement precision.
The present study nonetheless had several methodological strengths. Foremost, IBUD is a novel medication for addictions with support from theoretical rationale and strong preclinical data. The randomized, placebo-control, crossover design reduced study bias and error variance because participants served as their own controls. The design additionally included observation of medication adherence and a standardized meal prior to alcohol administration. Another strength of the design is measurement of the effect of IBUD on tonic food craving as well as food craving following stress and alcohol administration. Calls in the medical field have emphasized that studying multiple behavior intersections in treatment may increase health benefits, maximize health promotion, and reduce health care costs (Prochaska & Prochaska, 2011). Understanding intersections between eating and alcohol use—and intersections in the context of medication effects—may be important to promoting overall health in addiction treatment.
Conclusions
IBUD appears safe with regard to changes in appetitive responses. The observed secondary effects on high-fat/high-sugar food craving were small, limited to the context of psychological stress reactivity, and were found only for those who scored lower in depressive symptomatology. In contrast, IBUD versus PLAC improved mood and reduced tonic alcohol craving as well as attenuated the stimulant and mood-altering effects of alcohol for those who scored higher in depressive symptomatology (Ray et al., 2017a). Taken together, these findings show the potential utility of IBUD as a pharmacotherapy for AUD independent of altering other appetitive responses. Trials with larger samples, samples with comorbid diagnoses, and longer durations may better address this question. In addition, food craving may change if AUD becomes more severe or when individuals seek treatment and decrease drinking, all which IBUD could affect. Indeed, prior research has typically tested effects of addiction medication on food craving in samples of those abstaining from substance use (de Zwaan & Mitchell, 1992) but research has shown that when individuals decrease drinking or practice abstinence from alcohol they often increase eating (Cummings & Tomiyama, 2018). This might mask any reduction in food cravings provided by addiction medications. Moreover, those with severe alcoholism may become malnourished (Barboriak et al., 1978) and recent research has highlighted other important differences between treatment and non-treatment seekers with AUD (Ray et al., 2017b; Rohn et al., 2017). Future trials on the clinical efficacy of IBUD may uncover long-term benefits or harm on appetitive responses.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism to LAR (R21AA022214). This funding source had no other role than financial support. Support for this study was also provided by grants from the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. Portions of the study procedure were completed at the UCLA CTSI. This funding source had no other role in the current research report.
Footnotes
Controlling for marijuana use (as indicated by toxicology tests) did not affect the significance of the reported results.
Conflicts of Interest: Study medication was provided by Medicinova Inc. LAR has received study medication from Pfizer and consulted for GSK. None of the authors have conflicts of interest to disclose.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Osei SY. Molecular regulation of eating behavior: New insights and prospects for therapeutic strategies. Trends Mol Med. 2001;7(5):205–213. doi: 10.1016/s1471-4914(01)01989-x. [DOI] [PubMed] [Google Scholar]
- Barak S, Wang J, Ahmadiantehrani S, et al. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addict Biol. 2015;20(4):626–642. doi: 10.1111/adb.12152. [DOI] [PubMed] [Google Scholar]
- Barboriak JJ, Rooney CB, Leitschuh TH, Anderson AJ. Alcohol and nutrient intake of elderly men. J Am Diet Assoc. 1978;72(5):493–495. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, Tx: Psychological Corporation; 1996. [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17(1):108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton SJ, Ball M, Ahern A, Hetherington MM. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004;81(1):51–58. doi: 10.1016/j.physbeh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Rose A, Randall-Smith L, Hardman CA. Alcohol’s acute effect on food intake is mediated by inhibitory control impairments. Heal Psychol. 2016;35(5):518–522. doi: 10.1037/hea0000320. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Pavlicova M, et al. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol. 2016;21(4):895–903. doi: 10.1111/adb.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JR, Tomiyama AJ. Bidirectional associations between eating and alcohol use during restricted intake. Current Addiction Reports. 2018 doi: 10.1007/s40429-018-0180-4. Epub ahead of print Available from: [DOI]
- de Zwaan M, Mitchell JE. Opiate antagonists and eating behavior in humans: A review. J Clin Pharmacol. 1992;32(12):1060–1072. [PubMed] [Google Scholar]
- DeYoung DZ, Heinzerling KG, Swanson A-N, et al. Safety of intravenous methamphetamine administration during ibudilast treatment. J Clin Psychopharmacol. 2016;36(4):347–354. doi: 10.1097/JCP.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eiler WJA, Džemidžić M, Case KR, et al. The apéritif effect: Alcohol’s effects on the brain’s response to food aromas in women. Obesity. 2015;23(7):1386–1393. doi: 10.1002/oby.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Tomiyama AJ, Mason AE, et al. The reward-based eating drive scale: A self-report index of reward-based eating. PLoS One. 2014;9(6):e101350. doi: 10.1371/journal.pone.0101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington MM, Cameron F, Wallis DJ, Pirie LM. Stimulation of appetite by alcohol. Physiol Behav. 2001;74(3):283–289. doi: 10.1016/s0031-9384(01)00598-4. [DOI] [PubMed] [Google Scholar]
- Hofmann W. Impulses got the better of me: Alcohol moderates the influence of implicit attitudes toward food cues on eating behavior. J Abnorm Psychol. 2008;117(2):420–427. doi: 10.1037/0021-843X.117.2.420. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Matsuda K, Iwaki Y. Ibudilast for the treatment of drug addiction and other neurological conditions. Clin Investig (Lond) 2014;4(3):269–279. [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(1) doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen H, Mannisto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54(3):473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Busch EL, O’Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl) 2012;220(3):559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Mason AE, Laraia B, Daubenmier J, et al. Putting the brakes on the “drive to eat”: Pilot effects of naltrexone and reward-based eating on food cravings among obese women. Eat Behav. 2015;19:53–56. doi: 10.1016/j.eatbeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav. 1996;59(1):179–187. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- Metz VE, Jones JD, Manubay J, et al. Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology. 2017:1–8. doi: 10.1038/npp.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppitt SD, Eckhardt JW, McGonagle J, Murgatroyd PR, Prentice AM. Short-term effects of alcohol consumption on appetite and energy intake. Physiol Behav. 1996;60(4):1063–1070. doi: 10.1016/0031-9384(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. Am J Lifestyle Med. 2011;5(3):208–221. doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K. Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: A randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology. 2017a:1–13. doi: 10.1038/npp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: Do they matter? Am J Drug Alcohol Abuse. 2017b;0(0):1–8. doi: 10.1080/00952990.2017.1312423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Kahler CW, et al. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: A pilot study. Psychopharmacology (Berl) 2007;193(4):449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rohn MCH, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L. Differences between treatment-seeking and nontreatment-seeking alcohol-dependent research participants: An exploratory analysis. Alcohol Clin Exp Res. 2017;41(2):414–420. doi: 10.1111/acer.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Hardman CA, Christiansen P. The effects of a priming dose of alcohol and drinking environment on snack food intake. Appetite. 2015;95:341–348. doi: 10.1016/j.appet.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Schrieks IC, Stafleu A, Griffioen-Roose S, et al. Moderate alcohol consumption stimulates food intake and food reward of savoury foods. Appetite. 2015;89:77–83. doi: 10.1016/j.appet.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addict Biol. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, Finch LE, Cummings JR. Did that brownie do its job? Stress, eating, and the biobehavioral effects of comfort food. In: Scott RA, Kosslyn SM, editors. Emerging Trends in the Social and Behavioral Sciences. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2015. [Google Scholar]
- Worley MJ, Heinzerling KG, Roche DJO, Shoptaw S. Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend. 2016;162:245–250. doi: 10.1016/j.drugalcdep.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR. Short term effects of alcohol on appetite in humans. Effects of context and restrained eating. Appetite. 2010;55(3):565–573. doi: 10.1016/j.appet.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Phillips MF. Failure to reduce short-term appetite following alcohol is independent of beliefs about the presence of alcohol. Nutr Neurosci. 2002;5(2):131–139. doi: 10.1080/10284150290019008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.