Abstract
Background:
Researchers have long sought to understand how individuals respond to alcohol in social settings with the aim of elucidating pathways of risk for alcohol use disorder (AUD). But studies that incorporate a social context are still outnumbered by those that examine alcohol’s subjective effects among participants drinking alcohol in isolation. Further, perhaps due to the challenges of capturing automatic affective processes in these settings, prior studies of alcohol response in social context have relied mainly on self-report measures, and so relatively little is known about alcohol’s psychophysiological effects in social settings.
Methods:
Using a novel paradigm that integrated alcohol-administration procedures, startle methodology, and social context, the current study examined the impact of alcohol and social context on startle eyeblink reflex among 40 social drinkers.
Results:
Results indicated that there was a significant effect of group presence, indicating that startle magnitude was larger when people were alone than with others. There was a significant group presence by alcoholic beverage interaction, with the effect of alcohol being significantly larger when people were alone versus with others. These effects were found both for the startle habituation data and during the picture viewing task.
Conclusions:
Results of this study highlight the importance of considering the presence of other individuals for understanding alcohol response and mechanisms of AUD risk. Findings are discussed in light of both emotional and also cognitive correlates of startle reflex magnitude. Future research should examine these effects within larger samples of participants and further explore mechanisms that might underlie these effects.
Keywords: alcohol, social context, startle, emotion, cognition
Introduction
Researchers have long been interested in understanding individuals’ responses to alcohol in social settings (Harford, 1983; Pliner & Cappell, 1974; Lindman, 1982; Aan Het Rot et al., 2008). Importantly, the vast majority of alcohol consumption in everyday life takes place in social context (Bourgault and Demers, 1997; Senchak et al., 1998; Single and Wortley, 1993). Further, Studies have found that people drink more and report greater mood enhancement from alcohol when they drink in group settings versus when they drink alone (Doty and de Wit, 1995; Pliner and Cappell, 1974). The belief that alcohol enhances social situations represents a robust correlate of heavy drinking and predictor of later transition into problematic drinking patterns (Jones et al., 2002; see also Fairbairn and Sayette, 2014; Fairbairn et al., in press). Thus, understanding alcohol’s effects in social context may be critical to understanding factors that drive alcohol consumption and, ultimately, to helping trace the roots of alcohol use disorder.
Although outside the laboratory, most alcohol consumption takes place in social context, within laboratory investigations of alcohol’s effects, most paradigms have examined individuals drinking in isolation (see Fairbairn and Sayette, 2014 for a review). Furthermore, among the minority of laboratory studies that have examined alcohol response in social context, the measurement of alcohol’s effects on subjective experience has been limited, relying overwhelmingly on self-reports (Fairbairn and Sayette, 2014). The current study seeks to fill this gap by expanding the range of measures used to gauge alcohol’s effects on subjective experience in social context. More specifically, the current study represents the first to employ startle methodology—a method with powerful advantages for capturing individuals’ automatic responses—to examine the impact of social context on responses to alcohol.
Startle Responses, Emotion, and Cognition
Prior studies examining alcohol response in group settings have mainly relied on self-reports to capture emotional responses. An understanding of acute emotional response to alcohol—including the extent to which alcohol has the ability to relieve negative affective states—has been thought to be key to understanding factors that reinforce drinking and thus make individuals vulnerable to developing alcohol use disorder (Conger, 1956; Sher, 1987). Self-reports of emotion have several well-known advantages, particularly when consciously accessible emotional states are the subject of interest, as they offer a relatively low-cost, straightforward means of measuring internal subjective responses (Mauss and Robinson, 2009). Nonetheless, self-reports may not offer a complete picture of emotions experienced in social contexts, since emotional experiences in social contexts may shift frequently from one moment to the next and, further, emotions may often be beyond awareness such that they may not easily be captured via explicit self-reports (Kirsch & Lynn, 1999; Locke, 2005).
In an effort to build on the information provided by self-reports of emotion, researchers have sought measures that are capable of assessing more automatic, instantaneous emotional experiences. Among these methods, startle methodology has emerged as one powerful tool for capturing emotional responses. The majority of studies employing startle methodology have examined the influence of foreground images on the magnitude of muscle responses to sudden noise probes. Vrana, Spence, and Lang, (1988) were among the first to demonstrate that the magnitude of human startle reflex is influenced by the valence and arousal of the foreground images. Lang’s subsequent studies consistently supported this theory by replicating the finding that human startle reflex is potentiated during emotionally unpleasant and highly arousing slides and attenuated during emotionally pleasant and highly arousing slides (Bradley et al., 1990; Lang et al., 1990; although see Kaye et al., 2016 for psychometric issues with the paradigm). Researchers have argued that startle magnitude in the context of foreground stimuli can often tap into participants’ automatic and instantaneous emotional dispositions and thus represent a powerful method for assessing internal affective experience. In fact, startle methods have been used in alcohol research to understand situational factors that affect stress dampening (Curtin, et al., 1998); however, to our knowledge these methods have not been used to study the social aspects of alcohol.
In addition to measuring startle magnitude during the presentation of foreground images, some studies have examined the habituation of the startle response without the presentation of foreground stimuli (Lane et al., 2013; LaRowe et al., 2006; Verona and Curtin, 2006). Similar to startle research using foreground stimuli, prior research has shown that the rate of habituation is correlated with individual differences in emotional responding (e.g., Blanch et al., 2014; LaRowe et al., 2006) and can be affected by state manipulations related to emotional responding (e.g., stress; (Schicatano, 1998; Verona & Curtin, 2006). Habituation of the startle response, however, has been less well studied than responses to foreground stimuli, and so, for this and other reasons, the precise mechanisms underlying individual differences in overall startle response during habituation are less well understood.
Although startle methods have emerged as a key tool for understanding affective experience, it has become clear that the magnitude of startle responses may reflect elements of experience beyond emotion. More specifically, researchers have argued that startle methods may often capture elements of cognition or, more specifically, the allocation of attention (Anthony & Graham, 1985; Hackley & Graham, 1983; Silverstein et al., 1981). In Graham’s studies, findings indicated that when attention was directed away from the startle-eliciting stimulus and towards a distraction, the amplitude of the startle reflex was diminished. In other words, in these studies, as more attention was drawn to the distraction, startle reflexes were smaller, whereas reflexes were larger when the distraction was not present (Anthony & Graham, 1985; Hackley & Graham, 1983; Silverstein et al., 1981). Research on the habituation of the startle response suggest that it may also tap into aspects of cognitive processing (e.g., Braff et al., 1992). These results suggest an interaction between emotion and attention. Questions surrounding the allocation of attention have historically been an area of great interest to alcohol researchers, since theories of alcohol’s effects have postulated that a core element of alcohol’s effects is its tendency to decrease individuals’ ability to divide attention between tasks (Steele & Josephs, 1990). In the current study, we aim to take a first step towards understanding alcohol’s impact on startle in a social context—an ecologically valid drinking setting with known effects on emotion and cognition—in order to further explore alcohol’s effects on psychological processes.
Understanding Startle Responses in a Social Context
Prior studies have examined the effect of social context using a variety of methods and measures, including studies examining whether the presence of others influences facial expressions during the viewing of photographic slides (Flores & Berenbaum, 2014) and neural responses during fMRI scanning or EEG recording (Coan et al., 2006). To our knowledge, however, no prior study has incorporated a “real” social context into a startle paradigm (for an example of virtual social situations see Cornwell et al., 2006). Social contexts are not only a setting with relatively high ecological validity for understanding alcohol’s effects, but they represent an area with decades of psychological research. Social contexts can exert potent influences on both emotion and cognition, effects that may vary depending on the nature of the social context in question. The presence of other individuals might either increase anxiety (e.g., intergroup interactions; Plant & Devine, 2003) or decrease anxiety (e.g., interactions with a trusted friend/partner; Clark and Lemay, 2010). Similarly, while all social contexts tend to draw attentional resources, some tend to be more cognitively demanding than others (e.g., interracial interactions; Mendes et al., 2008; Richeson and Shelton, 2007). Further, even in the absence of direct social interaction or engagement in shared activity, the mere presence of other individuals in the same physical space can have key effects on psychological processes (e.g., Zajonc, 1965). The goal of the current study was to examine the interaction of alcohol (known to both narrow attention and decrease anxiety) and social context on the magnitude of startle responses. Specifically, in an effort to promote precise mechanistic understanding and isolate potential mechanisms that might underlie social contextual effects on startle response, we have designed a paradigm that isolates the effect of the “mere presence” of other individuals on alcohol’s effects on startle.
The primary goal of the current study was to take an initial step towards understanding alcohol’s effects on startle responses within a social context. As the current study is, to our knowledge, the first study to incorporate a social context into a startle paradigm, we tested two competing hypotheses. To the extent to which startle represented a pure index of emotion in this study, we anticipated we might find an increased effect of alcohol on startle response in groups of unfamiliar individuals vs alone (e.g., Pliner & Cappell, 1974). In contrast, to the extent to which startle responses capture distraction, or the allocation of attention away from the startle stimulus and towards other individuals present in the room, we might anticipate a different pattern of findings (Anthony & Graham, 1985; Hackley & Graham, 1983). In order to gain a deeper understanding of the relative influence of emotion and attention allocation on startle responses in our study, we conducted analyses examining the magnitude of effects within interracial groups—contexts known to increase anxiety and also draw attention. Our secondary goal of this study was to further characterize the habituation of startle magnitude. Given the smaller body of research on startle habituation, we thought identifying the effects of alcohol and social context would represent an addition to the literature. Taken together, these examinations are aimed at gaining a deeper understanding of the effects of alcohol on automatic cognitive and emotional processes across contexts and to move towards expanding our repertoire of experimental paradigms capable of addressing such questions.
Materials and Methods
Participants
Participants consisted of 60 healthy social drinkers, aged 21 to 28, recruited via advertisements and posted notices in the local community. In order to meet the eligibility criteria for the current study, participants were required to report consuming at least 2 drinks on at least 2 occasions per month, or at least 4 drinks on at least 1 occasion per month, over the past 12 months. Individuals who successfully completed an initial phone screening interview were invited to the Alcohol Research Laboratory for further screening. Exclusion criteria included medical conditions that contraindicated alcohol consumption, a diagnosis of alcohol use disorder as indexed by the Diagnostic and Statistical Manual of Mental Disorders (5th ed.), pregnancy in women and being uncomfortable with study drinking requirements. Participants with a body mass index (BMI) less than 19 or greater than 27 were also excluded, due to alcohol dosing requirements. Participants who met the eligibility criteria were invited to participate in the experiment. Of these participants, 30 were male and 30 were female. 57% were European American, 12% were African American, 5% were Hispanic, and 20% were Asian. Participants reported drinking on average two to three times per week and consuming 4.07 (SD = 1.94) drinks per occasion. Of the 60 participants, all engaged in alcohol-administration procedures and were involved in the “group” startle condition, and 40 (50% MALE, average age=22.73) served as primary startle participants (see Table 1 for descriptive characteristics; see also below for description of startle procedures).
Table 1.
Descriptive Characteristics of the 60 participants in the Study Sample. Data are expressed as mean SD or frequency (%).
| Variable | All participants (n=60) | Primary Startle Participants (n=40) | Non-startle Participants (n=20) |
|---|---|---|---|
| Age | 22.47 (1.87) | 22.73 (2.01) | 21.95 (1.47) |
| Sex (% Female) | 50 | 50 | 50 |
| % White | 57 | 50 | 70 |
| % Black | 12 | 15 | 5 |
| % Asian | 20 | 22.5 | 15 |
| % Hispanic | 5 | 2.5 | 10 |
| Number of Drinking Occasions in the past 30 days | 10.43 (5.44) | 9.93 (4.92) | 11.45 (6.38) |
| Number of Drinks per Occasion | 4.07 (1.94) | 3.82 (1.84) | 4.55 (2.09) |
Of the 60 participants, all engaged in alcohol-administration procedures and were involved in the “group” startle condition, and 40 (50% MALE, average age=22.7) served as primary startle participants (see above for description of startle procedures).
Procedures
All participants attended two beverage administration visits – one alcohol beverage administration session and one control beverage administration session – in same-gender groups of three, a drinking configuration chosen to reflect a reasonably common real-world social drinking setting (Sayette et al., 2012). Participants attended all visits with the same group of individuals to ensure that all conditions were held constant across the experimental beverage-administrations sessions as much as possible, aside from beverage content. Measures were taken to ensure that all participants were unacquainted prior to study participation (see procedures in (Fairbairn & Sayette, 2013).
The two beverage-administration sessions started at approximately 3 pm. The second laboratory session was held 3 days after the first session. During one of these experimental sessions, groups were administered an alcoholic beverage and, during the other session, they were administered a control beverage. The order of sessions was counterbalanced across groups. A placebo condition, in which participants are given a non-alcoholic beverage but informed that they would be receiving alcohol, was not used in this study (Balodis et al., 2011) because placebo manipulations can lead to unanticipated compensatory effects (Testa et al., 2006); in addition, a within-subject design makes placebo manipulations challenging, as it would have been difficult to deceive participants regarding the content of their beverages on the second beverage-administration session.
Upon arrival, participants were brought into separate rooms and asked to provide a breath sample to assess blood alcohol concentration (BAC). They also completed a variety of self-report questionnaires. The three participants then moved to the beverage administration room to consume drinks. On the alcohol beverage administration session, participants were informed that they would be receiving alcohol and that the dose would be about the legal driving limit. The alcoholic beverage was 100-proof vodka and 3.5 parts cranberry juice cocktail. The difference between men and women in rates of alcohol metabolism was accounted by adjusting the dose of alcohol according to gender. Men were administered a 0.82-g/kg dose of alcohol, while women were administered a 0.74-g/kg dose. On the control beverage administration session, participants were given the same volume of cranberry juice. Participants remained seated for a total of 36 min while beverages were administered in three equal parts at 0 min, 12 min, and 24 min. They were instructed to drink their beverages evenly during the 12-min intervals. Immediately after the drinking period, we recorded participants’ BACs and had them complete a set of questionnaires that are unrelated to the current research questions.
Startle.
Startle procedure (a picture viewing task) began approximately 20 minutes after the completion of beverage administration. Among the three participants in each group, one participant was assigned to the Alone condition in which the individual completed the startle task alone on both alcohol and control sessions. Another participant was assigned to the Group condition, in which the individual completed the startle task in the presence of the other two participants on both alcohol and control sessions.1 In the Group condition, the startle participant was facing the computer screen, while the other two group members were seated on either side of this participant, facing the other direction (i.e., they could not see the images). The room was 7 feet by 7 feet and participants were approximately 12 inches from each other. This measure was taken to ensure that only the presence or absence of others—and not also the knowledge of shared viewing—varied across Alone and Group conditions. Participants in the Group condition were told not to talk during the task. Group/Alone condition assignment was held constant for each participant across sessions because there are large individual differences in startle magnitude and this allowed us to make our key comparison (the effect of alcohol vs. control for Group and Alone) within-subjects, which made individual differences irrelevant as each person served as their own control. The roles were randomly assigned for each group. The order of two startle sessions (Alone, Group) was counterbalanced across groups. For one group, there was no startle data for the alcohol session due to a computer error.
After the startle procedure, participants completed other tasks unrelated to the current research questions. Individuals were paid $160 for participation. In the alcohol condition, participants were dismissed only after their BACs were reduced to .03% or below.
Measures
Startle.
Participants completed a computer task in which they viewed neutral and unpleasant IAPS images (e.g., Bradley et al., 2007; Sadeh & Verona, 2013).2 Prior to the picture-viewing task, 11 habituation probes were presented to reduce the effect of abnormally large blinks (Lane et al., 2013). These trials were later used in the habituation analyses. A total of 60 images, split into two sets of 30, were used in the current study. In each session, 28 images (14 from each category) were presented along with a probe and 2 were presented without a probe to decrease predictability of the startle probe.3 Prior research suggests that 7 blinks are sufficient for a reliable startle response (Lieberman et al., 2017). In any given session, both participants saw the same set of images. Presentation order was counterbalanced across participants. Images were presented between 4.25 and 5.25 seconds, with an intertrial interval of 2 seconds. The probes were played at 2.5, 3.0, or 3.5 seconds after picture onset. Acoustic startle probes (each a 105-dB, 50-ms burst of white noise with an instantaneous rise time) were administered binaurally over earphones to elicit a blink response. Due to concerns about participant burden and the timing of the peak of the BAC curve, participants did not rate the images on valence and arousal, as is common in these paradigms (Bradley et al., 2007).
Two 4-mm Ag-AgCl electrodes were placed on the orbicularis oculi muscle under the left eye, in order to record the eyeblink component of the startle reflex. Electrode impedance was kept below 10kΩ. The signal was amplified using a Neuroscan Synamps and digitized online at 2000Hz using a 24-bit A/D converter. Data were processed using the Physbox add-on toolbox to EEGlab in Matlab (Curtin, 2011; Delorme and Makeig, 2004). The data were first high-pass filtered (28 Hz butterworth filter), then rectified and low-pass filtered (30 Hz butterworth filter). Startle magnitude, the peak response between 20-100 ms post-probe relative to the mean of the 50 ms baseline, was calculated by an automated procedure. Negative values were set to zero.
We took several steps to ensure the integrity of our data. First, trials where the magnitude did not exceed the maximum value of the baseline period, including trials set to zero (13% of trials), and trials with peak baseline values that exceeded the absolute value of 10μV (3% of trials) were excluded from all analyses. Second, we examined the number of usable trials for each participant for the smallest cell of interest. In the habituation portion of the data, participants had on average 8.88 (out of 11) usable trials (SD = 2.93). Six participants had fewer than seven useable trails for one cell. In the task portion of the data, participants had on average 11.72 (out of 14) usable trials (SD = 2.49). Four participants had fewer than seven trials for one cell. Our results were largely the same when we excluded the cells from participants that had fewer than seven trials as when we included all usable trials. Based on this and the fact that multi-level modeling is robust to missing data (Judd et al., 2012; Quené and van den Bergh, 2004), we report results with all usable trials included. Finally, we examined the presence of outliers. Outliers (2% of trials) were defined as values greater than +/− 2.5 SD from the participants mean across trials for that portion of the task (i.e., habituation trials or task trials). Outlier values were replaced with the corresponding +/− 2.5 SD value.
Data Analysis
Because of the nested nature of our data (i.e., people nested within groups and trials nested within people), we used multi-level modeling to analyze our data. Multi-level modeling has three major advantages over repeated measures ANOVA (see Judd et al., 2012; Quené, & van den Berg, 2004 for reviews). First, it allows for the explicit modeling of different sources of variation, which allows for more statistical power. Second, it allows for more flexible modeling of the variance/covariance matrix than repeated measures ANOVA, which requires sphericity. This flexibility has been shown to protect against Type-I errors. Finally, multi-level modeling is robust to unbalanced data (i.e., participants having different numbers of trials). This allowed us to use all possible data. An additional advantage of multi-level modeling for startle magnitude data, is that it allows for the research to include linear and quadratic effects to adjust for habituation of the startle response to the noise probe during the task, further strengthening the statistical power. Although most startle research has used repeated measures ANOVA and mixed model ANOVAs, there are examples of uses of multi-level modeling (e.g., Bresin & Verona, 2016; Verona et al., 2013).
Habituation analysis.
To examine the effect of group presence (Alone, Group) and alcoholic beverage (control, alcohol) on habituation startle, we used a three-level multi-level model to understand variation in the first 11 habituation probes. Because startle magnitude generally decreases during the habituation period, it is important to consider time in this analysis (Lane et al., 2013). Therefore, we first started by modeling startle magnitude as a function of trial number to understand the normative habituation of startle magnitude across trials. Based on previous research (Lane et al., 2013), we also included a quadratic effect of trial. The Level 1 (within-person, within-group) modeled startle magnitude as a function of trial number and trial number squared. Trial was centered on the average trial number so that the intercept represented the average startle magnitude during the habituation period and the slopes represent changes from that average. The Level 2 (between-person, within-group) and Level 3 (between-groups) models only contained a random effect for variation in the intercept. After establishing the normative trend, we added group presence, and alcoholic beverage along with relevant interactions (e.g., group presence × alcoholic beverage, trial number × group presence, trial number × group presence × alcoholic beverage) to see how those factors moderated the normative change.
Image analysis.
To examine whether valence (unpleasant, neutral) affected startle magnitude in the main task, we used a similar model to that above. First, we included trial and trial2 as covariates to account for continued habituation to the noise probe throughout the task (e.g., Bresin & Verona, 2016). The Level 1 (within-person, within-group) modeled startle magnitude as a function of valence, alcoholic beverage, and all interactions. The Level 2 (between-person, within-group) model contained group presence and the Level 3 (between-groups) model only contained a random effect for variation in the intercept. All models were run in SAS using full information maximum likelihood with degrees of freedom estimated with the between function.
Results
Manipulation Check
All participants in both conditions had zero BAC upon arrival to the lab. BAC peaked just after the startle task (M = .07%, SD = .001). During the control session, all participants had a zero BAC after the drink period.
Habituation
Consistent with previous research (e.g., Lane et al., 2013), we found a significant linear decrease in startle across the habituation period, γ = −.98, t(672) = −4.72, p < .001 and a quadratic increase, γ = .16, t(673) = 2.36, p =.018. Taken together, this suggests that in general there is an initial steep linear decrease that levels out in later trials. When group presence, alcoholic beverage, and interactions were added to the model, there were several significant effects. There was a significant effect of alcohol, such that startle magnitude was lower overall in the alcohol condition, γ = −6.32, t(675) = −2.51, p = .012. There was a marginal effect of group presence, indicating that startle magnitude was larger when people were alone versus with others, γ = 12.87, t(51.5) = 1.94, p = .058. These effects were qualified by a significant group presence × alcoholic beverage interaction, γ = −7.70, t(675) = −2.12, p = .034. To decompose this interaction, we looked at the effect of alcohol within group presence (see Figure 1 top panel). The effect of alcohol was significant when people were alone, F(1, 680) = 100.14, p < .001, d = .38, and with others, F(1, 677) = 15.88, p < .001, d = .15; however, the interaction indicated that the effect of alcohol was significantly bigger when people were alone than when they were with others. Because time is centered on the average trial number, the effect sizes only apply to the average startle magnitude during the habituation period.
Figure 1.
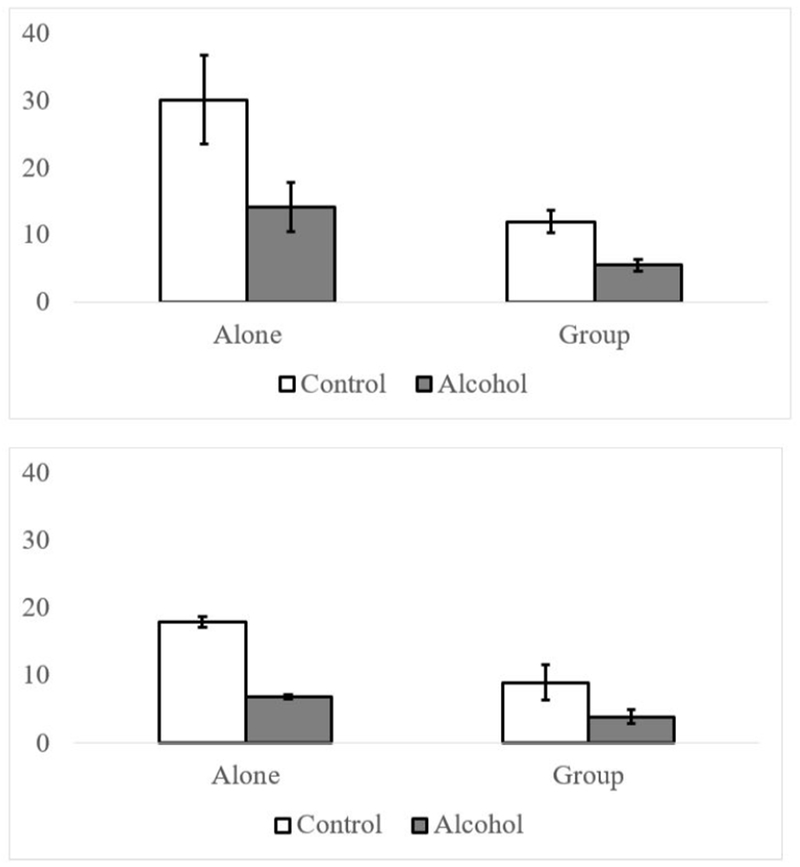
Top Panel: Habituation Startle Magnitude as a Function of Group Presence (Group, Alone) and Alcoholic Beverage (Alcohol, Control). Bottom Panel: Task Startle Magnitude as a Function of Group Presence (Group, Alone) and Alcoholic Beverage (Alcohol, Control).
Although alcoholic beverage did not interact with trial, group presence interacted with the linear, γ = −1.94, t(672) = −3.68, p < .001, and quadratic γ = .43, t(672) = 2.29, p = .025 components. These significant interactions indicate that the change in startle magnitude overtime is influenced by the presence (versus absence) of group members. Figure 2 shows the estimated growth curves for each condition. Two things are worth noting. First, when people were in the presence of others there was a significant linear decrease, γ = −.35, t(336) = −2.90, p = .004, but not a significant quadratic effect, γ = .04, t(337) = .92, p = .360. When people were alone, however, both effects were significant, linear: γ = −1.65, t(337) = −4.56, p < .001; quadratic: γ = .32, t(336) = 2.43, p = .016. Moreover, the linear decrease in the Alone condition was 5 times larger than the Group condition. Second, it appears that part of the reduced decrease in the Group condition was possibly influenced by the fact that they began with relatively lower startle magnitude.
Figure 2.
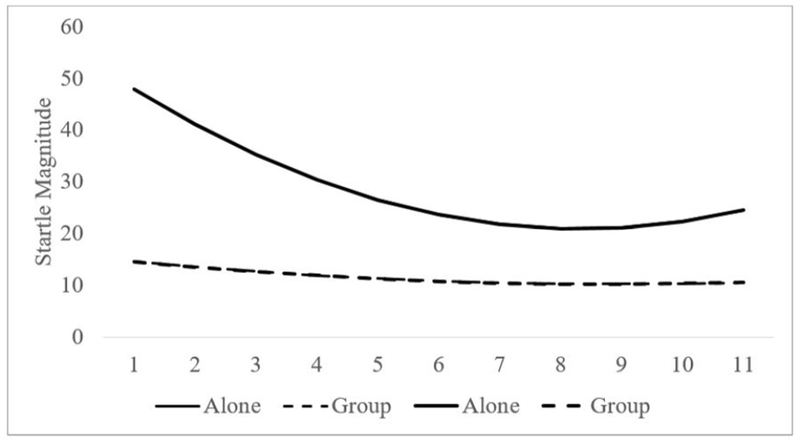
Habituation Startle Magnitude as a Function of Group Presence (Group, Alone) and Trial Number.
Image Task
Similar to the results from the habituation trials, there was a significant quadratic effect for trial number during the picture viewing task, γ = −.01, t(1767) = 2.41, p = .016. There was also a trend for a linear effect, γ = −.06, t(1776) = −1.50, p = .134. Thus, we included both as covariates. The results from this analysis did not show any effect of valence or interactions involving valence (p’s range from .617 - .941). There was a main effect of alcohol, such that startle magnitude was lower in the alcoholic beverage condition than the control beverage condition, F(1, 1735) = 295.22, p < .001. There was also a significant interaction between alcoholic beverage and group presence, F(1,1735) = 58.60, p < .001. As above, we looked at the effect of alcohol according to group presence condition. When people were alone, there was a significant effect of alcohol, F(1, 1736) = 304.25, p < .001, d = .42, with smaller blinks while intoxicated (M = 4.58) compared to sober (M = 17.11). There was a similar effect in the Group condition (Alcohol: M = 3.40; Control: M = 8.21); however this effect was significantly smaller, F(1, 1733) = 46.00, p < .001, d = .16.
Exploratory Follow-up Analysis
Given that our results were somewhat ambiguous as to whether they reflected emotion or attention, we conducted further exploratory analyses. Because our results showed that the effect of alcohol on startle magnitude was smaller in the Group condition, we wanted to explore whether a factor that varied within groups showed a similar effect. The number of out-group members (as compared to the participant) was one factor that varied within groups. Therefore, we looked at whether the number of out-group members (as compared to the participant) influenced the effect of alcohol on the startle magnitude. Prior research has shown that intergroup interactions tend to increase anxiety, an effect that extends across divergent group racial compositions and varying attitudes towards outgroup members (e.g., Richeson and Shelton, 2007; Stephan and Stephan, 1985, 1989). Thus, if the effects are on emotion, you might expect diminished effects of alcohol when more out-group members were present. If the effect was larger when more out-group members were present, however, it would be more consistent with an attentional allocation effect (i.e., out-group members are distracting attention away from the startle probe). Note that, as these follow-up analyses were not the main focus of our research, this particular study was not well powered for these models, and so our analysis of outgroup members should be considered preliminary.
For this analysis, we only considered participants in the Group condition. The number of out-group members was calculated by comparing the self-reported race of the participant viewing the images to that of the other participants in the room. Group composition was analyzed as a continuous variable ranging from 0 (neither of the other 2 individuals in the room with the image-viewing participant were racial out-group members) to 2 (both of the other 2 individuals in the room were racial out-group members). Of the 20 groups used in the analysis, 3 contained no out-group members, 5 contained one out-group member, and 12 had 2 out-group members. Although a comparison of racial subcategories (i.e., beyond in-group and out-group) is not within the scope of this paper, our sample did include representation from a variety of racial categories racial configurations—the participants who were viewing images were 30% White, 20% African American, 25% Asian, 5% Hispanic, 5% Pacific Islander, and 15% Other; individuals sitting in the same room with them were 67.5% White, 7.5% African American, 17.5% Asian, 5% Hispanic, and 2.5% Other.
As with the main results, separate analyses were run for the habituation period and the picture viewing period. The models were similar to those above, with the major difference being that instead of group presence, the number of out-group members was included as a predictor of startle magnitude. Because zero out-group members had different meanings for the Alone condition as compared to the all in-group members condition, we only look at participants in the Group condition. Therefore, the key parameter of interest was the alcoholic beverage × number of out-group members’ interaction.
In the analysis for the habituation period, there was an effect of alcohol, F(1, 303) = 84.65, p < .001, which was clarified by a significant alcoholic beverage × number of out-group members interaction, F(2, 303) = 6.91, p = .001. The effect of alcohol was larger when there were no out-group members γ = −11.70, t(59) = −4.71, p < .001, d = .61, and one out-group member, γ = −8.97, t(76) = −5.26, p < .001, d = .60, than when there were two out-group members, γ = −4.23, t(173) = −4.69, p < .001, d = .35. Given that there were so few participants per cell, we also looked at the relation between the number of out-group members and startle magnitude within each session. In the alcohol session, the number of out-group members was not significantly related to startle magnitude, γ = −.42, t(16.7) = −.44, p = .662, and for the control session, there was a marginal negative relation between the number of out-group members and startle magnitude, γ = −4.45, t(17.2) = −1.76, p = .095.
Very similar results were found during the task. Among those in the Group condition, there was a significant interaction between alcohol and number of out-group members, F(2, 791) = 53.75, p < .001. The effect of alcohol was bigger when there were no out-group members, γ = −11.59, t(156) = −8.59, p < .001, d = .68, or one out-group member, γ = −7.90, t(183) = −8.27, p < .001, d = .6, than two out-group members, γ = −1.65, t(456) = −4.28, p < .001, d = .20. In the alcohol session, the number of out-group members was not significantly related to startle magnitude, γ = .61, t(19) = .72, p = .479, but was significantly negatively related in the control session, γ = −4.35, t(18.4) = −2.27, p = .035. Together these results are more consistent with out-group members distracting participants from attending to the startle probes, which combined with our other results might suggest that the effects of group are more about cognitive resources than affect per se.
Discussion
The current study offers a novel paradigm for understanding alcohol’s reinforcing effects, for the first time examining how social context and alcohol interact to predict startle responses. This paradigm made it possible to capture effects of alcohol on automatic psychological processes and to examine how these processes vary according to social setting—a setting with relatively high ecological validity for understanding alcohol’s effects. Our results indicate that there was a significant effect of group presence, indicating that startle magnitude was larger when people were alone versus with others. In addition, there was a significant group presence by alcoholic beverage interaction, with the effect of alcohol being significantly larger when people were alone than when they were with others. These effects were found both for the startle habituation data and during the picture viewing task.
Prior research examining the effect of alcohol and social context on emotion has produced results that would appear to diverge from the results of this study, with findings of these studies indicating that alcohol’s effects on emotion are larger in social context (Doty and de Wit, 1995; Pliner & Cappell, 1974). Of note, these prior studies have focused on neutral or explicitly pleasurable social stimuli (e.g., conversing in a room with books and games, composing cartoon captions), and the emotion outcomes did not explicitly target stress processes. One possible explanation for the results of the current study is that, given this somewhat uncomfortable and sometimes distressing experimental procedures/stimuli in our study (darkened room, experimental electrodes, distressing images), people were feeling more comfortable in the presence of another person (albeit a relatively unfamiliar individual) than they were when they were alone. Some past studies, i.e., Flores & Berenbaum (2017), indicate that even holding the hand of a stranger can be comforting when individuals are presented with a stressful situation. Thus, alcohol and social context may have been performing similar functions of alleviating anxiety in this paradigm. In this regard, alcohol might have less of an effect in reducing anxiety in the group setting because another source of comfort – the presence of others – was already available.
A second possible explanation for our results is that the presence of others served as a distraction, which pulled attention away from the startle probes. The current study focused on overall startle response during the habituation and task period, rather than emotion modulated startle associated with the viewing of different emotionally laden slides. Overall startle response is likely influenced by a variety of different psychological processes, not all of which are well characterized within the literature (Kaye et al., 2016). Of note, the presence of other individuals was already a cognitively demanding experience for participants, and thus they might have had less attentional resources to focus on the task in the Group condition (Anthony & Graham, 1985; Hackley & Graham, 1983; Silverstein et al., 1981). This was supported by the fact that startle magnitude was not significantly influenced by the presence of affective foreground images. Moreover, the follow-up analysis on interracial groups appear to provide some support, although preliminary, for this cognitively-focused interpretation of findings. Interracial interactions serve both to increase anxiety and also to usurp attentional resources. Thus, were startle magnitude in the social context serving primarily as an index of emotion (i.e., anxiety), we might expect enhanced startle magnitude in interracial contexts whereas, were it serving primarily as an index of attention allocated away from the startle procedures, we might expect diminished startle magnitude. In fact, the results indicated that the effect of alcohol on the startle reflex was larger when there were no out-group members than when there was one out-group member, or two out-group members. These results appear to provide support for a cognitive (i.e., attention allocation) explanation of our group presence by alcoholic beverage interaction. Importantly, however, power was low for these between-group analyses, and these models further examined effects of intergroup contexts across a range of group racial compositions. Findings examining effects of intergroup context should therefore be viewed as preliminary.4 Future research might examine effects within specific racial pairings (e.g., Black-White interactions) and among individuals higher in automatic racial bias, and could further examine attention allocation effects by combining startle with event-related potentials (e.g., P300; Drislane et al., 2013).
We did not find evidence of the well-replicated effect of increased startle magnitude for unpleasant (versus neutral) images during the task. There are several possible reasons for this. First, it is possible that the manipulations and procedures of the task were such that they distracted participants from fully attending to the images, even when they were alone in the control condition. It is also possible that the manipulations influenced how participants appraised the images. We did not have participants rate the valence and arousal of the pictures, which may have provided some insights into why we did not find the common valence effects. Although the lack of valence effect is difficult to interpret, this is not a problem with the habituation data, which showed an identical pattern of effects as the task data. There is not a consensus as to what the startle habituation means; however, many studies suggest a cognitive component (e.g., Braff et al., 1992) further supporting our interpretation that our manipulations had effects on cognition.
The results of this study might ultimately have implications for understanding alcohol response and risk for AUD. For decades, alcohol researchers have been seeking to understand the emotional and also cognitive effects of alcohol with a view to better elucidating the factors that make people vulnerable to developing AUD. As the majority of alcohol consumption occurs in social context and people mainly drink together with other people regardless of age or problem drinking status, it is important to understand how social factors and alcohol act in combination in order to fully understand alcohol’s rewarding effects (Fairbairn & Sayette, 2014). Nevertheless, perhaps partially due to the methodological challenges regarding the adoption of social context into studying the effects of alcohol, relatively little is known about alcohol’s rewarding effects in social settings. In this regard, the current research may have important methodological implications as it investigated the reinforcing effects of alcohol in social context. Specifically, the current study is the first step towards capturing the automatic emotional and cognitive effects of alcohol in a social context, using a startle methodology.
Another important contribution of the current study is that, to our knowledge, this is the first study to incorporate a social context into a startle paradigm. As noted previously, while many past studies have attempted to examine the effect of social context on psychological processes using a variety of methods and psychophysiological measures (Donohue et al., 2007), to our knowledge, no study has employed startle methodology in social context. Social contexts are complex, impacting both emotional and cognitive processes with high relevance for physical and mental wellbeing (Boyce et al., 1998). In this regard, the current study is intended to take an initial step towards the integration of a social context into startle paradigms, and, in broader terms, to help further develop various ecologically valid startle paradigms (Dunsmoor et al., 2014; Mühlberger et al., 2008).
Limitations and future directions should be noted. First, in this study of the impact of social context on startle response, we examined the influence of social context on responses to a slide viewing task. We chose a slide paradigm as the most widely studied and well understood context for examining startle, which we concluded was a useful starting place for the study of social context effects. Unlike much prior startle research, however, the current study did not produce a significant effect of slide valence on the startle magnitude—possibly due to the salience of other manipulations employed in the current study (e.g., beverage manipulation, social context, etc.). Assessing alcohol’s effects on emotional responses to more diverse stimuli, i.e., audiovisual stimuli (Gerdes et al., 2014), might be a direction for future studies. Another possibility is that null effects are attributable to the relatively low reliability for emotion modulated startle measured via slide viewing tasks when compared with overall startle responses (Kaye et al., 2016). Second, we also acknowledge that the design of the current study was highly complex, and some of the positive and negative findings should further be studied in more parsimonious designs to clarify their meaning. Future studies could simplify the study design by investigating the effect of alcohol alone or the effect of social situation alone, on the startle magnitude. Third, another limitation of note in the current study is that the follow-up analysis exhibited low power to detect significant between-group differences. Although we speculate about attention and affect changes that could covary with number of outgroup members, our interpretations are one of many processes that could have varied across groups with different numbers of outgroup members. Further, the sample size was very small for interracial groups, leading to low power and a higher possibility for false positive effects. Future research would be indicated to examine these effects in designs with better statistical power to detect effects for these supplemental analyses. Fourth, a limitation of the alcohol administration procedure is that a placebo condition was not used. As mentioned previously in this paper, placebo manipulations can lead to unanticipated compensatory effects (Testa et al., 2006), which is why we chose to use a control comparison in the current study. Nonetheless, future studies might consider incorporating a comparison group that accounts for alcohol expectancy effects. Fifth, individuals with alcohol use disorder were excluded from the current study. The National Institute on Alcohol Abuse and Alcoholism indicates concerns associated with administering alcohol to individuals with problematic drinking patterns (National Advisory Council on Alcohol Abuse and Alcoholism, 1989). Nonetheless, future research might consider investigating whether the findings can be generalized not only to social drinkers but also to individuals with alcohol use disorder. Sixth, another aspect of the current research is our choice to examine social context by exploring the effects of the mere presence of other individuals, rather than examining active social exchange or engagement in a joint activity. We made this choice for several reasons: 1) Accurate psychophysiological measurement precludes the kind of activity involved in some natural social exchange; 2) Social interactions involve multiple complex psychological processes, and the current design allowed us to more accurately pinpoint mechanisms underlying effects of social context. Although the current paradigm mirrors aspects of some common social drinking contexts (e.g., bar patrons seated in very close proximity but engaged in separate conversations), future research might alter our current design to more closely mirror features of everyday social drinking settings. Finally, the current study employed only one dose of alcohol—a “moderate” dose. Future studies should examine whether effects generalize to higher or lower doses.
In summary, in the present study, we integrated methods and theory from multiple fields to examine the impact of alcohol in a social context. Findings provide initial evidence for an interaction between alcohol and social context in predicting the magnitude of startle responses. More generally, the present study might offer new directions for understanding the effects of alcohol on automatic cognitive and emotional processes across contexts.
Figure 3.
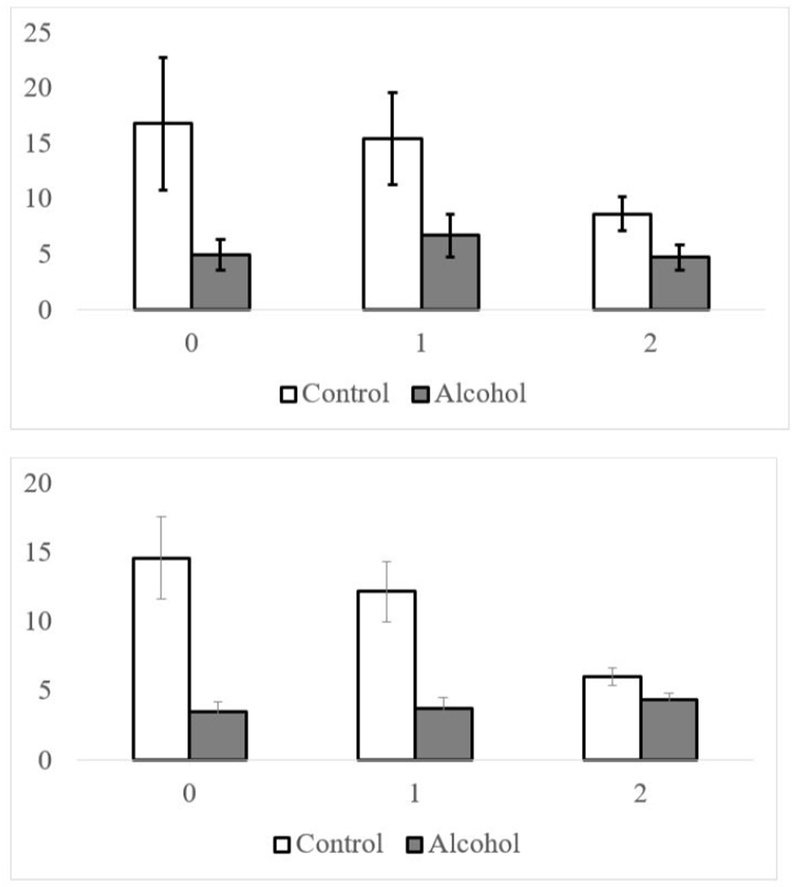
Top Panel: Habituation Startle Magnitude as a Function of Number of Out-group Members and Alcoholic Beverage (Alcohol, Control) For the Group condition. Bottom Panel: Task Startle Magnitude as a Function of Number of Out-group Members and Alcoholic Beverage (Alcohol, Control for the Group condition).
Footnotes
The third group member was not a participant in the startle task (i.e., no startle data were collected from them). This was done because the number of group members and conditions did not lead to a balanced design. This group member, however, did participate in the group condition
As an exploratory factor, the images also varied in complexity (i.e., figure-ground [low complexity] or scenes [high complexity]; Bradley et al., 2007). Within valence, there images were matched on valence and arousal, so concerns about collapsing across complexity level are minimized. Moreover, there were not any significant effects of interactions involving complexity.
IAPS numbers for the images: 7150, 2210, 7190, 7175, 2810, 7211, 7705, 7224, 6150, 2271, 7100, 2221, 7110, 2230, 2383, 7550, 9210, 7234, 3210, 6000, 2752, 2480, 7495, 7700, 2749, 2870, 2518, 7590.
Some prior research has examined the interaction between alcohol and distraction (i.e., attention allocation) in a startle context. For example, unlike the current study, Curtin and colleagues (Curtin et al., 1998) found no interaction between alcohol and attention allocation. One difference between the current study and that of Curtin is the presumed congruity of the distracting stimulus and the probe stimulus. In Curtin’s study, startle probes were auditory and distractions were visual (e.g., slides). While we also used an auditory probe, in the current study, given that group mates were outside of the sight line of our participants, it is more likely that distractions would have been experienced as auditory in form. While the participants in the current study were instructed not to talk during the task, it is possible that other forms of auditory distractions (i.e., adjusting body position, shifting chairs, etc.) took place. Some research suggests that the extent to which probes and distractions match in modality can have significant effects on startle responses (e.g., Anthony & Graham, 1985). Thus, the mismatched probe-distraction modality in Curtin’s work and the matched probe-distraction modality in our own work may account for our divergent findings here. However, the exact nature of potential distraction in the current study is unclear (e.g., auditory, visual, other). Future work is needed to clarify whether the match or mismatch in stimulus modality has direct effects on startle responses.
References
- Aan Het Rot M, Russell JJ, Moskowitz DS, Young SN, 2008. Alcohol in a social context: findings from event-contingent recording studies of everyday social interactions. Alcohol. Clin. Exp. Res 32, 459–471. 10.1111/j.1530-0277.2007.00590.x [DOI] [PubMed] [Google Scholar]
- Anthony BJ, Graham FK, 1985. Blink reflex modification by selective attention: Evidence for the modulation of ‘automatic’ processing. Biol. Psychol 21, 43–59. 10.1016/0301-0511(85)90052-3 [DOI] [PubMed] [Google Scholar]
- Balodis IM, Wynne-Edwards KE, Olmstead MC, 2011. The stress–response-dampening effects of placebo. Horm. Behav 59, 465–472. 10.1016/j.yhbeh.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Blanch A, Balada F, Aluja A, 2014. Habituation in acoustic startle reflex: Individual differences in personality. Int. J. Psychophysiol 91, 232–239. 10.1016/j.ijpsycho.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Bourgault C, Demers A, 1997. Solitary drinking: A risk factor for alcohol-related problems? Addiction 92, 303–312. 10.1111/j.1360-0443.1997.tb03200.x [DOI] [PubMed] [Google Scholar]
- Boyce WT, Frank E, Jensen PS, Kessler RC, Nelson CA, Steinberg L, 1998. Social context in developmental psychopathology: Recommendations for future research from the MacArthur Network on Psychopathology and Development. Dev. Psychopathol 10, 143–164. 10.1017/S0954579498001552 [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ, 1990. Startle reflex modification: emotion or attention? Psychophysiology 27, 513–522. 10.1111/j.1469-8986.1990.tb01966.x [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ, 2007. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology 44, 364–373. 10.1111/j.1469-8986.2007.00520.x [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA, 1992. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 49, 206–215. [DOI] [PubMed] [Google Scholar]
- Bresin K, Verona E, 2016. Pain, Affect, and Rumination: An experimental test of the emotional cascade theory in two undergraduate samples. J. Exp. Psychopathol 10.5127/jep.047715 [DOI] [Google Scholar]
- Clark MS, Lemay EP, 2010. Close relationships, in: Fiske ST, Gilbert DT, Lindzey G (Eds.), Handbook of Social Psychology. McGraw Hill, Boston. [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ, 2006. Lending a hand: Social regulation of the neural response to threat. Psychol. Sci 17, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Conger JJ, 1956. Reinforcement theory and the dynamics of alcoholism. Q. J. Stud. Alcohol 17, 296–305. [PubMed] [Google Scholar]
- Cornwell BR, Johnson L, Berardi L, Grillon C, 2006. Anticipation of public speaking in virtual reality reveals a relationship between trait social anxiety and startle reactivity. Biol. Psychiatry 59, 664–666. 10.1016/j.biopsych.2005.09.015 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, 2011. PhysBox: The Psychophysiology Toolbox. An open source toolbox for psychophysiological data reduction within EEGLAB.
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WGK, 1998. Alcohol and fear-potentiated startle: The role of competing cognitive demands in the stress-reducing effects of intoxication. J. Abnorm. Psychol 107, 547–557. 10.1037/0021-843X.107.4.547 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, Lang AR, 2007. Intoxication level and emotional response. Emotion 7, 103–112. 10.1037/1528-3542.7.1.103 [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H, 1995. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl.) 118, 19–27. 10.1007/BF02245245 [DOI] [PubMed] [Google Scholar]
- Drislane LE, Vaidyanathan U, Patrick CJ, 2013. Reduced cortical call to arms differentiates psychopathy from antisocial personality disorder. Psychol. Med 43, 825–835. 10.1017/S0033291712001547 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Ahs F, Zielinski DJ, LaBar KS, 2014. Extinction in multiple virtual reality contexts diminishes fear reinstatement in humans. Neurobiol. Learn. Mem 113, 157–164. 10.1016/j.nlm.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Briley DA, Kang D, Fraley RC, Hankin BL, Ariss T, in press A meta-analysis of attachment security and substance use. Psychol. Bull [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Sayette MA, 2014. A social-attributional analysis of alcohol response. Psychol. Bull 140, 1361–1382. 10.1037/a0037563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE, Sayette MA, 2013. The effect of alcohol on emotional inertia: A test of alcohol myopia. J. Abnorm. Psychol 122, 770–781. 10.1037/a0032980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores LE, Berenbaum H, 2017. The social regulation of emotion and updating negative contents of working memory. Emotion 17, 577–588. 10.1037/emo0000265 [DOI] [PubMed] [Google Scholar]
- Flores LE, Berenbaum H, 2014. Desired emotional closeness moderates the prospective relations between levels of perceived emotional closeness and psychological distress. J. Soc. Clin. Psychol 33, 673–700. [Google Scholar]
- Gerdes ABM, Wieser MJ, Alpers GW, 2014. Emotional pictures and sounds: a review of multimodal interactions of emotion cues in multiple domains. Front. Psychol 5 10.3389/fpsyg.2014.01351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackley SA, Graham FK, 1983. Early selective attention effects on cutaneous and acoustic blink reflexes. Physiol. Psychol 11, 235–242. 10.3758/BF03326801 [DOI] [Google Scholar]
- Harford TC, 1983. A contextual analysis of drinking events. Int. J. Addict 18, 825–834. 10.3109/10826088309033050 [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K, 2002. A review of expectancy theory and alcohol consumption. Addiction 96, 57–72. 10.1046/j.1360-0443.2001.961575.x [DOI] [PubMed] [Google Scholar]
- Judd CM, Westfall J, Kenny DA, 2012. Treating stimuli as a random factor in social psychology: A new and comprehensive solution to a pervasive but largely ignored problem. J. Pers. Soc. Psychol 103, 54–69. 10.1037/a0028347 [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ, 2016. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Lynn SJ, 1999. Automaticity in clinical psychology. Am. Psychol 54, 504–515. 10.1037/0003-066X.54.7.504 [DOI] [PubMed] [Google Scholar]
- Lane ST, Franklin JC, Curran PJ, 2013. Clarifying the nature of startle habituation using latent curve modeling. Int. J. Psychophysiol 88, 55–63. 10.1016/j.ijpsycho.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 1990. Emotion, attention, and the startle reflex. Psychol. Rev 97, 377–395. 10.1037/0033-295X.97.3.377 [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Patrick CJ, Curtin JJ, Kline JP, 2006. Personality correlates of startle habituation. Biol. Psychol 72, 257–264. 10.1016/j.biopsycho.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ, Weinberg A, Sarapas C, Huggins AA, Shankman SA, 2017. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. Int. J. Psychophysiol 114, 24–30. 10.1016/j.ijpsycho.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke EA, 2005. Why emotional intelligence is an invalid concept. J. Organ. Behav 26, 425–431. 10.1002/job.318 [DOI] [Google Scholar]
- Mauss IB, Robinson MD, 2009. Measures of emotion: A review. Cogn. Emot 23, 209–237. 10.1080/02699930802204677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J, 2008. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. J. Pers. Soc. Psychol 94, 278–291. 10.1037/0022-3514.94.2.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Pauli P, 2008. Darkness-enhanced startle responses in ecologically valid environments: A virtual tunnel driving experiment. Biol. Psychol 77, 47–52. 10.1016/j.biopsycho.2007.09.004 [DOI] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism, 1989. Recommended council guidelines on ethyl alcohol administration on human experimentation. [DOI] [PMC free article] [PubMed]
- Plant EA, Devine PG, 2003. The antecedents and implications of interracial anxiety. Pers. Soc. Psychol. Bull 29, 790–801. [DOI] [PubMed] [Google Scholar]
- Pliner P, Cappell H, 1974a. Modification of affective consequences of alcohol: A comparison of social and solitary drinking. J. Abnorm. Psychol 83, 418. [DOI] [PubMed] [Google Scholar]
- Pliner P, Cappell H, 1974b. Modification of affective consequences of alcohol: A comparison of social and solitary drinking. J. Abnorm. Psychol 83, 418–425. 10.1037/h0036884 [DOI] [PubMed] [Google Scholar]
- Quené H, van den Bergh H, 2004. On multi-level modeling of data from repeated measures designs: a tutorial. Speech Commun 43, 103–121. 10.1016/j.specom.2004.02.004 [DOI] [Google Scholar]
- Richeson JA, Shelton JN, 2007. Negotiating interracial interactions costs, consequences, and possibilities. Curr. Dir. Psychol. Sci 16, 316–320. [Google Scholar]
- Sadeh N, Verona E, 2013. Erratum to: Visual complexity attenuates emotional processing in psychopathy: implications for fear-potentiated startle deficits. Cogn. Affect. Behav. Neurosci 13, 584–585. 10.3758/s13415-013-0163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Kirchner TR, Levine JM, Moreland RL, 2012. Alcohol and group formation: A multimodal investigation of the effects of alcohol on emotion and social bonding. Psychol. Sci 23, 869–878. 10.1177/0956797611435134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicatano E, 1998. The effects of caffeine and directed attention on acoustic startle habituation. Pharmacol. Biochem. Behav 59, 145–150. 10.1016/S0091-3057(97)00384-5 [DOI] [PubMed] [Google Scholar]
- Senchak M, Leonard KE, Greene BW, 1998. Alcohol use among college students as a function of their typical social drinking context. Psychol. Addict. Behav 12, 62–70. 10.1037/0893-164X.12.1.62 [DOI] [Google Scholar]
- Sher KJ, 1987. Stress Response Dampening, in: Blane HT, Leonard KE (Eds.), Psychological theories of drinking and alcoholism. Guilford Press, New York, pp. 227–271. [Google Scholar]
- Silverstein LD, Graham FK, Bohlin G, 1981. Selective attention effects on the reflex blink. Psychophysiology 18, 240–247. 10.1111/j.1469-8986.1981.tb03026.x [DOI] [PubMed] [Google Scholar]
- Single E, Wortley S, 1993. Drinking in various settings as it relates to demographic variables and level of consumption: Findings from a national survey in Canada. J. Stud. Alcohol Drugs 54, 590–599. https://doi.org/10.15288/jsa.1993.54.590 [DOI] [PubMed] [Google Scholar]
- Steele CM, Josephs RA, 1990. Alcohol myopia: Its prized and dangerous effects. Am. Psychol 45, 921–933. 10.1037/0003-066X.45.8.921 [DOI] [PubMed] [Google Scholar]
- Stephan WG, Stephan CW, 1989. Antecedents of intergroup anxiety in Asian-Americans and Hispanic-Americans. Int. J. Intercult. Relat 13, 203–219. [Google Scholar]
- Stephan WG, Stephan CW, 1985. Intergroup anxiety. J. Soc. Issues 41, 157–175. [Google Scholar]
- Testa M, Fillmore MT, Norris J, Abbey A, Curtin JJ, Leonard KE, Mariano KA, Thomas MC, Nomensen KJ, George WH, 2006. Understanding alcohol expectancy effects: Revisiting the placebo condition. Alcohol. Clin. Exp. Res 30, 339–348. 10.1111/j.1530-0277.2006.00039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Bresin K, Patrick CJ, 2013. Revisiting psychopathy in women: Cleckley/Hare conceptions and affective response. J. Abnorm. Psychol 122, 1088–1093. 10.1037/a0034062 [DOI] [PubMed] [Google Scholar]
- Verona E, Curtin JJ, 2006. Gender differences in the negative affective priming of aggressive behavior. Emotion 6, 115–124. 10.1037/1528-3542.6.1.115 [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ, 1988. The startle probe response: A new measure of emotion? J. Abnorm. Psychol 97, 487–491. 10.1037/0021-843X.97.4.487 [DOI] [PubMed] [Google Scholar]
- Zajonc RB, 1965. Social facilitation. Science 149, 269–274. [DOI] [PubMed] [Google Scholar]


