Abstract
Background
Cognitive and structural brain abnormalities range from mild to severe in psychosis. The relation of specific cognitive functions to specific brain structures across the psychosis spectrum is less certain.
Methods
Participants (n=678) with bipolar, schizoaffective, or schizophrenia psychoses, and healthy controls, were recruited via the Bipolar-Schizophrenia Network for Intermediate Phenotypes. The Schizo-Bipolar Scale was used to create a psychosis continuum (from purely affective to purely nonaffective). Canonical correlation between 14 cognitive measures and structural brain measures (volume, thickness, surface area, and local gyrification indices) for 68 neocortical regions yielded constructs that defined shared cognition-brain structure relationships. Canonical discriminant analysis was used to integrate these constructs and efficiently summarize cognition-brain structure relationships across the psychosis continuum.
Results
General cognition was associated with larger volumes and thicker cortices, but smaller surface area, in frontal/parietal regions. Working memory was associated with larger volume and surface area in frontal/temporal regions. Faster response speed was associated with thicker frontal cortices. Constructs that captured general cognitive ability and working memory and their relationship to cortical volumes primarily defined an ordered psychosis spectrum (purely affective, least abnormal through purely nonaffective, most abnormal). A construct that captured general cognitive ability and its relationship to cortical surface area differentiated purely affective cases from other groups.
Discussion
General cognition and working memory with cortical volume deviations characterized more nonaffective psychoses. Alternatively, affective psychosis cases with general cognitive deficits had deviations in cortical surface area, perhaps accounting for heterogeneous findings across previous studies.
Keywords: sMRI, Cognition, Psychosis, Canonical Correlation Analysis, Schizo-Bipolar Scale, Multivariate Statistics, Canonical Discriminant Analysis
Introduction
There is significant overlap of clinical and biological features across bipolar disorder with psychosis, schizoaffective disorder, and schizophrenia (1). One feature is cognitive impairment (2–6), which is present before disease onset (7), reasonably stable throughout the course of illness (8–10), and predicts functional outcome (11, 12). The range of cognitive impairment observed in patients with psychotic disorders includes disruptions in behavioral inhibition, working memory, context processing, problem solving and reasoning, processing speed, and verbal memory (13–18). Another feature is structural brain abnormalities. In general, people with psychosis show reductions in regional volumes and cortical thickness compared to healthy controls, although findings in bipolar cases tend to be less clear (19–21).
Understanding the structural correlates of neurocognition could give insight into the etiology and treatment for psychotic disorders. In healthy controls, better cognition is generally associated with, larger brain volumes (22, 23), thicker cortices (24), larger surface area (25), and greater gyrification (26, 27). The direction of cognition/structure relationships in schizophrenia tend to mirror that in healthy controls; poor cognition is often associated with reduced frontal and temporal volumes (28), thinner cortex (24, 29–31) and lower gyrification indices (27, 32). Studies in bipolar disorder are less common and consistent than those in schizophrenia, with some studies reporting altered relationships between cognition and brain structure in the frontal lobes (related to volume) (33, 34) and cingulate and temporal regions (related to cortical thickness and gyrification measures) (35, 36). Findings in bipolar disorder, however, remain unclear given that psychosis status is inconsistently reported and studies are more likely to focus on deep structures like the hippocampus, amygdala, thalamus, and basal ganglia (36–39). Studies in schizoaffective disorder are sparse with problems arising from the inclusion of schizoaffective samples with schizophrenia samples (40). Combination of these two groups is at least consistent with a recent meta-analysis concluding that volumetric and cognitive deficits in schizoaffective disorder may be closer to those seen in schizophrenia (41, 42) than those seen in bipolar disorder (43).
There are additional factors that have significantly impacted the evaluation of cognition/structure relationships in psychosis. One factor is symptom overlap across and symptom heterogeneity within diagnoses, which can complicate distinctions between psychosis syndromes. A second factor is the use of extensive univariate strategies that adopt a one cognitive test to one brain region approach, which is not optimal given that one brain region is unlikely to underlie the various operations required for completion of complex cognition assessments. Measures of complex cognition work well for quantifying brain dysfunction because they rely on distributed brain structures for their successful performance. There are many paths to dysfunction, so many syndromes can have phenotypic similarity on measures of complex cognition; what is needed is a means for differentiating distinct brain correlates of phenotypically similar cognitive dysfunction within psychosis.
In general, studies of cognition-structure relationships have been constrained by limited sample sizes, limited cognitive assessment, and selective focus on a particular structural measure or brain region of interest in psychosis groups with overlapping symptomatology. The purpose of this study was to define the relationships between cognition and brain structure, independent of specific syndromal definitions (i.e. a DSM diagnosis), using a multivariate data-driven approach in a large sample of psychosis and healthy participants. This method allowed for simultaneous analysis of multiple cognitive domains and structural brain measures (volume, cortical thickness, surface area, and gyrification) in order to define bi-directional relationships between them. We then determined how these bi-directional relationships differ along an affective-nonaffective psychosis continuum using a quasi-dimensional scale. We hypothesized that, in general, better cognition would be associated with larger volumes, thicker cortex, larger surface area and greater gyrification (22–27), although altered relationships between cognition and brain structure may be present in bipolar disorder (33–36). In terms of inter-related deficits in cognition and brain structure, we expected nonaffective psychosis cases to be the most and affective cases to be the least deviant on cognition-cortical brain structure constructs.
Materials and Methods
Participants
Patients with bipolar disorder (BP) with psychosis, schizoaffective disorder (SAD), or schizophrenia (SZ) (as defined by the DSM IV-TR) and healthy controls (HC) were recruited as part of the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP)(44). Six hundred and seventy eight participants (438 people with psychosis and 240 healthy controls) had complete datasets (scores on all cognitive and brain structure measures) (see Table 1 and Supplementary Methods for further details). This study was approved by the Institutional Review Boards at all sites and all participants provided written informed consent.
Table 1.
Demographic Information
| HC (N=240) |
SBS 0–1 (N=92) |
SBS 2–4 (N=99) |
SBS 5–7 (N=98) |
SBS 8–9 (N=134) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Statistics | Findings |
| Age (yrs) | 37.5 | 12.4 | 35.8 | 13.7 | 34.7 | 11.5 | 35.8 | 12.6 | 36.0 | 12.0 | F(4, 658) = 1.04 | |
| Education (yrs) | 15.1 | 2.4 | 14.6 | 2.3 | 13.4 | 2.1 | 13.0 | 2.1 | 13.0 | 2.4 | F(4, 655) = 26.3a | HC, SBS 0–1 > SBS 2–4, SBS 5–7, SBS 8–9 |
|
| ||||||||||||
| N | % | N | % | N | % | N | % | |||||
|
| ||||||||||||
| Male | 115 | 47 | 31 | 33 | 31 | 31 | 46 | 46 | 92 | 68 | χ2(4)= 41.5a | SBS 8–9 >all(/br)HC>SBS 2–4 |
| Race | ||||||||||||
| Caucasian | 160 | 66 | 74 | 80 | 57 | 57 | 45 | 45 | 62 | 46 | χ2(4)= 39.3 | HC, SBS 0–1 > SBS 5–7, SBS 8–9(/br)SBS 0–1 >SBS2–4 |
| African American | 62 | 25 | 12 | 13 | 39 | 39 | 48 | 48 | 67 | 50 | χ2(4)= 51.1a | SBS 0–1 <all(/br)HC<SBS 5–7, SBS 8–9 |
| Other | 18 | 7 | 6 | 6 | 3 | 3 | 5 | 5 | 5 | 3 | χ2(4)= 3.9 | |
|
| ||||||||||||
| Clinical Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
|
| ||||||||||||
| Illness duration (yrs) | – | – | 17.1 | 12.4 | 15.8 | 11.7 | 16.6 | 12.6 | 14.1 | 11.2 | F (2, 401) = 1.38 | |
| Total | – | – | 53.7 | 14.4 | 60.6 | 16.1 | 64.5 | 14.9 | 66.8 | 16.6 | F (2, 411) = 13.7a | SBS 0–1 < SBS 2–4, SBS 5–7, SBS 8–9(/br)SBS 2–4 < SBS 8–9 |
| Positive | – | – | 12.6 | 4.3 | 15.3 | 5.4 | 17.1 | 5.0 | 17.2 | 5.5 | F (2, 413) = 17.5a | SBS 0–1 < SBS 2–4, SBS 5–7, SBS 8–9(/br)SBS 2–4 < SBS 8–9 |
| Negative | – | – | 12.3 | 4.3 | 13.6 | 4.6 | 14.9 | 4.23 | 17.1 | 6.1 | F (2, 413) = 18.5a | SBS 0–1, SBS 2–4, SBS 5–7 < SBS 8–9(/br)SBS 0–1 < SBS 5–7 |
| YMRS | – | – | 6.2 | 6.9 | 6.9 | 6.6 | 6.8 | 6.0 | 5.3 | 4.9 | F (2, 408) = 1.7 | |
| MADRS | – | – | 10.0 | 9.4 | 13.4 | 10.3 | 12.5 | 9.2 | 8.6 | 7.7 | F (2, 411) = 6.5a | SBS 2–4>SBS 0–1, SBS 8–9(/br)SBS 5–7>SBS 8–9 |
|
| ||||||||||||
| Medications | N | % | N | % | N | % | N | % | ||||
|
| ||||||||||||
| Antipsychotic | – | – | 62 | 67 | 75 | 75 | 90 | 91 | 126 | 94 | χ2(6)= 38.8a | SBS 0–1, SBS 2–4< SBS 5–7, SBS 8–9 |
| Lithium | – | – | 28 | 30 | 20 | 20 | 10 | 10 | 8 | 5 | χ2(6)= 31.2a | SBS 0–1, SBS 2–4> SBS 8–9(/br)SBS 0–1 >SBS 5–7 |
Demographic measures for the groups determined using the Shizo-Bipolar Scale. Fifteen subjects (5 from each psychosis group) did not have Schizo-Bipolar scores. Results of statistical tests of group differences on demographic measures are reported in the Statistics and Findings columns. PANSS= Positive and Negative Symptom Scale, YMRS=Young Mania Rating Scale, MADRS=Montgomery Asberg Depression Rating Scale
Statistical test with significant effects
Cognitive Assessment
Cognitive assessments included the reading sub-test of the Wide Range Achievement Test 4th edition (WRAT IV) (45), Brief Assessment of Cognition in Schizophrenia (BACS) battery (46), the spatial span of the Weschler Memory Scale (WMS-III) (47), the Dot Pattern Expectancy task (DPX) (48, 49), and antisaccades (AS) (50). Procedures and findings for each cognitive measure from the B-SNIP study are available in previous reports (16, 17, 51, 52). Cognitive tests that did not yield scores based on normative data (DPX and AS) were normed to the healthy sample that underwent extensive screening (53) and did not have elevated Cluster A personality disorder traits (within 1 symptom of disorder). See Table S1 for descriptive data for cognitive measures.
MRI Structural Imaging
Brain gray matter volume (GMV), cortical thickness (CT), cortical surface area (CSA), and local gyrification indices (LGI) were obtained from 68 regions of interest from high-resolution T1-weighted scans (see list in Table S2). MRI acquisition parameters and findings with morphometric parameters used in this study are available in prior reports (54–56). Further details of MRI protocols and pre-processing are in Supplementary Methods.
Statistical Analysis
To evaluate the bi-directional relationships between cognition and neocortical brain structure, we performed canonical correlation analyses (CCAs) across all groups using Statistic Analysis System (SAS) software (SAS Institute Inc., Cary, NC). CCA is a data-driven, multivariate approach that identifies the relationship between two sets of variables by maximizing correlations between ‘predictor’ and ‘criterion’ variable sets (57). CCA is particularly useful when there are high inter-correlations within variable sets and the relationship between variable sets is non-directional/bi-orthogonal (57). Results of a CCA are correlated pairs of latent variates. Each pair is independent and composed of weighted sums of the predictor variables that maximally correlate with the weighted sums of the criterion variables. Interpretation of what the latent variates represent and how they are related to each other can be determined by the weighted sums or loadings of individual measures on the latent structure, much like principal components analysis.
In the present study, variable sets were 14 cognitive measures (listed in Table S1) and structural measures extracted from each of 68 ROIs (listed in Table S2). There were four types of structural measures (GMV, CSA, CT, LGI); we conducted a separate CCA for each type. Cognitive measures were adjusted for age, sex, and race. Parameter estimates of age, race, and sex on cognitive measures were obtained in the healthy group and subsequently applied to adjust cognitive measures in all psychosis subgroups, an approach we have taken in previous B-SNIP publications (17, 51, 52, 56, 58). A similar adjustment procedure (with the addition of intracranial volume-ICV) was performed for structural measures (for each ROI) when relationships with these variables were significant (uncorrected threshold of p < .05) in the healthy group (59). Cognitive and structural measures also were standardized before insertion into the CCA to eliminate differences in scale from contributing to the outcome. The multivariate nature of CCA does not require multiple testing within a CCA analysis, although multiple testing across the four CCA analyses does and was accounted for using Bonferroni correction with the threshold for significance set at p = .0125 (.05/4 CCA analyses).
To evaluate the consistency of the models produced by the CCA solutions and latent variate pairs, we used a resampling method implementing a delete-n jackknife procedure (Lee 2007). We conducted delete-2, 4, 8, and 16 jackknife analyses with 10,000 replicates constructed using random sampling without replacement. The CCA was then conducted on each replicate. Variates were deemed to be consistent and valid for interpretation 1) if they reached significance in the original analysis and 2) if the individual measures that loaded the highest in the original analysis did not include “0” in the 99% confidence interval across all jackknife outcomes.
The subsequent set of analyses evaluated the unique contribution of the significant CCA pairs (cognition-structure constructs) across an affective-nonaffective psychosis symptom continuum. The psychosis continuum was defined using the Schizo-Bipolar Scale (SBS) as in prior studies (60, 61). The SBS ordinal scale ranges from 0–9 and reflects the proportion of non-affective psychosis symptoms and affective symptoms in relation to total illness duration as well as which mood symptoms (manic vs. depressive) are predominant when present. SBS scores closer to 0 indicate more BP-like and affective psychosis presentations whereas scores closer to 9 indicate more SZ-like and nonaffective psychosis presentations (62) (see Supplementary Methods for details). To gain the advantage of an ordered psychosis continuum, we parceled the continuum into 4 groups defined by SBS score (0–1, 2–4, 5–7, 8–9). Four groups sufficiently retained the nature of the continuum but also provided enough observations per group for further analysis.
Canonical discriminant analysis (CDA) (63, 64) with group membership as the criterion (healthy, SBS 0–1, SBS 2–4, SBS 5–7, SBS 8–9) and significant CCA variates as predictors was used to quantify cognition-brain structure features across the psychosis continuum. CDA creates a linear combination of the predictors that have the highest possible within-group correlations and returns canonical variables (a linear combination of the predictors). The nature of significant canonical variables (i.e. which of the predictors contribute most) can be determined by inspecting standardized coefficients and group differences can be evaluated by plotting the group means of the scores generated by CDA. A general linear model with factors for group membership, sex, and sex by group membership was performed on significant canonical variables from the CDA. If there were significant effects in the omnibus general linear model, group means were compared using Tukey’s HSD.
Results
Canonical Correlation Variate Pairs
CCA latent variate pairs were retained for further analysis if their correlation was significant (p ≤ .0125) and if the loadings of individual variables was stable, as determined by the jackknife outcomes. These criteria were met for 1) the first canonical correlation pairs for all four structural analyses and 2) the second pairs for the GMV, CT, and CSA analyses (See Table 2 and Figure S1). All significant canonical correlations were positive (ranging from r = .42 to r = .55), meaning higher scores on latent cognitive variates were associated with higher scores on latent structural variates (See Figure S2 for an example).
Table 2.
Significance of CCA variate pairs
| CCA | Canonical Correlation | Squared Canonical Correlation | Eigenvalue | Wilk’s Lambda | F(num df, den df) | p-value | Jackknife criteria met? |
|---|---|---|---|---|---|---|---|
| GMV | |||||||
|
| |||||||
| Pair 1 | .54 | .29 | .41 | .14 | 1.4 (952, 8243.7) | < .001 | yes |
| Pair 2 | .46 | .22 | .28 | .19 | 1.2 (871,768.1) | < .001 | yes |
|
| |||||||
| CT | |||||||
|
| |||||||
| Pair 1 | .56 | .31 | .45 | .15 | 1.3 (952, 8243.7) | < .001 | yes |
| Pair 2 | .49 | .24 | .32 | .22 | 1.1 (871,768.1) | .01 | yes |
|
| |||||||
| CSA | |||||||
|
| |||||||
| Pair 1 | .54 | .29 | .40 | .15 | 1.3 (952, 8243.7) | < .001 | yes |
| Pair 2 | .46 | .21 | .27 | .21 | 1.2 (871,768.1) | .002 | yes |
|
| |||||||
| LGI | yes | ||||||
|
| |||||||
| Pair 1 | .54 | .3 | .42 | .15 | 1.2 (952, 7791) | < .001 | yes |
Table shows significant results for latent pairs of each CCA analysis and information about the jackknife criteria. CCA = canonical correlation analysis; GMV = Volume Analysis; CT= Cortical Thickness Analysis; CSA= Surface Area Analysis; LGI= Gyrification Analysis.
Variate Loadings
For each CCA pair, loading strength of individual measures on the latent variate were used to define their nature (see Figure 1). Signs of loadings (positive or negative) were used to interpret how scores on individual measures with moderate-strong loadings (beyond −.3 or .3) related to the latent variates: positive values indicate higher scores on individual measures; negative values indicate lower scores on individual measures. Loadings, therefore, indicate what aspect of cognition is captured in each analysis, the cortical structural characteristics with which they are associated, and the nature of the relationship between them. The pattern of loadings across the GMV, CT, CSA, and LGI analyses in Figure 1 also indicates the extent to which structural parameters capture similar or different aspects of associations with cognition (See also Table 3). A visual summary of the cognitive variates and the spatial distribution of cortical regions associated with them (regions with an absolute loading beyond .3) can be seen in Figure S3 and Table S5.
Figure 1. Loadings.
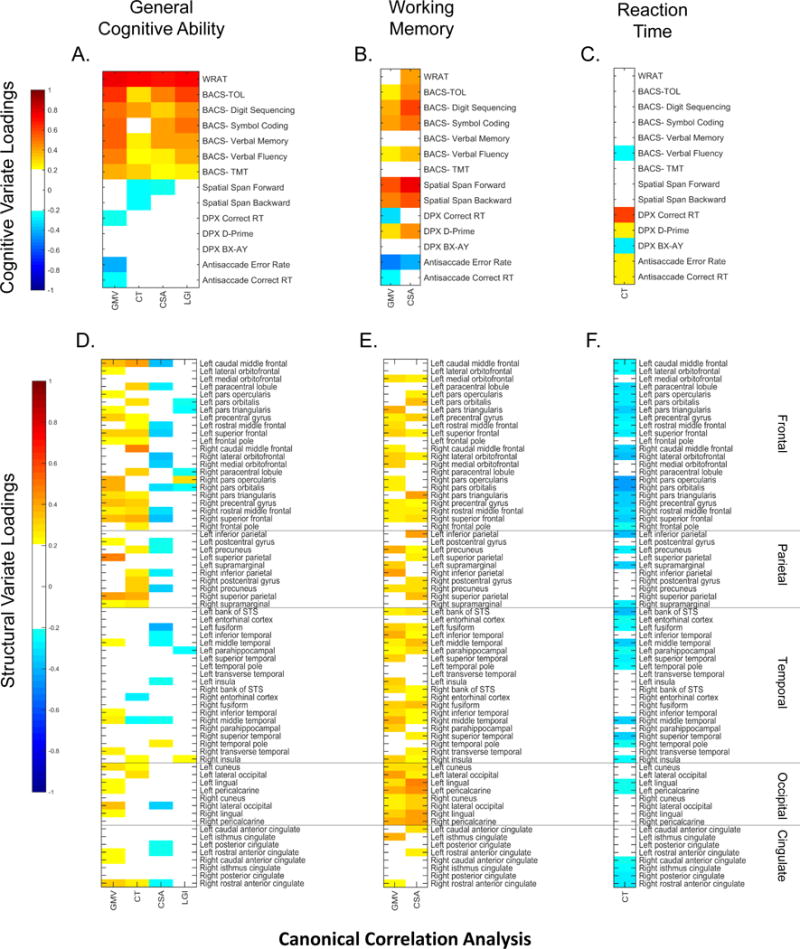
Heat map colors show the loading strength of individual cognitive (A, B, C) and structural measures (D, E, F) on their respective latent variates for each CCA analysis (volume (GMV), thickness (CT), surface area (CSA), and gyrification (LGI). Heat maps are grouped based on the cognitive domain they represent (General Cognitive Ability, Working Memory, and Reaction Time). Warmer colors indicate stronger positive loadings (higher scores on an individual measure), cooler colors indicate stronger negative loadings (lower scores on an individual measure). Cognitive measures are ordered by assessment protocol; structural measures are ordered by lobe. For clarity loadings between −.2 and .2 are shown in white. Loadings above −.3 and .3 are used for interpretation of latent variates. WRAT= Wide Range Achievement Test; BACS= Brief Assessment of Cognition in Schizophrenia; TOL= Tower of London; TMT= Token Motor Task; DPX= Dot Pattern Expectancy task; RT= Reaction Time; GMV= Volume Analysis; CT= Cortical Thickness Analysis; CSA= Surface Area Analysis; LGI= Gyrification Analysis.
Table 3.
Correlations between CCA Constructs
| GMV Pair1 | CT Pair 1 | CSA Pair 1 | LGI Pair1 | GMV Pair 2 | CT Pair 2 | CSA Pair 2 | |
|---|---|---|---|---|---|---|---|
| GMV Pair1 | – | ||||||
| CT Pair 1 | 0.70718 | – | |||||
| CSA Pair 1 | 0.79775 | 0.75687 | – | ||||
| LGI Pair1 | 0.76696 | 0.7367 | 0.7917 | – | |||
| GMV Pair 2 | −0.00694 | −0.3081 | −0.2467 | −0.1462 | – | ||
| CT Pair 2 | −0.16974 | 0.00349 | 0.02098 | 0.01553 | −0.12662 | – | |
| CSA Pair 2 | 0.20308 | −0.0581 | 0.00578 | 0.08608 | 0.77634 | 0.21521 | – |
Given all CCA correlations were positive, we averaged latent scores over cognitive and structural variates for each individual within pair. Table shows pair-wise correlations between averaged latent scores for each CCA pair. GMV = Volume Analysis; CT= Cortical Thickness Analysis; CSA= Surface Area Analysis; LGI= Gyrification Analysis.
Pair 1 variates assessed highly related constructs (See Table 3). The latent cognitive variate from the first CCA pair of each analysis (GMV, CT, CSA, and LGI) was composed of higher scores on measures that represented general cognitive ability (65, 66) (Figure 1A). Better general cognitive ability was associated with larger volumes, thicker cortex, and smaller surface area in mostly frontal/parietal regions (Figure 1D). The negative loadings for CSA in pair one may indicate that bigger surface area is not always better for cognitive performance. Although the LGI pair was significant, structural loadings were weak, with no regions loading beyond −.3 or .3.
Pair 2 variates of the GMV and CSA analysis also captured similar constructs (See Table 3) but were related to more specific cognitive domains. The latent cognitive variate was composed of higher scores on measures that represent working memory (Figure 1B). Better working memory ability was associated with larger volume and surface area in frontal and temporal regions (Figure 1E). The second cognitive variate in the CT analysis was composed of higher scores on measures that represent reaction time (Figure 1C). Slower reaction times were associated with thinner cortex in lateral frontal and temporal regions (Figure 1F).
Psychosis Continuum Analyses
The seven significant CCA constructs were used as predictors in the CDA. Associations between the CCA latent scores, symptom measures, and medication were negligible and were not considered further (see Supplementary Results and Table S4). Because each canonical variate from the CCA indexes a single cognition-brain structure construct (all positive correlations-refer to Figure S2), cognition and brain structure variate scores within each significant CCA pair and within each subject were averaged before entering them into the CDA as predictors.
The CDA returned 2 significant canonical variables (CV1: Λ = .47, F(16, n = 663) = 7.1, p < .001; CV2: Λ = .20, F(16, n = 663) = 1.9, p =.010). The first canonical variable was highly associated with the first and second CCA constructs in the GMV analysis (larger volume associated with better scores on general cognitive ability and working memory). The second canonical variable was largely accounted for by the first CCA construct in the CSA analysis (smaller surface area associated with better general cognitive ability). Loading coefficients for each CCA construct on CDA canonical variables are reported in Table 4. There was a main effect of sex for both canonical variables (M<F; CV1: F(1,620)= 4.2, p= .04; CV2: F(1,620)=13.9, p= .002), but no significant sex by diagnosis interactions.
Table 4.
CDA Standardized Coefficients
| CCA Construct | Cognitive Pattern | Structural Pattern | Canonical Variable 1 | Canonical Variable 2 |
|---|---|---|---|---|
| GMV Pair 1 | ↑ General Cognitive Ability | ↑ GMV | 0.85 | −0.60 |
| CT Pair 1 | ↑ General Cognitive Ability | ↑ CT | 0.37 | −0.49 |
| CSA Pair 1 | ↑ General Cognitive Ability | ↓ CSA | −0.30 | 0.94 |
| LGI Pair 1 | ↑ General Cognitive Ability | – | 0.14 | −0.37 |
| GMV Pair 2 | ↑ Working Memory | ↑ GMV | 0.86 | 0.38 |
| CT Pair 2 | ↑ Reaction Time | ↓ CT | −0.10 | −0.58 |
| CSA Pair 2 | ↑ Working Memory | ↑ CSA | −0.38 | 0.33 |
Table shows original CCA pairs, the effect they represent, and the standardized coefficients for the averaged CCA constructs in the CDA analysis. Bolded values indicate those coefficients that were the strongest for each canonical variable. GMV = Volume Analysis; CT= Cortical Thickness Analysis; CSA= Surface Area Analysis; LGI= Gyrification Analysis.
The first canonical variable described a psychosis continuum (See Figure 2). The healthy group had the highest scores (best cognition and largest brain volumes) with canonical scores decreasing incrementally as scores on the SBS increased. The most purely nonaffective psychosis cases had the worst cognition and smallest cortical volumes. Low scores on the second canonical variable (worse general cognition associated with larger surface area) were peculiar to the most purely affective psychosis cases (SBS 0–1). The SBS 0–1 cases significantly differed from all groups, but the other groups did not significantly differ from each other (see Figure 2).
Figure 2. Group Differences in CDA Variables.
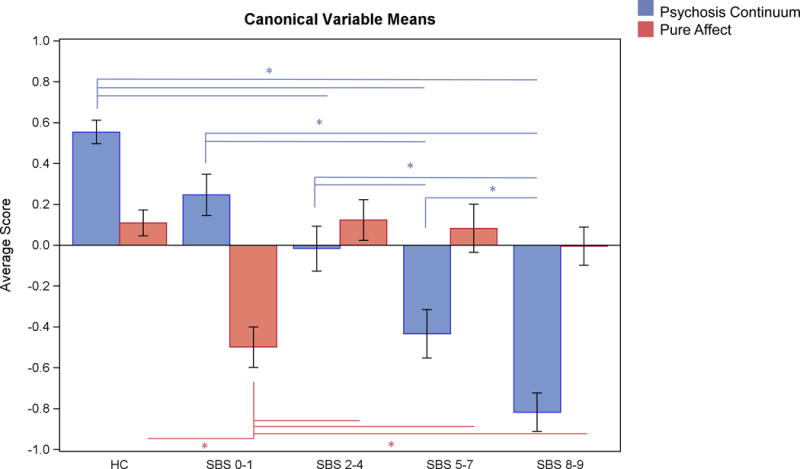
Bars show SBS group means (SE) on the significant canonical variables from the CDA. The first canonical variable (blue bars) best distinguishes groups along a psychosis continuum (nonaffective cases lowest scores, affective cases higher scores), whereas the second canonical variable (red bars) distinguishes more affective cases from all other groups. Lines and asterisks represent significant pair-wise comparisons using Tukey’s HSD.
Discussion
Behavior-brain relations in psychosis are of considerable interest, but associations between cognitive deviations and indices of brain structure had yet to be examined using multivariate methods in a sufficiently large sample. Previous strategies had limited coverage of cognitive functions and their association to a small number of brain regions, yielding an interesting but incomplete picture of cognition-brain structure relationships. In addition, considering relationships at one level of analysis (e.g. within cognition alone or within brain structure alone) independent of a different but related level of analysis (e.g. cognitive functioning is associated with distributed brain structure) may have provided incomplete, perhaps incorrect, answers to pressing questions in psychosis research. In the present project, we used a means for differentiating distinct structural brain correlates of phenotypically similar cognitive dysfunction. The outcome may advance our understanding of differences between the mechanisms associated with cognitive deviations observed between more purely affective and more purely nonaffective psychosis manifestations.
The initial canonical correlation analyses, which constructed bi-orthogonal constructs relating general and specific cognitive domains to cortical structure, generally supported hypotheses about projected cognition/structure relationships except for surface area. Cognitive variates in the first pair of each CCA captured general cognitive ability given the high loadings of the WRAT and BACS. This is consistent with a previous study that reported a unitary factor of generalized cognitive ability underlying the BACS (66). General cognitive ability was associated with brain structure in particularly frontal/parietal cortices (see Figure S3), regions consistently linked to diverse cognitive functions (67–69). Correlations between general cognitive ability and GMV and CT were positive and consistent with studies of healthy individuals (25, 27, 36), whereas correlations between general cognitive ability and CSA were negative. Studies correlating CSA and cognition yield inconsistent results, with some showing positive correlations (25, 36, 70) and others showing negative (71) or no correlations between the two measures in healthy samples (72). The use of ICV as an adjustment variable may contribute to the negative relationship between general cognitive ability and CSA. Without ICV adjustment, CSA loadings were positive for the first pair (data not shown). Negative relationships between CSA and other structural measures like CT have been reported elsewhere (73), so it is not unexpected that larger volumes and thicker cortices associated with smaller surface areas. It could be that more neuronal bodies (as measured by larger volume and thicker cortices) in a smaller space result in a more efficient brain. Such a brain configuration may enhance local neural processing because local neural signals travel a shorter distance to support the required operations.
The second variates captured relationships between brain structure and more specific cognitive domains. For the GMV and CSA analyses, the CCA variates in the second pair indexed working memory constructs, given the high loading of spatial span, digit sequencing scores, and antisaccade error rate. While spatial span and digit sequencing are not equivalent (74), they both capture aspects of working memory (46, 75). Those with higher working memory capacity also typically display lower antisaccade error rate (76, 77), which is consistent with its negative loading on these constructs. Working memory abilities were associated with structural measures in lateral and medial frontal and in parietal-temporal cortices, all of which support working memory abilities (78–82). For the CT analysis, the second cognitive variate indexed reaction time given the singular high loading of DPX reaction time; this was paired with volumetric measures predominantly in frontal cortex, which passes information to motor outputs to guide behavior initiation (83–85).
An important consideration when interpreting cortical regions with high variate associations, particularly those that share the same cognitive constructs, is that structural measures are not necessarily, or even likely fully independent. Volume measures correlate with both cortical thickness and surface area measures (20) in that larger volumes can result from thicker cortices, larger surface area or both. The magnitude of the inter-correlations between the cognition-brain structure constructs can be seen in Table 3 (see also Figure S4). For illustration, in CCA analyses capturing general cognitive ability (left panel in Figure S3), most of the regions that loaded the highest in the volume analysis also loaded the highest in other analyses (purple, orange, and pink regions). Regions in pink are of interest because they show overlap among GMV, CT, and CSA measures. Given that structural loadings were positive for GMV and CT and negative for CSA, it could mean that pink regions loaded highly in the volume analysis due to thicker cortices and not surface area. The subsequent canonical discriminant analysis that used the seven significant cognition-brain structure constructs to differentiate groups across the affective-nonaffective psychosis continuum allowed for the determination of which of the cortical quantification approaches best captures psychosis associated deviations. Using multiple cortical quantification schemes was important because each reflects at least some distinct neurobiological processes (86), so the outcome of such comprehensive investigations can inform future cognition-brain structure projects while also informing particular neurobiological theories.
After deriving cognition-brain structure constructs, we used canonical discriminant analysis to determine which of the constructs most efficiently captured deviations across an affective-nonaffective psychosis continuum. There were two important outcomes, and their differences highlight the possible importance of multi-level approaches to understanding the neurobiology of the psychoses. The first canonical variable from the CDA captured an affective to nonaffective psychosis continuum. This canonical variable was associated with primarily cortical volumes and their relation to general cognition and working memory. Healthy persons had the highest scores on this variable. The most purely affective psychosis cases were the least and the most purely nonaffective psychosis cases were the most deviant on this variable, with an ordered continuum describing the intermediate psychosis groups. Such a pattern is consistent with previous studies of global cognitive and structural deficits in psychosis (5, 28, 87–89) and the Kraepelinian-type distinction between schizophrenia-type disorders and bipolar disorders (90). Such a pattern also suggests that cognition-cortical volumetric deviations are scalable and have illness severity consequences across the psychosis spectrum.
The second canonical variable, which was primarily associated with a poor general cognition-larger cortical surface area construct, differentiated the mostly affective psychosis cases (SBS 0–1) from all other groups. This could suggest that general deficits in cognition in individuals with BP-like features are more a consequence of deviations in surface area rather than deviations in volume, as in those with SZ-like features. Such differentiations between psychosis individuals could provide information about the etiology of cognitive impairment and suggest different physiological and biochemical mechanisms underlying them. Differential patterns in BP-like individuals are also consistent with altered associations between cognition and structure in studies of bipolar disorder, although previous studies have focused on volume, thickness, and gyrification (33–36).
Limitations of the study include the possible effects of medication on both cognition and brain structure. Effect sizes between daily CPZ dose and all variates were small and consistent with existing literature (5, 10, 56, 58, 91, 92), making drug effects an unlikely confound. Association between variates and symptoms were similarly small and previous studies of relationships between symptoms and brain structure have produced inconsistent results (58, 93, 94). This project also mostly included chronic and clinically stable cases, so the similarities to cases in other stages of illness are uncertain. Multivariate procedures are further dependent on the included variables. B-SNIP included a reasonably comprehensive battery covering multiple cognitive domains of known relevance to psychosis. Although the cognitive measures were normed on different samples (published norms for BACS and WMS and sample based norms for the remaining measures), it seemed prudent to use published norms when possible. Correspondence between our healthy sample norms and published norms have been calculated for the BACS in a previous publication with correlations >.98 (16). Although the correlations between our healthy sample and published norms is high, this does not rule out that there may be a systematic bias in using norms from different groups, so the specificity of test-based results may not hold up in other samples. Another factor could be shared methods variance in some tests, such as the Spatial Span forward and backward. We conducted the CCA with the Spatial Span measures collapsed and the same regions and cognitive measures loaded above .3, so this does not appear to have influenced our results. We also acknowledge that some brain regions (e.g. amygdala and basal ganglia) known to be involved in cognition in psychotic disorders were not included in our analysis. This stemmed from our desire to consider regions that could be measured on all four structural measures. Future studies could usefully extend similar investigations to more subcortical brain structures.
This study used multivariate analyses to quantify relationships between cognition and brain structure across an affective to nonaffective psychosis symptom spectrum to address a major unanswered question about deviations in behavior-brain systems in psychosis. Multivariate cortical volumes and their relationships to general cognitive ability and working memory best described a psychosis continuum of increasing deviation from affective to nonaffective cases. Additionally, poor general cognitive ability and its association with larger cortical surface area uniquely characterized more pure affective psychosis cases. The former pattern is consistent with the thesis that lower cortical density, probably secondary to reduced synaptic connectivity, is particularly import for describing a neurocognitive severity continuum in psychosis (5, 95). Alternatively, the latter pattern indicates that deviations in neocortical communication, rather than reductions in cortical tissue may account for cognitive deviations observed in more pure affective psychosis cases. Analyses integrating data across level of analysis was critical to identifying these different cognition-brain structure relationships. Different structural deviations across the psychosis spectrum may yield phenotypically similar cognitive deviations; such information may be critical for developing more effective and targeted treatments for psychosis subtypes.
Supplementary Material
Acknowledgments
The authors would like to thank Emma Auger for her assistance on this project. The authors also would like to thank Brad Witte and Gaurav Poudyal for their contributions to data management and the volunteer study participants who contributed their time and effort to the B-SNIP study. This study was funded by NIMH MH077851, MH078113, MH077945, MH077852, and MH077862.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Keshavan is a consultant to Forum Pharmaceuticals and is the editor of Schizophrenia Research. Dr. Sweeney reports serving on an advisory board for Takeda. Dr. Tamminga has consulted for Intracellular Therapies (ITI, Inc.), PureTech Ventures, Eli Lilly Pharmaceuticals, Sunovion, Astellas, and Merck. She is also an unpaid volunteer for NAMI, Deputy Editor of the American Psychiatric Association, and an expert witness for Finnegan Henderson Farabow Garrett & Dunner, LLP. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Whalley HC, Papmeyer M, Sprooten E, Lawrie SM, Sussmann JE, McIntosh AM. Review of functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disorders. 2012;14(4):411–431. doi: 10.1111/j.1399-5618.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 2.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry. 1990;27(11):1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- 4.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 5.Hill SK, Reilly JL, Harris MSH, Rosen C, Marvin RW, DeLeon O, et al. A Comparison of Neuropsychological Dysfunction in First-Episode Psychosis Patients with Unipolar Depression, Bipolar Disorder, and Schizophrenia. Schizophr Res. 2009;113(2–3):167–175. doi: 10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen C, Marvin R, Reilly JL, DeLeon O, Harris MS, Keedy SK, et al. Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses. 2012;6(3):145–151A. doi: 10.3371/CSRP.6.3.6. [DOI] [PubMed] [Google Scholar]
- 7.Weickert TW, Goldberg TE. The course of cognitive impairment in patients with schizophrenia Cognition in Schizophrenia: Impairments, Importance and Treatment Strategies, In: Sharma T and Harvey P, editors. Oxford University Press; New York, NY: 2000. pp. 3–15. [Google Scholar]
- 8.Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry. 2001;158(9):1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- 9.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 10.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naı̈ve patients with schizophrenia. Schizophr Res. 2004;68(1):49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 11.Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophr Bull. 2007;33(5):1247–56. doi: 10.1093/schbul/sbl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. [PubMed] [Google Scholar]
- 13.Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26(1):25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 14.Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill S, Keefe RSE, et al. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res. 2014;159(2–3):491–498. doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill SK, Beers SR, Kmiec JA, Keshavan MS, Sweeney JA. Impairment of verbal memory and learning in antipsychotic-naıve patients with first-episode schizophrenia. Schizophr Res. 2004;68(2):127–136. doi: 10.1016/S0920-9964(03)00125-7. [DOI] [PubMed] [Google Scholar]
- 16.Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological Impairments in Schizophrenia and Psychotic Bipolar Disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. Am J Psychiatry. 2013;170(11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly JL, Hill SK, Gold JM, Keefe RS, Clementz BA, Gershon E, et al. Impaired Context Processing is Attributable to Global Neuropsychological Impairment in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill SK, Buchholz A, Amsbaugh H, Reilly JL, Rubin LH, Gold JM, et al. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166(1):310–315. doi: 10.1016/j.schres.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Rimol LM, Nesvåg R, Hagler DJ, Jr, Bergmann Ø, Fennema-Notestine C, Hartberg CB, et al. Cortical Volume, Surface Area, and Thickness in Schizophrenia and Bipolar Disorder. Biol Psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Arnone D, Cavanagh J, Gerber D, Lawrie S, Ebmeier K, McIntosh A. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 22.Gignac G, Vernon PA, Wickett JC. The Scientific Study of General Intelligence. Pergamon; Oxford: 2003. Chapter 6 - Factors Influencing the Relationship Between Brain Size and Intelligence A2 - Nyborg, Helmuth; pp. 93–106. [Google Scholar]
- 23.Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, et al. The Relationship Between Frontal Gray Matter Volume and Cognition Varies Across the Healthy Adult Lifespan. Am J Geriatr Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- 24.Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Research: Neuroimaging. 2010;182(2):123–133. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Walhovd KB, Krogsrud SK, Amlien IK, Bartsch H, Bjørnerud A, Due-Tønnessen P, et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci U S A. 2016;113(33):9357–9362. doi: 10.1073/pnas.1524259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 2015;287:331–339. doi: 10.1016/j.bbr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh AM, Moorhead TWJ, McKirdy J, Hall J, Sussmann JED, Stanfield AC, et al. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2009;119(3):192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- 28.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70(2):117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Fears SC, Schür R, Sjouwerman R, Service SK, Araya C, Araya X, et al. Brain structure–function associations in multi-generational families genetically enriched for bipolar disorder. Brain. 2015;138(7):2087–2102. doi: 10.1093/brain/awv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133(1–3):68–76. doi: 10.1016/j.schres.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisler D, Walton E, Naylor M, Roessner V, Lim KO, Schulz SC, et al. Brain structure and function correlates of cognitive subtypes in schizophrenia. Psychiatry Research: Neuroimaging. 2015;234(1):74–83. doi: 10.1016/j.pscychresns.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnici HM, William T, Moorhead J, Stanfield AC, Harris JM, Owens DG, et al. Pre-frontal lobe gyrification index in schizophrenia, mental retardation and comorbid groups: An automated study. Neuroimage. 2007;35(2):648–654. doi: 10.1016/j.neuroimage.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 33.Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Pschopharmacol. 2008:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman ME, DelBello MP, Getz GE, Shear PK, Strakowski SM. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disorders. 2006;8(3):281–288. doi: 10.1111/j.1399-5618.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 35.Nenadic I, Maitra R, Dietzek M, Langbein K, Smesny S, Sauer H, et al. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J Affect Disord. 2015;185:104–107. doi: 10.1016/j.jad.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Hartberg C, Sundet K, Rimol L, Haukvik U, Lange E, Nesvåg R, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc. 2011;17(06):1080–1093. doi: 10.1017/S1355617711001081. [DOI] [PubMed] [Google Scholar]
- 37.Ali OS, Denicoff KD, Altshuler LL, Hauser P, Li X, Conrad AJ, et al. A preliminary study of the relation of neuropsychological performance to neuroanatomic structures in bipolar disorder. Cogn Behav Neurol. 2000;13(1):20–28. [PubMed] [Google Scholar]
- 38.Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An mri study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55(7):663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 39.Killgore WD, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cogn Behav Neurol. 2009;22(1):28–37. doi: 10.1097/WNN.0b013e318192cc67. [DOI] [PubMed] [Google Scholar]
- 40.Hoptman MJ, Volavka J, Weiss EM, Czobor P, Szeszko PR, Gerig G, et al. Quantitative MRI Measures of Orbitofrontal Cortex in Patients with Chronic Schizophrenia or Schizoaffective Disorder. Psychiatry Res. 2005;140(2):133–145. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amann BL, Canales-Rodríguez EJ, Madre M, Radua J, Monte G, Alonso-Lana S, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133(1):23–33. doi: 10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madre M, Canales-Rodríguez EJ, Ortiz-Gil J, Murru A, Torrent C, Bramon E, et al. Neuropsychological and neuroimaging underpinnings of schizoaffective disorder: a systematic review. Acta Psychiatr Scand. 2016;134(1):16–30. doi: 10.1111/acps.12564. [DOI] [PubMed] [Google Scholar]
- 43.Getz GE, DelBello MP, Fleck DE, Zimmerman ME, Schwiers ML, Strakowski SM. Neuroanatomic characterization of schizoaffective disorder using MRI: a pilot study. Schizophr Res. 2002;55(1–2):55–59. doi: 10.1016/s0920-9964(01)00210-9. [DOI] [PubMed] [Google Scholar]
- 44.Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and Schizophrenia Network for Intermediate Phenotypes: Outcomes Across the Psychosis Continuum. Schizophr Bull. 2014;40(Suppl 2):S131–S137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jastak SR, Wilkinson GS. Wide Range Achievement Test: WRAT-R. Western Psychological Services; 1984. [Google Scholar]
- 46.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler memory scale (WMS-III) Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 48.Henderson D, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, et al. Optimization of a Goal Maintenance Task for Use in Clinical Applications. Schizophr Bull. 2012;38(1):104–113. doi: 10.1093/schbul/sbr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones JA, Sponheim SR, MacDonald AW., 3rd The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22(1):131–41. doi: 10.1037/a0017828. [DOI] [PubMed] [Google Scholar]
- 50.Hallett P. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 51.Kristian Hill S, Buchholz A, Amsbaugh H, Reilly JL, Rubin LH, Gold JM, et al. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166(1–3):310–315. doi: 10.1016/j.schres.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS, et al. Elevated Antisaccade Error Rate as an Intermediate Phenotype for Psychosis Across Diagnostic Categories. Schizophr Bull. 2014;40(5):1011–1021. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 54.Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1285–1296. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivleva EI, Clementz BA, Dutcher AM, Arnold SJ, Jeon-Slaughter H, Aslan S, et al. Brain Structure Biomarkers in the Psychosis Biotypes: Findings From the Bipolar-Schizophrenia Network for Intermediate Phenotypes. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nanda P, Tandon N, Mathew IT, Giakoumatos CI, Abhishekh HA, Clementz BA, et al. Local Gyrification Index in Probands with Psychotic Disorders and Their First-Degree Relatives. Biol Psychiatry. 2014;76(6):447–455. doi: 10.1016/j.biopsych.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lambert ZV, Wildt AR, Durand RM. Redundancy analysis: An alternative to canonical correlation and multivariate multiple regression in exploring interset associations. Psychol Bull. 1988;104(2):282. [Google Scholar]
- 58.Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 2015;41(1):154–162. doi: 10.1093/schbul/sbu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney JA, Pearlson GD, et al. Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology. 2014;51(4):348–357. doi: 10.1111/psyp.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nanda P, Tandon N, Mathew IT, Padmanabhan JL, Clementz BA, Pearlson GD, et al. Impulsivity across the psychosis spectrum: Correlates of cortical volume, suicidal history, and social and global function. Schizophr Res. 2016;170(1):80–86. doi: 10.1016/j.schres.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 61.Narayanan B, O’Neil K, Berwise C, Stevens MC, Calhoun VD, Clementz BA, et al. Resting State Electroencephalogram Oscillatory Abnormalities in Schizophrenia and Psychotic Bipolar Patients and Their Relatives from the Bipolar and Schizophrenia Network on Intermediate Phenotypes Study. Biol Psychiatry. 2014;76(6):456–465. doi: 10.1016/j.biopsych.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophr Res. 2011;133(1–3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klecka WR. Discriminant analysis. Sage; 1980. [Google Scholar]
- 64.Lawley D. Tests of significance in canonical analysis. Biometrika. 1959:59–66. [Google Scholar]
- 65.Jensen AR. Psychometric g: Definition and substantiation The general factor of intelligence: How general is it, In: Sternberg R and Grigorenko E, editors Layrence. Erlbaum Associates; Mahwah, New Jersey: 2002. pp. 39–53. [Google Scholar]
- 66.Hochberger WC, Hill SK, Nelson CLM, Reilly JL, Keefe RSE, Pearlson GD, et al. Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophr Res. 2016;170(1):156–161. doi: 10.1016/j.schres.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brass M, Ullsperger M, Knoesche TR, Cramon DYv, Phillips NA. Who Comes First? The Role of the Prefrontal and Parietal Cortex in Cognitive Control. J Cogn Neurosci. 2005;17(9):1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- 68.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11(2):157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 69.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vuoksimaa E, Panizzon MS, Chen CH, Fiecas M, Eyler LT, Fennema-Notestine C, et al. The Genetic Association Between Neocortical Volume and General Cognitive Ability Is Driven by Global Surface Area Rather Than Thickness. Cereb Cortex. 2015;25(8):2127–2137. doi: 10.1093/cercor/bhu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haring L, Müürsepp A, Mõttus R, Ilves P, Koch K, Uppin K, et al. Cortical thickness and surface area correlates with cognitive dysfunction among first-episode psychosis patients. Psychol Med. 2016;46(10):2145–2155. doi: 10.1017/S0033291716000684. [DOI] [PubMed] [Google Scholar]
- 72.Gutiérrez-Galve L, Wheeler-Kingshott CAM, Altmann DR, Price G, Chu EM, Leeson VC, et al. Changes in the Frontotemporal Cortex and Cognitive Correlates in First-Episode Psychosis. Biol Psychiatry. 2010;68(1):51–60. doi: 10.1016/j.biopsych.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009:bhp026. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilde NJ, Strauss E, Tulsky DS. Memory Span on the Wechsler Scales. J Clin Exp Neuropsychol. 2004;26(4):539–549. doi: 10.1080/13803390490496605. [DOI] [PubMed] [Google Scholar]
- 75.Millis SR, Malina AC, Bowers DA, Ricker JH. Confirmatory Factor Analysis of the Wechsler Memory Scale-III. J Clin Exp Neuropsychol. 1999;21(1):87–93. doi: 10.1076/jcen.21.1.87.937. [DOI] [PubMed] [Google Scholar]
- 76.Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007;114(1):104. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- 77.Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. J Exp Psychol Learn Mem Cogn. 2004;30(6):1302. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- 78.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 79.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. The Journal of Neuroscience. 1994;14(5):2775. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21(1):1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 81.Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The Role of Parietal Cortex in Verbal Working Memory. The Journal of Neuroscience. 1998;18(13):5026. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254(5036):1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 83.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pujol J, Vendrell P, Deus J, Junqué C, Bello J, Martί-Vilalta JL, et al. The Effect of Medial Frontal and Posterior Parietal Demyelinating Lesions on Stroop Interference. Neuroimage. 2001;13(1):68–75. doi: 10.1006/nimg.2000.0662. [DOI] [PubMed] [Google Scholar]
- 85.Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, et al. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43(3):396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 87.Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: A quantitative review. Schizophr Res. 2005;80(2):137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and Specific Neurocognitive Deficits in Prodromal Schizophrenia. Biol Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, et al. Specific and Generalized Neuropsychological Deficits: A Comparison of Patients With Various First-Episode Psychosis Presentations. Am J Psychiatry. 2010;167(1):78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- 90.Möller H-J, Bottlender R, Groß A, Hoff P, Wittmann J, Wegner U, et al. The Kraepelinian dichotomy: preliminary results of a 15-year follow-up study on functional psychoses: focus on negative symptoms. Schizophr Res. 2002;56(1):87–94. doi: 10.1016/s0920-9964(01)00252-3. [DOI] [PubMed] [Google Scholar]
- 91.Seidman LJ, Shapiro DI, Stone WS, et al. Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the north american prodrome longitudinal study. JAMA Psychiatry. 2016;73(12):1239–1248. doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuepbach D, Hill SK, Sanders RD, Hell D, Keshavan MS, Sweeney JA. Early treatment-induced improvement of negative symptoms predicts cognitive functioning in treatment-naive first episode schizophrenia: a 2-year followup. Schizophr Bull. 2004;30(4):837–848. doi: 10.1093/oxfordjournals.schbul.a007136. [DOI] [PubMed] [Google Scholar]
- 93.Flaum M, O’Leary DS, Swayze VW, II, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res. 1995;29(4):261–276. doi: 10.1016/0022-3956(94)00046-t. [DOI] [PubMed] [Google Scholar]
- 94.Barta P, Pearlson G, Powers R, Richards S, Tune L. Reduced volume of superior temporal gyrus in schizophrenia; relationship to auditory hallucinations. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 95.Reilly JL, Sweeney JA. Generalized and Specific Neurocognitive Deficits in Psychotic Disorders: Utility for Evaluating Pharmacological Treatment Effects and as Intermediate Phenotypes for Gene Discovery. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


