Abstract
ST6Gal1 is a critical sialyltransferase enzyme that controls the addition of α2,6-linked sialic acids to the termini of glycans. Attachment of sialic acids to glycoproteins as a posttranslational modification influences cellular responses, and is a well-known modifier of immune cell behavior. ST6Gal1 activity impacts processes such as: effector functions of immunoglobulin G via Fc sialylation, hematopoietic capacity by hematopoietic stem and progenitor cell surface sialylation, and lymphocyte activation thresholds though CD22 engagement and inhibition of galectins. This review summarizes recent studies that suggest α2,6 sialylation by ST6Gal1 has an immunoregulatory effect on immune reactions.
Keywords: Sialylation, Glycosylation, Immunoregulation, Antibody, IgG, Leukocytes, B cell, T cell
1. Introduction
Due to their charge and location on the terminus of glycan structures, sialic acids are poised at the interface between the cell surface and an interaction partner (cell-matrix, cell-cell, cell-self, cell-protein, etc.) making them an attractive target for study. The addition of sialic acids is a well-known immunomodulatory mechanism, and has been extensively reviewed [1–4]. The focus of the discussion herein are recent studies which provide evidence that α2,6 sialylation by ST6Gal1 is an anti-inflammatory modification, and acts as an agent to limit the magnitude of the immune response. Following a brief review of the mechanism of action by ST6Gal1 and parameters that influence this enzymatic reaction, recent efforts investigating the in vivo mechanisms that modulate IgG sialylation are discussed. The final section outlines the immunomodulatory effects of α2,6 sialylation on stem cells, B cells, T cells, and macrophages.
2. Mechanism of sialylation by ST6Gal1
Sialic acids are a family of monosaccharides that are negatively charged, have a 9-carbon backbone, terminally decorate complex carbohydrates [5], and are found in α2,6 or α2,3 or α2,8 linkages [6]. Each linkage type results in a unique epitope [7], thus many sialic acid binding proteins specifically bind one particular linkage. One prominent example is the general preference of the hemagglutinin of human influenza virus particles for α2,6 sialic acids [8]. The α2,8 linkage is specific to polysialic acid, is associated with neural cell adhesion molecule (NCAM), and is only found in the neuronal compartment and on certain cancers [9]. A family of six members (ST3Gal1 though ST3Gal6) adds α2,3 sialic acids to lactosamine, whereas only 2 enzymes add α2,6-linked sialic acids to the same underlying sugar. β-galactoside α2,6-sialyltransferase 1 (ST6Gal1) is a membrane-bound enzyme that mediates the attachment of α2,6-linked sialic acids from donor CMP-sialic acid to its acceptor Galβ(1,4)GlcNAc, located on growing oligosaccharide chains of glycoproteins [10;11], and is the first member of a family of two genes sharing this function [12]. ST6Gal1 is expressed widely amongst all tissues, with particularly high amounts found in the liver [12–14]. ST6Gal2 expression is exclusively restricted to the neuronal compartment [13], thus α2,6 sialic acids found outside of neural tissues are solely derived from the action of ST6Gal1. Correspondingly, complete ST6Gal1 knockout mice show total loss of α2,6-linked sialic acids outside of the central nervous system [15], which confirms the exclusivity of ST6Gal1 in this enzymatic process and the absence of other compensatory mechanisms or enzymes [16]. For a comprehensive review of sialyltransferases, please see Bhide et. al. [6].
ST6Gal1 is a type II membrane protein containing a small cytosolic tail, a transmembrane domain, and a proteolytic-susceptible stem followed by a large catalytic domain [17;18]. ST6Gal1 and its α2,6 sialylated glycoproteins have long been known as acute phase reactants, as increased enzymatic activity was found in the blood stream during inflammatory events nearly 40 years ago [19;20]. The ST6Gal1 found in circulation is a cleaved version of the enzyme, which lacks the transmembrane region and cytosolic tail, having been cut away from the membrane by BACE1 and released into the Golgi lumen [21;22]. Importantly, this soluble form of ST6Gal1 retains its enzymatic potential, and is capable of sialylating additional targets. Once thought to simply be a byproduct of sialylating secreted hepatic glycoproteins, there is mounting evidence that released ST6Gal1 has important functions as part of an extracellular glycosylation pathway. Multiple studies demonstrate the impact of extracellular ST6Gal1 in hematopoiesis and immunity [23–27], and are discussed later in this review. It has been speculated that cleaved ST6Gal1 may have broader specificity from the full-length form due to the lack of a membrane tether, but whether it sialylates the same targets as the membrane-bound form is still an open question [28]. In addition to membrane release, generation of α2,6 sialylation by ST6Gal1 activity is influenced by several other factors. This enzyme has a highly regulated expression profile mediated by a promoter region that contains multiple transcriptional starts [29;30]. ST6Gal1 has a naturally occurring isoform, present at least in the liver, that has altered secretion kinetics and catalytic activity [31]. This enzyme is also able to form dimers that abrogate sialyltransferase activity, but retain the ability to bind to glycan acceptors [32]. Since ST6Gal1 is a glycoprotein, it may be influenced by its own resident glycans [33]. Finally, as with any glycosyltransferase, the enzymatic activity is dependent on the concentration of nucleotide-sugar donor [34] and the presence of ST6Gal1’s glycan acceptor. Notably, both donor (CMP-sialic acid) and acceptor (lactosamine) are built by multiple enzymes in the glycosylation pathway, each potentially being regulated. Despite seeming like a simple target for studying sialylation, there are many regulatory systems involved in directing the enzymatic activity of ST6Gal1. Remaining considerate of these regulatory processes, in addition to the biological ramifications of α2,6 sialylation, is valuable when evaluating ST6Gal1.
3. Modulation of IgG α2,6 sialylation
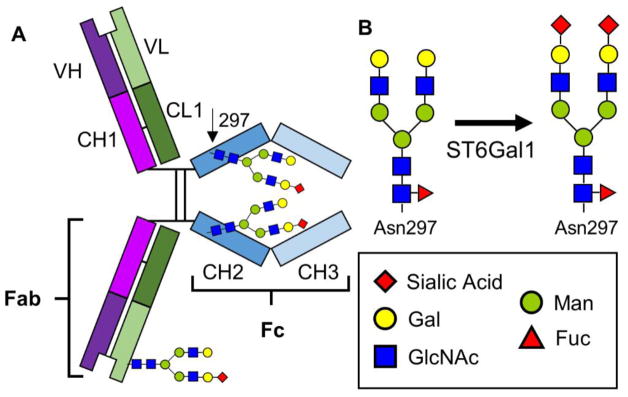
The central role antibodies play in protection from invading microbes is universally recognized. Antibodies perform a number of functions, which include opsonization and antibody-dependent cell-mediated cytotoxicity (ADCC), neutralization of target antigens, and complement deposition [35]. IgG is the dominant antibody class found in circulation and effective immunizations are defined by measuring IgG reactivity to specific targets. These glycoproteins contain variable antigen specificity-determining regions (Fab) and a constant region, the crystallizable fragment (Fc). The Fc portion of IgG contains a single highly conserved site of glycosylation at Asn297, which carries complex N-glycans required for Fc receptor binding. These Fc glycans have a key structural role in stabilizing the conformation of the Fc region [36] (Figure 1a). Over thirty glycan variants have been detected at this site, but they are mostly limited to bi-antennary structures with varying levels of α2,6-linked sialylation [37] (Figure 1b). Differences in glycan structures are associated with modulating the binding preferences of the Fc region to Fcγ receptors (FcγR) by inducing differential confirmations of the Fc barrel. FcγRs are found on leukocytes and are further defined into three classes FcγRI, FcγRII and FcγRIII; for a review of FcγRs, see Nimmerjahn et. al. [38]. Specific Fc glycoforms have particular effects on FcγR binding, for instance: core fucosylated antibodies are associated with decreased binding to FcyRIIIA and decreased ADCC [39–41], while those with bisecting GlcNAc have increased affinity to FcyRIIIA [42]. For yet unexplained reasons, IgG Fc is predominantly found with α2,6 and not α2,3 sialic acids, despite α2,3-sialylatransferase expression in B cells [43]. When Fc regions do contain α2,6 sialic acids they have reduced binding to FcγRs and instead bind to the C-type lectin DC-SIGN (SIGN-R1 in mice), which induces an IL-33 and IL-4 mediated signaling cascade ultimately upregulating the anti-inflammatory receptor FcyRIIB on inflammatory cells [44–46]. There remain some questions over the precise steps in this mechanism, arising from differing results between studies in mice versus those in humans, the differential expression profiles of DC-SIGN versus SIGN-R1, and the affinity of DC-SIGN for sialic acids. These topics have been the subject of recent reports and reviews [47–51]. Additional studies are needed to address the exact pathway of their effect, however; glycans in the Fc region clearly modulate IgG function.
Figure 1. Visualization of IgG glycosylation.
A) Schematic of sites of glycosylation on IgG, either in the Fab region or in the Fc portion at the conserved Asn297 residue. B) N-glycan structures showing the action of ST6Gal1 converting galactosylated forms to fully sialylated forms. The key shows symbolic names for each monosaccharide.
Although Fc glycans have received the bulk of recent research efforts, it is worth noting that an estimated 15% of IgGs are N-glycosylated on the Fab region as well as the Fc, which may introduce an added variable during IgG analysis [52–55]. Fab glycosylation may be located on either the heavy or light chains, and can potentially modulate antigen binding. Fab carbohydrates may be positioned such that: they have no role in antigen interactions, they facilitate or impede binding, or they hinder access to the antigen binding site. In addition to being able to influence Fab binding, the presence of desialylated Fab N-glycans may also impact antibody half-life by mediating hepatic clearance via interactions with the asialoglycoprotein receptor [56]. For a specific review of Fab glycans, please see van de Bovenkamp et. al. [57].
Delivered at high dosages (1–2 g/kg), pooled human preparations of IgG have been used as a therapy for inflammatory and autoimmune patients, and is given as intravenous immunoglobulin (IVIg) [58–60]. The discovery that the Fc-sialylated portion of IVIg is the active component that serves to suppress autoimmunity [37;44;45;61] holds distinct significance for the treatment of diseases in the clinic, recently reviewed by Seeling et. al. [62]. It implies that control of IgG sialylation is a dynamic part of the normal immune response, and that a pathway exists that switches IgG function between pro-inflammatory and anti-inflammatory functions in order to modulate resolution of inflammation. This discovery has led to an invigoration into IgG glycosylation research, and it has become evident that IgG sialylation is a regulated process. Classical disorders associated with changes in Fc glycans such as rheumatoid arthritis [63] and Wegener’s granulomatosis [64] are now joined by additional disease states like HIV infection [65], smoking [66], inflammatory bowel disease [67], poor metabolic health [68], susceptibility to relapse during Wegener’s [69], myeloma progression [70], and diabetes [71] to name a few.
While these new studies link modulation of IgG glycosylation to a wide spectrum of diseases, there remain few publications investigating the specific in vivo conditions in which IgGs are sialylated. Summarized here are the most recent findings into how the immune system may control Fc sialylation. Ohmi et. al. showed that in a model of collagen induced arthritis (CIA), mice have lowered Fc sialylation similar to that seen in humans. Of interest, the murine Fc regions remained galactosylated, whereas IgGs in arthritic patients lack galactose, thought to be due to the increased duration of human disease. Investigating ST6Gal1 involvement, they report that conditional knock-out mice for ST6Gal1 in activated B cells (AIDCRExST6Gal1f/f) have more severe arthritis than wild-types. They further demonstrate that injection of sialylated anti-citrullinated protein antibodies were able to ameliorate arthritis severity as opposed to delivery of either the desialylated variant or sialylated but non-specific antibodies [72]. These results suggest that the anti-inflammatory mechanism in arthritis acts though B cell sialylated antigen-specific IgGs.
A study conducted by Wang et. al., elaborates a novel sialylated IgG-CD23 (Type-II FcR) dependent mechanism of affinity maturation. B cells, after primed with influenza specific immune complexes containing sialylated IgGs, had increased inhibitory FcγRIIB expression. With additional FcγRIIB on the surface, these cells had an increased BCR activation threshold resulting in improved affinity maturation compared to cells receiving desialylated immune complexes [73]. These data provide evidence of possible regulation of B cells though sialylated IgG feedback loops.
Pfeifle et. al. have recently demonstrated a mechanism of T cell dependent modulation of sialylated glycans. In a model of CIA, they show that IL-23-activated Th17 cells were able to suppress ST6Gal1 expression in developing plasmablasts and plasma cells in an IL-21 and IL-22 dependent manner. The result of ST6Gal1 downregulation was a measured decrease in IgG sialylation, which correlated with the initiation of arthritis in mice [74]. This demonstrates that Th17 cells and their cytokines are capable of altering the glycosylation machinery of B cells to modulate IgG glycosylation. Moreover, Oefner et. al. showed that mice administered with T cell dependent antigens under a tolerance inducing protocol are able to generate antigen specific IgGs with sialylation states comparable to baseline IgG, whereas administration of antigen with adjuvant resulted in decreased antigen specific IgG sialylation [75]. In similar studies by Hess et. al., immunization with a T cell independent antigen also elicited antigen specific IgGs sialylated to a similar degree as resting animals. The impact of inflammatory cytokines on IgG sialylation was also demonstrated, as IFNγ−/−IL-17−/− mice administered antigen in adjuvant resulted in ~13% sialyated IgGs, as compared to 8% seem in the single knockout animals and 4% in WT animals with the same treatment [76]. Together these studies demonstrate that T cell responses are a critical element in determining the glycosylation state of IgGs.
By examining the role of circulatory ST6Gal1, Jones et. al. describe a B cell independent mechanism by which IgG sialylation may be controlled. They report that B cell specific knockouts of ST6Gal1 (CD19CRExST6Gal1f/f) have similar levels of sialylated IgGs as compared to WT mice both at rest and after inflammatory stimulus. Considering that ST6Gal1 and IgGs may interact in the bloodstream, they provide evidence for extracellular sialylation of IgGs demonstrating that: ST6Gal1 is cleaved and secreted from the central veins of the liver, released ST6Gal1 retains its enzymatic activity, and the nucleotide-sugar donor CMP-sialic acid is released in sufficient quantity from the granules of activated platelets to drive IgG sialylation. This study establishes that B cells are not the final determinant of IgG glycans, and that control of IgG sialylation is a systemic mechanism involving multiple organs [26]. Pagan et. al. harness this mechanism of extracellular glycosylation for the purposes of IgG glycan remodeling. By attaching both recombinant human galactosyltransferase B4GALT1 and ST6GAL1 to a single IgG1 Fc fragment, they show that injection of this combination protein is capable of ameliorating inflammation in a K/BxN model of arthritis and in NTN-driven nephritis. They further confirm that their multi-enzyme Fc fusions act to sialylate IgGs deposited at sites of inflammation due to proximal platelet release of nucleotide-sugar donors [77]. This report provides an elegant example by which extracellular glycosylation can be engineered to modulate endogenous IgG glycans.
These research efforts show the ever-growing role of IgG sialylation in disease states, immunity, and inflammation. They reveal that there is still room for additional study in order to understand the precise inflammatory cues and molecular mechanisms governing IgG glycosylation. There is tremendous potential in controlling in vivo IgG glycosylation, where any advances impact not just disease intervention but also vaccine and therapeutic design.
4. ST6Gal1 in leukocyte function
The role that glycans play in modulating leukocyte behavior is well established, with prominent examples including selectin-mediated leukocyte migration [78], CD22 (Siglec-2) acting as a negative regulator of B cell activation [79], integrin sialylation modulating adhesiveness [80], and galectin regulation of T cell signaling [81]. Indeed, many prominent immunological proteins like the B and T cell receptors, cytokines, and even compliment are glycoproteins. Unfortunately, the first step in obtaining protein crystal structures is typically to remove the carbohydrates, thus the influence of glycosylation is often disregarded in these studies [82]. Additionally, as glycans are not template driven, a single site of glycosylation on a protein can yield multiple glycoforms, which sometimes draws concern over the relationship between modification and function. However, this criticism is precisely the strength that glycans play in immunity; through regulation of the glycosylation machinery the conformation or behavior of a protein can be altered by changing the size or charge of its attached glycans. Thus protein modulation can occur in response to stimuli on a per cell basis, and is faster than evolving a new protein or regulatory element [83]. Discussed below are recent studies where ST6Gal1 and α2,6 linked sialic acids are involved in immunomodulation.
ST6Gal1 has an apparent role in hematopoiesis as hematopoietic stem and progenitor cells (HSPCs) have been shown to have a high degree of α2,6 sialic acids on their cell surface, which decreases as the they become more differentiated [84–86]. As evidence that there is functionality to surface sialylation, induced pluripotent stem cells have been reported to differentiate toward neural precursors when treated with neuraminidase [87]. Wang et. al. indicate that human pluripotent stem cells (hPSCs) have more ST6Gal1 and are more sialylated than non-pluripotent cells. Upon shRNA knockdown of ST6Gal1, hPSCs downregulated genes associated with pluripotency and upregulated those associated with differentiation. They additionally demonstrate that after induced pluripotency treatment ST6Gal1 knockdown cells had significantly reduced reprogramming efficiency [88]. These experiments indicate that ST6Gal1 and its sialylated targets play a role in maintaining pluripotency and may impact iPSC strategies. In a series of experiments, the Lau group has established that circulating ST6Gal1 is a key regulator of myelopoiesis. They observed increased granulopoiesis in animals with a targeted defect in the liver specific promoter of ST6Gal1, attributed to a deficiency in circulating ST6Gal1 [89;90]. Utilizing ST6Gal1−/− mice they then confirm that knockout HSPCs are α2,6 sialylated by extracellular ST6Gal1 when transplanted into WT backgrounds [91]. Further, ex vivo experiments with both mouse and human HSPCs showed inhibition of early granulocytic development when supplemented with recombinant ST6Gal1 [92]. These results demonstrate that the α2,6 sialylation state of HSPCs affects lineage fate and hematopoietic potential, and thus exerts influence at early stages of leukocyte development.
Siglecs (sialic acid recognizing Ig-superfamily lectins) are a large family of sialic acid binding cell surface receptors that typically contain an ITIM (immunoreceptor tyrosine-based inhibitor motif), making them negative regulators of cell signaling [93]. They are found on the surface of many immune cells including B cells, eosinophils, DCs, and macrophages [94]. As these cells typically have high levels of surface α2,6 sialylation, Siglecs are usually bound in ‘cis’ interactions to sialylated glycans on the same cell surface [79]. It is also possible that they bind to glycoproteins on other cells, deemed ‘trans’ interactions. Trans binding is expected to occur when high affinity and avidity targets outcompete available cis targets, and is an area of current research [95]. An extensive review of Siglecs and their impact on the immune system can be found by Macauley et. al. [96]. Of the Siglec family, human and mouse CD22 (Siglec-2) as well as Human Siglec-10 (mouse Siglec-G) bind to α2,6 sialic acids, although Siglec-G also binds α2,3 sialic acids. CD22 is a well-known associate with the B cell receptor [97], and acts to increase activation thresholds, meaning that surface α2,6 sialic acids act as negative regulators of B cell activation [98], and are thought to mediate B cell tolerance and prevent autoimmunity [99]. Siglecs provide an example of how surface sialic acid, from both adjacent cells and the same cell, can mediate cell signaling in a linkage specific fashion.
Macauley et. al. describe a CD22 and Siglec-G based mechanism by which antigen specific B cells are deleted. When CD22 on the B cell surface is recruited to the immunological synapse by α2,6 sialylated ligands on an antigen bearing cell, it initiates a signaling cascade involving Lyn and BIM that induce apoptosis in the B cell. In experiments utilizing antigen bearing lymphocytes, they demonstrate that deletion of antigen specific B cells occurs in a CD22 dependent manner. Additionally, when treated animals were subsequently challenged with the same antigen, they failed to mount an antibody response [100]. This implies that α2,6 sialylated ligands are critical participants during antigen presentation to B cells, contributing to antigen specific cell death and thus tolerance. However, in order for this system to work CD22 must be found in a trans configuration to interact with sialic acids on the antigen presenting cell, at a point before B cell maturation. Addressing this point in a subsequent study, they demonstrate that when naïve B cells migrate to the germinal center (GC) and differentiate into GC B cells, their cell surface CD22 ligands are modulated, shifting from high to low affinity. By decreasing the affinity for CD22 cis interactions and encouraging trans ligand interactions, GC B cells have a greater possibility for CD22 interaction with other cells and stimuli. Upon leaving the GC and differentiating into memory B cells, high affinity ligands are restored, thereby re-engaging cis interactions and ‘masking’ CD22, as seen in naïve B cells. This process was found to occur in both mice and in humans, although slightly different ligands were utilized by each species [101]. Chappell et. al. show that CD22−/− mice generate GC B cells, but that they do not develop into memory B precursor cells, putatively though lack of trans CD22-CD22L interactions and altered B cell signaling [102]. Harnessing the therapeutic potential of this pathway by utilizing siglec tolerizing antigenic liposomes (STALs), Orgel et. al. present data that sensitization to peanut allergen Ah2 can be prevented by treatment with Ah2-STAL in mice [103]. Further refinements to this STAL-based system by inclusion of additional immunomodulatory molecules, like rapamycin are underway [104]. These data indicate that cell surface α2,6 linked sialic acids are actively regulated during B cell maturation and that there is an expanding immunomodulatory role for trans CD22 signaling, both contributing to the avoidance of autoreactivity.
Galectins are a family of over 15 galactose binding lectins that have been of particular interest in T cell and macrophage biology. Galectins are both intra- and extra-cellular, with some cell surface galectins forming a ‘lattice’ thought to organize the cell surface for optimal receptor spacing and signaling [81]. This lattice seems to have a significant effect on T cell function by controlling the threshold of activation of the TCR. The Mgat5−/− mouse lacks the gene that initiates N-glycan branching on glycoproteins, including the TCR, and thus these mice are severely lacking in galectin epitopes. T cells in these animals displayed a decreased threshold for activation and an increased proclivity to autoimmune disorders like immune complex deposition [105]. Galectin-1 and galectin-3 are known initiators of T cell apoptosis [106;107], with galectin-1 being reported to preferentially kill Th1 cells over Th2 cells [108]. Since sialylation of galectin ligands significantly reduces binding [109], this skewing phenomenon is at least partially explained by Th2 cells having higher levels of surface α2,6 sialic acids and ST6Gal1 expression than Th1 cells and are thus protected from galectin-mediated death. This may also be true for regulatory T cells (Tregs), which are reported to be high in surface α2,6 sialic acids [110]. The disruption of galectin mediated apoptosis by surface α2,6 sialic acids directly leads to the idea that sialic acids are negative regulators of galectin binding and function [111]. If galectins can skew a T cell population toward Th2 cells, it follows that α2,6 linked sialic acids and ST6Gal1 expression may also be primary mediators of T cell polarization. In a dedicated galectin-sialic acid interaction review, Zhuo et. al. additionally point out that lectin array evidence shows some galectins, like Galectin-3, may be able to bind to internal galactoses on α2,6 sialylated N-glycan arms containing polyLacNAc [111], implying that increased number of LacNAc repeats may correlate with diminished α2,6 galectin inhibition, a topic for further study.
Lymphocytes are not the only immune cell type impacted by α2,6 sialylation, as multiple groups have described the reduction of cell surface sialic acids during the maturation of THP-1 and U937 monocytes into macrophages after PMA stimulation [112;113]. This effect is also seen in human primary monocytes [114]. Liu et. al. further indicate this loss is associated with decreased ST6Gal1 levels and induction of tumor necrosis factor α receptor (TNFR1)-mediated apoptosis. Interestingly, this process is mediated by the degree of α2,6 sialylation on TNFR1 as data from both in vitro overexpression and ex vivo studies from mice constitutively overexpressing ST6Gal1 demonstrated abrogation of TNFR1 mediated macrophage apoptosis [113]. In experiments with recombinant cytokine polarized macrophages, Wang et. al. revealed that M2 macrophages, which are associated with anti-inflammatory effects, have increased ST6Gal1 production compared to pro-inflammatory M1 macrophages [115]. These data show that α2,6 sialic acids are involved with macrophage apoptosis, maturation, and responses.
5. Conclusion
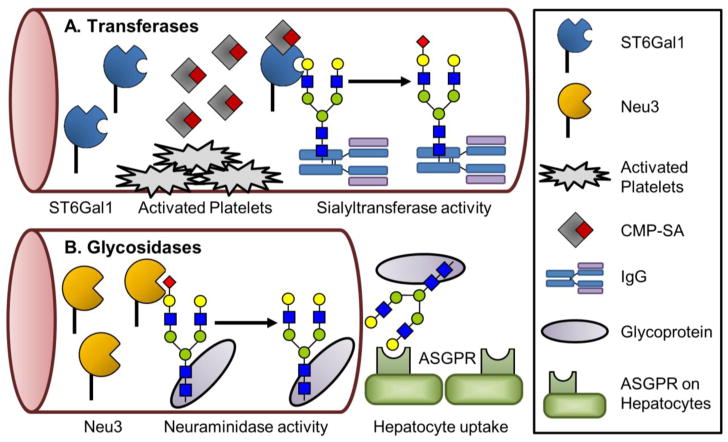
ST6Gal1 is ubiquitously but differentially expressed throughout mammalian tissues [14], and its product, α2,6-linked sialic acid, has a common theme of dampening inflammatory responses. However, sialylation is not limited to immunological targets and is only one example of many biological processes in which glycosylation is able to modulate protein and cellular function. Recent studies have identified additional diseases in which sialylation may play a role, increasing the number of possible model systems and targets for study. Investigators have also generated tools that take advantage of sialic acid biology, such as: sialylated Fc therapy, STALs for B cell manipulation, modulation of hematopoiesis, and site-specific IgG sialylation. Importantly, there is a growing appreciation of the novel glycosylation mechanism of extracellular sialylation. Glycosylation is not restricted to occurring in the ER/Golgi, as there is now a recognized means by which protein and cell surface glycans can be altered by ST6Gal1 and potentially other glycosyltransferases (Figure 2a). Derived from tissues distal to their site of action, these extracellular enzymes hold the potential to reveal further roles of glycan-based regulation. A report from Yang et. al. demonstrates that the half-life of circulating proteins is mediated by soluble glycosidases which influence hepatic scavenging (Figure 2b) [116]. Added to the mounting evidence of platelets as nucleotide-sugar donors [26;77;117;118], these studies together establish that the bloodstream is primed for modifying glycans through building and/or deconstructing structures. Harnessing glycan remodeling by extracellular glycosylation, or deglycosylation, may have an impact on existing therapies; for example, the half-life of a biological or monoclonal antibody therapeutic may be extended or truncated mid-treatment by adding or removing sialic acids to engage or inhibit uptake by the asialoglycoprotein receptor, respectively. There are clearly additional novel biological roles to discover for ST6Gal1, and further applications of the current technologies forthcoming from this field. Continued investigation into the precise processes that regulate ST6Gal1, especially in response to various inflammatory cues, will yield critical insight into how the immune system is regulated.
Figure 2. Methods of extracellular glycosylation in circulation.
Two illustrated examples of extracellular sialyltransferase and neuraminidase activity. A) Soluble ST6Gal1 interacts with CMP-sialic acid released by activated platelets to transfer a sialic acid to an acceptor, shown here as IgG [26]. B) The neuraminidase, Neu3, when secreted binds and removes a sialic acid from a glycoprotein exposing the underlying galactose [116]. This causes glycoproteins to be scavenged by hepatocytes due to interaction with the ASGPR.
Highlights.
Sialylation, a form of glycosylation, modulates immune responses.
IgGs become anti-inflammatory when the Fc region is sialylated.
Leukocyte α2,6 sialylation has immunoregulatory effects on cell behavior.
Extracellular glycosylation is an emerging regulatory pathway.
Acknowledgments
We thank Dr. Brian Cobb, Douglas Oswald, and Julie Zhou for their insight during manuscript preparation. This work was supported by the National Institutes of Health grant F32 [AI114109] to MBJ. We also offer our apologies to any authors and investigators inadvertently not included.
Abbreviations
- PMA
phorbol myristate acetate
- Siglec
sialic acid recognizing Ig-superfamily lectins
- STAL
siglec tolerizing antigenic liposome
- GC
germinal center
- IgG
immunoglobulin G
- NCAM
neural cell adhesion molecule
- ADCC
antibody dependent cell mediated cytotoxicity
- CIA
collagen induced arthritis
- CMP
cytidine monophosphate
- HSPC
hematopoietic stem and progenitor cell
- hPSC
human pluripotent stem cell
- iPSC
induced pluripotent stem cell
- ITIM
immunoreceptor tyrosine-based inhibitor motif
- ASGPR
asialoglycoprotein receptor
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bi S, Baum LG. Sialic acids in T cell development and function. Biochim Biophys Acta. 2009;1790:1599–1610. doi: 10.1016/j.bbagen.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, van KY, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253:1–15. doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol. 2017;147:149–174. doi: 10.1007/s00418-016-1520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu R, de Vries RP, Zhu X, Nycholat CM, McBride R, Yu W, Paulson JC, Wilson IA. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science. 2013;342:1230–1235. doi: 10.1126/science.1243761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Air GM. Influenza virus-glycan interactions. Curr Opin Virol. 2014;7:128–133. doi: 10.1016/j.coviro.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charter NW, Mahal LK, Koshland DE, Jr, Bertozzi CR. Biosynthetic incorporation of unnatural sialic acids into polysialic acid on neural cells. Glycobiology. 2000;10:1049–1056. doi: 10.1093/glycob/10.10.1049. [DOI] [PubMed] [Google Scholar]
- 10.Datta AK, Paulson JC. The sialyltransferase “sialylmotif” participates in binding the donor substrate CMP-NeuAc. J Biol Chem. 1995;270:1497–1500. doi: 10.1074/jbc.270.4.1497. [DOI] [PubMed] [Google Scholar]
- 11.Datta AK, Sinha A, Paulson JC. Mutation of the sialyltransferase S-sialylmotif alters the kinetics of the donor and acceptor substrates. J Biol Chem. 1998;273:9608–9614. doi: 10.1074/jbc.273.16.9608. [DOI] [PubMed] [Google Scholar]
- 12.Petit D, Mir AM, Petit JM, Thisse C, Delannoy P, Oriol R, Thisse B, Harduin-Lepers A. Molecular phylogeny and functional genomics of beta-galactoside alpha2,6-sialyltransferases that explain ubiquitous expression of st6gal1 gene in amniotes. J Biol Chem. 2010;285:38399–38414. doi: 10.1074/jbc.M110.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehoux S, Groux-Degroote S, Cazet A, Dhaenens CM, Maurage CA, Caillet-Boudin ML, Delannoy P, Krzewinski-Recchi MA. Transcriptional regulation of the human ST6GAL2 gene in cerebral cortex and neuronal cells. Glycoconj J. 2010;27:99–114. doi: 10.1007/s10719-009-9260-y. [DOI] [PubMed] [Google Scholar]
- 14.Dalziel M, Huang RY, Dall’Olio F, Morris JR, Taylor-Papadimitriou J, Lau JT. Mouse ST6Gal sialyltransferase gene expression during mammary gland lactation. Glycobiology. 2001;11:407–412. doi: 10.1093/glycob/11.5.407. [DOI] [PubMed] [Google Scholar]
- 15.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 17.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 18.Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- 19.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan HA, Woloski BM, Hellman M, Jamieson JC. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta1,4GlcNAc alpha2,6 sialyltransferase from liver. J Biol Chem. 1983;258:11505–11509. [PubMed] [Google Scholar]
- 21.Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci USA. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitazume S, Nakagawa K, Oka R, Tachida Y, Ogawa K, Luo Y, Citron M, Shitara H, Taya C, Yonekawa H, Paulson JC, Miyoshi E, Taniguchi N, Hashimoto Y. In vivo cleavage of alpha2,6-sialyltransferase by Alzheimer beta-secretase. J Biol Chem. 2005;280:8589–8595. doi: 10.1074/jbc.M409417200. [DOI] [PubMed] [Google Scholar]
- 23.Dougher CWL, Buffone A, Jr, Nemeth MJ, Nasirikenari M, Irons EE, Bogner PN, Lau JTY. The blood-borne sialyltransferase ST6Gal-1 is a negative systemic regulator of granulopoiesis. J Leukoc Biol. 2017;102:507–516. doi: 10.1189/jlb.3A1216-538RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MB, Nasirikenari M, Feng L, Migliore MT, Choi KS, Kazim L, Lau JT. Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J Biol Chem. 2010;285:25009–25017. doi: 10.1074/jbc.M110.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones MB, Nasirikenari M, Lugade AA, Thanavala Y, Lau JT. Anti-inflammatory IgG production requires functional P1 promoter in beta-galactoside alpha2,6-sialyltransferase 1 (ST6Gal-1) gene. J Biol Chem. 2012;287:15365–15370. doi: 10.1074/jbc.M112.345710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. B-cell-independent sialylation of IgG. Proc Natl Acad Sci USA. 2016;113:7207–7212. doi: 10.1073/pnas.1523968113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manhardt CT, Punch PR, Dougher CWL, Lau JTY. Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J Biol Chem. 2017;292:13514–13520. doi: 10.1074/jbc.C117.795138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto I, Futakawa S, Oka R, Ogawa K, Marth JD, Miyoshi E, Taniguchi N, Hashimoto Y, Kitazume S. Beta-galactoside alpha2,6-sialyltransferase I cleavage by BACE1 enhances the sialylation of soluble glycoproteins. A novel regulatory mechanism for alpha2,6-sialylation. J Biol Chem. 2007;282:34896–34903. doi: 10.1074/jbc.M704766200. [DOI] [PubMed] [Google Scholar]
- 29.Appenheimer MM, Huang RY, Chandrasekaran EV, Dalziel M, Hu YP, Soloway PD, Wuensch SA, Matta KL, Lau JT. Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology. 2003;13:591–600. doi: 10.1093/glycob/cwg066. [DOI] [PubMed] [Google Scholar]
- 30.Wuensch SA, Huang RY, Ewing J, Liang X, Lau JT. Murine B cell differentiation is accompanied by programmed expression of multiple novel beta-galactoside alpha2, 6-sialyltransferase mRNA forms. Glycobiology. 2000;10:67–75. doi: 10.1093/glycob/10.1.67. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Qian R, Rausa FM, III, Colley KJ. Two naturally occurring alpha2,6-sialyltransferase forms with a single amino acid change in the catalytic domain differ in their catalytic activity and proteolytic processing. J Biol Chem. 1997;272:672–679. doi: 10.1074/jbc.272.1.672. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Colley KJ. A disulfide-bonded dimer of the Golgi beta-galactoside alpha2,6-sialyltransferase is catalytically inactive yet still retains the ability to bind galactose. J Biol Chem. 1996;271:7758–7766. doi: 10.1074/jbc.271.13.7758. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Colley KJ. Minimal structural and glycosylation requirements for ST6Gal I activity and trafficking. Glycobiology. 2000;10:531–583. doi: 10.1093/glycob/10.5.531. [DOI] [PubMed] [Google Scholar]
- 34.Pels Rijcken WR, Overdijk B, Van den Eijnden DH, Ferwerda W. The effect of increasing nucleotide-sugar concentrations on the incorporation of sugars into glycoconjugates in rat hepatocytes. Biochem J. 1995;305(Pt 3):865–870. doi: 10.1042/bj3050865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin EC. Structure and function of immunoglobulins. Acta Endocrinol Suppl (Copenh) 1975;194:77–95. doi: 10.1530/acta.0.080s077. [DOI] [PubMed] [Google Scholar]
- 36.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 39.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci USA. 2017;114:3485–3490. doi: 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 41.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, Shitara K, Satoh M. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–118. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 42.Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng. 2001;74:288–294. [PubMed] [Google Scholar]
- 43.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 44.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwab I, Lux A, Nimmerjahn F. Pathways Responsible for Human Autoantibody and Therapeutic Intravenous IgG Activity in Humanized Mice. Cell Rep. 2015;13:610–620. doi: 10.1016/j.celrep.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, Prestegard JH. NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry. 2012;51:4618–4626. doi: 10.1021/bi300319q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holla A, Skerra A. Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng Des Sel. 2011;24:659–669. doi: 10.1093/protein/gzr016. [DOI] [PubMed] [Google Scholar]
- 49.Leontyev D, Katsman Y, Ma XZ, Miescher S, Kasermann F, Branch DR. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion. 2012;52:1799–1805. doi: 10.1111/j.1537-2995.2011.03517.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 51.von GS, Shoenfeld Y, Blank M, Branch DR, Vassilev T, Kasermann F, Bayry J, Kaveri S, Simon HU. IVIG pluripotency and the concept of Fc-sialylation: challenges to the scientist. Nat Rev Immunol. 2014;14:349. doi: 10.1038/nri3401-c1. [DOI] [PubMed] [Google Scholar]
- 52.Anumula KR. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J Immunol Methods. 2012;382:167–176. doi: 10.1016/j.jim.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Huang L, Biolsi S, Bales KR, Kuchibhotla U. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal Biochem. 2006;349:197–207. doi: 10.1016/j.ab.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Lim A, Reed-Bogan A, Harmon BJ. Glycosylation profiling of a therapeutic recombinant monoclonal antibody with two N-linked glycosylation sites using liquid chromatography coupled to a hybrid quadrupole time-of-flight mass spectrometer. Anal Biochem. 2008;375:163–172. doi: 10.1016/j.ab.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology. 2015;25:1325–1334. doi: 10.1093/glycob/cwv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stockert RJ, Morell AG, Ashwell G. Structural characteristics and regulation of the asialoglycoprotein receptor. Targeted Diagn Ther. 1991;4:41–64. [PubMed] [Google Scholar]
- 57.van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J Immunol. 2016;196:1435–1441. doi: 10.4049/jimmunol.1502136. [DOI] [PubMed] [Google Scholar]
- 58.Ballow M, Allen C. Intravenous immunoglobulin modulates the maturation of TLR 4-primed peripheral blood monocytes. Clin Immunol. 2011;139:208–214. doi: 10.1016/j.clim.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med. 1992;326:107–116. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- 60.Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson RP, Jr, Patel DD, Secord E, Sorensen RU, Wasserman RL, Cunningham-Rundles C. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seeling M, Bruckner C, Nimmerjahn F. Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat. Rev Rheumatol. 2017;13:621–630. doi: 10.1038/nrrheum.2017.146. [DOI] [PubMed] [Google Scholar]
- 63.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Chereau C, Pagnoux C, Michalski JC, Guillevin L, Weill B, Batteux F, Guilpain P. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2011;63:2105–2115. doi: 10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- 65.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wahl A, Kasela S, Carnero-Montoro E, van IM, Stambuk J, Sharma S, van den Akker E, Klaric L, Benedetti E, Razdorov G, Trbojevic-Akmacic I, Vuckovic F, Ugrina I, Beekman M, Deelen J, van HD, Heijmans BT, Wuhrer M, Plomp R, Keser T, Simurina M, Pavic T, Gudelj I, Kristic J, Grallert H, Kunze S, Peters A, Bell JT, Spector TD, Milani L, Slagboom PE, Lauc G, Gieger C Consortium BIOS. IgG glycosylation and DNA methylation are interconnected with smoking. Biochim Biophys Acta. 2017;1862:637–648. doi: 10.1016/j.bbagen.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Miyoshi E, Shinzaki S, Fujii H, Iijima H, Kamada Y, Takehara T. Role of aberrant IgG glycosylation in the pathogenesis of inflammatory bowel disease. Proteomics Clin Appl. 2016;10:384–390. doi: 10.1002/prca.201500089. [DOI] [PubMed] [Google Scholar]
- 68.Plomp R, Ruhaak LR, Uh HW, Reiding KR, Selman M, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, Wuhrer M. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci Rep. 2017;7:12325. doi: 10.1038/s41598-017-12495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kemna MJ, Plomp R, van PP, Koeleman CAM, Jansen BC, Damoiseaux JGMC, Cohen Tervaert JW, Wuhrer M. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. EBioMedicine. 2017;17:108–118. doi: 10.1016/j.ebiom.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mittermayr S, Le GN, Clarke C, Millan MS, Larkin AM, O’Gorman P, Bones J. Polyclonal Immunoglobulin G N-Glycosylation in the Pathogenesis of Plasma Cell Disorders. J Proteome Res. 2017;16:748–762. doi: 10.1021/acs.jproteome.6b00768. [DOI] [PubMed] [Google Scholar]
- 71.Keser T, Gornik I, Vuckovic F, Selak N, Pavic T, Lukic E, Gudelj I, Gasparovic H, Biocina B, Tilin T, Wennerstrom A, Mannisto S, Salomaa V, Havulinna A, Wang W, Wilson JF, Charutvedi N, Perola M, Campbell H, Lauc G, Gornik O. Increased plasma N-glycome complexity is associated with higher risk of type 2 diabetes. Diabetologia. 2017;60:2352–2360. doi: 10.1007/s00125-017-4426-9. [DOI] [PubMed] [Google Scholar]
- 72.Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, Narazaki M, Shoda H, Takahashi N, Ohkawa Y, Ji S, Sugiyama F, Fujio K, Kumanogoh A, Yamamoto K, Kawasaki N, Kurosaki T, Takahashi Y, Furukawa K. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 2016;7:11205. doi: 10.1038/ncomms11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang TT, Maamary J, Tan GS, Bournazos S, Davis CW, Krammer F, Schlesinger SJ, Palese P, Ahmed R, Ravetch JV. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell. 2015;162:160–169. doi: 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Bohm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lonnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Bluml S, Nimmerjahn F, Schett G, Kronke G. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 2017;18:104–113. doi: 10.1038/ni.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, Schommartz T, Petzold D, Bitterling J, Schoen AL, Stoehr AD, Vu VD, Darcan-Nikolaisen Y, Blanchard V, Schmudde I, Laumonnier Y, Strover HA, Hegazy AN, Eiglmeier S, Schoen CT, Mertes MM, Loddenkemper C, Lohning M, Konig P, Petersen A, Luger EO, Collin M, Kohl J, Hutloff A, Hamelmann E, Berger M, Wardemann H, Ehlers M. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol. 2012;129:1647–1655. doi: 10.1016/j.jaci.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 76.Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MM, Schoen CT, Tiburzy B, Herrmann A, Kohl J, Manz RA, Madaio MP, Berger M, Wardemann H, Ehlers M. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest. 2013;123:3788–3796. doi: 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pagan JD, Kitaoka M, Anthony RM. Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Cell. 2017 doi: 10.1016/j.cell.2017.11.041. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 1998;95:7469–7474. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bellis SL. Variant glycosylation: an underappreciated regulatory mechanism for beta1 integrins. Biochim Biophys Acta. 2004;1663:52–60. doi: 10.1016/j.bbamem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Li H, Dimasi N, McCormick JK, Martin R, Schuck P, Schlievert PM, Mariuzza RA. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity. 2001;14:93–104. doi: 10.1016/s1074-7613(01)00092-9. [DOI] [PubMed] [Google Scholar]
- 83.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Tateno H, Toyota M, Saito S, Onuma Y, Ito Y, Hiemori K, Fukumura M, Matsushima A, Nakanishi M, Ohnuma K, Akutsu H, Umezawa A, Horimoto K, Hirabayashi J, Asashima M. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286:20345–20353. doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satomaa T, Heiskanen A, Mikkola M, Olsson C, Blomqvist M, Tiittanen M, Jaatinen T, Aitio O, Olonen A, Helin J, Hiltunen J, Natunen J, Tuuri T, Otonkoski T, Saarinen J, Laine J. The N-glycome of human embryonic stem cells. BMC Cell Biol. 2009;10:42. doi: 10.1186/1471-2121-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hasehira K, Tateno H, Onuma Y, Ito Y, Asashima M, Hirabayashi J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol Cell Proteomics. 2012;11:1913–1923. doi: 10.1074/mcp.M112.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alisson-Silva F, de Carvalho RD, Vairo L, Asensi KD, Vasconcelos-dos-Santos A, Mantuano NR, Dias WB, Rondinelli E, Goldenberg RC, Urmenyi TP, Todeschini AR. Evidences for the involvement of cell surface glycans in stem cell pluripotency and differentiation. Glycobiology. 2014;24:458–468. doi: 10.1093/glycob/cwu012. [DOI] [PubMed] [Google Scholar]
- 88.Wang YC, Stein JW, Lynch CL, Tran HT, Lee CY, Coleman R, Hatch A, Antontsev VG, Chy HS, O’Brien CM, Murthy SK, Laslett AL, Peterson SE, Loring JF. Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells. Sci Rep. 2015;5:13317. doi: 10.1038/srep13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nasirikenari M, Chandrasekaran EV, Matta KL, Segal BH, Bogner PN, Lugade AA, Thanavala Y, Lee JJ, Lau JT. Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J Leukoc Biol. 2010;87:457–466. doi: 10.1189/jlb.1108704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones MB, Nasirikenari M, Feng L, Migliore MT, Choi KS, Kazim L, Lau JT. Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J Biol Chem. 2010;285:25009–25017. doi: 10.1074/jbc.M110.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nasirikenari M, Veillon L, Collins CC, Azadi P, Lau JT. Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialyltransferase. J Biol Chem. 2014;289:7178–7189. doi: 10.1074/jbc.M113.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dougher CWL, Buffone A, Jr, Nemeth MJ, Nasirikenari M, Irons EE, Bogner PN, Lau JTY. The blood-borne sialyltransferase ST6Gal-1 is a negative systemic regulator of granulopoiesis. J Leukoc Biol. 2017;102:507–516. doi: 10.1189/jlb.3A1216-538RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36:1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 94.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann N Y Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Reilly MK, Paulson JC. Multivalent ligands for siglecs. Methods Enzymol. 2010;478:343–363. doi: 10.1016/S0076-6879(10)78017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268:7019–7027. [PubMed] [Google Scholar]
- 98.Nitschke L. CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance. Glycobiology. 2014;24:807–817. doi: 10.1093/glycob/cwu066. [DOI] [PubMed] [Google Scholar]
- 99.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 100.Macauley MS, Paulson JC. Siglecs induce tolerance to cell surface antigens by BIM-dependent deletion of the antigen-reactive B cells. J Immunol. 2014;193:4312–4321. doi: 10.4049/jimmunol.1401723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macauley MS, Kawasaki N, Peng W, Wang SH, He Y, Arlian BM, McBride R, Kannagi R, Khoo KH, Paulson JC. Unmasking of CD22 Co-receptor on Germinal Center B-cells Occurs by Alternative Mechanisms in Mouse and Man. J Biol Chem. 2015;290:30066–30077. doi: 10.1074/jbc.M115.691337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chappell CP, Draves KE, Clark EA. CD22 is required for formation of memory B cell precursors within germinal centers. PLoS One. 2017;12:e0174661. doi: 10.1371/journal.pone.0174661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orgel KA, Duan S, Wright BL, Maleki SJ, Wolf JC, Vickery BP, Burks AW, Paulson JC, Kulis MD, Macauley MS. Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2. J Allergy Clin Immunol. 2017;139:366–369. doi: 10.1016/j.jaci.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pang L, Macauley MS, Arlian BM, Nycholat CM, Paulson JC. Encapsulating an Immunosuppressant Enhances Tolerance Induction by Siglec-Engaging Tolerogenic Liposomes. Chembiochem. 2017;18:1226–1233. doi: 10.1002/cbic.201600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 106.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 107.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142:107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 109.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jenner J, Kerst G, Handgretinger R, Muller I. Increased alpha2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp Hematol. 2006;34:1212–1218. doi: 10.1016/j.exphem.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 111.Zhuo Y, Bellis SL. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J Biol Chem. 2011;286:5935–5941. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Delannoy CP, Rombouts Y, Groux-Degroote S, Holst S, Coddeville B, Harduin-Lepers A, Wuhrer M, Elass-Rochard E, Guerardel Y. Glycosylation Changes Triggered by the Differentiation of Monocytic THP-1 Cell Line into Macrophages. J Proteome Res. 2017;16:156–169. doi: 10.1021/acs.jproteome.6b00161. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z, Swindall AF, Kesterson RA, Schoeb TR, Bullard DC, Bellis SL. ST6Gal-I regulates macrophage apoptosis via alpha2–6 sialylation of the TNFR1 death receptor. J Biol Chem. 2011;286:39654–39662. doi: 10.1074/jbc.M111.276063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woodard-Grice AV, McBrayer AC, Wakefield JK, Zhuo Y, Bellis SL. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J Biol Chem. 2008;283:26364–26373. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang D, Ozhegov E, Wang L, Zhou A, Nie H, Li Y, Sun XL. Sialylation and desialylation dynamics of monocytes upon differentiation and polarization to macrophages. Glycoconj J. 2016;33:725–733. doi: 10.1007/s10719-016-9664-4. [DOI] [PubMed] [Google Scholar]
- 116.Yang WH, Aziz PV, Heithoff DM, Mahan MJ, Smith JW, Marth JD. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci USA. 2015;112:13657–13662. doi: 10.1073/pnas.1515464112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wandall HH, Rumjantseva V, Sorensen AL, Patel-Hett S, Josefsson EC, Bennett EP, Italiano JE, Jr, Clausen H, Hartwig JH, Hoffmeister KM. The origin and function of platelet glycosyltransferases. Blood. 2012;120:626–635. doi: 10.1182/blood-2012-02-409235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee MM, Nasirikenari M, Manhardt CT, Ashline DJ, Hanneman AJ, Reinhold VN, Lau JT. Platelets support extracellular sialylation by supplying the sugar donor substrate. J Biol Chem. 2014;289:8742–8748. doi: 10.1074/jbc.C113.546713. [DOI] [PMC free article] [PubMed] [Google Scholar]