Abstract
Lung stereotactic body radiation therapy (SBRT) is a novel and effective modality for treatment of early stage non-small cell lung cancer (NSCLC), with expanding indications in locally advanced and metastatic disease. Herein, we will review current treatment recommendations for early stage NSCLC, detail treatment planning of SBRT, and discuss future directions.
Introduction
Stereotactic body radiotherapy (SBRT) is a specialized delivery of external beam radiation therapy. It is defined by the American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) as a “method used to very precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions”.1 The technology delivers a high dose of radiation to a target with a rapid dose fall-off, maximizing dose to the tumor while minimizing dose to normal structures. While this strategy has a long history of successful use in intracranial lesions for more than 30 years, the ability to deliver similar treatments outside the brain is a more recent development. The first reports of extracranial stereotactic radiotherapy treatments were published in the 1990s. A seminal study from Sweden reported the results of their first 31 patients treated in a stereotactic body frame to extracranial sites of disease, including five patients treated to metastases in the lungs, with local control of 80%.2 Lately, its use in treating thoracic tumors has seen a particularly rapid expansion in use as technology and understanding of the biology associated with such treatments has improved.
Background and Clinical Uses
Lung cancer is the most common cause of cancer death in the United States, with an estimated 159,260 deaths in 2014.3 In Missouri each year, there will be an estimated 5,370 new cases and 3,950 deaths of cancer of the lung and bronchus.4 Currently, 15% of non-small cell lung cancer (NSCLC) cases are diagnosed as early stage.5 As CT-based screening for lung cancer in high risk patients increases, the percentage of lung cancers diagnosed as early stage may increase up to 33%.6 This increase in the incidence of early stage NSCLC, coupled with the parallel increase in proportion of older (>65 years) adults 7 that may not tolerate aggressive surgical therapy, necessitates alternative treatment strategies.
For appropriate surgical candidates, the standard treatment for early stage NSCLC is surgical resection, often consisting of lobectomy, yielding five-year overall survival (OS) rates of up to 70%.8 If a patient does not have the pulmonary reserve to tolerate a lobectomy, a sublobar resection such as a wedge resection or segmentectomy may also be offered for select patients, though older randomized data suggests this has inferior outcomes to lobectomy.9 However, patients may be deemed inoperable due to pulmonary or cardiac comorbidities, advanced age, poor functional status, or patient refusal, among other reasons. In these situations, definitive doses of radiation can be used for tumor control. Historically, patients were treated to 60 to 70 Gray (Gy) in 30–35 daily fractions of 1.8–2.0 Gy per fraction, yielding five-year OS rates of only 5–10% with 50–70% of patients failing locally.10, 11 Older studies also treated patients to larger volumes, including the primary tumor as well as elective nodal coverage, which could include the ipsilateral and contralateral mediastinum, ipsilateral and contralateral hila, and/or supraclavicular lymph node areas. The large treatment volume is one source of increased toxicity in this patient population. As radiation advanced from two-dimensional (2D) technique to three dimensional (3D) conformal radiotherapy, radiation was able to be delivered more precisely, and five-year OS improved from 10% for the 2D patients to 36% for patients treated with 3D radiation.12 However, outcomes were still worse when compared to surgical treatment of early stage NSCLC.
More recently, SBRT has been used increasingly for treatment of early stage NSCLC. SBRT uses higher doses of radiation per fraction, often delivered in five fractions or fewer, and targets a volume using 3D localization. Since the target is more precisely localized, higher doses per fraction can be safely delivered while minimizing dose delivered to normal tissue. The outcomes for early stage NSCLC in medically inoperable patients treated with SBRT have been promising. After completing a phase I dose escalation trial, a phase II trial from Indiana evaluated SBRT for T1-2N0 NSCLC in 70 patients and found a three-year local control rate of 88% and three-year OS of 43%.13 While this OS is lower than historical surgical controls, this patient population treated with SBRT also has more medical comorbidities that contribute to lower OS. In the multi-institutional phase II trial RTOG 0236, which excluded centrally located tumors within 2 centimeters of the proximal bronchial tree, three-year local control was 91% and three-year overall survival was 56%.14 These early promising results led to an expanded role of lung SBRT.
The advantage of delivering a higher dose per fraction is based on an increased cytotoxic effect which can translate to an increased probability of tumor control. However, the location of the tumor and proximity to critical normal structures plays a role in determining the ideal dose that can be delivered safely. In a phase II trial where all patients received 60 to 66 Gy total in 3 fractions, two-year freedom from severe toxicity for peripheral tumors was 83% compared to 54% for centrally located tumors.15 Doses used at Washington University most commonly are 54 Gy in 3 fractions for peripheral thoracic tumors and 50–55 Gy total in 5 fractions for centrally located thoracic tumors.
At our institution, there is a phase I/II trial exploring SBRT dose escalation of central tumors. The phase I dose escalation level portion of the trial began at 9 Gy for 5 fractions for a total dose of 45 Gy and was escalated to 10 Gy x 5 fractions, 11 Gy x 5 fractions, and 12 Gy x 5 fractions. There were no local failures in the 50 Gy, 55 Gy, or 60 Gy cohorts. It was determined to proceed to the phase II portion of the trial using 55 Gy.16 As these data mature, further tumor control and toxicity profile will be available for centrally located tumors. Currently there is a national phase I/II trial, Radiation Therapy Oncology Group (RTOG 0813), that also looks to evaluate optimal dosing for early stage, centrally located NSCLC.
For more peripheral tumors, a higher dose can be delivered; however, the ideal dose for these tumors has yet to be determined. There is a wide range of acceptable doses that provide excellent tumor control while minimizing normal tissue toxicities. RTOG 0915 was a phase II trial that compared 34 Gy in one fraction to 48 Gy total in 4 fractions; both arms had similar rates of adverse events and local control.17 From RTOG 0236, 60 Gy in 3 fractions was delivered safely to peripheral lung tumors. At our institution, it is common practice to treat peripherally located tumors to 54 Gy in 3 fractions.
Treatment Planning
Prior to planning a patient for SBRT, the patient undergoes a computed tomography (CT) simulation within our radiation oncology department. This simulation includes a free breathing scan and a four-dimensional CT (4DCT) scan. The 4DCT acquires images throughout the breathing cycle, allowing an assessment of tumor motion. Patients are placed in a body frame to facilitate patient immobilization. As the target lies within the lung parenchyma, there is often significant tumor motion secondary to the respiratory cycle. It is important to monitor these motion changes in order to adequately cover the tumor. Images from the CT simulation are then used to define the gross target volume (GTV) and assess tumor motion. Generally, 1 centimeter or less of motion in any direction is considered acceptable. One method of decreasing tumor motion is to apply abdominal compression. Tumor motion can also be monitored using respiratory gating or patient breath holding. An additional margin is added to account for set-up inaccuracies called the planning target volume (PTV). After the tumor is contoured and the final target volume determined (i.e., the PTV), a plan is generated utilizing beams from various directions to cover the target adequately (Figure 1). On the day of each treatment, the patient is immobilized identically to the simulation session. Set-up is verified, which includes in-room imaging with a CT scanner built into the radiation treatment machine. Treatment is then delivered as planned with images reviewed prior to delivery of radiation (See Figure 2)
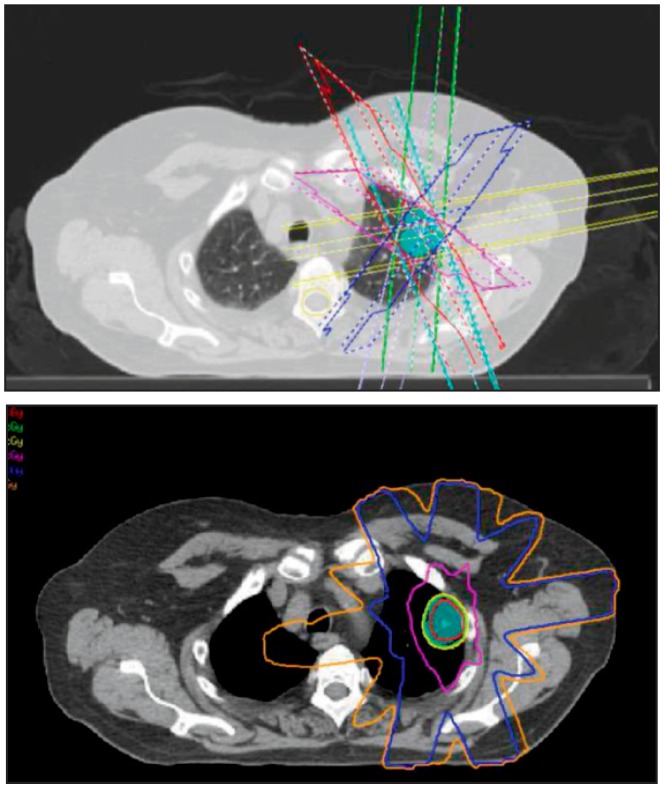
Figure 1.
A Top) A left upper lobe lesion (shown in cyan colorwash) being treated to 5400 centigray (cGy) total in three fractions. The various beam angles to target the lesion are shown.
B Bottom) The dosimetric coverage of the lesion. The 100% isodose line (5400 cGy) covers the tumor. There is a rapid dose fall-off to the 50% isodose line (2700 cGy).
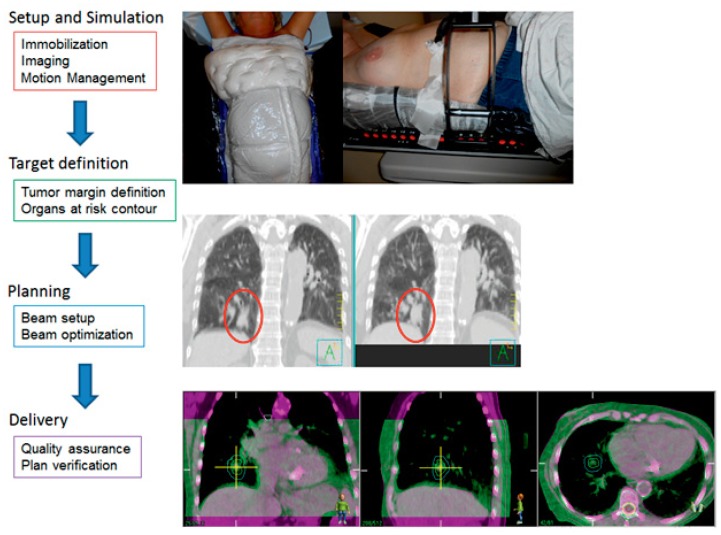
Figure 2. The Process of Stereotactic Body Radiation Therapy.
A) Two types of immobilization devices are shown. In the left panel, the patient is immobilized with BodyFIX. It utilizes vacuum technology to create a mold of the patient’s body contour. In the right panel, the patient is immobilized with a custom fitted foam frame with a paddle on the patient’s abdomen to minimize tumor motion with the respiratory cycle.
B) During simulation, a four-dimensional or respiratory CT scan is obtained. It allows for visualization of structures throughout the respiratory cycle. The scan on the left is during the exhale portion of respiration when the tumor motion is typically minimal. The right panel shows the maximum intensity projection (MIP) images. MIP is a surrogate for where the tumor is moving during respiration. The red oval encircles the tumor; the tumor appears fuller and larger on the MIP images.
C) Prior to delivering each fraction, a cone beam CT (CBCT) is performed on the treatment machine. The purple shade CT is the scan from the simulation. The green shade scan is from the CBCT prior to treatment. Depending on the location of the tumor, the CBCT can be matched to the simulation CT by bony landmarks or soft tissue to ensure the tumor is lining up properly prior to delivery of treatment.
Toxicity
While delivering high doses of radiation per fraction has been shown to provide good local control, there are toxicities associated with the treatment from damage to nearby normal tissue. While overall toxicity rates after SBRT are exceptionally low, the most common adverse events include chest wall pain and radiation pneumonitis. In the RTOG 0236 phase II trial, which included 55 evaluable patients, 24% of patients developed a moderately severe adverse event and 4% of patients developed a severe adverse event.14 The majority of moderately severe adverse events were related to the pulmonary or upper respiratory tract or musculoskeletal or soft tissue. Severe adverse events included hypocalcemia and decrease in pulmonary functions.
Specifically regarding chest wall toxicity, in an analysis from our institution, Creach et al. reported 16% of patients developed CW pain at a median time of 12.6 months after completion of SBRT. Dosimetric factors predictive for developing chest wall pain included chest wall volume receiving ≥30 Gy (V30), while clinical factors predictive included high BMI and connective tissue disease (18). This chest wall pain can be treated with anti-inflammatories and narcotic medications, similar to how chest wall toxicity from a surgical resection would be treated.
Radiation-induced pneumonitis (RP) is another reported toxicity. It consists of inflammation of the lung parenchyma which is often asymptomatic or easily treatable; however, rare cases may be fatal. On RTOG 0236, the rate of grade 1–3 RP was 16%. When grade 1 (asymptomatic but radiograhically diagnosed) RP was excluded, the rate of symptomatic radiation pneumonitis was less than 10%. There was no severe pneumonitis.19 At Washington University, our overall rate of symptomatic RP in a recent analysis of 321 patients was only 5%.20
With careful planning and awareness of normal structures at risk, lung SBRT can be safely delivered and risk of acute and long term side effects can be minimized.
Expanding Role of SBRT
SBRT is an accepted therapy for early stage NSCLC, but it also has expanding roles such as for patients who present with multiple primary lung nodules. It is often difficult to ascertain if these patients have multiple primary lung cancers or intrapulmonary metastases. Those treated with SBRT for multiple lung cancers at presentation are classified as undergoing synchronous treatment. Alternatively, patients may relapse in the future with a new lung lesion which may represent a recurrence or a new primary and are again treated with SBRT (metachronous treatment). Data from our institution reveal two-year OS for synchronous and metachronous tumors was 50% and 61%, respectively.21 However, patients with centralized tumors did worse than those with peripheral tumors in this cohort, supporting the need for optimized strategies for this patient population.
Lung SBRT has also gained popularity in patients with limited number and sites of metastatic disease (i.e. oligometastases), where potential for long term disease control is possible. Singh et al. reported results of 34 patients treated with SBRT for oligometastatic cancer to the lung.22 Patients were treated with doses ranging from 40 to 60 Gy total in 5 fractions. Patients included in the study had up to five lung metastases, local control of primary tumor, and no other sites of active metastatic disease. As with primary lung cancer, a high rate of local control of the metastases was achieved (one- and three-year local control of 93% and 80%, respectively). Our institutional experience in SBRT for oligometastases has shown one- and two-year local control rates of 93% and 77%, respectively (unpublished data). This data lends support to the role of lung SBRT in the setting of oligometastatic disease with an emphasis on carefully selecting patients that would benefit from this treatment modality. At our institution, this is becoming more common practice.
As the overall United States patient population ages and is often frail, patients more commonly present without the capability of safely undergoing biopsy to confirm malignancy. Particularly in Missouri, the differential diagnosis for a newly diagnosed pulmonary nodule includes fungal infection from histoplasmosis or blastomycosis. However, with the proper selection criteria, this patient population can be safely treated even without a histopathologic diagnosis for a presumed early stage NSCLC. The diagnosis of NSCLC is favored when patients have growing, positron emission tomography (PET) avid nodules with an appropriate clinical history. At Washington University, 22% of patients have been treated with lung SBRT without a pathologic diagnosis based on clinical grounds alone. Our recently updated institutional data shows equivalent local control and OS between patients with and without a biopsy, confirming our practice to treat carefully selected patients without a pathologic diagnosis.23
Future Directions
Lung SBRT has had an expanding role in the treatment of medically inoperable lung cancer. However, there may also be a role for lung SBRT in patients with medically operable disease. RTOG 0618 was a phase II trial that studied the outcomes of SBRT for operable early stage lung cancer patients. Preliminary results showed a two-year primary tumor failure rate of 8% and local failure (including primary tumor and lobar failure) rate of 19%. Two year OS was 84%. The authors concluded that SBRT provided a high rate of tumor control with survival comparable to historic surgical series.24
While lung SBRT is most often used for early stage lung cancer, it can also be used in the setting of a boost for more advanced disease. Currently, there is a phase I/II alternative radiation fractionation trial for locally advanced NSCLC open at our institution. Patients are treated with standard 2 Gy per fraction to 44 Gy with concurrent carboplatin and paclitaxel. The residual gross disease then receives a SBRT dose (using a dose escalation schema). The trial is exploring delivery of a higher total effective dose with this alternative radiation fractionation schema.
As the role of lung SBRT expands in the setting of oligometastases, this has also become an important area of research, and we co-developed and recently opened a phase I trial (NRG BR001) exploring the safety and efficacy of SBRT for ≤4 oligometastases in patients with breast, prostate, and lung cancer. Once the results are available, this study will provide more information about the safety of treating multiple sites of metastatic disease.
As technology continues to evolve, new ways of delivering SBRT will continue to improve. At our institution, we have started to treat patients on the ViewRayTM system, the world’s first MRI-guided radiation therapy machine. Using image guidance with MRI can help guide radiation treatments and evaluate tumor motion, and possibly reduce margins as well as adapting treatment if tumor location or size changes. We will wait for our clinical data to mature as we continue to use this new technology.
Conclusion
Lung SBRT offers a safe and effective method of treating thoracic tumors using high doses of radiation in less than five treatments. It is most often used in early stage NSCLC, but it has an expanding role in other types of malignant thoracic diseases as well. Tremendous progress has been made in our understanding of the technology and clinical effects of lung SBRT, and with continuing research, there is potential to continue to expand its clinical indications.
Biography
Clifford G. Robinson, MD, (above), Assistant Professor of Radiation Oncology and Chief of Service, Stereotactic Body Radiation Therapy; Jeffrey Bradley, MD, FACR, Professor of Radiation Oncology, the S. Kling Endowed Chair of Radiation Oncology, Director, Proton Center, and Chief, Thoracic Service; Sana Rehman, MD; and Michael C. Roach, MD, are all at the Washington University School of Medicine in St. Louis.
Contact: crobinson@radonc.wustl.edu

Footnotes
Disclosure
J.B. has received a clinical trial grant from ViewRay, Inc. C.R. is a PI of a Varian master research agreement, for which Washington University receives funds. He has also received funds from Varian for travel and speaking.
References
- 1.Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology. (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:326–332. doi: 10.1016/j.ijrobp.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Blomgren H, Naslund LI, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–70. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, Ga: American Cancer Society; 2014. [Google Scholar]
- 5.Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute; Bethesda, MD: 2007. (SEER Program, NIH Pub. No. 07-6215). [Google Scholar]
- 6.Roth JA, Sullivan SD, Ravelo A, et al. Low-dose computed tomography lung cancer screening in the Medicare program: Projected clinical, resource, and budget impact. Poster session presented at: American Society of Clinical Oncology J Clin Oncol. 2014;32(5s) (suppl; abstr 6501) [Google Scholar]
- 7.Administration on Aging [Internet] Washington DC: [Accessed December 17, 2014]. Available from: http://www.aoa.acl.gov/Aging_Statistics/index.aspx. [Google Scholar]
- 8.Adebonojo SA, Bowser AN, Moritz DM, et al. Impact of revised stage classification of lung cancer on survival: a military experience. Chest. 1999;115:1507–13. doi: 10.1378/chest.115.6.1507. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung Cancer Study Group Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 10.Dosoretz DE, Katin MJ, Blitzer PH, et al. Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys. 1992;24:3–9. doi: 10.1016/0360-3016(92)91013-d. [DOI] [PubMed] [Google Scholar]
- 11.Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517–23. doi: 10.1016/0360-3016(93)90374-5. [DOI] [PubMed] [Google Scholar]
- 12.Fang LC, Komaki R, Allen P, et al. Comparison of outcomes for patients with medically inoperable stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:108–16. doi: 10.1016/j.ijrobp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 16.Bradley JD, Robinson C, Parikh P, et al. Prospective phase I dose escalation results of SBRT for centrally-located stage I NSCLC. American Society for Therapeutic Radiology and Oncology (ASTRO) meeting; Miami Beach, FL. 04 Oct 2011; Oral presentation. [Google Scholar]
- 17.Videtic GM, Hu C, Singh A, et al. Radiation Therapy Oncology Group (RTOG) Protocol 0915: A randomized phase 2 study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:S3. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creach KM, El Naga I, Bradley JD, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol. 2012;104:23–7. doi: 10.1016/j.radonc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early-stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys. 2014;88:1092–9. doi: 10.1016/j.ijrobp.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chundury A, Rehman S, Roach M, et al. Radiation pneumonitis with stereotactic body radiotherapy: effects of angiotensin converting enzyme inhibitors. Poster presentation at: European Society for Radiotherapy and Oncology (ESTRO) meeting; Barcelona, Spain. 27 Apr 2015; ESTRO Young Scientists Poster Session. [Google Scholar]
- 21.Roach M, Rehman S, DeWees T, et al. Stereotactic body radiation therapy for the treatment of multiple primary non-small cell lung carcinoma. Digital poster discussion at: American Society for Therapeutic Radiology and Oncology (ASTRO) meeting; San Francisco, CA. 14 Sept 2014. [Google Scholar]
- 22.Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis. 2014;6:369–74. doi: 10.3978/j.issn.2072-1439.2013.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehman S, Roach M, Mullen D, et al. Stereotactic body radiotherapy for histopathologically confirmed vs. presumed early stage NSCLC. Poster presentation at: European Society for Radiotherapy and Oncology (ESTRO) meeting; Barcelona, Spain. 25 Apr 2015. [Google Scholar]
- 24.Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. Poster presentation at: American Society of Clinical Oncology (ASCO) meeting; Chicago, IL. June 2013. [Google Scholar]