Abstract
While surgical management is a common approach for gynecologic malignancies; often disease is locally advanced such that surgery is precluded or surgical pathology reveals disease extent that mandates adjuvant treatment. Gynecologic brachytherapy is an important tool for both definitive and adjuvant treatment of cervical and endometrial cancers. Brachytherapy enables high radiation doses to a target with rapid fall-off to protect adjacent normal structures. This paper aims to detail the usage of brachytherapy in gynecologic cancers with a focus on advances in technique.
Introduction
Of the 810,320 malignancies expected to be diagnosed in females in the United States in 2014, gynecologic malignancies are estimated to total 94,990 cases. Over half of these diagnoses will be cancers of the uterine corpus. Table 1 shows the expected distribution of gynecologic malignancies among the various subsites including uterine cervix, uterine corpus, ovary, vulva and vagina.1 In Missouri in 2014, the estimated number of new diagnoses of uterine cancer is 1,090, while there are expected to be 240 new cases of cervical cancer.1 Cervical cancer incidence rates have declined in the past several decades and have now stabilized among women younger than 50, and continue to decline in women age 50 and older. Uterine cancer incidence rates continue to increase among women both over and under age 50.
Table 1.
Data from American Cancer Society. Cancer Facts and Figures 2014. Available at www.cancer.org
| Diagnoses | Deaths | |
|---|---|---|
|
| ||
| All sites | 810,320 | 275,710 |
| All Gynecologic sites | 94,990 | 28,790 |
| Uterine cervix | 12,360 | 4,020 |
| Uterine corpus | 52,630 | 8,590 |
| Ovary | 21,980 | 14,270 |
| Vulva | 4,850 | 1,030 |
| Vagina | 3,170 | 880 |
The primary presenting symptom of both cervical and uterine cancer is abnormal vaginal bleeding. The majority of patients are treated by surgical approaches, typically via hysterectomy. However, some patients have risk factors found on surgical pathology that predict for increased risk of recurrence, warranting further adjuvant treatment with radiation and/or chemotherapy. Additionally, particularly in locally advanced malignancies, a definitive management approach with chemotherapy and radiation is recommended. Brachytherapy is a particularly attractive approach for radiation treatment of the gynecologic malignancies by the principles of source proximity to treatment target as well as an ability to generate rapid dose fall-off to protect surrounding organs at risk. It should be emphasized that brachytherapy is part of the standard of care for the treatment of locally advanced cervical cancers. In fact, recent studies have emphasized the importance of brachytherapy in the primary management of this disease.2
History and Physics of Gynecologic Brachytherapy
The first report of the use of gynecologic intracavitary brachytherapy was in 1903 by Margaret Cleaves, MD, in an effort to treat a patient with a locally advanced cervical squamous cell carcinoma with bladder and rectal invasion and extension to the introitus. After standard treatment with UV light and intracavitary x-rays, Dr. Cleaves elected to attempt application of radium bromide acquired from a local chemistry lab in attempt for deeper penetration of the tissues. After application for 10 minutes on day 1, and 5 minutes on day 2, an intense reaction of the mucous membranes was noted. However, several days later the tissues had healed without bleeding, odor, and discharge with a grossly normal appearance.3
The term brachytherapy is derived from the Greek brakhus, meaning “short.” In this radiation therapy technique, sealed radioactive sources are placed near or in direct contact with target tissues. Brachytherapy sources are typically unstable gamma-emitting radioisotopes, classically radium (which is no longer in clinical use) as well as isotopes of cesium or iridium. Brachytherapy source physics allows a high dose concentration immediately surrounding the radioactive source, with rapid dose fall-off within adjacent tissues. There are a variety of different delivery methods for brachytherapy including intracavitary, in which the applicator is placed within an existing body cavity and interstitial, in which sources are placed directly into tissue. Of the gynecologic subsites, cervical and endometrial cancers are most amenable to treatment via intracavitary brachytherapy, while the vagina and vulvar subsites are more suited for interstitial brachytherapy.
An additional variable in brachytherapy is the concept of dose rate. Traditionally, treatments were low-dose rate (LDR) and occurred over a period of 40–60 hours. The patient is nonambulatory during the duration of treatment, and as such the treatments would require inpatient hospitalization with regional or even general anesthesia. The clinical usage of high-dose rate (HDR) brachytherapy has dramatically increased, significantly decreasing treatment times. As a result, these treatments can occur on an outpatient basis with patient preparation, implantation procedure, imaging and treatment being completed within a few hours. Patient risks such as prolonged immobilization and hospitalization are therefore minimized and as such the treatments are more convenient and potentially more cost-effective. Additionally, there are opportunities to perform adaptive treatment planning and dosimetry with each fraction. From a radiation biology perspective, HDR has been proposed to be superior if adequate time is allotted between fractions for normal tissue to repair sublethal damage between fractions.4
A final modern improvement in gynecologic brachytherapy has arisen from the development of computer-controlled remote afterloading devices for brachytherapy. These devices allow automated loading of the radioactive source while the patient is within the shielded treatment room. This eliminates radiation exposure to health care workers in contrast to manual application or manual afterloading of radioactive sources in which the operator must manually place the radioisotope.
Cervical Cancer
The widespread use of the Papanicolaou screening has greatly reduced the incidence of cervical cancer in the United States; however worldwide this disease remains quite prevalent. Treatment of cervical cancer largely depends on the stage at diagnosis, which is determined by a clinical stage established by the International Federation of Gynecology and Obstetrics (FIGO). FIGO staging depends on the physical examination with tumor size, extension past the cervix into the vagina or parametria, involvement of pelvic sidewall or hydronephrosis being the factors that upstage a tumor. Radiographic studies such as CT, MRI or PET do not determine a patient’s tumor stage; however these modalities are used to make treatment management decisions. An early stage microinvasive cervical cancer may be treated with simple or radical hysterectomy. However, with increase in tumor size, the likelihood of a recommendation of post-operative radiation increases based on operative pathology. For example, in one study by the Gynecologic Oncology Group (GOG), patients with stage IB cervical cancer treated with radical hysterectomy and pelvic lymphadenectomy with at least two risk factors (>1/3 stromal invasion, lymphovascular space invasion, or large clinical tumor) were randomized to pelvic irradiation vs observation. Pelvic radiation was found to decrease cancer recurrence by 50% in this set of patients, although a risk of increase in treatment-related toxicities was noted.55 Additionally, in a subsequent GOG study, patients with pathologically positive parametria, pelvic lymph nodes or positive surgical margins after surgery were randomized to pelvic radiation vs. pelvic radiation with cisplatin-based chemotherapy.6 The combination of chemotherapy with radiation increased both progression-free survival and overall survival with increased risk of grade severe toxicity. An Italian study randomizing patients with bulky cervical cancer to primary RT vs radical hysterectomy showed no difference in five-year overall survival or progression-free survival.7 Greater than 50% of patients in the surgery arm warranted post-operative radiation based on surgical pathology findings. Complications and toxicities were significantly increased in the surgical arm.
Due to the high likelihood of need for postoperative radiation for patients with a bulky cervical cancer and the increased toxicities with this treatment, definitive chemoradiation is the treatment of choice for cervical cancers greater than 4 cm and those with extension past the cervix. PET has been shown to be more sensitive for detection of nodal metastases than CT scan8 and as such, a pre-treatment PET should be performed to guide treatment volumes. Presence of lymph node metastasis and degree of PET uptake within lymph nodes are prognostic factors in patients with advanced cervical cancer.9, 10 Typical treatment for a locally advanced cervical cancer involves external beam radiation to the pelvic lymph node regions to a dose of 50 Gy over five to six weeks. Cisplatin-based chemotherapy is typically administered concurrently with radiation, as randomized studies have shown improved overall survival, and progression-free survival in comparison with other regimen.11,12 Treatment breaks can decrease the acute toxicities of radiation, though they can decrease efficacy of treatment by accelerated repopulation of tumor clones. In cervical cancer, each additional day of treatment beyond 55 days has been shown decrease overall survival by 1%.13
Brachytherapy is an integral component of definitive treatment of locally advanced cervical cancer by providing high radiation dose to the primary cervical tumor while allowing relative sparing of the bladder and rectum. In a Surveillance, Epidemiology and End Results (SEER) analysis, Han et al. have demonstrated a dramatic decline in the use of brachytherapy in the definitive treatment of cervical cancer from 1988 – 2009. In this study, the use of brachytherapy was associated with significantly higher cause-specific survival and overall survival.2 Tanderup et al. detail the risks of attempting to utilize new external-beam radiation techniques (intensity modulated or stereotactic body radiotherapy) in lieu of brachytherapy; these risks include decreased local control and increased toxicities.14 These findings suggest that inclusion of brachytherapy in treatment planning for locally advanced cervical cancer is paramount for treatment success.
The components of brachytherapy for cervical cancer include an intrauterine applicator, called a tandem which is used in conjunction with intravaginal components such as vaginal ovoids (seen in conjunction with a tandem in Figure 1A), or a vaginal ring. Varieties of tandem lengths and cur vatures, as well as different ovoid diameters are available and are selected based on patient anatomy. Intravenous conscious sedation is utilized prior to implantation of the applicators. Gauze or vaginal balloons are used as packing anterior and posterior to the ovoids to decrease bladder and rectum dose. Timing of brachytherapy with external beam radiation is institution-dependent; at our institution, weekly HDR brachytherapy treatments are administered concurrently with external beam treatments. The patient does not receive an external beam radiation treatment on the day of brachytherapy. In an analysis of patterns of radiation therapy practice in the treatment of intact cervical cancer, Eifel et al. have estimated that only 8% of radiation therapy facilities in the United States treat more than three patients per year with intact cervical cancer.1515 Patients receiving their treatments at low volume centers are less likely to receive brachytherapy, likely receive a lower dose, and require a longer average time to complete treatment – all factors that are detrimental to the efficacy of treatment.
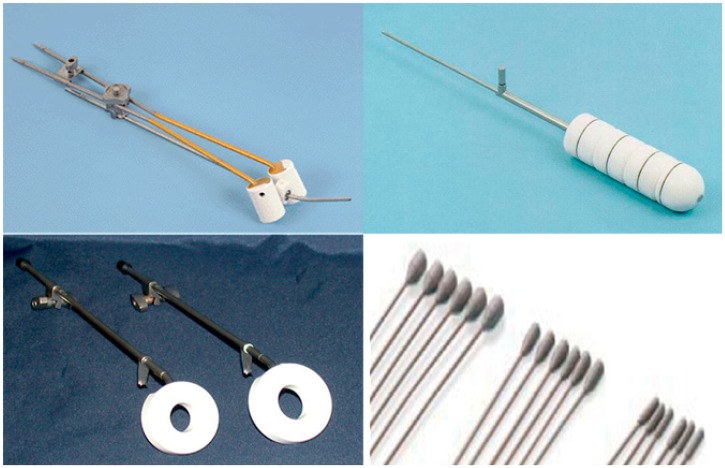
Figure 1.
Applicators used in gynecologic intracavitary brachytherapy. A: Tandem and ovoids, used in definitive treatment of cervical cancer. B: Vaginal cylinder, used in post-hysterectomy treatment of endometrial cancer to decrease vaginal cuff recurrence. C: Vaginal Ring, which can be used in conjunction with an intrauterine tandem for definitive cervix treatment or used alone for palliation of cervical tumor bleeding. D: Simon-Heyman capsules which are used with tandem and ovoids for early-stage medically inoperable endometrial cancer.
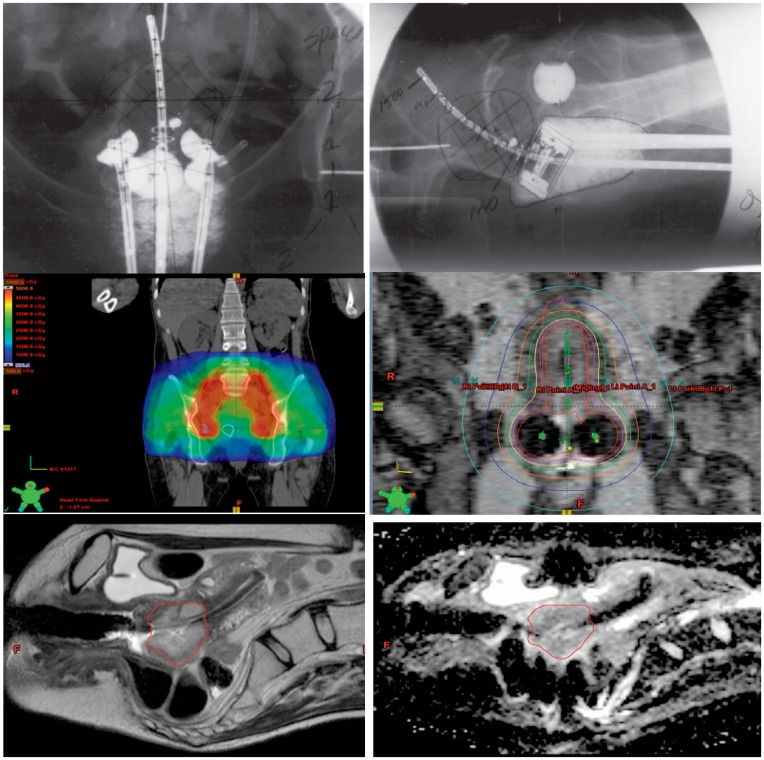
In brachytherapy treatments, the geometry of the implant is critical for optimizing dose to the tumor target. Traditional implant evaluation was via pelvic x-rays (Figure 2A and 2B), with radiation prescribed to Point “A” which is 2 cm lateral to the center of the uterine canal and 2 cm superior to the lateral fornices of the vagina. This point is to approximate where the uterine vessels cross the ureter and the dose represents the normal tissue tolerance to prevent necrosis. More modern 3D treatment planning, in which an MRI is performed immediately following each implantation and prior to each treatment fractions allows customized treatments based on implant geometry. A variety of HDR dose prescriptions are acceptable based on guidelines by the American Brachytherapy Society, consisting of four to six HDR treatments which, in conjunction with pelvic external beam treatment will yield an equivalent dose of 85 Gy to point A.16 External beam radiation treatment is performed in conjunction with tandem and ovoid brachytherapy, and in the external beam plan the pelvic lymph node regions receive 45 Gy while the metabolically active cervical tumor receives 20 Gy, shown in Figure 2C. Figure 2D depicts the planned isodose lines for a tandem and ovoid brachytherapy fraction. A composite plan is created with the external beam treatment plan and the brachytherapy fractions to assess total overall dose. In the Figure 2E and F, T2-weighted and diffusion-weighted sagittal MRI sequences of a brachytherapy implant are seen with the cervical tumor target for that fraction contoured in red. Adjacent normal structures (bladder, rectum and sigmoid colon) are contoured to generate radiation dose information for the organs at risk in addition to the tumor target. MRI-guided brachytherapy in conjunction with PET-CT guided intensity-modulated external beam radiation for definitive cervical cancer treatment is currently being actively investigated to determine optimal radiation distribution that correlates with tumor control. Recently, Dyk et al. have shown the dosimetric parameters D100 (minimum dose covering 100% of the cervical target), D90, and Dmean of the gross tumor volume (GTV) as defined by MRI are associated with improved local tumor control.17
Figure 2.
A and B: Posterior and lateral views of a tandem and ovoid implant. C. Coronal representations of an radiation plan delivering 45 Gy to the pelvic lymph nodes (red contour), and 20 Gy to the metabolically active primary cervical tumor (white contour) in a patient with FIGO stage IIb squamous cell carcinoma with pelvic lymph node involvement. D: Coronal isodose lines for a tandem and ovoid implant. The prescription isodose line is seen in green (650 cGy). E and F: Corresponding T2-weighted sagittal brachytherapy planning image with primary tumor in red (left), and corresponding diffusion-weighted sequence (right).
In addition to serving as a component of definitive treatment for locally advanced cervical cancer, brachytherapy plays a role in palliation of bleeding from cervical tumors. Two treatments of 500 cGy via a vaginal ring (Figure 1C) have been demonstrated to reduce the need for additional transfusion in 93% of patients without acute or long-term grade 3–5 toxicity.18 The dose conformality of these treatments minimizes dose to the rectum, bladder or Point A and, as such, the definitive treatment planning is not jeopardized or limited. Patients who present with symptomatic bleeding from a cervical tumor may undergo vaginal ring brachytherapy with improvement in symptoms prior to initiation of definitive treatment.
Endometrial Cancer
Endometrial cancer, the most common gynecologic malignancy, is typically diagnosed at an early stage after presentation with post-menopausal bleeding. Initial management generally includes total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node sampling and peritoneal cytology. FIGO staging of endometrial cancer is based on surgical pathology. In FIGO stage I patients, where involvement is limited to the endometrium or myometrium, stratification by adverse risk factors including age, lymphovascular space invasion, and depth of myometrial invasion is used to make adjuvant therapy recommendations. Randomized trials for low-intermediate risk endometrial cancer have demonstrated that the majority of recurrences in this group are limited to the vaginal cuff.19, 20 A subsequent trial randomized patients with high-intermediate risk endometrial cancer to pelvic external beam radiation versus vaginal cuff brachytherapy showing no difference in vaginal cuff relapse between the two groups.21 While the risk of pelvic nodal relapse was greater in the brachytherapy alone group, there was no overall survival difference between the two groups. Importantly, there was less acute toxicity after brachytherapy, and quality of life analysis showed significantly worse bowel symptoms and social functioning in patients who received external beam radiation.21, 22
Post-operative vaginal cuff HDR brachytherapy is performed via implantation of a vaginal cylinder (Figure 2B) for treatment of the upper vagina, with a target of the vaginal submucosal lymphatics with administration in three to six doses per American Brachytherapy Society guidelines.23 After implantation of the vaginal cylinder, CT images are obtained to verify implant position and to perform dosimetric analysis for each fraction (Figure 3B). Unlike tandem and ovoid brachytherapy, sedation is often not necessary for vaginal cuff treatments.
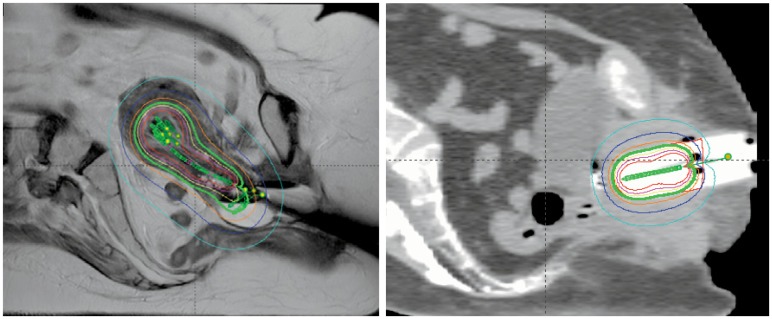
Figure 3.
A: Sagittal plane representative isodose lines for a tandem, ovoid and Simon-Heyman capsule brachytherapy. The prescription isodose line for a single brachytherapy treatment (375 cGy) is shown in green, while the 188cGy (dark blue) and 113 cGy (light blue) lines can also be seen. The isodose line in the center of the implant (red) is 750 cGy.
B: Sagittal plane representative isodose lines for a vaginal cylinder brachytherapy fraction. The prescription isodose line for a single brachytherapy fraction (400 cGy) is shown in green, while the 200cGy (dark blue) and 120 cGy (light blue) lines can also be seen. The isodose line in the center of the implant (red) is 800 cGy.
In contrast, stage I endometrial cancer in a medically inoperable patient may be treated with definitive brachytherapy. In these treatments, tandem and ovoid applicators are used similarly to definitive cervical cancer treatment. Radiation dose to the uterine serosa is achieved by the addition of Simon-Heyman capsules (Figure 3A), which are inserted through the dilated cervical os prior to placement of the intrauterine tandem. This technique is particularly useful in newly diagnosed endometrial cancer patients who cannot undergo surgery due to medical co-morbidities including obesity.
Endometrial cancer patients with extrauterine disease, such as a FIGO stage III patient with positive pelvic or para-aortic lymph nodes receive external beam radiation to the pelvis (and para-aortic region if involved) in addition to vaginal cuff brachytherapy. Chemotherapy may be given either prior to or concurrently with radiation depending on patient and pathologic factors.
Vulvar and Vaginal Cancers
Early-stage vulvar and vaginal cancers may be treated with surgical resection, though advanced or bulky tumors warrant a definitive approach of chemotherapy and radiation. These sites are generally not amenable for intracavitary brachytherapy, and in order to safely deliver high radiation doses to the tumor target, interstitial brachytherapy may be a component of treatment planning. In interstitial HDR brachytherapy, catheters are inserted directly into the tumor target while the patient is under conscious sedation. Catheter arrangement is determined by patient anatomy and tumor geometry. After catheter placement, treatment planning is done via a post-procedure CT. The catheters remain in place for four days during which the patient receives two brachytherapy fractions daily at six-hour inter vals. Due to the complexity of both the implantation procedure and treatment planning, patients who are candidates for vaginal or vulvar brachytherapy warrant referral to a tertiary care center specializing in these techniques.
Conclusions
Brachytherapy is one of the cornerstones of treatment for gynecologic malignancies due to the ability to deliver high target doses while minimizing normal tissue toxicity due to rapid dose fall-off. New advances in imaging techniques, 3D adaptive treatment planning, and dose-volume reporting are adding precision and versatility to this powerful treatment tool in the curative treatment for cervical and endometrial cancers.
Biography
Ashley A. Weiner, MD, PhD, and Julie K. Schwarz, MD, PhD, (above) Assistant Professor, are both at Washington University School of Medicine in St. Louis.
Contact: jschwarz@radon.wustl.edu

Footnotes
Disclosure
None reported.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. International journal of radiation oncology, biology, physics. 2013;87:111–9. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Aronowitz JN, Aronowitz SV, Robison RF. Classics in brachytherapy: Margaret Cleaves introduces gynecologic brachytherapy. Brachytherapy. 2007;6:293–7. doi: 10.1016/j.brachy.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Orton CG. High-dose-rate brachytherapy may be radiobiologically superior to low-dose rate due to slow repair of late-responding normal tissue cells. International journal of radiation oncology, biology, physics. 2001;49:183–9. doi: 10.1016/s0360-3016(00)00810-5. [DOI] [PubMed] [Google Scholar]
- 5.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecologic oncology. 1999;73:177–83. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 6.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 7.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 8.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 9.Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2108–13. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- 10.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116:1469–75. doi: 10.1002/cncr.24972. [DOI] [PubMed] [Google Scholar]
- 11.Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:2804–10. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 12.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 13.Petereit DG, Sarkaria JN, Chappell R, Fowler JF, Hartmann TJ, Kinsella TJ, et al. The adverse effect of treatment prolongation in cervical carcinoma. International journal of radiation oncology, biology, physics. 1995;32:1301–7. doi: 10.1016/0360-3016(94)00635-X. [DOI] [PubMed] [Google Scholar]
- 14.Tanderup K, Eifel PJ, Yashar CM, Potter R, Grigsby PW. Curative radiation therapy for locally advanced cervical cancer: brachytherapy is NOT optional. International journal of radiation oncology, biology, physics. 2014;88:537–9. doi: 10.1016/j.ijrobp.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Eifel PJ, Ho A, Khalid N, Erickson B, Owen J. Patterns of radiation therapy practice for patients treated for intact cervical cancer in 2005 to 2007: a quality research in radiation oncology study. International journal of radiation oncology, biology, physics. 2014;89:249–56. doi: 10.1016/j.ijrobp.2013.11.228. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52. doi: 10.1016/j.brachy.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyk P, Jiang N, Sun B, DeWees TA, Fowler KJ, Narra V, et al. Cervical Gross Tumor Volume Dose Predicts Local Control Using Magnetic Resonance Imaging/Diffusion-Weighted Imaging-Guided High-Dose-Rate and Positron Emission Tomography/Computed Tomography-Guided Intensity Modulated Radiation Therapy. International journal of radiation oncology, biology, physics. 2014 doi: 10.1016/j.ijrobp.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Grigsby PW, Portelance L, Williamson JF. High dose ratio (HDR) cervical ring applicator to control bleeding from cervical carcinoma. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2002;12:18–21. doi: 10.1046/j.1525-1438.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 19.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecologic oncology. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 21.Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3547–56. doi: 10.1200/JCO.2008.20.2424. [DOI] [PubMed] [Google Scholar]
- 22.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–23. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 23.Small W, Jr, Beriwal S, Demanes DJ, Dusenbery KE, Eifel P, Erickson B, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff bra. doi: 10.1016/j.brachy.2011.08.005. [DOI] [PubMed] [Google Scholar]