The Alvin J. Siteman Comprehensive Cancer Center opened a first-of-its-kind proton beam radiation therapy (PBT) center in December 2013. The S. Lee Kling Proton Therapy Center is jointly operated by Washington University School of Medicine and Barnes-Jewish Hospital to bring the latest technology to patients in the Midwestern region of the United States (U.S.). The Kling Proton Therapy Center is the 14th proton therapy center to open in the U.S. and is designed and developed by Mevion Medical, Inc. This PBT unit is the first of several smaller, less expensive proton therapy facilities that are scheduled to be installed worldwide. The unique first-of-its-kind features of this unit are; first single-room proton facility worldwide, first gantry-mounted cyclotron, and first proton unit to use a six-degree robotic couch. The rotating gantry function allows the proton beam to enter the room from 190 degrees. The robotic couch allows the patient to be positioned while small adjustments can be made in six dimensions (three translational, three rotational) so that the proton beam can precisely reach the tumor from any angle. The treatment room is shown in Picture 1.
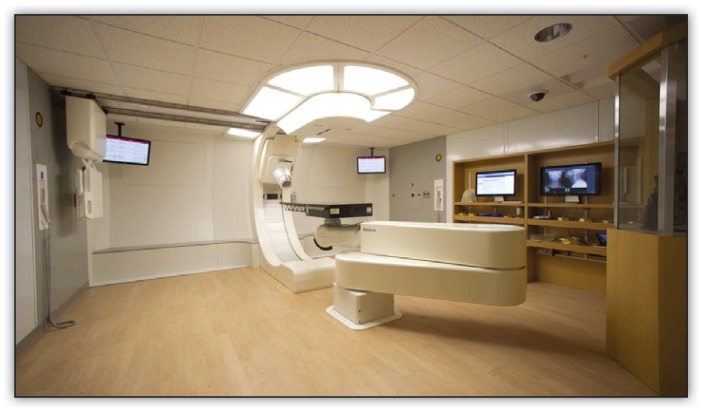
Picture 1.
This picture of the proton treatment room at Barnes-Jewish Hospital shows the inner gantry with applicator, where the protons enter the room, and the six-degree robotic couch.
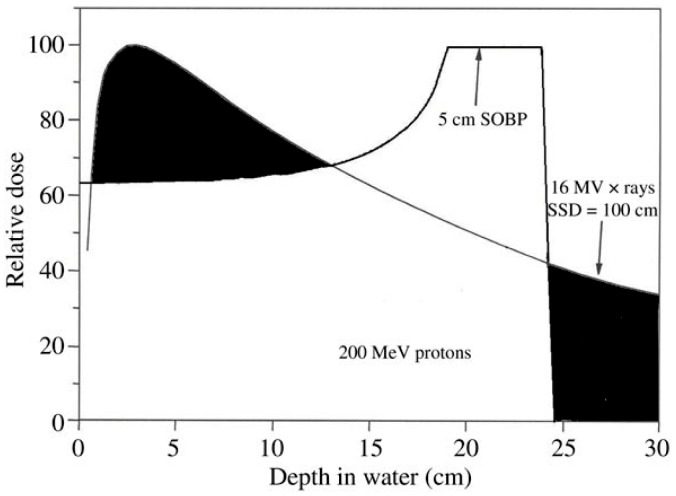
PBT is a useful tool to treat many types of cancer in adults and children. The main advantage to PBT is the ability to precisely localize radiation dose to the tumor, while minimizing dose to adjacent normal tissue. An advantage of PBT compared to x-rays is that protons have a charge and mass. Protons travel a finite distance in tissue, depending on the beam energy (and other factors) and do not have an exit dose deposition beyond the tumor. The scientific term for this feature is called the Bragg peak (see Figure 1), named after physicist William Henry Bragg’s discovery in 1903. A composite of Bragg peaks deposited at various depths delivers a spread-out-Bragg-peak (SBP). Compared to x-rays, protons have a lower entrance dose (to skin) and do not have an exit dose beyond the tumor target. The major advantage to PBT is a reduction in side effects, enabled by the ability to spare adjacent normal tissues.
Figure 1.
Depth-dose curve for x-rays and for protons. As x-rays enter tissue, they have a quick buildup just below the skin surface and deposit radiation dose from entrance through exit. As protons enter the skin surface, they deposit a lower skin dose and build up to deposit most of their dose at the end of their depth range. Protons have no exit dose, which is a major advantage compared to x-rays.
Cancers commonly treated with protons include tumors in locations near critical structures, such as those near the brain, eye, base of skull, spinal cord, major nerve trunks, bowel, and heart. Protons are particularly useful in pediatric patients due to the desire to reduce radiation dose to developing brain or bone, which can lead to long-term cognitive, hormonal, and growth disturbances. In adults, protons can reduce dose to normal brain and the optic structures in patients with brain tumors. In the chest, protons can reduce dose to normal lung and heart. In the abdomen in pelvis, the goals are to reduce dose to bowel, kidneys, liver, and bladder.
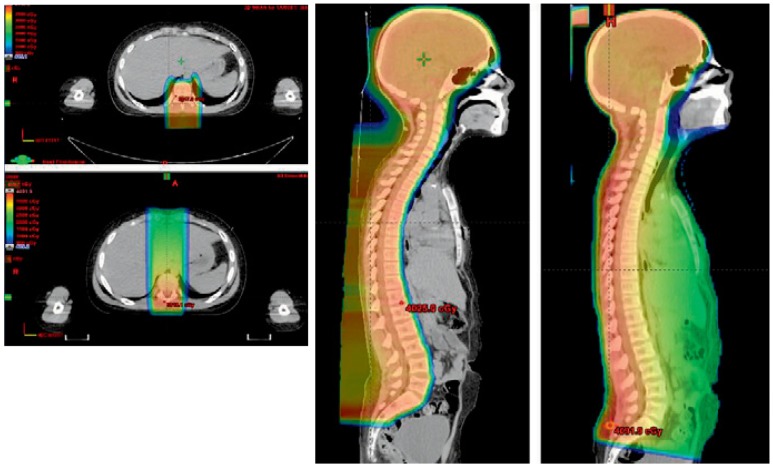
A classic example of the advantage for PBT over x-rays is shown in Figure 2. This image shows the radiation dosimetric plan of a pediatric patient receiving craniospinal radiation following resection of a medulloblastoma brain tumor. Patients with medulloblastoma are at risk for tumor recurrence throughout the craniospinal axis by seeding of the cerebrospinal fluid. In this case, PBT was used to treat both the brain and spinal canal. The advantage for protons is that there is no exit dose and the patient received no dose to the heart, lungs, or bowel.
Figure 2.
This pediatric patent received craniospinal radiation therapy using protons. Both axial and sagittal images for protons and x-rays are shown (Proton plan shown on upper left and leftmost sagittal images). Protons were used to treat both the brain and spine, encompassing all tissues bathed in cerebrospinal fluid for the proton plan compared to the x-ray plan. Note that there is no exit dose within the thorax or abdomen, sparing heart, lungs and bowel from unnecessary radiation.
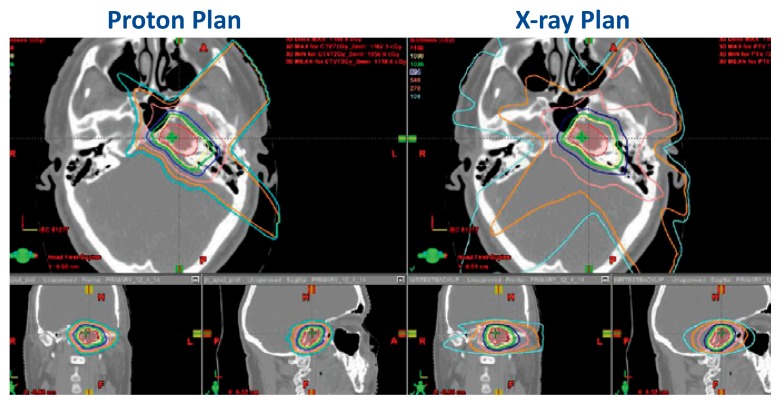
Another example of a plan comparison between protons and x-rays is shown in Figure 3. This is a middle-aged patient who was treated with PBT for a base of skull chondrosarcoma. The patient was initially recommended to have a primary resection which would have included a left orbital exenteration. He sought out other options which included PBT. The proton beam plan is on the left, and the x-ray plan is on the right. The red shaded area is the tumor target. The colored lines represent the radiation isodoses. The PBT plan spared the adjacent brain, left and right eyes, and optic nerves for this patient. This happened to be our very first patient!
Figure 3.
This figure shows a dosimetric plan comparison for PBT versus x-rays. The red shaded area represents the tumor volume of a chondrosarcoma located in the base of skull. The yellow line in both images represents the prescription dose. The other colored lines represent successive decreasing radiation doses that irradiate normal tissues. In this example, the proton beam plan (left) is more conformal to the shape of the tumor and is able to avoid radiation dose to the eyes and brain compared to the x-ray plan.
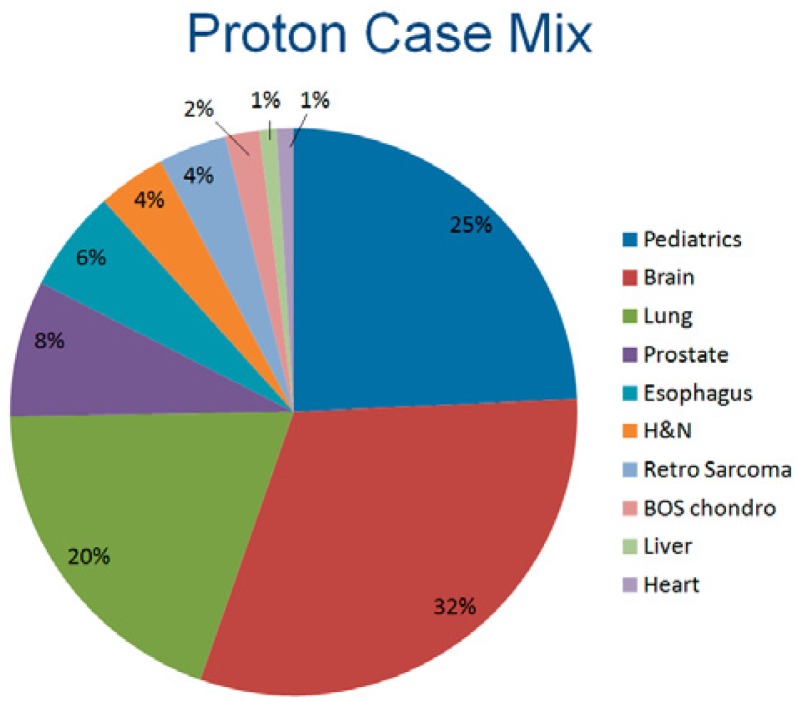
The Kling Proton Therapy Center opened in December 2013. We treated 110 patients during the first year. An example of the types of cancers treated are shown in Figure 4. Approximately 32% of patients have had brain tumors. About 25% of our patients have been children, referred through St. Louis Children’s Hospital and other regional pediatric oncologists. Another 20% have been patients with lung cancer. Our relationship with St. Louis Children’s Hospital is an important one. We have dedicated pediatric oncologists, a pediatric radiation oncologist, pediatric nursing and child-life specialists. Our dedicated staff provide care to our families on a personal level. During the first year of operation, our pediatric patients have come from a six state region to travel here for this specialized treatment.
Figure 4.
This figure shows the proton case mix at the Siteman Cancer Center for the first year of operation, 2014.
An important component of offering PBT is participation in prospective clinical trials. Many insurers cover PBT for classic proton therapy cases, such as for pediatrics, tumors in the brain, base of skull, brain, eye, and spine. However for other types of cancers such as prostate and lung, insurers consider protons still unproven and encourage participation in clinical trials or prospective registries. Each of the following studies are collecting scientific information evaluating the value of PBT. The Siteman Cancer Center houses an open prospective registry for every patient who wishes to participate. This registry will be open to many other proton centers that are planning to open in coming years. Pediatric patients are offered participation in a national pediatric patient registry operated through the Francis Burr Proton Center at Harvard University. We also have dedicated prospective clinical trials that are currently accruing patients with lung, brain, esophagus, and prostate cancers. RTOG 1308 is a phase III randomized trial funded through the National Cancer Institute (NCI) comparing x-rays to protons for patients with unresectable Stage III non-small cell lung cancer. NRG BR001 is an NCI-sponsored phase III randomized clinical trial comparing dose-intensified intensity modulated radiation therapy (IMRT with x-rays) compared to dose-intensified proton beam therapy for patients with glioblastoma multiforme brain tumors. Furthermore, men with low or intermediate-risk prostate cancer may participate in the NCI-sponsored trial entitled Prostate Advanced Radiation Technologies Investigating Quality of Life (PARTiQOL). This randomized trial compares patient-reported quality of life scores for bowel function for patients treated with IMRT versus PBT at participating centers. Due to ongoing interest in evaluating the efficacy and toxicity associated with PBT, other multi-institutional studies are being planned.
Biography
Jeffrey Bradley, MD, FACR, (right), Professor of Radiation Oncology, the S. Kling Endowed Chair of Radiation Oncology, Director, Proton Center, and Chief, Thoracic Service and Eric Klein, PhD, FAAPM, FASTRO, Professor of Radiation Oncology, are both at Washington University School of Medicine in St. Louis. Beth Bottani, CMD, Proton Therapy Manager is at Barnes-Jewish Hospital.
Contact: jbradley@radonc.wustl.edu

Footnotes
Disclosure
J. B. has received a clinical trial grant from ViewRay, Inc. E.K. has received travel reimbursement to scientific meetings from Mevion.