Abstract
Introduction
Adequate persistence to antidiabetic treatment is highly important to achieve proper glycemic control. In this study we evaluate the persistence to treatment with dipeptidyl peptidase-4 inhibitors, sodium-glucose co-transporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists in a nationwide cohort of patients with type 2 diabetes.
Methods
Using a central database in Hungary, we analyzed the persistence to the treatment with dipeptidyl peptidase-4 inhibitors (n = 59,900), sodium-glucose co-transporter-2 inhibitors (n = 26,052), and glucagon-like peptide-1 receptor agonists (n = 17,332) at treatment intensification between 2014 and 2016. We also compared the persistence of dipeptidyl peptidase-4 inhibitors (n = 9163) and sodium-glucose co-transporter-2 inhibitors (n = 1257) in initial therapy to that of metformin (n = 79,305) or sulfonylureas (n = 29,057). The rates of persistence to treatment and risk of non-persistence are reported.
Results
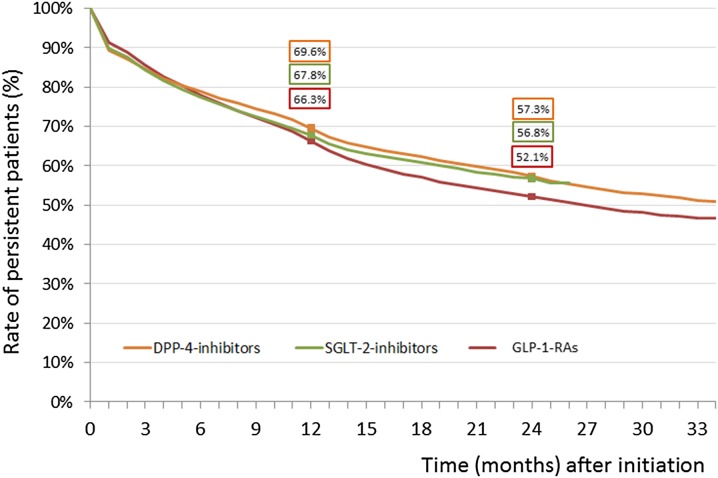
The persistence rates of dipeptidyl peptidase-4 inhibitors, sodium-glucose co-transporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists at treatment intensification were 69.6%, 67.8%, and 66.3% at year 1 which decreased to 57.3%, 56.8%, and 52.1% by year 2, respectively. The risk of non-persistence was higher by 6.6% (95% CI 3.6–9.6) for sodium-glucose co-transporter-2 inhibitors and by 8.3% (95% CI 5.0–11.5) for glucagon-like peptide-1 receptor agonists as compared to dipeptidyl peptidase-4 inhibitors. Novel oral antidiabetic drugs in fixed versus free add-on combinations with metformin had higher persistence. The persistence to treatment with novel oral antidiabetic drugs in initial therapy was better (dipeptidyl peptidase-4 inhibitors, 59.6% and 47.6%; sodium-glucose co-transporter-2 inhibitors, 61.9% and 47.0%) than that of initial monotherapy with metformin (47.0% and 39.1%) or sulfonylureas (52.4% and 41.8%) at years 1 and 2, respectively.
Conclusion
Analysis of persistence of treatment with novel glucose-lowering medications revealed differences between drug classes, favoring dipeptidyl peptidase-4 inhibitors vs. sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists. Persistence data of novel antihyperglycemic agents may be useful for guiding the decision at initiation of antidiabetic treatment.
Funding
Hungarian Diabetes Association.
Plain Language Summary
Plain language summary available for this article.
Keywords: Adherence, Antidiabetic treatment, Dipeptidyl peptidase-4 inhibitors, Glucagon-like peptide-1 receptor agonists, Incretin therapies, Persistence, Sodium-glucose co-transporter-2 inhibitors
Plain Language Summary
Although type 2 diabetes is a very frequent metabolic disease in adulthood and among the elderly, its manifestation is often symptomless. Patients with type 2 diabetes should be treated with glucose-lowering pills or injectable drugs (i.e., insulin or other agents) to achieve normalization of elevated blood glucose values. This treatment with medications alongside lifestyle modification may prevent or at least decrease the risk of late complications due to permanently high blood glucose values which may affect the eyes, kidneys, nerves, and cardiovascular system. Failure of adequate treatment may ultimately lead to increased morbidity (blindness, chronic kidney disease, amputation) and mortality. We care for our diabetic patients regularly for years because medications cannot definitively reverse the metabolic abnormalities. Persistence to treatment (duration of prescribed medication use) is of great importance in the long run as it may affect the outcome of the disease.
In the last couple of years, new and innovative glucose-lowering pills became available. In addition, when glucose levels remain too high there are new options for applying medication by a small needle at the abdominal wall or thighs. We investigated three new glucose-lowering drugs called dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors), sodium-glucose co-transporter-2 inhibitors (SGLT-2 inhibitors) (both are pills), and glucagon-like peptide-1 receptor agonists (GLP-1-RAs, an injectable medication) to assess whether patients follow the physicians’ instructions and use these medications accordingly. We used the database of the National Institute of Health Insurance Fund Management in Hungary. We found that patients were more likely to continue treatment with DPP-4 inhibitors as compared to SGLT-2 inhibitors or GLP-1 RAs. We suggest that it is reasonable for clinicians to consider—among others—persistence data when prescribing novel antidiabetic drugs to patients with type 2 diabetes mellitus.
Introduction
The burden of type 2 diabetes mellitus has been widely recognized. The recent prevalence rate and the estimated increase in incidence of type 2 diabetes create a severe global health problem [1]. The research activity in development of new antidiabetic drugs for treating type 2 diabetes was very successful in the last decade. New classes of drugs (DPP-4 inhibitors, GLP-1-RAs, and SGLT-2 inhibitors) became available and broadened the armamentarium of antidiabetic drugs for treating people with type 2 diabetes [2].
Persistence to treatment is a key element associated with the effectiveness of pharmacological therapies in patients with diabetes mellitus. The antihyperglycemic agents work in different ways to lower blood glucose levels. Besides patients’ characteristics, the mode of administration (oral, injectable), glycemic effect, and potential risks (hypoglycemia, weight gain, cardiovascular safety profile, side effects) of antihyperglycemic agents should be considered when deciding the pharmacological treatment for diabetes [3, 4]. The success of antidiabetic treatment may be influenced by several factors and persistence to treatment should be considered important in this regard [5]. Although persistence to a particular antidiabetic drug depends on the patient’s age, gender, ethnicity, social conditions, and comorbidities [6], specific characteristics of the treatment such as complexity of dosing regimen, safety, tolerability, and cost may also play an important role [7]. Even therapeutic inertia in the antihyperglycemic treatment can be a concern [8].
While several studies were earlier performed to assess the persistence to treatment with traditional oral antidiabetic drugs [metformin (MET) and sulfonylureas (SUs)] [9, 10], such data with novel agents are limited [11–20] and lacking from the Central-Eastern European countries. Therefore, the aim of our study was to evaluate the persistence of prescribed treatment with novel antidiabetic drugs (DPP-4 inhibitors, SGLT-2 inhibitors, and GLP-1-RAs) in a nationwide cohort of people with type 2 diabetes in Hungary.
Methods
In this study we used the database of the National Institute of Health Insurance Fund Management (Hungary). All data were anonymized at data extraction, and we used non-identifiable data at further analyses. In this central database, all antidiabetic drugs prescribed with reimbursement and dispensed in pharmacies nationwide are regularly registered. Actually, a near 3-year-long period from January 1, 2014 was investigated. The persistence of novel antidiabetic drugs was analyzed in two treatment options:
First, we focused on treatment intensification with novel antidiabetic drugs on failure with first treatment option. Accordingly, we captured the new antihyperglycemic agents either in monotherapy (switched from another former drug) or in add-on combinations with different former drugs and the persistence to the treatment with DPP-4 inhibitors (n = 59,900), SGLT-2 inhibitors (n = 26,052), and GLP-1-RAs (n = 17,332) was assessed. In a subanalysis, the persistence of fixed and free add-on combinations of DPP-4 inhibitors with MET (n = 21,167 and n = 1986, respectively) and those of SGLT-2 inhibitors with MET (n = 4286 and n = 2603, respectively) were separately investigated.
Secondly, we focused on the treatment initiation and the persistence of DPP-4 inhibitors (n = 9163) and SGLT-2 inhibitors (n = 1257) was assessed and compared to that of initial monotherapy with MET (n = 79,305) or SUs (n = 29,057).
In Hungary, five DPP-4 inhibitors (sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin) and two SGLT-2 inhibitors (dapagliflozin, empagliflozin) and their fixed combinations with MET are available. As for GLP-1-RAs, exenatide twice daily, exenatide once weekly, liraglutide, and lixisenatide can be prescribed. All these drugs are reimbursed at 70%. As for MET, different preparations exist and five SUs (glibenclamide, gliclazide, gliquidone, glimepiride, glipizide) are available in Hungary, all of which are reimbursed at 55%.
People starting with the particular antidiabetic therapy from January 1, 2014 were enrolled in this study. The year of 2013 was used as reference year in order to detect only the real starting therapy in 2014. For each person, dispensing of the prescribed drugs was followed until October 31, 2016, followed by a permissible gap of 180 days (grace period) until 30 April, 2017.
The persistence curves were obtained by shifting the individual treatments initiated during the entire study period into the zero point. The numerical value of persistence was determined as a percentage of the initial population that remained on therapy in each drug classes [21]. Treatment was considered persistent if the medicine was dispensed throughout the study period irrespective of other antidiabetic drugs, if any. Consequently, treatment was classified non-persistent if a patient started the antidiabetic therapy and then later—including the grace period—did not continue the treatment with the respective antidiabetic drug.
It was determined in advance for how many days the defined dosage forms of the medication would last for a person. On the basis of these results, we calculated for how long the dispensed quantities would be enough. A grace period of 180 days was applied according to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines often used in international literature [22]. The therapy was considered continuous if the individual still had medication 180 days before the end of the study period. The treatment was considered non-persistent if the last dispensed amount of medication had been used up, based on the earlier dosing, and within a further 180 days no repeated dispensing occurred. In other words, persistence was defined as the duration of time from initiation to discontinuation of the particular therapy and a permissible gap of 180 days (grace period) was allowed between the last day on therapy and the next refill of prescription. Analyses with shorter grace periods (120, 90, and 60 days) in our cohort revealed an approximately 3–4% decrease in persistence rate at year 1 and 2 but we decided to remain at the less strict 180-day grace period. Death cases were censored, meaning that they were registered but not counted for persistence analysis.
The numerical value of persistence of the actual drug therapy (the proportion of persistent people in the initial cohort) was determined at two predefined time points (year 1 and year 2, i.e., 12 months and 24 months after the initiation of the medication, respectively). In addition, Kaplan–Meier curves were generated with age- and gender-corrected values of length-of-therapy data and Cox proportional hazard analysis was used for evaluating hazard ratio with 95% confidence interval (HR; 95% CI) of non-persistence.
At treatment intensification we also assessed the persistence to treatment in patients with different age by decades. For statistical analysis we formed three age groups (≤ 50, 51–70, ≥ 71 years) and used the Tukey contrast method for making a comparison.
In this research we used non-identifiable data obtained from the database of the National Institute of Health Insurance Fund Management, Hungary. This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, informed consent was not required. A study license number was needed and provided (S04/168/2017) by the National Institute of Health Insurance Fund Management, Hungary.
Results
The persistence rates of DPP-4 inhibitors, SGLT-2 inhibitors, and GLP-1-RAs at treatment intensification were 69.6%, 67.8%, and 66.3% at year 1 which decreased to 57.3%, 56.8%, and 52.1% by year 2, respectively (Fig. 1). Regarding the entire period (24 months), the risk of non-persistence was significantly higher for SGLT-2 inhibitors (HR 1.066, 95% CI 1.036–1.096; p < 0.0001) and for GLP-1-RAs (HR 1.083, 95% CI 1.050–1.116; p < 0.0001) as compared to DPP-4 inhibitors.
Fig. 1.
Persistence to novel antidiabetic drugs [dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors), sodium glucose co-transporter-2 inhibitors (SGLT-2 inhibitors), glucagon-like peptide-1 receptor agonists (GLP-1-RAs)] used in treatment intensification on failure with the initial drug
At treatment intensification with DPP-4 inhibitors, the proportion of persistent patients was lower in patients aged ≤ 50 vs. ≥ 51 years (Table 1). Regarding the entire period (24 months), the risk of non-persistence was significantly higher in patients aged ≤ 50 vs. 51–70 years (HR 1.259, CI 1.203–1.318; p < 0.0001) but no difference in risk was found in patients aged ≥ 71 vs. ≤ 50 years (HR 1.013, CI 0.971–1.056; p = 0.734). In case of SGLT-2 inhibitors, the persistence was the best in patients aged between 41 and 60 years (Table 1). Regarding the entire period (24 months), there was no difference in risk of non-persistence between patients aged ≤ 50 vs. 51–70 years (HR 0.996, CI 0.927–1.070; p = 0.992) but the risk was higher in patients aged ≥ 71 vs. 51–70 years (HR 1.415, CI 1.290–1.551; p < 0.0001). In patients treated with GLP-1-RAs, the persistence was the best at age between 51 and 70 years (Table 1). Regarding the entire period (24 months), the risk of non-persistence was significantly higher in patients aged ≤ 50 vs. 51–70 years (HR 1.138, CI 1.065–1.217; p < 0.0001) and in patients with aged ≥ 71 vs. 51–70 years (HR 1.353, CI 1.197–1.529; p < 0.0001).
Table 1.
Persistence to treatment (proportion of persistent people according to age decades) with novel antihyperglycemic agents at treatment intensification in patients with type 2 diabetes
| Age (years) | DPP-4 inhibitors | SGLT-2 inhibitors | GLP-1-RAs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | At year 1 (%) | At year 2 (%) | Patients (n) | At year 1 (%) | At year 2 (%) | Patients (n) | At year 1 (%) | At year 2 (%) | |
| ≤ 30 | 291 | 50.9 | 35.4 | 115 | 54.5 | 149 | 43.2 | 33.4 | |
| 31–40 | 1858 | 59.2 | 40.5 | 1054 | 66.3 | 1154 | 62.2 | 42.8 | |
| 41–50 | 6764 | 68.2 | 51.8 | 4279 | 70.0 | 59.0 | 3626 | 67.1 | 48.7 |
| 51–60 | 17,910 | 69.8 | 56.6 | 10,256 | 70.2 | 60.3 | 6776 | 68.0 | 55.4 |
| 61–70 | 20,100 | 71.3 | 60.0 | 8281 | 66.3 | 55.0 | 4580 | 66.8 | 53.7 |
| ≥ 71 | 12,977 | 69.5 | 59.6 | 2067 | 58.0 | 44.9 | 1047 | 57.8 | 49.6 |
| Total | 59,900 | 69.6 | 57.3 | 26,052 | 67.8 | 56.8 | 17,332 | 66.3 | 52.1 |
DPP-4 dipeptidyl peptidase-4, SGLT-2 sodium-glucose co-transporter-2, GLP-1-RAs glucagon-like peptide-1 receptor agonists
Treatment with DPP-4 inhibitors or SGLT-2 inhibitors in fixed versus free add-on combinations with MET had higher persistence rates (Table 2). Regarding the entire period (24 months for DPP-4 inhibitors and 12 months for SGLT-2 inhibitors), the risk of non-persistence was considerably lower for fixed versus free add-on MET combinations with DPP-4 inhibitors (HR 0.549, 95% CI 0.513–0.587; p < 0.0001) or with SGLT-2 inhibitors (HR 0.550, 95% CI 0.498–0.607; p < 0.0001).
Table 2.
Persistence to treatment (proportion of persistent people) with novel antihyperglycemic agents in patients with type 2 diabetes
| Persistence analysis | People (n) | Age (years; x ± SD) | Women (%) | Persistence | |
|---|---|---|---|---|---|
| At year 1 (%) | At year 2 (%) | ||||
| Drugs in monotherapy or combinations after failing with first antihyperglycemic agents | |||||
| DPP-4 inhibitors | 59,900 | 63.6 ± 11.0 | 49.9 | 69.6 | 57.3 |
| SGLT-2 inhibitors | 26,052 | 60.4 ± 9.5 | 48.3 | 67.8 | 56.8 |
| GLP-1-RAs | 17,332 | 57.9 ± 10.0 | 47.3 | 66.3 | 52.1 |
| Drugs with fixed versus add-on free combinations | |||||
| DPP-4 inhibitors + MET fixed combination | 21,167 | 62.7 ± 10.8 | 47.3 | 65.4 | 52.1 |
| DPP-4 inhibitors + MET add-on free combination | 1986 | 65.3 ± 11.1 | 54.6 | 44.6 | 30.9 |
| SGLT-2 inhibitors + MET fixed combination | 4286 | 59.6 ± 9.5 | 47.5 | 75.6 | – |
| SGLT-2 inhibitors + MET add-on free combination | 2603 | 59.5 ± 9.4 | 49.5 | 55.7 | – |
| Drugs in initial treatment | |||||
| DPP inhibitors | 9163 | 59.6 ± 12.9 | 49.5 | 59.6 | 47.6 |
| SGLT-2 inhibitors | 1257 | 56.9 ± 11.1 | 47.6 | 61.9 | 47.0 |
| MET | 79,305 | 60.3 ± 12.8 | 52.5 | 47.0 | 39.1 |
| SUs | 29,057 | 66.4 ± 12.4 | 56.0 | 52.4 | 41.8 |
DPP-4 dipeptidyl peptidase-4, SGLT-2 sodium-glucose co-transporter-2, GLP-1-RAs glucagon-like peptide-1 receptor agonists, MET metformin, SUs sulfonylureas
The persistence rates of treatment with novel oral antidiabetic drugs (DPP-4 inhibitors, SGLT-2 inhibitors) in initial therapy were much better at year 1 and year 2 than those of MET or SUs (Table 2). Regarding the entire period (24 months), the risk of non-persistence was significantly lower for DPP-4 inhibitors versus MET (HR 0.771, 95% CI 0.746–0.797; p < 0.0001) or SUs (HR 0.812, 95% CI 0.782–0.842; p < 0.0001). Similarly, the risk of non-persistence was significantly lower for SGLT-2 inhibitors versus MET (HR 0.706, 95% CI 0.639–0.779; p < 0.0001) or SUs (HR 0.706, 95% CI 0.638–0.781; p < 0.0001).
Discussion
Our study demonstrates that at treatment intensification with novel antidiabetic drugs, DPP-4 inhibitors have the highest persistence rate followed by SGLT-2 inhibitors and GLP-1-RAs. Novel oral antidiabetic drugs in fixed combinations with MET have significantly higher persistence than that of free add-on combinations. The persistence to treatment with novel oral antidiabetic agents (DPP-4 inhibitors, SGLT-2 inhibitors) in initial therapy is much better than that with MET or SUs.
We used a nationwide database to determine persistence to treatment with different antihyperglycemic drug classes. It is widely accepted that pharmacy claims databases are a reliable source of data based on prescription refills. Using such database, one can perform an accurate analysis of persistence to a treatment in chronic diseases such as diabetes [23].
In our analysis we investigated a near 3-year-long period from 2014 to 2017. Although DPP-4 inhibitors became available 8–10 years ago, GLP-1-RAs reached the market later and SGLT-2 inhibitors could be prescribed only in the last couple of years. As our original aim was to perform a comparative analysis in the same period, the timeframe of availability of SGLT-2 inhibitors was taken into account.
In our study we used the term persistence. Although there is no clear distinction in some publications between the terms persistence and adherence, and sometimes they are used interchangeably in the literature, we maintain the term persistence—the duration of medication use—as an important indicator of adherence [24, 25].
We evaluated the rates of persistence to the treatment with particular antihyperglycemic drug classes at prespecified time points but we also assessed the hazard ratios of non-persistence by using persistence curves. Although persistence (or non-persistence) data can easily be followed in our study, we acknowledge that another method (i.e., evaluating proportion of days covered) is still in use in the literature [23].
Although persistence to diabetes medication is of great importance, non-persistence is common and varies according to classes of drugs [26]. Former retrospective database analyses from the USA and Germany documented that DPP-4 inhibitors have better persistence than traditional antidiabetic drugs such as SUs or thiazolidinediones [11, 12]. Similar results were found in another study which used a database from the UK [13]. In 2015, the persistence of oral antidiabetic drugs proved to be suboptimal in a meta-analysis [14]. A study from the USA recently documented a better persistence with the SGLT-2 inhibitor canagliflozin compared to dapagliflozin, GLP-1-RAs, or DPP-4 inhibitors [15]. Another study also from the USA reported on better persistence of initial SGLT-2 inhibitors than newly initiated treatment with SUs [16]. In a recent large comparative study from the UK, persistence to older and newer oral antidiabetic drugs was analyzed and the results suggested that medication class was the major influencing factor in medication persistence [17]. Among the GLP-1-RAs, dulaglutide had better persistence data than that of once-weekly exenatide or liraglutide [18]. To the best of our knowledge, our study is the first report from Central-Eastern Europe demonstrating the persistence rates of all novel antidiabetic drug classes either at treatment intensification or at initial treatment. The data from our region add to the current body of knowledge on persistence of novel antidiabetic drugs.
Regarding our results, two recent real-world studies from the USA are of particular interest. Using the same large database (US Centricity Electronic Medical Records), the authors reported on—among others—the discontinuation rate of DPP-4 inhibitors, GLP-1-RAs, and SGLT-2 inhibitors in patients with type 2 diabetes. When these drugs were used as initial treatment, the discontinuation rates at year 1 were 17%, 20%, and 25% resulting in persistence of 83%, 80%, and 75%, respectively. When these drugs were used as second treatment, the discontinuation rates at year 1 were 18%, 21%, and 25% resulting in persistence of 82%, 79% and 75%, respectively. Although the numbers differ, the ranking order of persistence of these novel antidiabetic drugs is very close to what we found in our study either at treatment intensification or at initial treatment [19]. In another publication the authors reported on the sustainability of desirable glycemic control over 2 years with major second-line antidiabetic therapies. They found that patients with second-line incretin-based drugs (DPP-4 inhibitors, GLP-1-RAs) had higher probability of achieving and sustaining acceptable glycemic control compared with those treated with SUs or insulin [20]. Unfortunately, we could not perform such analysis as no laboratory parameters were recorded in our database. Additionally, our data suggest that age of patients may have some influence on persistence (with DPP-4 inhibitors, persistence was higher in patients aged ≥ 51 vs. ≤ 50 years; with SGLT-2 inhibitors, the worst persistence data was observed in patients aged ≥ 71 years; while with GLP-1-RAs, the best persistence rate was observed in patients aged 51–70 years).
It was earlier documented that management of type 2 diabetes using fixed-dose combinations provides a compliance benefit relative to free add-on combinations [27, 28]. In this regard our study should be considered confirmatory with novel oral antidiabetic drugs with MET combinations.
Our results have to be interpreted within the context of their limitations. In our study no specific data were available about important covariates such as severity of the disease, comorbidities, glycemic control, HbA1c values, BMI, renal function, socioeconomic status, or incidence of side effects (especially hypoglycemic event rates) of ongoing antidiabetic treatment. While we acknowledge the importance of these cofounders, we feel that our results are meaningful for characterizing the persistence of treatment with novel antidiabetic drugs in our region. Nevertheless, the generalizability of our results is limited because the healthcare system and accesses to different treatment options are country-specific. In this regard it is noteworthy that our study was based a nationwide register which should be considered as a strength of the investigation. It is also of note that although the prices of novel drugs in different antidiabetic classes differ, all have the same (70%) reimbursement in our country.
Conclusion
The analysis of persistence of treatment with novel glucose-lowering medications revealed significant differences between drug classes, favoring DPP-4 inhibitors vs. SGLT-2 inhibitors and GLP-1-RAs, and between free and fixed-dose MET combinations, favoring the latter. These data should be useful in clinical practice and might be considered as a factor that help to guide the decision about antidiabetic treatment.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the Hungarian Diabetes Association. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors (G. Jermendy, Z. Kiss, G. Rokszin, Z. Abonyi-Tóth, I. Wittmann, P. Kempler) have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In this research we used non-identifiable data obtained from the database of the National Institute of Health Insurance Fund Management, Hungary. This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, informed consent was not required. A study license number was needed and provided (S04/168/2017) by the National Institute of Health Insurance Fund Management, Hungary.
Data Availability
The database of the National Institute of Health Insurance Fund Management (Hungary) can be accessed for research on reasonable request. The datasets generated during and/or analyzed during the current study are available from GR and ZA-T on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6876722.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Scheen AJ. Pharmacological management of type 2 diabetes: what’s new in 2017? Expert Rev Clin Pharmacol. 2017;10:1383–1394. doi: 10.1080/17512433.2017.1376652. [DOI] [PubMed] [Google Scholar]
- 3.Schnell O, Rydén L, Standl E, Ceriello A, D&CVD EASD Study Group Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2017;2017(16):128. doi: 10.1186/s12933-017-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheen A, Paquot N. Gliptin versus a sulphonylurea as add-on to metformin. Lancet. 2012;380:450–452. doi: 10.1016/S0140-6736(12)60859-9. [DOI] [PubMed] [Google Scholar]
- 5.Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32:725–737. doi: 10.1111/dme.12651. [DOI] [PubMed] [Google Scholar]
- 6.Guénette L, Moisan J, Breton MC, Sirois C, Grégoire JP. Difficulty adhering to antidiabetic treatment: factors associated with persistence and compliance. Diabetes Metab. 2013;39:250–257. doi: 10.1016/j.diabet.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 7.García-Pérez L-E, Álvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(Suppl 5A):27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Jermendy G, Wittmann I, Nagy L, et al. Persistence of initial oral antidiabetic treatment in patients with type 2 diabetes mellitus. Med Sci Monit. 2012;18:CR72–CR77. doi: 10.12659/MSM.882459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31:1287–1305. doi: 10.1007/s12325-014-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathmann W, Kostev K, Gruenberger JB, Dworak M, Bader G, Giani G. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013;15:55–61. doi: 10.1111/j.1463-1326.2012.01674.x. [DOI] [PubMed] [Google Scholar]
- 13.McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–1043. doi: 10.1111/dom.13160. [DOI] [PubMed] [Google Scholar]
- 14.Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31:1283–1296. doi: 10.1185/03007995.2015.1053048. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Divino V, Burudpakdee C. Adherence and persistence in patients with type 2 diabetes mellitus newly initiating canagliflozin, dapagliflozin, DPP-4s, or GLP-1s in the United States. Curr Med Res Opin. 2017;33:1317–1328. doi: 10.1080/03007995.2017.1320277. [DOI] [PubMed] [Google Scholar]
- 16.Bell KF, Cappell K, Liang M, Kong AM. Comparing medication adherence and persistence among patients with type 2 diabetes using sodium-glucose cotransporter 2 inhibitors or sulfonylureas. Am Health Drug Benefits. 2017;10:165–174. [PMC free article] [PubMed] [Google Scholar]
- 17.McGovern A, Hinton W, Calderara S, Munro N, Whyte M, de Lusignan S. A class comparison of medication persistence in people with type 2 diabetes: a retrospective observational study. Diabetes Ther. 2018;9:229–242. doi: 10.1007/s13300-017-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953–961. doi: 10.1111/dom.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the US real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41:69–78. doi: 10.2337/dc17-1414. [DOI] [PubMed] [Google Scholar]
- 20.Montvida O, Shaw JE, Blonde L, Paul SK. Long-term sustainability of glycaemic achievements with second-line antidiabetic therapies in patients with type 2 diabetes: a real-world study. Diabetes Obes Metab. 2018;20:1722–1731. doi: 10.1111/dom.13288. [DOI] [PubMed] [Google Scholar]
- 21.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 22.Barron TI, Bennett K, Feely J. A competing risks prescription refill model of compliance and persistence. Value Health. 2010;13:796–804. doi: 10.1111/j.1524-4733.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;4:1–13. doi: 10.1080/03007995.2018.1477747. [DOI] [PubMed] [Google Scholar]
- 24.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 25.Aronson JK. Compliance, concordance, adherence. Br J Clin Pharmacol. 2007;63:383–384. doi: 10.1111/j.1365-2125.2007.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simard P, Presse N, Roy L, et al. Persistence and adherence to oral antidiabetics: a population-based cohort study. Acta Diabetol. 2015;52:547–556. doi: 10.1007/s00592-014-0692-x. [DOI] [PubMed] [Google Scholar]
- 27.Schernthaner G. Fixed-dose combination therapies in the management of hyperglycaemia in type 2 diabetes: an opportunity to improve adherence and patient care. Diabet Med. 2010;27:739–743. doi: 10.1111/j.1464-5491.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 28.Han S, Iglay K, Davies MJ, Zhang Q, Radican L. Glycemic effectiveness and medication adherence with fixed-dose combination or coadministered dual therapy of antihyperglycemic regimens: a meta-analysis. Curr Med Res Opin. 2012;28:969–977. doi: 10.1185/03007995.2012.684045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database of the National Institute of Health Insurance Fund Management (Hungary) can be accessed for research on reasonable request. The datasets generated during and/or analyzed during the current study are available from GR and ZA-T on reasonable request.