Abstract
Introduction
Dulaglutide (Dula) is a once-weekly glucagon-like peptide-1 receptor agonist that efficiently reduces the level of glycated hemoglobin (HbA1c) in patients with type 2 diabetes (T2D). However, the durability of the glucose-lowering effect of the first injection of Dula (1st Dula) remains unclear.
Methods
This study had a retrospective, single-center, and single-arm design in a clinical setting and was conducted between April 2016 and March 2017. We investigated the changes and fluctuations in glucose level in 15 patients with T2D using a continuous glucose monitor, from 1 day before the first administration of Dula to 6 days thereafter.
Results
The mean glucose levels decreased significantly from 1 day before 1st Dula up to 5 days thereafter, whereas the standard deviation, mean amplitude of glucose excursion, and percentage of the glucose levels > 180 mg/dL were significantly improved only up to 3, 2, and 3 days after the 1st Dula, respectively, compared to those before administration.
Conclusion
The effect of blood glucose regulation after the 1st Dula did not continue for a whole week. These effects should be considered when adjusting for other hypoglycemic agents.
Electronic supplementary material
The online version of this article (10.1007/s13300-018-0474-5) contains supplementary material, which is available to authorized users.
Keywords: Continuous glucose monitoring, Dulaglutide, Type 2 diabetes
Introduction
Dulaglutide (Dula) is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1RA) that mimics the activation of endogenous GLP-1 [1], Its durable effects allow the frequency of injection to be reduced to once weekly. Dula also shows dose-dependent effects on both glycated hemogloblin (HbA1c) levels and body weight reduction [2], with its effect on the reduction of HbA1c levels found to be equal to that of liraglutide (Lira), a daily GLP-1RA [3]. For example, in phase 3 studies in Japanese patients with type 2 diabetes (T2D), the changes in HbA1c within 52 weeks after the administration of weekly Dula and daily Lira were − 1.39 ± 0.06% (mean ± standard error) and −1.19 ± 0.08%, respectively [3]. In another study on oral hypoglycemic agent (OHA)-naïve T2D patients, changes in HbA1c within 52 weeks after the administration of one-weekly Dula and daily Lira were − 1.45 ± 0.07% and − 1.21 ± 0.10%, respectively, and no cases of severe hypoglycemia were reported [4]. Notably, while Dula reduced body weight (BW) within 26 weeks of administration, insulin glargine increased it within the same time span [5]. Despite clinical studies reporting on the long-term effects of Dula, the durability of its glucose-lowering effect on first administration (1st Dula) has not yet been reported. We have therefore examined the changes and fluctuations in the levels of glucose within 1 week after the 1st Dula to determine both the immediate and persistent effects of Dula.
Methods
Study Design and Ethics
The present study had a retrospective, single-center, and single-arm design in a clinical setting. It was conducted at Center Hospital, National Center for Global Health and Medicine between April 2016 and March 2017.
All procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments and with the “Ethical guideline for medical and health research involving human subjects” published from the Ministry of Health, Labour and Welfare of Japan (Tokyo, Japan). The study protocol was approved by the ethical committee of the National Center for Global Health and Medicine (ID: NCGM-G-00247-00). Since this study was a retrospective observational study that used non-identifiable data obtained by the treating physicians, our ethical committee decided that it was not mandatory to register the study in the national database for clinical trials.
Participants and Study Assessment
Fifteen T2D patients who were admitted in our hospital for > 7 days between April 2016 and March 2017 participated in the study. They received dietary therapy during their hospitalization, and their daily calorie intake was calculated using the formula “ideal weight × 30 kcal.” Participants were instructed to self-monitor their blood glucose level at least before each meal and before going to bed. Treatment with dipeptidyl peptidase 4 (DPP-4) inhibitors was discontinued from 1 day before the 1st Dula and, if necessary, the dosage of insulin was adjusted to target the fasting blood glucose level of each patient individually (100–130 mg/dL). Patients with a serum C-peptide level of < 0.5 ng/mL were excluded from the study. Prior to the 1st Dula, we obtained values for the relevant metabolic parameters, such as BW, body mass index (BMI), HbA1c, glycoalbumin, serum C-peptide, and total daily dose of insulin (TDD) when the patients were treated with insulin. We also used a continuous glucose monitor (CGM) (CGM-Gold®, Medtronic, Dublin, Ireland) from 1 day before to 6 days after the 1st Dula to obtain data related to glucose fluctuation, such as average glucose levels, standard deviation (SD), mean amplitude of glycemic excursions (MAGE) [6], and the percentage of below and above the target range (70–180 mg/dL) of glucose (Electronic Supplementary Material [ESM] Fig. S1).
Statistical Analysis
Data are presented as median values and interquartile range (IQR). Changes in average glucose levels, SD, MAGE, and the percentage of below and above the target glucose range after the 1st Dula were compared with those 1 day before its administration. The Wilcoxon hazard test was used to test the difference between the two groups of values. For the analysis, a P of < 0.05 was considered to be statistically significant. Statistical analysis was determined using STATA software, version 14.0 (StataCorp LP, College Station, TX, USA).
Results
Baseline Characteristics
The baseline characteristics of the 15 patients with T2D who participated in this study are presented in Table 1. At baseline the median patient age was 74 (IQR 60–77) years, duration of diabetes was 17.0 (IQR 3.0–26.0) years, BW was 63.5 (IQR 52.0–82.6) kg, BMI was 26.2 (IQR 23.7–30.3) kg/m2, HbA1c was 9.4% (IQR 8.7–11.7%), and fasting serum C-peptide level was 2.23 (IQR 1.41–2.61) ng/mL, indicating that, on average, the participants tended to be overweight, and their endogenous insulin secretion was relatively preserved. Of the 15 patients, 14 were injected with insulin at baseline, and the TDD was 40.0 (IQR 15.0–45.0) units/day.
Table 1.
Clinical characteristics of the 15 patients with type 2 diabetes participating in the study
| Baseline clinical characteristics | Values |
|---|---|
| Sex (male/female) | 5/10 |
| Age (years) | 74.0 (60.0–77.0) |
| Diabetes duration (years) | 17.0 (3.0–26.0) |
| BW (kg) | 63.5 (52.0–82.6) |
| BMI (kg/m2) | 26.2 (23.7–30.3) |
| HbA1c (%) | 9.4 (8.7–11.7) |
| GA (%) | 25.9 (23.1–32.3) |
| Fasting serum C-peptide (ng/mL) | 2.33 (1.41–2.61) |
| TDD (units/day) | 40.0 (15.0–45.0) |
| OHA administration | |
| DPP-4i/BG/SGLT-2i/a-GI/Glinide/SU (Number of participants) | 7/2/1/3/3/1 |
Data are shown as the median value with the IQR in parenthesis, with the exception of Sex, which is shown as a number
BW body weight, BMI body mass index, GA glycoalbumin, TDD total daily insulin dose, OHA oral hypoglycemic agents, DPP-4i dipeptidyl peptidase-4 inhibitor, BG biguanide, SGLT-2i sodium glucose co-transportor-2 inhibitor, SU sulfonylurea
Durability of Glucose-Lowering Effect of the 1st Dula
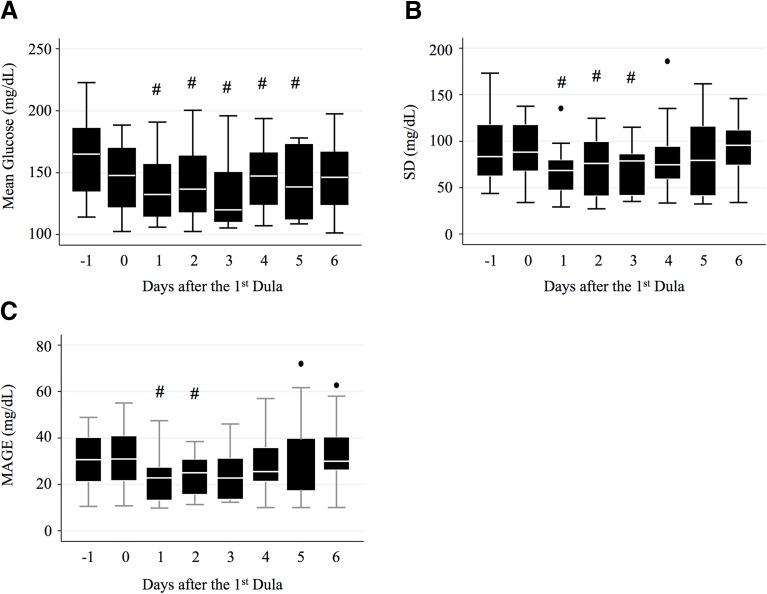
All of the 15 participants tolerated the administration of 0.75 mg of Dula. After starting Dula, the dosage of insulin was adjusted for each patient to target glucose levels of 100–130 mg/dL before each meal. Changes in the dosage of insulin are shown in ESM Fig. S2. DPP-4 inhibitors had been discontinued since 1 day before the 1st Dula. Compared to the mean glucose levels 1 day before the 1st Dula, mean glucose levels on days 1, 2, 3, 4, and 5 after the 1st Dula were significantly lower (P < 0.05 on each day) based on analysis of the CGM data (Fig. 1a). On the other hand, the SD of glucose and MAGE were significantly decreased only up to 3 and 2 days after the 1st Dula, respectively, compared to the respective values 1 day before administration the 1st Dula (Fig. 1b, c). Additionally, the percentage of patients with glucose levels of > 180 mg/dL were also significantly improved only up to 3 days (P < 0.05) after the 1st Dula (Table 2). No patient had a glucose level of < 70 mg/dL (Table 2).
Fig. 1.
Boxplot of changes in mean glucose levels (a), standard deviation (SD) of glucose levels (b), and mean amplitude of glycemic excursions (MAGE; c) after the first administration of dulaglutide (1st Dula). #P < 0.05 compared to the values of day 1 after the 1st Dula. Black dots indicate the values outside the error bars
Table 2.
Changes in the percentage of glucose levels below and above the target range
| Glucose valuesa | Study dayb | |||||||
|---|---|---|---|---|---|---|---|---|
| Day − 1 | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| % < 70 mg/dL | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| % > 180 mg/dL | 30 (2–55) | 27 (9–33) | 5 (0–30)# | 13 (0–23)# | 5 (1–39)# | 9 (4–32) | 10 (1–37) | 9 (2–41) |
Data are shown as median with the IQR in parenthesis
#P< 00.05 compared to the value on Day − 1
a% < 70 mg/dL, Percentage of glucose levels below 70 mg/dL; % > 180 mg/dL, percentage of glucose levels above 180 mg/dL.
bDay 0 indicates the day of the 1st administration of Dulaglutide
Discussion
Dulaglutide is a once-weekly GLP-1RA that reduces HbA1c and BW [2]. Dula was also been shown to improve glucose variability and reduce the number of insulin injections after four Dula injections, indicating that the concentration of Dula reached steady state after four weeks in the T2D patients in that study [7]. In the present study, we demonstrated the durability of the glucose-lowering effect of the 1st Dula for the first time using CGM in the clinical setting. In our study, the mean glucose levels and the parameters for glucose fluctuation, such as SD and MAGE, were significantly decreased even at 1 day after the 1st Dula, indicating that Dula exhibited a rapid glucose-lowering effect despite it being a once-weekly agent. Dula is a large molecule (with a molecular weight of approx. 63 kDa) consisting of two DPP-4-protected GLP-1 analogs covalently linked to a human immunoglobulin (Ig) G4-fragment crystallizable (Fc) heavy chain [1]. As a result of this engineering, Dula shows a flat prolonged concentration–time curve. For example, the authors of one study reported that following the administration of a 0.75 mg dose of Dula, the area under the plasma concentration–time curve from time zero to the end of the dosing interval at 168 h was 6730 (ng h/mL), and the apparent total body clearance of drug calculated after extra-vascular administration was 0.111 L/h [8]. In that study and another study, Dula was observed to reach its maximum concentration approximately 48 h after administration [3, 8]. Based on these results, the glucose-lowering effects of Dula appear to be effective even before it reaches its maximum concentration.
However, in our study, the significant reductions in the mean glucose level, SD, and MAGE were not maintained for a whole week after the 1st Dula. In previous pharmacokinetic analyses, Dula had a terminal elimination half-life of 4.5–5 days [3, 8], and steady state was achieved between the second and fourth doses [8]. The durability of the glucose-lowering effects in our study may be associated with the half-life of Dula. The lack of durability of the 1st Dula dose should be considered when adjusting for other OHAs.
In terms of drug safety, all 15 participants tolerated the 0.75 mg Dula dose and did not have any severe gastrointestinal symptoms. Notably, none of the patients complained of hypoglycemic symptoms and none showed glucose levels of < 70 mg/dL according to the CGM throughout the study period even with the combination of insulin therapy. These data are compatible with those from an earlier study showing that patients receiving the combination of basal insulin with a GLP-1RA showed lower glucose variability and fewer hypoglycemic events that those receiving the combination of basal insulin with oral drugs, premixed insulin, and basal-bolus insulin therapy, respectively [9].
There are a number of limitations to our study. First, the treatment pattern of the participants prior to the study was heterogeneous. Second, there was no wash-out period for DPP4 inhibitor users in this study. Of the 15 patients, nine were administrated DPP-4 inhibitors until 1 day before the initiation of the 1st Dula. It is possible that the overlapping of DPP-4 inhibitor dose and Dula dose may have helped maintain the glucose-lowering effect. Third, although we targeted glucose levels of 100–130 mg/dL before each meal, we did not use any strict algorithm for glucose adjustment. In addition, the dosage of insulin was adjusted according to the glucose levels during the study period. However, the effect of GLP-1RAs on the reduction of insulin doses has already confirmed in several clinical studies [7, 10], and such effects are remarkable, particularly after repeated administrations of Dula [7]. Therefore, despite these limitations, our data are important in determining the clinical effects of this agent. In future, prospective studies with more strict inclusion criteria, the duration of T2D and other relevant parameters based on the usage of OHAs should be examined.
Conclusion
Although the 1st Dula displayed rapid glucose-lowering effect, its regulation of the blood glucose level was not maintained for a whole week. These effects should be considered in the adjustment for other hypoglycemic agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S2 Changes in the total daily dose of insulin after the 1st Dula (PDF 33 kb)
Acknowledgements
We thank all of the investigators and patients who took part in this study. We also thank Takehiro Sugiyama, MD, PhD, for assistance in the statistical analyses.
Funding
No funding or sponsorship were received for this study or publication of this article. The article charges processing were funded by the authors. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
We’d like to thank Editage (Tokyo, Japan; http://www.editage.jp) for English language editing. This was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Maya Matsushita, Daisuke Chujo, Mie Tonoike, and Hiroshi Kajio have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This research used non-identifiable data obtained by the treating physicians and therefore on the basis of the decision from our local ethics committee of National Center for Global Health and Medicine (ID: NCGM-G-00247-00), informed consent was not required. Patients have the opportunity to object to the use of their data for retrospective scientific research, but none of the patients did.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6803789.
References
- 1.Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287–296. doi: 10.1002/dmrr.1080. [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Pantalone KM, Kwan AY, et al. Relationship between weight change and glycaemic control in patients with type 2 diabetes receiving once-weekly dulaglutide treatment. Diabetes Obes Metab. 2016;18:615–622. doi: 10.1111/dom.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyagawa J, Odawara M, Takamura T, et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;17:974–983. doi: 10.1111/dom.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odawara M, Miyagawa J, Iwamoto N, et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab. 2016;18:249–257. doi: 10.1111/dom.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki E, Inagaki N, Tanizawa Y, et al. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17:994–1002. doi: 10.1111/dom.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;15:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 7.Yajima Takahiro, Yajima Kumiko, Hayashi Makoto, Takahashi Hiroshi, Yasuda Keigo. Improved glycemic control with once-weekly dulaglutide in addition to insulin therapy in type 2 diabetes mellitus patients on hemodialysis evaluated by continuous glucose monitoring. Journal of Diabetes and its Complications. 2018;32(3):310–315. doi: 10.1016/j.jdiacomp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Geiser JS, Heathman MA, Cui X, et al. Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analyses of data from clinical trials. Clin Pharmacokinet. 2016;55:625–643. doi: 10.1007/s40262-015-0338-3. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj HS, Venn K, Ye C, et al. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION Study) Diabetes Care. 2017;40:194–200. doi: 10.2337/dc16-1582. [DOI] [PubMed] [Google Scholar]
- 10.Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7:565–573. doi: 10.1111/jdi.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2 Changes in the total daily dose of insulin after the 1st Dula (PDF 33 kb)
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.