Abstract
While the phrase ‘foraging bumblebee’ brings to mind a bumbling bee flying flower to flower in a sunny meadow, foraging is a complicated series of behaviors such as: locating a floral patch; selecting a flower-type; learning handling skills for pollen and nectar extraction; determining when to move-on from a patch; learning within-patch paths (traplining); and learning efficient hive-to-patch routes (spatial navigation). Thus the term ‘forager’ encompasses multiple distinct behaviors that rely on different sensory modalities. Despite a robust literature on bumblebee foraging behavior, few studies are directly relevant to sensory-guided search; i.e. how workers locate novel patches. The first step in answering this question is to determine what sensory information is available to searching bumblebees. This manuscript presents a computational model that elucidates the relative frequency of visual and olfactory cues that are available to workers searching for floral resources under a range of ecologically relevant scenarios. Model results indicate that odor is the most common sensory cue encountered during search flights. When the likelihood of odor-plume contact is higher, odor-encounter is ubiquitous. While integrative (visual + olfactory) cues are common when foragers are searching for larger flowers (e.g. Echinacea), they become rare when foragers are searching for small flowers (e.g. Penstemon). Visual cues are only encountered in isolation when foragers are seeking large flowers with a low odor-plume contact probability. These results indicate that despite the multisensory nature of floral signals, different modalities may be encountered in isolation during search-behavior, as opposed to the reliably multimodal signals encountered during patch-exploitation or nectar/ pollen acquisition.
Introduction
Bumblebee populations are sensitive to decreases in foraging efficiency
Bumblebees are critical pollinators in both agricultural and native ecosystems1–3. Unfortunately these keystone species have experienced alarming declines alongside the highly publicized drops in honeybee numbers4–7. Critical work exposing the negative effects of neonicitinoid pesticides on bumblebee fitness indicates that pesticide exposure lowers rates of reproduction due, at least in part, to a drop in foraging efficacy of both workers and the colony as a whole8,9. This provides a critical link showing that the modification of worker behavior scales up to impact colony level fitness – a result that is consistent with seminal work showing that a colony’s ability to produce reproductive individuals is directly correlated with their size10. Better foragers provide more resources to rear young at the hive, which can increase the size of a colony during a foraging season. Given the current environmental pressures on bumblebees, developing a deeper understanding of their foraging behavior is relevant to conservation efforts.
How do foragers search for flowers?
While the term “forager” can be defined as an animal locating and consuming food resources, it is a complicated series of behaviors. In bumblebees this includes: locating a floral patch11,12; selecting a flower-type13,14; learning handling skills for pollen and nectar extraction15; determining when to move-on from a patch16,17; learning within-patch paths (traplining)12,18; and learning efficient hive-to-patch routes (spatial navigation)11,12,19. Thus the term ‘forager’ encompasses multiple distinct behaviors that rely on different sensory modalities20–22. A critical component of foraging theory is the search phase23; which would be floral patch location in the case of pollinators. This phase is comprised of: (1) movement through the environment; and (2) recognition of resources, which should terminate the search. There is a wealth of literature analyzing forager search paths, from bumblebees to albatrosses24–27. While there is some controversy over the precise algorithms that accurately describe these search paths28–33, there is consensus that search paths can be reasonably represented with stochastic models of forward-biased motion (i.e. while turning events happen, complete direction reversal will be rare). Once a searching forager recognizes a resource, their behavior should transition from random-search to approach and feeding. In bumble bees the ability to recognize floral resources will be dependent upon perception of floral signals. Flowers provide complex sensory displays, including color, shape, nectar guides, odor and morphology34–37. In the case of pollinators searching for novel patches, only those sensory cues capable of operating at a distance will factor into recognition and subsequent sensory-guided navigation. Morphological cues are only relevant upon physical contact with flower and are thus not useful for search. Complex patterns on flowers, such as nectar guides or visible stamens, are only resolvable at close distances (4–45 cm)38. Thus shape, color and odor are the sensory signals most likely to be available for patch recognition.
Odor pollution impacts forager behavior, but the effects on foraging efficiency are unclear
Several studies over the past decade have indicated that anthropogenic odor pollution is both modifying floral odor plumes39 and subsequent behavioral responses of bees40,41. While this work is interesting from a neuroethological standpoint, it is currently unclear how drastically natural foraging populations are impacted by odor pollution. Understanding the potential impact of odor-pollution first requires an understanding of odor’s role in foraging.
There is a substantial body of work indicating that olfaction is important in patch exploitation; however, the precise role that odor plays is not completely understood. PER studies indicate that bumblebees are capable of associative odor learning42–44, generating the logical hypothesis that floral odor could be used to identify rewarding flowers. Multimodal studies investigating both vision and olfaction indicate that stimulation of odor pathways improves foraging accuracy, regardless of whether or not floral signals have differentiating odor stimuli45. Field experimentation on floral morphs showed that bumblebees prioritized visitation of a learned visual (color) signal over the learned odor46. These findings might imply that any odor is effective, and that precise odor identity might be irrelevant. However, work by Leonard et al. showed that when flowers differ in both visual and olfactory modalities, foraging accuracy was higher35 – pushing back against the idea that odor identity is unimportant. Social odor cues – tarsal scent deposits on flowers, reduce bumblebee visitation rates. This is an example of a ‘contaminating odor’ that increases energy gain by reducing visitation to recently emptied flowers. It is likely that scent marks are perceptually distinct from the floral odor, rather than modifying the floral blend-structure such that it becomes unrecognizable to the bumblebee, as behavioral data have been relatively consistent across multiple flower species47 and with unscented artificial flowers48. Therefore, it appears that the precise odor identification of tarsal scent-marks is quite important to foraging behavior. Given the contradictory nature of current data on odor usage, it is difficult to predict the effects of pollution on foraging efficiency during patch exploitation.
There is a paucity of work looking at the impact of odor on navigation to food resources in bumblebees. Several lab-based studies indicate that odor alone is sufficient to facilitate navigation40,49. However, the relative roles of odor and vision (which could have implications for how drastic the effects of odor pollution might be) have never been investigated at a spatial scale that would shed light on the role of odor in patch location. For example, lab studies are typically in arenas that are less than 3.6 m in their largest dimension13,35,40,48,50–59. However, the foraging range of a bumblebee can reach up to 1.75 km from their nest60– a distance that is orders of magnitude larger than typical sensory-behavior studies, even those that are based in the field46. An understanding of odor-pollution’s impacts requires a better understanding of the relative roles that vision and olfaction play in navigation to floral resources. If a searching-forager is consistently encountering an odor signal before a visual signal, it stands to reason that odor-guided navigation will bring that animal within visual range of a flower. Given that odor plumes are theoretically available at a much greater distance from a flower39,61 than visual cues38,62 this is a logical assumption. However, odor plume contact is stochastic, and some empirical measurements of odor-plumes indicate much shorter distances travelled63. This manuscript presents a computational model that moves beyond assumptions and asks – given the probabilistic nature of odor plume contact- what is the likelihood of a bumblebee encountering resolvable visual versus olfactory cues?
Methods
In order to determine which sensory cues are available to searching foragers this model creates a random search path for a bumblebee through a simulated meadow and at each step assesses whether or not the bumblebee has encountered a resolvable visual or olfactory cue from flowers populating the meadow. Meadow dimensions (70 m × 160 m) were based upon Google-satellite images of a clearing at Conrad W. Raker Sanctuary, a biological field station owned by Muhlenberg College (Fig. 1).
Figure 1.
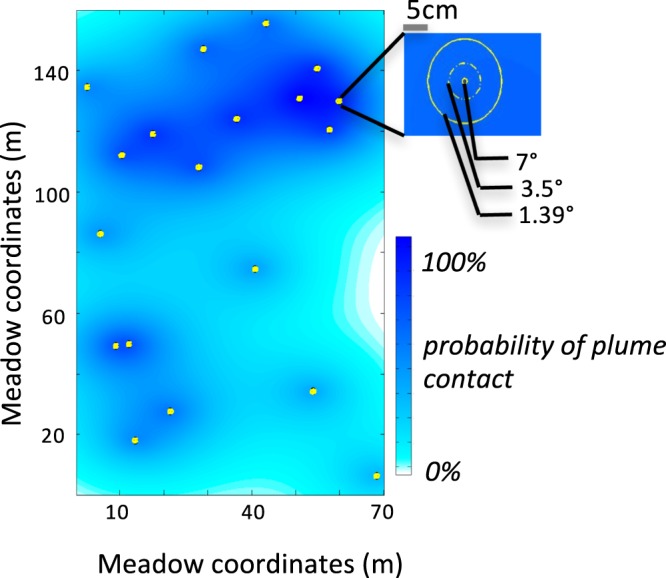
Sensory construction of the artificial meadow that ‘bumblebees’ searched within. The digitized meadow from a scenario utilizing single-flower Echinacea plants, a plant density of 1/600 m2, and a high odor probability. The probability of odor contact is represented by the blue contours. The visual resolvability is represented by the yellow circles; the inset labels the relationship between angular resolution of the searching bumblebee and the distance at which a flower becomes visible. The finest resolution (1.39 degrees) results in the greatest visual detection distance.
Bumblebee Movement
This model generated a search path for bumblebees in order to walk them through a digital meadow until they encountered a salient and resolvable floral sensory cue. These computational paths utilized a correlated random walk model (CRW) to generate motion in the latitude-longitude plane:
where: α is the current heading angle. β is the turning angle, where the probability of turning is based upon digitization of Heinrich’s canonical observations of bumblebee foraging behavior64 (Table 1). T is the time step – an iterative and scale-less variable whose actual value is represented by step length. x(t + T) gives the longitudinal position of the bumblebee in the next time step. y(t + T) gives the latitudinal position of the bumblebee in the next time step. l(t) is a step length of 0.3 m; determined by the product of bumblebee flight speed (3 m/s65) and the interspike interval of motor neurons (approx. 0.1 s66). This represents a reasonable estimate of how rapidly the flight system could change course.
Table 1.
Turning probability digitized from Fig. 3 in Heinrich64.
| Turn angle (degrees) | Probability (0–1) |
|---|---|
| −135 | 0.025 |
| −90 | 0.065 |
| −45 | 0.19 |
| 0 | 0.44 |
| 45 | 0.19 |
| 90 | 0.065 |
| 135 | 0.025 |
This method of search-path computation deviates slightly from the more commonly-referenced Levy walk19,24,26–28,67,68 in that the step length is constant, rather than pulled from a power-law distribution29. Maintaining this constant step length allows the model to be tightly parameterized to known flight-speed measurements (as in Becher et al.69) rather than incorporate occasional large step lengths that imply biologically implausible flight speeds. Work by James et al. indicates that efficiency in resource location by searching foragers has little to do with the search algorithm and is predominantly driven by the density of food resources70; if so the use of a CRW in this model should not corrupt the results. In addition, the basis for Levy-flights/walks being a behaviorally accurate method of modelling forager-search behavior has recently been called into question31,33. However, given the prevalence of Levy flights in foraging literature, it is worthwhile to confirm that using this method would not significantly modify conclusions about floral sensory encounter. Thus, a subset of model-conditions were run with variable step lengths drawn from a Levy Distribution67:
where: P(l) is the probability of a particular step length. l is the corresponding step length. l0 is the minimum step length, set to 0.3 m (see justification above). μ is an exponential constant such that if it is between 1 and 3 the distribution meets the requirements for a Levy flight/walk. In this case it is set to 2, which produces an optimal search strategy71.
Individual model runs started with bumblebees entering the meadow at a randomized edge location – mimicking arriving at the meadow from adjacent wooded territory. In all cases individual model runs continued until the bumblebee encountered a resolvable sensory signal (see Sensory Performance of Bumblebees) or completed 5000 steps – the equivalent of 1.5 km in the CRW, a value selected because it falls in the upper range of measured foraging distances60. Given the variable step lengths in the Levy-flight condition, each model run has a unique potential maximum distance travelled; the mean for 1000 runs is 3 km.
Because this model ends its runs upon floral-signal encounter, it is investigating what sensory information is available to searching bees and does not explicitly simulate floral approach. However, given that the visual acuity measurements are derived from behavioral rather than physiological experiments38,62 bumblebees should be able to visually navigate to a “found” flower. Likewise, there is a body of work indicating that bumblebees are capable of using odor cues at a distance to locate food resources40,49. It is therefore plausible that searching bumblebees would be capable of acting upon salient and resolvable sensory stimuli; i.e. workers would be able to pick up where the model leaves off. This model does not model floral approach because it is outside the scope of our current question.
Floral Parameters
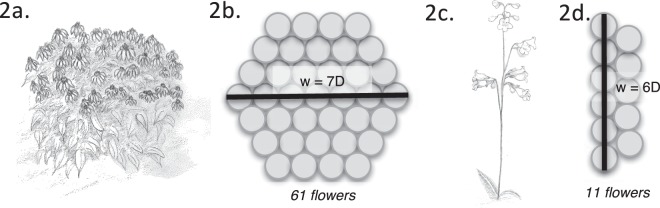
Given that bumblebees are likely to encounter environmental variation in the field, the model varied plant density, plant size, and inflorescence size. Floral parameters were based upon published data from Echinacea and Penstemon spp, two common native genuses with wide ranging distributions that are readily pollinated by bumblebees72,73. These species provide an ecologically relevant range of bloom sizes, ranging from 0.7 cm diameter (Penstemon74) to 7.6 cm (Echinacea). Previous work on Echinacea spp indicates a wide range of naturally occurring densities (0.001 to 3 plants/m2, estimated from nearest neighbor data in Wagenius and Lyon72). For the purpose of this study, which is interested in navigation to novel patches, I tested a realistic range of low densities (i.e. situations where the next nearest patch was not likely to be within visual range of the first): 1 plant/6 m2 (0.17), 1 plant/60 m2 (0.017), and 1 plant/ 600 m2 (0.0017). Field data on Penstemon indicated higher density tendencies, with a range of 0.16–1.64 plants/m2. Thus the total set of tested densities was 0.0017, 0.017, 0.17, 0.89, 1.64. Floral patches were then randomly distributed throughout the meadow based upon the overall density (the number of plants per square meter). A brief survey of Echinacea purpurea plants revealed a high variability in number of blooms per plant (3–62). Therefore, I tested five different display sizes based on number of observed flowers: 1, 7, 19, 37, and 61. To determine the diameter of these displays flowers were polygon packed, resulting in diameters of 0.076, 0.228, 0.38, 0.532, and 0.684 meters respectively (Fig. 2a,b). Penstemon plants have a different growth habit than Echinacea, presenting flowers vertically on spikes; therefore, I used a two line packing of multiple blooms to estimate display size from the largest dimension (Fig. 2c,d). Recent work has shown the mean daily number of flowers for Penstemon digitalis to be 575– the tested number of flowers were 3, 5, 7, 9, and 11 to encompass a range around this mean; resulting in display sizes of 0.014, 0.021, 0.028, 0.035, and 0.042 meters respectively.
Figure 2.
The relationship between flower number and the size of visual stimulus (w). The morphology of Echinacea plants (a) lends itself to polygon packing of blooms (b) while the upright habit of Penstemon (c) makes a double row arrangement (d) a more logical choice for that species.
Sensory Performance of Bumblebees
Bumblebee size has a marked effect on visual acuity62. Given the large variability in worker size that is likely to occur in natural populations, the model was tested with three different visual acuity values (1.39, 3.5, and 7 degrees) representing the range of values in the literature for two bumblebee species (Bombus terrestris62 and Bombus impatiens38). Flowers were considered to be resolvable if their angular size from the bumblebee’s current position was equal to or greater than the visual acuity value. Angles were calculated as:
where: θ is the angle subtended by the floral display. w is the width of the floral display. D is the bumblebee’s distance to the floral display calculated via the Pythagorean theorem.
While the CRW generates planar motion, bumblebees clearly forage in three dimensional environments. Therefore, distance calculations assumed bees were flying 1 m above vegetation.
While variation in body size of workers also impacts olfactory performance49, the resolution of olfactory stimuli is based upon the likelihood of encountering an intact (i.e. not well mixed and therefore diluted) and resolvable plume-filament. Probability of plume contact is derived from studies investigating odor plume availability in field conditions; this model is making the assumption that an odor filament strong enough to be measured by laboratory equipment would be strong enough to stimulate a response. Given work by Murlis et al.61 – where they found a 1:1 relationship between antennal response in Manduca sexta and presence of a measured plume – this is a physiologically reasonable assumption. As existing field measurements show variability across environments, I tested two different olfactory probability functions (Fig. 3). The ‘high’ probability function was based upon field measurements by Murlis et al. taken in mid-July in an open field near Amhurst, MA61. This study provided field measurements of contact probability up to a distance of 20 m. However, odor plumes tend to be highly mixed and thus undetectable by a distance of 100 M76. Thus I bookended the Murlis data with two constraints: a value of 1 at 0 meters, representing the maximum probability of plume contact; and a value of 0.0001 at 100 meters – given that the model was repeated 1000 trials per condition this value functionally represents zero. These data were then fit with an exponential in excel (y = 0.54e−0.086×, R2 = 0.99, Fig. 3). The ‘low’ probability was taken directly from the exponential fit from Riffell et al.’s work measuring plume structure in a high alpine desert63 (Fig. 3). Odor probabilities operated in a radially symmetric fashion around floral displays (Fig. 1). Both of these probabilities are based upon data from studies on the hawkmoth Manduca sexta, a model organism in the study of odor-guided flight77 and olfactory processing78–80. Hawkmoths have both a larger body size and antennal length than Bombus species81,82. Given that body size in bumblebees correlates with greater olfactory sensitivity49, there is the possibility that odor-encounter probabilities for Manduca over-estimate bumblebee olfactory capabilities. Interestingly, a comparison of the odor-behavior literature shows that bumblebee experiments are typically run at much lower odor concentrations (1:1000) than Manduca experiments (neat extracts)40,49,80,83. In addition, bumblebee electroantennogram experiments (EAGs) require significantly higher odor concentrations (1:10–1:100) than bumblebee behavior experiments49 (Sprayberry unpub data), likely due to the noisy nature of electrophysiology recordings requiring a stronger stimulus to create a favorable signal: noise ratio. This is relevant because the “high” probability odor encounter plume is derived from EAG recordings, and thus likely underestimates the actual sensing ability of insect antennae. Therefore, while in-vivo hawkmoths may have higher odor sensitivity than bumblebees, the anthropogenically-derived odor probabilities are likely applicable to both insect groups. In addition, even the high-probability fit is conservative when compared to calculations of distance travelled by floral odorants, which indicate a less than 50% loss of volatiles at distances of 100 m39 (Fig. 3). McFrederick et al.’s computational analysis does not consider plume structure – those remaining molecules may be well mixed and thus at physiologically irrelevant concentrations; however, it does imply that this model is unlikely to overestimate olfactory contact.
Figure 3.
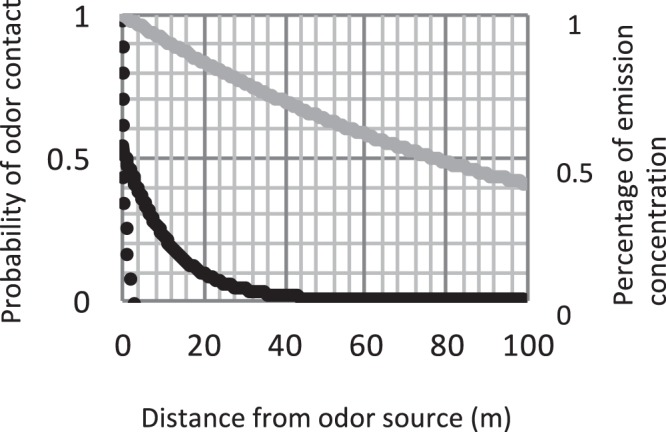
The black lines represent the relationship between distance from flower (odor source) and the likelihood of plume contact, with the solid line representing a ‘high’ probability derived from Murlis et al.64 and the dashed line representing a ‘low’ probability derived from Riffell et al.67. The grey line is an estimated fit derived from McFrederick et al.’s48 calculations on distance travelled by floral odorants, represented by percent of emission concentration. By these calculations common floral odorants do not drop below 80% of original concentration until 25 m from source, indicating the odor probabilities used in this model are quite conservative. Additionally, comparing the concentration decay with the model’s odor probabilities indicates that when bumblebees have a 10% probability of plume contact, that plume is still at >80% original concentration, thus that plume is likely physiologically salient.
Results and Discussion
This model explored the sensory signals available to bumblebee foragers searching for novel resources by calculating the relative probability of workers encountering the visual and/or olfactory signal from a floral resource while searching in a relatively low-resource environment. The parameters varied in this model were: plant density, number of blooms (and thus the strength of sensory signals from an individual plant), the probability of odor plume encounter, and the visual acuity of the searching “bumblebee”.
Olfaction is the dominant sensory modality available to searching bees
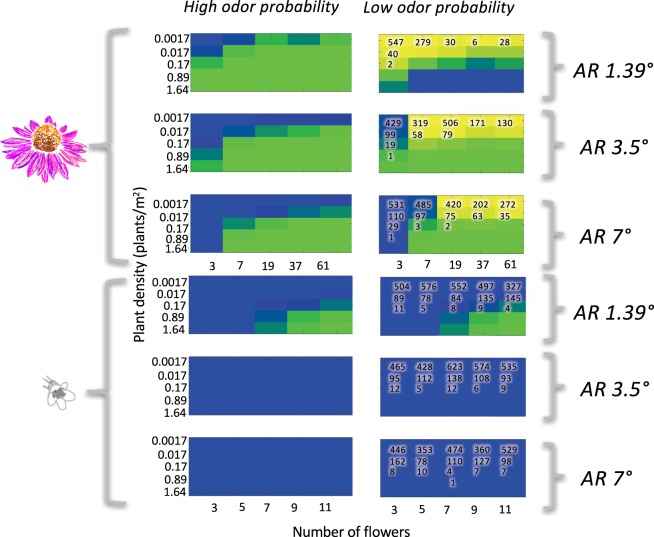
Looking holistically at all tested scenarios we see that odor dominated as the available sensory modality; with odor alone representing floral sensory encounter in 179/350 scenarios, an integrated odor-visual signal available in 136/350 scenarios, and vision alone as the dominate modality in only 35/350 (Figs 4 and 5). Odor information is therefore available for decision making in 90% of successful floral encounters, while visual information is only present in 49%. While there is substantial work indicating that vision is vitally important for patch exploitation behaviors35,46,84, it is likely that odor is crucial in patch location behavior.
Figure 4.
Heat maps indicating the relative likelihood of encountering a resolvable olfactory (blue), visual (yellow), or integrated olfactory and visual (green) sensory signal. These likelihoods were calculated for:1. multiple plant sizes, indicated by a variable number of flowers on the x axes; 2. multiple plant densities, indicated on the y axes; 3. two different plant species, Echinacea (top diagram) and Penstemon (bottom diagram); 4. two different odor probabilities, with high encounter probability represented in the left row and low on the right; and 5. three different visual acuities, labelled with their angular resolution on the right hand side of the figure. Each model scenario was run 1000 times. The number of failures – runs where a bee searched for 1.5 km without encountering a sensory signal- are indicated on the plots themselves. The absence of a number means that all 1000 runs resulted in a successful sensory encounter.
Figure 5.
Results of model runs using a Levy-walk distribution of step lengths for bumblebees with a visual resolution of 3.5° searching for Echinacea. Despite the difference in search-path calculation methods, the results are nearly identical to those depicted in Figs 4 and 6. Levy-walk searches do lead to a slight reduction in failure rates for low-odor probability scenarios.
Odor landscapes are changing, which could have a considerable impact on bumblebee foraging behavior
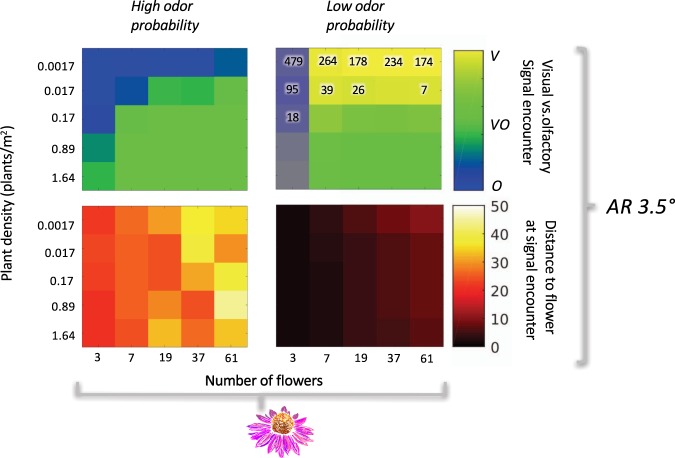
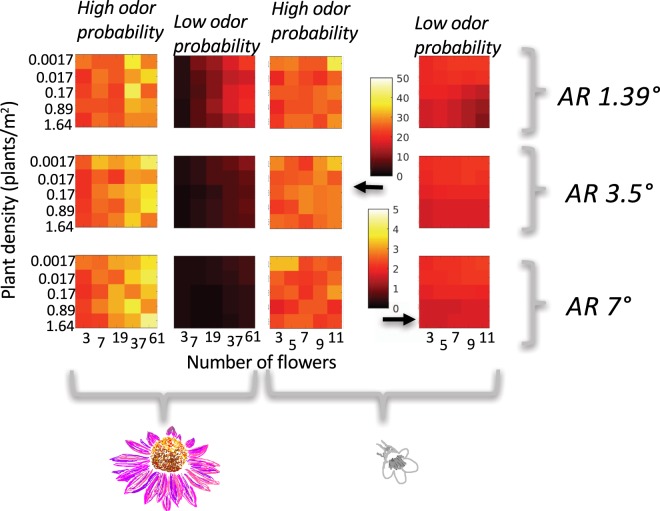
Model runs with a higher probability of odor contact demonstrated a larger discovery distance, with bumblebees contacting a resolvable sensory signal in the range of 25–40 meters, as opposed to 2–20 meters (Fig. 6). Additionally, decreased probability of odor-contact drastically increased the likelihood that forager searches would end in failure (Figs 4 and 5). Failure rates overall were higher for the smaller bloom size (Penstemon), as the larger plant and flower size of Echinacea afforded a better ability to transition to visual navigation when odor was unavailable. These computational results are commensurate with laboratory investigations on visual search time in bumblebees, where bees who have been restricted to solely visual information have higher search times to locate smaller flowers85. The low-odor probability tested in this model decays rapidly, transitioning to zero before 10 meters from the point source (Fig. 3)63. This empirical measurement may be underestimating plume strength due to environmental conditions: previous work has shown that odor plumes can rise in altitude86 and the Riffell et al. measurements were taken at a consistent elevation from the ground. However; the results from this odor fit are relevant to consider in light of work examining the impacts of anthropogenic pollution on floral odor-plumes. Seminal work by McFrederick et al. indicates that environmental pollutants can interact with floral odorants, reducing their distance travelled by an order of magnitude drop: odorants that historically could travel 1000 m before dropping to 80% of their original concentration would only make it 100 m in worst case scenarios. While McFrederick’s study was computational, subsequent experimental studies have been equally concerning. Girling et al. found that diesel exhaust degrades select floral odorants, modifying odor blend structure87. Likewise Farre-Armengol et al. found that ozone decreases floral odorant concentrations41. Based on our model results it is reasonable to hypothesize that bumblebees will experience higher failure rates in locating flowers when searching in polluted environments, particularly if available floral resources are comprised of plants with smaller bloom size and lower bloom number. Indeed, failure to locate a floral signal only occurred in the low-probability odor scenario - when odor information is readily available searching events are universally successful.
Figure 6.
Heat maps indicating average distance at which a resolvable sensory signal was encountered in successful model runs. These likelihoods were calculated for: 1. multiple plant sizes, indicated by a variable number of flowers on the x axes; 2. multiple plant densities, indicated on the y axes; 3. two different plant species, Echinacea (two left columns) and Penstemon (two right columns); 4. two different odor probabilities (labelled by column); and 5. three different visual acuities, labelled with their angular resolution on the right hand side of the figure.
Bloom size, number of blooms per plant and plant density impact both available sensory modality and distance at which plants are found
Unsurprisingly, plant size and density impact the likelihood that bumblebees will encounter a resolvable sensory signal (Figs 4 and 5). Increased plant density reduced failure rate in low odor probability situations for both large (Echinacea) and small (Penstemon) flowers. However, when plants with small bloom sizes are in low density patches they were only reliably ‘found’ in model runs with a higher probability of odor plume contact. Echinacea simulations were moderately less susceptible to density effects as they can be seen from a greater distance, but higher flower number was still associated with an increased discovery distance. Interestingly, field data on Penstemon indicated that they were typically found at the higher densities this model tested – the lower densities tested here were included purely for comparative purposes. In light of anthropogenic modulation of odor environments, bumblebees may passively select for larger bloom size and higher plant density in polluted environments by virtue of not being able to locate smaller flowers, or those with larger nearest-neighbor distances.
Effects of search-path type
The outcome of model runs using a power-law distribution for step lengths (Levy-walk) (Fig. 5) is nearly identical to the results from constant step lengths (Fig. 4). Odor information is ubiquitous in the high-odor probability scenarios, with visual information not being encountered in isolation until the low-odor probability scenarios. As in Figs 4 and 6, a shift to low-odor probability both decreases the distance at which flower-signals are encountered and increases failure rates in search flights. The predominant difference between the two search-path methods is a slight decrease in failure rates when using variable step lengths, a finding that is consistent with the fact that the latter method ran for approximately double the distance, creating a longer search path.
Limitations and Future Directions
It is worth emphasizing that this experiment was done in silica. As such it is limited by the assumptions used to generate model results. These model results are strongly driven by visual and olfactory resolution: on the plant side from the strength of floral signal, and on the pollinator side from sensory sensitivity. While all of these variables were parameterized based on the ecology and physiology of the relevant plant-pollinator relationships, the absolute values returned by the model are less relevant than the trends. These trends raise interesting questions for future experimental work. For example, the indication that bumblebees with lower visual acuity first encounter smaller floral displays via odor plumes begets the question, will bumblebees searching for novel resources navigate with odor information alone? This phenomenon has previously been demonstrated on a small spatial scale40,49, but remains to be shown at field-realistic scales. The substantial number of model runs finishing with simultaneous odor and visual signal encounter raises the question, does odor information make a minimally resolvable visual cue more salient? Again, work on small spatial scales shows improved learning and recognition of food resources with multimodal sensory information51,88, but how this operates on large spatial scales is less clear. Finally, this computational model provides an alarming context for recent work on odor pollution in bee behavior. While that work has largely focused on laboratory investigations, decreasing plume distance is likely to have profound impacts on foraging efficiency in bumblebees and other odor-guided pollinators. These results, in combination with recent computational findings on air pollution decreasing distance travelled by floral scent67, strongly indicate that relevant field work to ground-truth theoretical concerns is necessary.
Acknowledgements
J.S. thanks Muhlenberg College for research and travel funding that contributed to the development of this work. J.S. thanks the anonymous reviewers for valuable feedback on this manuscript.
Author Contributions
J.S. constructed the computational model, made the figures, wrote the manuscript and reviewed the manuscript.
Data Availability
Data generated by model runs are available upon request.
Competing Interests
The author declares no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hegland SJ, Nielsen A, Lazaro A, Bjerknes AL, Totland Ø. How does climate warming affect plant‐pollinator interactions? Ecol Letters. 2009;12:184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 2.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc Biol. 2008;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Dohzono I, Hiei K. Evolution of pollinator generalization in bumblebee-pollinated plants. Plant Spec Biol. 2007;22:141–159. doi: 10.1111/j.1442-1984.2007.00187.x. [DOI] [Google Scholar]
- 4.Williams PH, Osborne JL. Bumblebee vulnerability and conservation world-wide. Apidologie. 2009;40:367–387. doi: 10.1051/apido/2009025. [DOI] [Google Scholar]
- 5.Cameron SA, et al. Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA. 2011;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- 7.Potts SG, et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Whitehorn PR, O’Connor S, Wackers FL, Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336:351–352. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- 9.Gill RJ, Raine NE. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct Ecol. 2014;28:1459–1471. doi: 10.1111/1365-2435.12292. [DOI] [Google Scholar]
- 10.Owen RE, Rodd FH, Plowright RC. Sex ratios in bumble bee colonies: complications due to orphaning? Behav Ecol Sociobiol. 1980;7:287–291. doi: 10.1007/BF00300669. [DOI] [Google Scholar]
- 11.Woodgate JL, Makinson JC, Lim KS, Reynolds AM, Chittka L. Life-long radar tracking of bumblebees. Plos One. 2016;11:e0160333. doi: 10.1371/journal.pone.0160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lihoreau M, et al. Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes over large spatial scales. Plos Biol. 2012;10:e1001392. doi: 10.1371/journal.pbio.1001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunau K. Innate recognition of flowers by bumble bees: orientation of antennae to visual stamen signals. Can. J. Zool. 1992;70:2139–2144. doi: 10.1139/z92-288. [DOI] [Google Scholar]
- 14.Cnaani J, Thomson JD, Papaj DR. Flower Choice and Learning in Foraging Bumblebees: Effects of Variation in Nectar Volume and Concentration. Ethology. 2006;112:278–285. doi: 10.1111/j.1439-0310.2006.01174.x. [DOI] [Google Scholar]
- 15.Heinrich B. “Majoring” and ‘Minoring’ by Foraging Bumblebees, Bombus Vagans: An Experimental Analysis. Ecology. 1979;60:245–255. doi: 10.2307/1937652. [DOI] [Google Scholar]
- 16.Cartar RV. Resource tracking by bumble bees: responses to plant-level differences in quality. Ecology. 2004;85:2764–2771. doi: 10.1890/03-0484. [DOI] [Google Scholar]
- 17.Ott JR, Real LA, Silverfine EM. The effect of nectar variance on bumblebee patterns of movement and potential gene dispersal. Oikos. 1985;45:333–340. doi: 10.2307/3565568. [DOI] [Google Scholar]
- 18.Saleh N, Scott AG, Bryning GP, Chittka L. Distinguishing signals and cues: bumblebees use general footprints to generate adaptive behaviour at flowers and nest. Arthropod-Plant Inte. 2007;1:119–127. doi: 10.1007/s11829-007-9011-6. [DOI] [Google Scholar]
- 19.Osborne JL, et al. The ontogeny of bumblebee flight trajectories: from naïve explorers to experienced foragers. Plos One. 2013;8:e78681. doi: 10.1371/journal.pone.0078681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham P, Cheng K. Ants use the panoramic skyline as a visual cue during navigation. Curr Biol. 2009;19:R935–R937. doi: 10.1016/j.cub.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Knaden M, Graham P. The sensory ecology of ant navigation: from natural environments to neural mechanisms. Annu Rev Entomol. 2015;61:63–76. doi: 10.1146/annurev-ento-010715-023703. [DOI] [PubMed] [Google Scholar]
- 22.Dacke M, Jundi elB, Smolka J, Byrne M, Baird E. The role of the sun in the celestial compass of dung beetles. Phil Trans R Soc Biol. 2014;369:20130036. doi: 10.1098/rstb.2013.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyke GH. Optimal foraging theory: a critical review. Annu Rev Ecol Syst. 1984;15:523–575. doi: 10.1146/annurev.es.15.110184.002515. [DOI] [Google Scholar]
- 24.Viswanathan GM, et al. Lévy flight search patterns of wandering albatrosses. Nature. 1996;381:413–415. doi: 10.1038/381413a0. [DOI] [PubMed] [Google Scholar]
- 25.Osborne JL, et al. A landscape‐scale study of bumble bee foraging range and constancy, using harmonic radar. J Appl Ecol. 1999;36:519–533. doi: 10.1046/j.1365-2664.1999.00428.x. [DOI] [Google Scholar]
- 26.Cole BJ. Fractal time in animal behaviour: the movement activity of Drosophila. Anim Behav. 1995;50:1317–1324. doi: 10.1016/0003-3472(95)80047-6. [DOI] [Google Scholar]
- 27.Ramos-Fernández G, Mateos JL, Miramontes O. Lévy walk patterns in the foraging movements of spider monkeys (Ateles geoffroyi) Behav Ecol Sociobiol. 2004;55:223–230. doi: 10.1007/s00265-003-0700-6. [DOI] [Google Scholar]
- 28.Reynolds AM, Smith AD, Reynolds DR, Carreck NL, Osborne JL. Honeybees perform optimal scale-free searching flights when attempting to locate a food source. J Exp Biol. 2007;210:3763–3770. doi: 10.1242/jeb.009563. [DOI] [PubMed] [Google Scholar]
- 29.Lenz F, Chechkin AV, Klages R. Constructing a Stochastic Model of Bumblebee Flights from Experimental Data. Plos One. 2013;8:e59036. doi: 10.1371/journal.pone.0059036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James A, Plank MJ, Edwards AM. Assessing Levy walks as models of animal foraging. J Roy Soc Interface. 2011;8:1233–1247. doi: 10.1098/rsif.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyke GH. Understanding movements of organisms: it’s time to abandon the Lévy foraging hypothesis. Methods Ecol Evol. 2015;6:1–16. doi: 10.1111/2041-210X.12298. [DOI] [Google Scholar]
- 32.Bartumeus F, da Luz MGE, Viswanathan GM, Catalan J. Animal search strategies: a quantitative random-walk analysis. Ecology. 2005;86:3078–3087. doi: 10.1890/04-1806. [DOI] [Google Scholar]
- 33.Edwards AM, et al. Revisiting Lévy flight search patterns of wandering albatrosses, bumblebees and deer. Nature. 2007;449:1044–1048. doi: 10.1038/nature06199. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, A. S., Dornhaus, A. & Papaj, D. R. In Evolution of Plant-Pollinator Relationships (ed. Patiny, S.) 279–300 (books.google.com, 2011).
- 35.Leonard AS, Dornhaus A, Papaj DR. Flowers help bees cope with uncertainty: signal detection and the function of floral complexity. J Exp Biol. 2011;214:113–121. doi: 10.1242/jeb.047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruedenauer FA, Spaethe J, Leonhardt SD. Hungry for quality—individual bumblebees forage flexibly to collect high-quality pollen. Behav Ecol Sociobiol. 2016;70:1–9. doi: 10.1007/s00265-016-2129-8. [DOI] [Google Scholar]
- 37.Goyret J. The role of mechanosensory input in flower handling efficiency and learning by Manduca sexta. J Exp Biol. 2006;209:1585–1593. doi: 10.1242/jeb.02169. [DOI] [PubMed] [Google Scholar]
- 38.Macuda T, Gegear RJ, Laverty TM, Timney B. Behavioural assessment of visual acuity in bumblebees (Bombus impatiens) J Exp Biol. 2001;204:559–564. doi: 10.1242/jeb.204.3.559. [DOI] [PubMed] [Google Scholar]
- 39.McFrederick QS, Kathilankal JC, Fuentes JD. Air pollution modifies floral scent trails. Atmos Environ. 2008;42:2336–2348. doi: 10.1016/j.atmosenv.2007.12.033. [DOI] [Google Scholar]
- 40.Sprayberry JDH, Ritter KA, Riffell JA. The effect of olfactory exposure to non-insecticidal agrochemicals on bumblebee foraging behavior. Plos One. 2013;8:e76273. doi: 10.1371/journal.pone.0076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farré-Armengol G, et al. Ozone degrades floral scent and reduces pollinator attraction to flowers. New Phytol. 2015;209:152–160. doi: 10.1111/nph.13620. [DOI] [PubMed] [Google Scholar]
- 42.Riveros AJ, Gronenberg W. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften. 2009;96:851–856. doi: 10.1007/s00114-009-0532-y. [DOI] [PubMed] [Google Scholar]
- 43.Sommerlandt FMJ, Rössler W, Spaethe J. Elemental and non-elemental olfactory learning using PER conditioning in the bumblebee, Bombus terrestris. Apidologie. 2013;45:106–115. doi: 10.1007/s13592-013-0227-4. [DOI] [Google Scholar]
- 44.Toda NRT, Song J, Nieh JC. Bumblebees exhibit the memory spacing effect. Naturwissenschaften. 2009;96:1185–1191. doi: 10.1007/s00114-009-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunze J, Gumbert A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav Ecol. 2001;12:447–456. doi: 10.1093/beheco/12.4.447. [DOI] [Google Scholar]
- 46.Odell, E., Raguso, R. A. & Jones, K. N. Bumblebee foraging responses to variation in floral scent and color in snapdragons (Antirrhinum: Scrophulariaceae). Am Midl Nat (1999).
- 47.Goulson, D., Stout, J. C. & Langley, J. Identity and function of scent marks deposited by foraging bumblebees. J Chem Ecol (2000).
- 48.Wilms J, Eltz T. Foraging scent marks of bumblebees: footprint cues rather than pheromone signals. Naturwissenschaften. 2007;95:149–153. doi: 10.1007/s00114-007-0298-z. [DOI] [PubMed] [Google Scholar]
- 49.Spaethe J, Brockmann A, Halbig C, Tautz J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 50.Raine NE, Chittka L. The adaptive significance of sensory bias in a foraging context: floral colour preferences in the bumblebee Bombus terrestris. Plos One. 2007;2:e556–8. doi: 10.1371/journal.pone.0000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulahci IG, Dornhaus A, Papaj DR. Multimodal signals enhance decision making in foraging bumble-bees. Proc R Soc Biol. 2008;275:797–802. doi: 10.1098/rspb.2007.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molet M, Chittka L, Raine NE. How floral odours are learned inside the bumblebee (Bombus terrestris) nest. Naturwissenschaften. 2008;96:213–219. doi: 10.1007/s00114-008-0465-x. [DOI] [PubMed] [Google Scholar]
- 53.Witjes S, Eltz T. Influence of scent deposits on flower choice: experiments in an artificial flower array with bumblebees. Apidologie. 2007;38:12–18. doi: 10.1051/apido:2006048. [DOI] [Google Scholar]
- 54.Suchet C, et al. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav Ecol Sociobiol. 2010;65:1015–1027. doi: 10.1007/s00265-010-1106-x. [DOI] [Google Scholar]
- 55.Hudon TM, Plowright CMS. Trapped: assessing attractiveness of potential food sources to bumblebees. J Insect Behav. 2010;24:144–158. doi: 10.1007/s10905-010-9243-7. [DOI] [Google Scholar]
- 56.Dyer AG, Spaethe J, Prack S. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J Comp Physiol A. 2008;194:617–627. doi: 10.1007/s00359-008-0335-1. [DOI] [PubMed] [Google Scholar]
- 57.Leadbeater E, Chittka L. A new mode of information transfer in foraging bumblebees? Curr Biol. 2005;15:R447–8. doi: 10.1016/j.cub.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Ings TC, Raine NE, Chittka L. A population comparison of the strength and persistence of innate colour preference and learning speed in the bumblebee Bombus terrestris. Behav Ecol Sociobiol. 2009;63:1207–1218. doi: 10.1007/s00265-009-0731-8. [DOI] [Google Scholar]
- 59.Renner MA, Nieh JC. Bumble bee olfactory information flow and contact-based foraging activation. Insect. Soc. 2008;55:417–424. doi: 10.1007/s00040-008-1021-6. [DOI] [Google Scholar]
- 60.Walther Hellwig K, Frankl R. Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agricultural landscape. J Appl Entomol. 2000;124:299–306. doi: 10.1046/j.1439-0418.2000.00484.x. [DOI] [Google Scholar]
- 61.Murlis J, Willis MA, Carde RT. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol Entomol. 2000;25:211–222. doi: 10.1046/j.1365-3032.2000.00176.x. [DOI] [Google Scholar]
- 62.Spaethe J, Chittka L. Interindividual variation of eye optics and single object resolution in bumblebees. J Exp Biol. 2003;206:3447–3453. doi: 10.1242/jeb.00570. [DOI] [PubMed] [Google Scholar]
- 63.Riffell JA, et al. Flower discrimination by pollinators in a dynamic chemical environment. Science. 2014;344:1515–1518. doi: 10.1126/science.1251041. [DOI] [PubMed] [Google Scholar]
- 64.Heinrich B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia. 1979;40:235–245. doi: 10.1007/BF00345321. [DOI] [PubMed] [Google Scholar]
- 65.Heinrich, B. Bumblebee economics (Harvard University Press, 2004).
- 66.Mulloney B. Impulse patterns in the flight motor neurones of Bombus californicus and Oncopeltus fasciatus. J Exp Biol. 1970;52:59–77. doi: 10.1242/jeb.52.1.59. [DOI] [PubMed] [Google Scholar]
- 67.Fuentes JD, Chamecki M, Roulston T, Chen B, Pratt KR. Air pollutants degrade floral scents and increase insect foraging times. Atmos Environ. 9999;141:361–374. doi: 10.1016/j.atmosenv.2016.07.002. [DOI] [Google Scholar]
- 68.Wolf S, et al. Optimal search patterns in honeybee orientation flights are robust against emerging infectious diseases. Sci. Rep. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becher MA, et al. BEESCOUT: A model of bee scouting behaviour and a software tool for characterizing nectar/pollen landscapes for BEEHAVE. Ecol Model. 2016;340:126–133. doi: 10.1016/j.ecolmodel.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.James A, Pitchford JW, Plank MJ. Efficient or Inaccurate? Analytical and Numerical Modelling of Random Search Strategies. Bull. Math. Biol. 2009;72:896–913. doi: 10.1007/s11538-009-9473-z. [DOI] [PubMed] [Google Scholar]
- 71.Viswanathan GM, et al. Optimizing the success of random searches. Nature. 1999;401:911–914. doi: 10.1038/44831. [DOI] [PubMed] [Google Scholar]
- 72.Wagenius S, Lyon SP. Reproduction of Echinacea angustjfolia in fragmented prairie is pollen-limited but not pollinator-limited. Ecology. 2010;91:733–742. doi: 10.1890/08-1375.1. [DOI] [PubMed] [Google Scholar]
- 73.Zorn-Arnold B, Howe HF. Density and seed set in a self-compatible forb, Penstemon digitalis (Plantaginaceae), with multiple pollinators. Am J Bot. 2007;94:1594–1602. doi: 10.3732/ajb.94.10.1594. [DOI] [PubMed] [Google Scholar]
- 74.Parachnowitsch Amy L., Kessler André. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytologist. 2010;188(2):393–402. doi: 10.1111/j.1469-8137.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- 75.Parachnowitsch AL, Raguso RA, Kessler A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 2012;195:667–675. doi: 10.1111/j.1469-8137.2012.04188.x. [DOI] [PubMed] [Google Scholar]
- 76.Riffell JA, Abrell L, Hildebrand JG. Physical processes and real-time chemical measurement of the insect olfactory environment. Journal Of Chemical Ecology. 2008;34:837–853. doi: 10.1007/s10886-008-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willis MA, Ford EA, Avondet JL. Odor tracking flight of male Manduca sexta moths along plumes of different cross-sectional area. J Comp Physiol A. 2013;199:1015–1036. doi: 10.1007/s00359-013-0856-0. [DOI] [PubMed] [Google Scholar]
- 78.Hansson BS, Carlsson MA, Kalinovà B. Olfactory activation patterns in the antennal lobe of the sphinx moth, Manduca sexta. J Comp Physiol A. 2003;189:301–308. doi: 10.1007/s00359-003-0403-5. [DOI] [PubMed] [Google Scholar]
- 79.Martin JP, Lei H, Riffell JA, Hildebrand JG. Synchronous firing of antennal-lobe projection neurons encodes the behaviorally effective ratio of sex-pheromone components in male Manduca sexta. J Comp Physiol A. 2013;199:963–979. doi: 10.1007/s00359-013-0849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riffell JA, Lei H, Hildebrand JG. Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc Natl Acad Sci USA. 2009;106:19219–19226. doi: 10.1073/pnas.0910592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. 2006;5:16. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spaethe J, Weidenmüller A. Size variation and foraging rate in bumblebees (Bombus terrestris) Insect. Soc. 2002;49:142–146. doi: 10.1007/s00040-002-8293-z. [DOI] [Google Scholar]
- 83.Fraser AM, Mechaber WL, Hildebrand JG. Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J Chem Ecol. 2003;29:1813–1833. doi: 10.1023/A:1024898127549. [DOI] [PubMed] [Google Scholar]
- 84.Lunau K. Innate flower recognition in bumblebees (Bombus terrestris, B. lucorum; Apidae): optical signals from stamens as landing reaction releasers. Ethology. 1991;88:203–214. doi: 10.1111/j.1439-0310.1991.tb00275.x. [DOI] [Google Scholar]
- 85.Spaethe, J., Chittka, L. & Skorupski, P. Visual search and decision making in bees: time, speed, and accuracy. Int J Comp Psychol (2006).
- 86.Girling RD, Higbee BS, Carde RT. The plume also rises: trajectories of pheromone plumes issuing from point sources in an orchard canopy at night. J Chem Ecol. 2013;39:1150–1160. doi: 10.1007/s10886-013-0341-9. [DOI] [PubMed] [Google Scholar]
- 87.Girling, R. D., Lusebrink, I., Farthing, E., Newman, T. A. & Poppy, G. M. Diesel exhaust rapidly degrades floral odours used by honeybees. Sci Rep3 (2013). [DOI] [PMC free article] [PubMed]
- 88.Leonard AS, Masek P. Multisensory integration of colors and scents: insights from bees and flowers. J Comp Physiol A. 2014;200:463–474. doi: 10.1007/s00359-014-0904-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated by model runs are available upon request.