Abstract
Microbial survival in dynamic environments requires the ability to successfully respond to abrupt changes in osmolarity. The mechanosensitive channel of large conductance (MscL) is a ubiquitous channel that facilitates the survival of bacteria and archaea under severe osmotic downshock conditions by relieving excess turgor pressure in response to increased membrane tension. A prominent structural feature of MscL, the cytoplasmic C-terminal domain, has been suggested to influence channel assembly and function. In this report, we describe the X-ray crystal structure and electrophysiological properties of a C-terminal domain truncation of the Mycobacterium tuberculosis MscL (MtMscLΔC). A crystal structure of MtMscLΔC solubilized in the detergent n-dodecyl-β-D-maltopyranoside reveals the pentameric, closed state-like architecture for the membrane spanning region observed in the previously solved full-length MtMscL. Electrophysiological characterization demonstrates that MtMscLΔC retains mechanosensitivity, but with conductance and tension sensitivity more closely resembling full length EcMscL than MtMscL. This study establishes that the C-terminal domain of MtMscL is not required for oligomerization of the full-length channel, but rather influences the tension sensitivity and conductance properties of the channel. The collective picture that emerges from these data is that each MscL channel structure has characteristic features, highlighting the importance of studying multiple homologs.
Introduction
Mechanosensitive (MS) channels transduce mechanical stimuli into a variety of cellular responses. MS channels are widely distributed through all kingdoms of life, including bacteria and archaea, where they play a prominent role in maintaining proper osmoregulation1–5. Sudden changes in osmolarity, such as osmotic downshock during exposure to hypotonic conditions, increases turgor pressure across the cell membrane. This in turn increases the tension within the lipid bilayer and activates a network of MS channels which respond by opening typically nonselective pores in the membrane to release cell contents and alleviate the pressure1,5–7. Bacterial MS channels are characterized by their relatively high and non-selective conductances, ranging from ~0.1 nS to ~3 nS under defined experimental conditions6–8, which is up to several orders of magnitude greater than typical for ion-selective channels3,6,9. Of these channels, the mechanosensitive channel of large conductance (MscL) exhibits the largest conductance, with an estimated open state pore diameter of ~25–35 Å - large enough for the passage of molecules up to 9 kDa4,7,10,11. In view of the high conductance yet small subunit size of MscL (Escherichia coli MscL (EcMscL) has 136 residues; Fig. 1), the oligomeric nature of this channel was appreciated from its initial discovery11. Considerable efforts have been made to establish the molecular mechanism of MscL gating3,12, employing a variety of techniques including electrophysiology, biochemical and genetic studies, molecular dynamics simulations, and X-ray crystallography. Interest in understanding the factors controlling the high tension sensitivity and conductance of MscL has been heightened by reports utilizing this channel as a tension sensitive element in nanodevices and drug delivery systems, as well as a role for MscL in the uptake of antibiotics by bacteria13–16.
Figure 1.
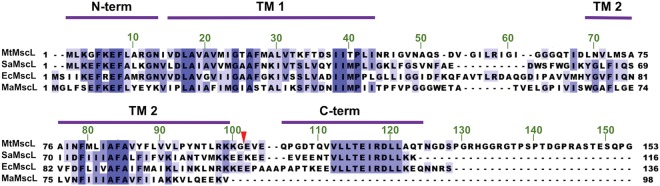
Alignment of MscL sequences. Full-length sequences of Mycobacterium tuberculosis MscL, Staphylococcus aureus MscL, Escherichia coli MscL and Methanosarcina acetivorans MscL were aligned using Clustal Omega. The sequence identities between pairs of sequences are MtMscL to SaMscL 40.5%, MtMscL to EcMscL 35.2%, and MtMscL to MaMscL 29.2%. The red triangle over the sequence alignment depicts the location of the (C) terminal truncation after residue 101 of MtMscLΔC. The blue shadings represent sequence identity gradients. The locations of the secondary structure regions are labeled based on the structure for MtMscL, PDBID 2OAR. The residue number legend in green along the top of the alignment corresponds to MtMscL, while the residue numbers on either side of the sequences correspond to that particular homolog. High variability is evident in the periplasmic loop among the homologs.
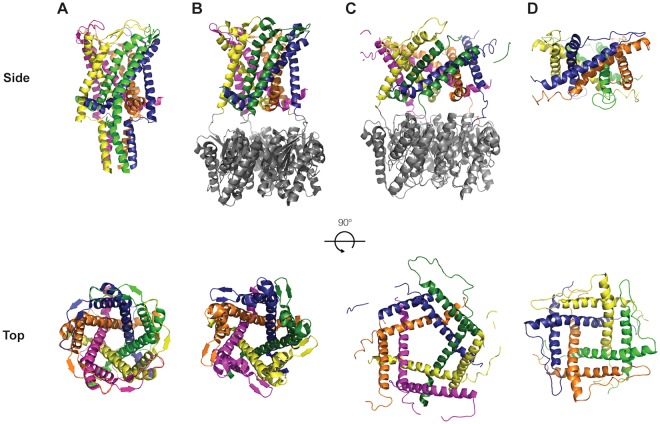
The first crystal structure determination of MscL was obtained for the Mycobacterium tuberculosis homolog (MtMscL), solved to 3.5 Å resolution17,18. This structure revealed a pentameric channel with a relatively simple subunit architecture consisting of a cytoplasmic N-terminal amphipathic helix, two transmembrane helices (TM1 and TM2) connected by a periplasmic loop, and a cytoplasmic C-terminal helix (Fig. 2). Several key structural features, including TM1 helices that form the permeation pathway and TM2 helices that form the channel–membrane interface, have been observed in the subsequently determined MscL structures from Staphylococcus aureus (SaMscL19) and Methanosarcina acetivorans (MaMscL20) (Fig. 2). The assembled channel can be considered to be composed of two major elements: the membrane spanning region that forms the high conductance permeation pathway in the open state, and the C-terminal cytoplasmic region. The narrowest opening of the permeation pathway in the MtMscL crystal structure was found to be ~3 Å, and formed through a constriction provided by the sidechains of Ile 17 and Val 21 from TM1. Since the minimum diameter required for the passage of water or ions through hydrophobic pores is calculated to be 9 and 13 Å21, respectively, the MtMscL structure was classified as a non-conducting (closed) conformation. Estimates of the open state diameter range from ~25–35 Å based on the large conductance and the sizes of molecules that can pass through the open channel10,22. These conclusions are supported by FRET observations23, and models for the open state have been developed through modeling and molecular dynamics simulations24,25. To date, however, the structure of the open state of MscL remains elusive.
Figure 2.
Crystal structures of MscL. Side and top views of the available crystal structures of MscL in a ribbon diagram, depicting (A) native (full length) MtMscL PDBID 2OAR, (B) MaMscL closed PDBID 4Y7K, (C) MaMscL expanded PDBID 4Y7J, and (D) tetrameric SaMscLΔC PDBID 3HZQ. The individual subunits in each structure are colored separately. Side views in panels (B) and (C) include the MjRS protein (colored in grey) that was fused to MaMscL for crystallization. The top row corresponds to a side view with the periplasmic side of the proteins facing up and the cytoplasmic side of the proteins facing down. The bottom row corresponds to a top periplasmic view of the channels.
While the membrane spanning region of MscL serves as the tension sensor and provides the gated permeation pathway, the role of the C-terminal domain is not as well understood. One challenge in defining a role for the C-terminal domain is that, while the sequence corresponding to the cytoplasmic helix is generally conserved, MscL function is largely insensitive to mutagenesis26,27 or truncation19,28,29 of the C-terminal region. Indeed, MscL homologs exist that lack this entire domain such as MaMscL (Fig. 1). Potential roles for the C-terminal domain include regulating the oligomeric state of the channel and/or tuning the mechanosensitivity of MscL19,30. Evidence for these functions may be summarized as follows:
-
(i)
A proposed role for the C-terminal domain in defining the MscL oligomeric state was motivated by the observation that a C-terminal truncation of SaMscL (SaMscLΔC) formed a tetramer (Fig. 2D)19. The subsequent report that the X-ray structure of the isolated C-terminal domain of EcMscL was pentameric31 suggested that the C-terminal domain might itself direct the oligomeric state of the entire MscL channel17,31,32. Biochemical and biophysical studies testing this proposal, however, have yielded conflicting results. While in vitro studies have demonstrated that removal of the C-terminus can perturb the oligomeric state33–36, the strong effect of detergent on the stability of oligomers confounds these analyses. The available data on the in vivo oligomeric state are consistent with a pentamer for both full length and truncated versions of EcMscL in the membrane37.
-
(ii)
A role for the C-terminal domain in regulating mechanosensitivity is supported by the observation that the EcMscL channel with a C-terminal domain truncation requires slightly higher tensions to gate than the wild type, and fails to exhibit subconducting states during both opening and closing gating events1,28,29. This is also observed with SaMscLΔC, which shows more stable gating events than the flickering events observed for full length SaMscL19,37. Some molecular dynamic simulations have suggested that the closed-to-open transition is accompanied by dissociation of the C-terminal helix bundle, but this is not universally observed23,24. Experimentally, stabilization of the C-terminal helix bundle with disulfide bonds does not impair function29,30, indicating that the bundle remains intact during gating, consistent with a thermodynamic analysis of the helix stability of the isolated C-terminal peptide31. Truncation of the C-terminal sequence does not significantly perturb the open state conduction, although shortening of the TM2 - helix linker does reduce the conductance30.
To generate a more complete picture of the role of the C-terminal domain, we endeavored to structurally and functionally characterize the C-terminal domain truncation of MtMscL. To date, MtMscL is the only full-length MscL with a crystallographically determined structure17. The crystal structure of MtMscLΔC presented in this work demonstrates that the C-terminal domain is not essential for assembly of a pentameric membrane pore domain, although indirect crystallographic evidence suggests that truncation may perturb the equilibrium between oligomeric states. Electrophysiological characterization in E. coli spheroplasts demonstrates that while MtMscLΔC remains mechanosensitive, it exhibits both a higher conductance and a higher tension sensitivity when compared to full-length MtMscL. Our results thus establish that while the C-terminal domain is not necessary for forming a functional state of MtMscL or mechanosensitivity, it does influence channel properties.
Results
The effects of C-terminal truncation on the structure of MtMscL
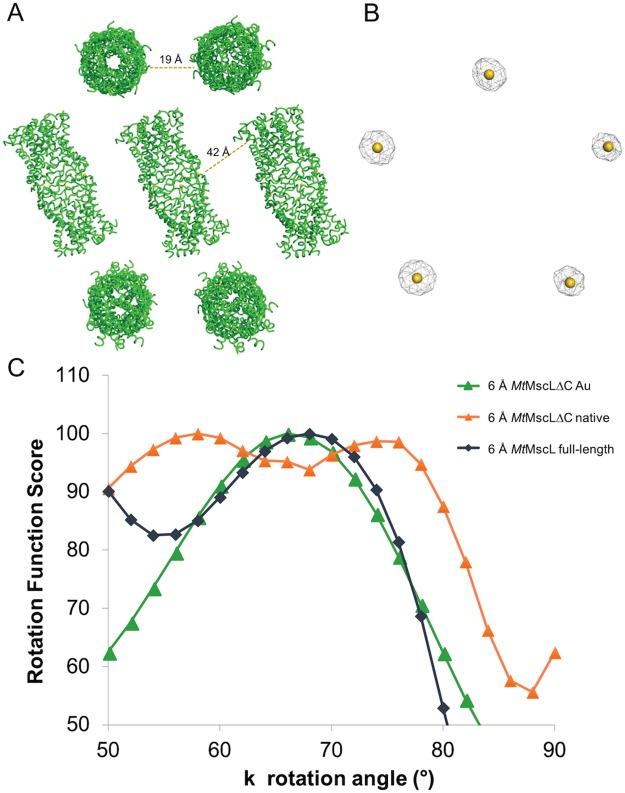
After screening several constructs, a variant of MtMscL was identified that expressed and purified well. This construct, truncated after residue 101 (Fig. 1, red triangle), was therefore selected for further analysis and is hereafter referred to as MtMscLΔC. Although this construct crystallized readily, the crystals typically diffracted poorly, to ~9 Å resolution. After screening ~2000 crystals, a crystal was found that diffracted to ~5 Å resolution. The diffraction data collected from this crystal was used to initially solve the structure by molecular replacement, using as the search model the pentameric MtMscL full-length structure with the C-terminal domain computationally truncated. Using this search model in Phaser MR, a TFZ score of 17.7 and an LLG score of 304.1 were obtained, supporting the validity of the molecular replacement solution. The asymmetric unit for this solution consisted of two pentameric channels, corresponding to a Matthews coefficient of 5.0 Å3/Dalton and a ~75% solvent content. The major crystal contacts were mediated by the head-to-head association of two pentamers through the periplasmic loops such that five-fold axes were coincident; similar contacts were observed in the full-length MtMscL structure (Fig. 3A). The remaining contacts required to form a three-dimensional lattice were not readily identified, as the minimal separations between adjacent pairs of pentamers are ~19 Å, precluding obvious contacts involving protein-protein interactions (Fig. 3A). This suggests that contacts between detergent micelles, rather than protein, mediate lattice formation along these directions, plausibly contributing to the relatively poor overall diffraction quality.
Figure 3.
Crystallographic analysis of MtMscLΔC. (A) Crystal packing of MtMscLΔC, depicting the head to head packing arrangement between adjacent pentamers. The distances of closest approach between channels along the different lattice directions are indicated; as they are too long for protein-protein contacts they presumably must be mediated at least in part by detergent micelles. (B) Anomalous difference Fourier map calculated at 6 Å resolution of the Au derivative using SAD phases reveal the pentameric arrangement (C) An analysis of self rotation functions as a function of the κ rotation angle about the direction of the 5-fold axis for MtMscLΔC Au data (green), MtMscLΔC native crystals (orange) and full length, wildtype MtMscL 2OAR (dark blue). The data were analyzed with a 6 Å resolution cutoff.
To verify the molecular replacement data described above, a heavy atom derivative was prepared with sodium aurothiosulfate. This gold compound was used in the original MtMscL structure determination and was found to bind to the periplasmic loops at the crystal contact between adjacent pentamers. A dataset was collected just above the Au L-II edge at 13815 eV and processed to 5.8 Å resolution. The single wavelength anomalous diffraction (SAD) data were used to search for the heavy atom positions using molecular replacement - SAD phasing in the Phaser program from the Phenix crystallographic programming suite38,39. These results identified 5 gold sites sandwiched between the periplasmic loops of two adjacent pentamers, at the same locations observed in the full-length protein (shown in Fig. 3B). To minimize model bias, SAD phases were calculated from the Au data alone with Phaser. These phases were improved using the CCP4 program DM to iteratively 10-fold average the electron density over the two pentamers related by non-crystallographic symmetry. The resulting electron density revealed the permeation pathway for MtMscLΔC to be in the closed conformation, with density covering TM1 and the N-terminal part of TM2, although the individual helices were not defined.
Crystallographic structure refinement was challenging, undoubtedly due to the low resolution and associated high temperature factors. Additionally, a rotation function analysis suggested heterogeneity in the crystal packing (Fig. 3C), possibly due to variability in the rotational orientation of pentamers around the 5-fold axis, or to the presence of multiple protein oligomeric states in the crystal. The self-rotation function calculated from the diffraction data obtained from the gold derivatized MtMscLΔC crystal (Supplementary Fig. S1) exhibits characteristic features of 522 point group non-crystallographic symmetry, with a 5-fold axis peak in the κ = 72° section near (ϕ, ω) = (69°, 54°) and a series of prominent peaks in the corresponding plane perpendicular to that axis in the κ = 180° section. The 5-fold peak arises from the pentameric arrangement of MtMscLΔC, while the perpendicular two-fold axes are generated from the non-crystallographic symmetry arising from the two pentamers packed in head-to-head fashion. In the native data set (Supplementary Fig. S2), however, the five-fold peak is broader and the two-fold section, while prominent, no longer has well defined peaks. This behavior suggested variability in either the orientation or the oligomeric state of MtMscLΔC in the native crystal as noted above.
To probe this possibility in more detail, the value of the self-rotation function along the channel rotation axis ((ϕ, ω) = (69°, 54°)) was calculated as a function of the rotation angle κ using the CCP4 program Polarrfn (Fig. 3C). The gold data set exhibited a broad peak near κ = 67° that, while displaced from that expected for a five-fold, matches well that observed using the diffraction data for the full length MtMscL structure (Fig. 3C). Interestingly, the native MtMscLΔC data set produced two peaks at κ = 58° and 74°. Both pentamers and hexamers could be present in the crystal, or perhaps that there is a single pentameric state that can vary in orientation around the 5-fold axis. Given the limited resolution of the data sets, it was deemed unlikely that these possibilities could be convincingly resolved. Consequently, the refinement proceeded with the gold data set, since the self-rotation function is indicative of a pentameric state, validated by the location of five Au sites in an anomalous difference Fourier map (Fig. 3B).
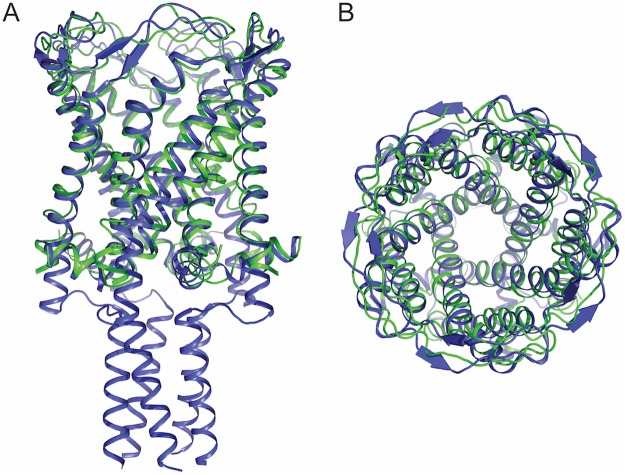
To refine the structure, MtMscL was truncated from residues 102–151 in silico; after initial refinement, C-terminal residues 97–101 were removed as they were not resolved in the refined density. The final refinement statistics are Rwork/free = 0.37/0.38 (Table 1). An alignment of MtMscLΔC with full length MtMscL gave a root mean squared deviation (RMSD) in the Cα positions of 1.4 Å, indicating that the structure remained relatively unchanged as a consequence of C-terminal truncation (Fig. 4). Coordinates for MtMscLΔC were deposited to the RSCB Protein Data Bank under PDBID 6CTD.
Table 1.
Data collection and refinement statistics for MtMscLΔC.
| Sodium Aurothiosulfate | Native | |
|---|---|---|
| Data collection | ||
| X-ray source | SSRL BL 12-2 | SSRL BL 12-2 |
| X-ray wavelength (Å) | 0.8974 | 0.9795 |
| Space group | P 21 | P 21 |
| Cell dimensions a, b, c (Å), β (°) | 84.7, 110.3, 136.8, 91.2 | 86.8, 111.9, 139.4, 92.2 |
| Resolution (Å)a | 35.0-5.5 (5.5–6.15) | 35.0-6.0 (6.0–6.7) |
| No. of unique reflections | 7936 | 6697 |
| Rmeasa (%) | 5.2 (124.7) | 3.3 (128.4) |
| Rmergea (%) | 4.8 (115.8) | 3.0 (126.4) |
| <I/σ I>a | 12.4 (1.8) | 17.7 (1.8) |
| Completeness (%)a | 96.0 (98.6) | 98.7 (98.5) |
| <Redundancy> | 7.0 | 6.9 |
| Refinement | ||
| Resolution (Å) | 23-5.8 | — |
| No. of reflections | 6049 | — |
| Rwork/Rfree (%) | 0.37/0.38 | — |
| <B-factor (Å2)> | 567 | — |
| Bond lengths (Å) | 0.007 | — |
| Bond angles (°) | 1.12 | — |
| Ramachandran Plot (%)b | — | |
| Favored regions | 94 (90) | — |
| Allowed regions | 6 (10) | — |
| PDB ID | 6CTD | — |
aIndicates high resolution parameters presented in parentheses.
bValue given by Coot outside of parenthesis; value given by PDB validation report, in parentheses.
Figure 4.
Structural superposition of MtMscLΔC with MtMscL full-length. MtMscLΔC is colored in green and MtMscL full-length is colored in blue. (A) Depicts the view parallel, and (B) depicts a view perpendicular to the five-fold axis, illustrating the structural similarity of the transmembrane domains in these two structures.
MtMscLΔC has a higher conductance than that of MtMscL, similar to that of EcMscL
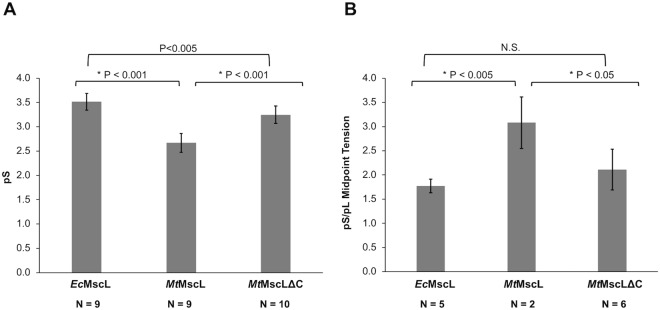
We used single channel patch clamp electrophysiology to characterize the channel properties of MtMscL and MtMscLΔC expressed in E. coli, and to compare them to EcMscL. Giant E. coli spheroplasts were generated from BL21 DE3 ΔmscL cells expressing MtMscL, MtMscLΔC or EcMscL. Inside-out patches were subjected to a pressure ramp protocol wherein membrane tension is increased in response to suction in the patch pipette. Representative traces from giant spheroplasts of E. coli expressing each of the three constructs are shown in Supplementary Fig. S3. The average unitary conductance of EcMscL at −20 mV was observed to be 3.5 ± 0.1 nanoSiemens (nS) (Fig. 5A); this result is in accordance with previous reports5. Full length MtMscL exhibited a conductance of 2.7 ± 0.2 nS, while MtMscLΔC had a significantly larger conductance of 3.3 ± 0.2 nS (p < 0.001, Student’s t-test) (Fig. 5A).
Figure 5.
Unitary conductance and MscL/MscS midpoint ratios. (A) Average unitary conductance from the indicated number of patches from BL21 DE3 ΔmscL cells expressing EcMscL WT, MtMscL WT, or MtMscLΔC. Membrane potential was clamped at −20 mV. (B) MscL/MscS midpoint ratios for EcMscL, MtMscL WT, and MtMscLΔC from the indicated number of patches derived from BL21 DE3 ΔmscL cells. Significant differences between pairs, based on Student’s t-test are reported above the charts.
To assess the relative tensions required for gating of these three channels, we calculated the midpoint pressure for channel opening relative to the endogenously expressed EcMscS (typically referred to as pL/pS40). Midpoint pressures are defined as the pressure where half of the channels of the population under consideration are open. Measurements of relative midpoint pressures therefore require saturation of both the EcMscS and MscL currents. The pL/pS ratios for EcMscL full-length, MtMscL full-length, and MtMscLΔC were 1.63 ± 0.06, 3.04 ± 0.38, and 1.93 ± 0.22, respectively (Fig. 5B). MtMscLΔC gated at a significantly lower tension than MtMscL (p < 0.05, Student’s t-test) while the pL/pS of EcMscL and MtMscLΔC were not significantly different. We note that we were only able to reach current saturation with WT MtMscL before the patch ruptured in 3 experiments out of 12, which is not surprising as MtMscL is reported to gate at the tensions approaching lytic tensions of the patch41. Taken together, the data in Fig. 5 indicate that MtMscLΔC more closely resembles EcMscL than full-length MtMscL, both in terms of conductance and tension sensitivity.
Inferring the conductance of a channel from the structure
The functional state of a channel cannot be directly assessed from the structure, but rather must be indirectly inferred from what is known about the properties of different states. Qualitatively, the conductance of a channel will reflect the geometry of the permeation pathway, with a wider pathway typically viewed as corresponding to a state of greater conductance. As an example, MtMscL and MtMscLΔC are assigned to representing the closed state on the basis of a pathway through the channel which is too narrow to support the high conductances observed in the fully open state. The conductances corresponding to the structures of the expanded states observed for SaMscLΔC and MaMscL are not known. While the permeation pathways in these two structures are too narrow to correspond to the fully open state, they also do not appear to be completely closed in SaMscLΔC and MaMscL expanded states. To address the conformational states available to MscL and their functional significance, a comparative analysis of the permeation pathway geometries of the available MscL structures, including our newly analyzed MtMscLΔC (summarized in Figs 2 and 4) was conducted.
MscL structures may be classified into two major conformational states through an analysis of the crossing angles of the transmembrane helices19,20. There are three primary helix-helix interfaces that can be used to describe the MscL structures, namely the crossing angles between TM1s in adjacent subunits (TM1-TM1′), between TM1 and TM2 in the same subunit (TM1-TM2), and between TM1 and TM2 in adjacent subunits (TM1-TM2′). Helix crossing angles for all available MscL structures are presented in Table 2. As noted previously19, the TM1-TM2′ contact is conserved in these structures, with a nearly antiparallel crossing angle of ~166° providing an extensive interface. In contrast, the TM1-TM1′ and TM1-TM2 crossing angles fall into two groups, representative of closed (MtMscL, MtMscLΔC and MaMscL closed) and expanded (SaMscLΔC and MaMscL expanded) conformations, respectively (Table 2). The closed conformation is characterized by TM1-TM1′ and TM1-TM2 angles of ~45° and 130°, respectively, while the corresponding values for the expanded state are ~60° and 112°, respectively (Supplementary Fig. S4).
Table 2.
Transmembrane helix crossing angles and RMSD between MscL channel structures.
| Closed | Expanded | ||||
|---|---|---|---|---|---|
| MscL Homologue and PDBID | Mt ∆C 6CTD | Mt 2OAR | Maclosed 4Y7K | Sa ∆C 3HZQ | Maexpanded 4Y7J |
| Crossing Angle TM1-TM2′ | 170° | 166° | 158° | 169° | 164° |
| Crossing angle TM1-TM2 | 130° | 130° | 130° | 112° | 112° |
| Crossing Angle TM1-TM1′ | 43° | 43° | 47° | 65° | 57° |
| RMSD (Å) Subunit A | — | 1.0 | 1.8 | 6.2 | 5.7 |
| RMSD (Å) TM helices in pentamers | — | 1.4 | 2.3 | — | 3.8 |
The crossing angles between TM1-TM2′, and between TM1-TM2 (in the same subunit) and TM1-TM1′ in different subunits are reported for the MscL structures solved to date. The root mean square deviation (RMSD) between MtMscLΔC and other MscL structures are reported for a single subunit (subunit A), and for all the TM helices in the pentameric MscL structures.
To assess the functional significance of these conformational states, the permeation pathways in the different MscL structures were characterized with the program HOLE42, which evaluates pore geometry and estimates the corresponding conductances. For comparison, a reference set of 4 proteins of high (~nS) conductance were also analyzed, three β-barrel proteins (two porins and VDAC) and E. coli MscS (EcMscS) in an open conformation43–46. Since channel conductances are sensitive to the solution conductivity, the measured conductances for the open state of MscL were approximately corrected to 1 M KCl, the conditions reported for the electrophysiological characterization of the porins, VDAC and EcMscS. The solutions typically used for MscL electrophysiology experiments have conductivities of ~30 mS cm-1 47 or ~0.27 times that of 1 M KCl (~110 mS cm-1;48. Since the conductance of EcMscL does not exhibit current saturation up to 2 M KCl1, the MscL conductances were corrected to the corresponding values in 1 M KCl by dividing by 0.27.
For this reference set, the relationship between the conductances calculated with HOLE, Gcalc, and the experimentally observed values, Gobs (corrected to 1 M KCl), may be approximated by a linear relationship (Fig. 6A):
| 1 |
Figure 6.
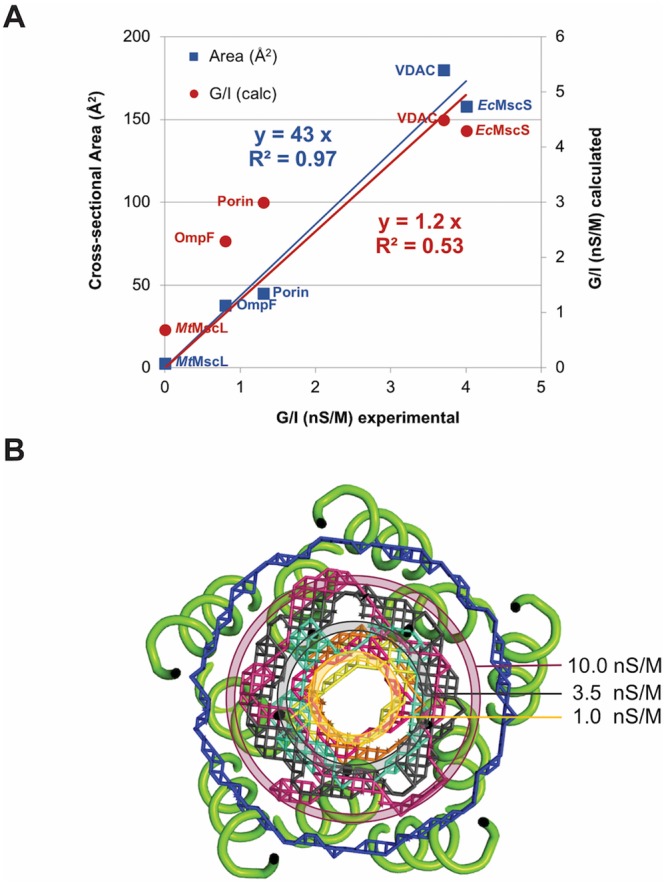
Analysis of pore area in relation to conductance in various MS channels. (A) Calibration curve between the experimental conductance, the conductance calculated by HOLE and the minimum cross-sectional area evaluated for four high conductance channels of known structure: OmpF, Porin, MscS, and VDAC. Data for this figure is presented in Table 3, with the point near the origin provided by the values for MtMscL in the closed conformation with a conductance of 0 nS. Linear fits were constrained to pass through the origin. (B) Structure of MtMscLΔC with mesh representation of the permeation pathway at the point of greatest restriction for channels of different conductances. Mesh colors: EcMscL model open- blue, SaMscLΔC (3HZQ) magenta (inner), MaMscL expanded (4Y7J)- cyan, MscS open (2VV5)- gray, porin (1PRN)- orange, porin (2OMF)- yellow, VDAC (3EMN) - warm pink (outer). The rings depict pore diameters at the restriction point for channels of indicated conductance, evaluated from equation (3).
While the largest conductance channels (VDAC and EcMscS) are closely replicated by the HOLE calculation, the conductances for the porins are overestimated as previously observed42; these effects contribute to a modest R2 value for the fit (0.53). Using this calibration curve, the conductances of the conformations of the expanded SaMscLΔC and MaMscL structures determined crystallographically are estimated as ~ 2–3 nS M−1.
The permeation pathways of these channels have large openings at one or both membrane surfaces tapering to a relatively narrow region and hence may be approximately described as funnel or hour-glass shaped. As a result, the overall channel conductance will be dominated by this constriction when an Ohm’s law-type model is used (see47). Consequently, the HOLE calculation was supplemented with an estimate of the cross-sectional area at the constriction point, as approximated by the number of grid points in a plane approximately perpendicular to the permeation pathway (Table 3). For the reference set, the minimum cross-sectional area, A (in Å2), and the experimentally determined specific conductances (Gobs, the conductance in nS corrected to 1 M KCl) are empirically related by the linear expression (Fig. 6A):
| 2 |
Table 3.
Conductance and permeation pathway properties of MscLs and calibration channels.
| Protein | PDB | Ref. | Gobs nS (Crystal structure) | G/I (obs) nS M−1 | G/I (calc) nS M−1 (HOLE) | Area (Å2) (at pore constriction) | Calc G/I nS M−1 (Eq. 2) |
|---|---|---|---|---|---|---|---|
| MscL Structures | |||||||
| MtMscL closed | 2OAR | 17This work | 0 | 0 | 0.7 | 3 | 0.1 |
| MtMscLΔC closed | 6CTD | This work | 0 | 0 | 0.7 | 1 | 0.0 |
| SaMscLΔC expanded | 3HDZ | 19 | — | — | 2.2 | 29 | 0.7 |
| MaMscL expanded | 4Y7J | 20 | 0.25 (open) | 0.9 | 3.5 | 85 | 2.0 |
| EcMscL open | model | 49 | 3.5 | 13.0 | 31 | 604 | 14.0 |
| Calibration Channels | |||||||
| VDAC | 3EMN | 45 | 3.7 | 3.7 | 4.5 | 180 | 4.2 |
| OmpF | 2OMF | 54 | 0.8 | 0.8 | 2.3 | 38 | 0.9 |
| Porin | 1PRN | 44 | 3.9 (per trimer) | 1.3 | 3.0 | 45 | 1.0 |
| EcMscS Open | 2VV5 | 46, 48 | 4.0 | 4.0 | 4.3 | 158 | 3.7 |
The observed and calculated conductances are tabulated for MscLs of defined structure, along with nS conductance calibration channels. The observed conductances for the open states of the corresponding channels, G(obs), are corrected for the ionic strength of the conductance measurements (eff. I (M)) to yield the G/I (obs) for the open state structures, reported in the concentration independent units of nS M−1 (‘G/I (obs) nS M−1’ column). The conductances calculated by the default mode of HOLE42 are listed in the ‘G/I (calc) nS M−1 (HOLE)’ column, along with an estimate of the conductance derived from the cross section area at the pore constriction, using the empirical parameterization provided in Eq. 2 (‘Calc G/I ns M−1 (Eq. 2)’ column). The relationships between the observed, HOLE-derived conductances, and the cross-sectional area at the pore constriction are depicted in Fig. 6A.
This fit is improved relative to equation (1) (R2 = 0.97), with an estimated standard deviation for the linear coefficient of 43 ± 2. The effective pore radius at the constriction point of a channel of specified conductance can be extracted from the cross-sectional area assuming a circular geometry:
| 3 |
The equivalent radii, R, of circular pores corresponding to conductances of 1, 3.5 and 10 nS M−1, (3, 7.0 and 12 Å, respectively) are illustrated in Fig. 6B and approximate the observed conductances of porins, and the open states of MscS and MscL, respectively. From this empirical calibration curve, the corresponding conductances of the available MscL structures and the computational model for the open state49 may be estimated (Table 3). The structures observed for MtMscL and MtMscLΔC are expected to exhibit minimal conductance, consistent with the assignment of these conformations to the closed state. More surprisingly, the expanded conformations observed in the crystal structures of SaMscLΔC and MaMscL are calculated to have conductances of ~1 nS M−1 (0.7 and 2.0 nS M−1 respectively) or ~0.3 nS under the typical conditions used for MscL electrophysiology. While this is well below the ~10 nS M−1 observed for the fully open conformation of EcMscL or MtMscL, it is close to that observed for MaMscL (0.25 nS20). Thus, this expanded conformation observed for MaMscL and SaMscLΔC may correspond to a sub-conductance state of other MscL channels.
Discussion
The crystal structure of MtMscLΔC reported in this paper establishes that removal of the C-terminal sequence after residue 101 has little consequence for the overall structure of the membrane domain relative to MtMscL. While many MscL homologs have been shown to maintain a pentameric state in DDM, the detergent used to purify and crystallize MtMscLΔC12,28,33, changes in oligomeric state have been observed when using alternate detergents. In particular, when using C8E4 and LDAO34,36, mass spectrometry studies of MscL homologs34 and the crystal structure analysis of SaMscLΔC show alternate oligomeric states19. Indeed, there is also evidence in our crystallographic analysis that oligomeric states other than a pentamer may be present (Fig. 3C), although this could not be definitively established.
The conformational space of MscL has been crystallographically explored through the use of different homologs, detergents and constructs. The two states of MscL that have been characterized to date correspond to closed (such as MtMscL full length and MtMscLΔC) and expanded conformations (such as SaMscLΔC and MaMscL expanded), where the latter likely corresponds to a sub-conductance state. Yet to be structurally captured is the fully open state of MscL, which in the absence of applied tension should be of sufficiently higher energy than the closed state to prevent the spontaneous opening of the channel. The challenge for the structural characterization of MscL is to identify conditions where the fully open state is stable and amenable to high resolution structural studies, either by crystallography or, as seems increasingly likely, the single particle cryo-electron microscopy methods which are revolutionizing structural biology.
We have shown that the function of MtMscL expressed in E. coli BL21 DE3 ∆mscL cells can be analyzed by patch clamp electrophysiology, in accordance with the methodology used on the recent study of MaMscL20. This expression system allowed us to increase the amount of protein expressed in the cell compared to previous methods that utilize a low expression vector, pB10b41 and increase our chances of observing a gating event. This was critical for our efforts, particularly when working with MtMscL, which has a shifted activation curve that reaches the lytic limits of E. coli. Electrophysiological characterization of MtMscLΔC indicates that its basic properties (unitary conductance and MscL: MscS activation midpoint ratio) are closer to those of EcMscL than MtMscL. Overall, these results support the hypothesis that the C-terminal domain is not essential for the intrinsic mechanosensitivity of MtMscL. The C-terminus, however, clearly plays a role in the detailed electrophysiological characteristics of the channel, in particular modulation of gating tension and conductance. The C-terminal domain of MtMscL could undoubtedly play other functional roles, perhaps interacting with other proteins or serving as a filter to limit the release of cellular contents during gating of MscL4,50. If used as filter, it would parallel with the role for the C-terminal domain of EcMscS, where it serves as a pre-filtering domain51. In addition, it would shed light on the observed higher conductance and disappearance of sub-conducting states in C-terminal truncations observed in EcMscL and SaMscL, respectively.
Our observation that truncation of the C-terminal domain in MtMscL results in a decreased tension requirement for gating contrasts with previous observations of EcMscL and SaMscL, where C-terminal truncation increases the tension threshold for gating19,29,52. The underlying mechanistic basis for these distinctions is not evident. The inescapable conclusion from an analysis of the data available for MscL channels from different species is that despite major structural and sequence similarities across MscL homologs, they do not all function identically, particularly with regards to the role of the C-terminus. Consequently, it is of high importance to study multiple MscL homologs to obtain a balanced view of structure and function relationships, rather than assuming they all look and function similarly.
Materials and Methods
Protein cloning, expression, and purification
Using site-directed mutagenesis on a plasmid prepared for previous MtMscL studies17, two mutations were introduced to MtMscL in pET-19b, one adding a tryptophan at the hexa-his tag linker (to produce MGWSHHHHHH), and the other introducing a stop codon at position E 102 STOP in the C-terminal domain to create the desired truncations. As MtMscL naturally lacks tryptophan, this residue was added to facilitate quantitation of this protein by the UV absorption at 280 nm. The constructs were inserted into a pET 19b vector carrying ampicillin resistance and transformed into E. coli BL21 DE3 mscL-, which was the strain previously developed for over-expression of MscL for structural and biochemical studies17. Protein was overexpressed by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to 1 mM once the culture reached an OD600nm of 2–2.5 in Terrific-Broth. After harvesting, cells were lysed using osmotic downshock. Cells were suspended in high osmolarity buffer (50 mM Tris HCl pH 7.5, 200 mM NaCl, 1 mM EDTA, 0.5 μg/mL lysozyme) at a 10 mL buffer per gram of cells ratio with stirring at 4 °C for 1 hour. Thereafter, cells were harvested by centrifugation at 6000 RPM using a JLA 16.250 rotor at 4 °C for 45 minutes, the cell pellet was then quickly re-suspended in low osmolarity buffer at a 10 mL buffer per gram of cells ratio (10 mM Tris HCl, pH 7.5, 20 mM NaCl, 1 mM MgCl2, 0.05 µg/mL DNase) at 4 °C for 1 hour to lyse the cells. The lysed cells were harvested by centrifugation at 12500 RPM using a JLA 16.250 rotor at 4 °C for 45 minutes, and then solubilized by homogenizing the pellet and stirring with 20 mM Tris HCl pH 7.5, 100 mM NaCl, 30 mM imidazole pH 8.0, 1% n-dodecyl-β-D-maltopyranoside (DDM) overnight at 4 °C. Cellular debris was removed by ultracentrifugation using rotor Type 45 Ti at 31,500 RPM for 1 hour at 4 °C. MscL constructs were purified using Ni-column affinity chromatography with Qiagen Superflow NiNTA resin (2 mL of a 50% solution of resin per 100 mL of lysate) using the following buffers – Equilibration (5 column volumes): 20 mM Tris HCl pH 7.5, 150 mM NaCl, 10 mM imidazole, 0.05% DDM, Wash (1 column volume): 20 mM Tris HCl pH 7.5, 500 mM NaCl, 25 mM imidazole, 0.05% DDM, Elution (3 column volumes): 20 mM Tris HCl pH 7.5, 150 mM NaCl, 300 mM imidazole, 0.05% DDM. This was followed by Source 30 S anion exchange chromatography using the following buffers – Low salt: 20 mM Tris HCl pH 7.5, 20 mM NaCl, 0.05% DDM, High salt: 20 mM Tris HCl pH 7.5, 1 M NaCl, 0.05% DDM, using a gradient of low salt and high salt buffers for elution. The final step was size exclusion chromatography through a Superdex 200 16/60 column, run with 20 mM Tris HCl pH 7.5, 150 mM NaCl, 0.05% DDM. The purified protein was concentrated with a molecular weight cutoff filter of 50 kDa to a final concentration of 25 mg/mL, as measured by UV280 nm readings using extinction coefficient Abs 0.1% = 0.649.
Crystallization and structure determination
Crystallization trays were set up using both sitting drop and hanging drop vapor diffusion at 4 °C. MtMscLΔC crystallized in 2 µL + 2 µL drops of protein and crystallization solution. The crystallization solution contained 0.1 M Tris HCl pH 8, 0.15 M CaCl2, 25% PEG 400, with 10% of 1 M glycine as an additive directly added to the crystallization drops. After 1.5 weeks, crystals typically grew to 0.4 × 0.3 × 0.1 mm3 and were cryo-protected using PEG 400. To provide phase information and to help validate the structure determination, full size crystals were soaked for one week with 5 mM sodium aurothiosulfate (used as a heavy atom derivative in the original MtMscL structure determination). Following soaking, the crystals were briefly crosslinked by adding a 2 µL drop of 25% glutaraldehyde next to the crystal drop and allowing for a 5-minute equilibration period. The derivatized crystals were then harvested and cryoprotected. The native crystals were cryoprotected with 40% PEG 400, whereas the gold derivative crystals were cryoprotected with a mixture of 12.5% diethylene glycol, 25% ethylene glycol, 12.5% 2-methyl-2,4-pentanediol, 12.5% glycerol, 12.5% 1,2-propanediol, 12.5% nondetergent sulfobetaine, 3-(1-pyridino)-1-propane sulfonate from the Molecular Dimensions CryoProtX kit. All diffraction data was collected at SSRL BL 12-2 on a Pilatus 6 M detector.
Data processing and refinement were conducted using the CCP4 and Phenix programming suites38,39. Diffraction datasets were processed using XDS-GUI and scaled using AIMLESS in the CCP4i suite. The structure was solved by molecular replacement using Phaser MR in CCP4i, and MR-SAD and SAD phase information was obtained from the gold-derivative dataset using Phaser in the Phenix suite. Structure refinement utilized REFMAC5 in CCP4i and Phenix.refine in Phenix39,53. Helix crossing angles were calculated with the PyMOL extension, AnglesBetweenHelices. Data collection details and refinement statistics are found in Table 1.
Permeation pathway analysis
The permeation pathways of the available MscL structures were quantitatively characterized with the program HOLE42, with calculated conductances and Rmin, the effective minimal radius, evaluated in default mode. As an alternative measure, the cross-sectional area at narrowest constriction of the permeation pathway was determined by summing the number of points inside the channel on a 1 Å × 1 Å grid on a plane perpendicular to the pore; to approximate the van der Waals surface of pore, all points in the channel were at least 2.8 Å from the nearest protein atom. To provide an empirical calibration between conductance and pore geometry, four proteins with ~nS conductances in 1 M KCl were used as a reference set:
Rhodopseudomonas blastica porin (PDB 1PRN, conductance55 and structure44).
mouse Voltage Dependent Anion Channel (VDAC1) (PDB 3EMN, conductance and structure45).
To correct for the effect of salt concentration, the measured MscL conductances were corrected to ~1 M KCl as described in the text.
Patch Clamp Electrophysiology
Giant spheroplasts were prepared using established protocols56, with several important modifications. Briefly, a culture of E. coli BL21 DE3 ΔmscL containing the construct of interest was treated with cephalexin for 1.5 hours and induced with 1 mM IPTG for 30 minutes. Spheroplasts were then prepared by lysozyme treatment at room temperature for 18–20 min. The spheroplast suspension was centrifuged through 7 mL column of 1 M sucrose at 4 °C and re-suspended in 300 µL 1 M sucrose. Aliquots were stored at −80 °C.
Patch-clamp experiments were carried out using pipette buffer (200 mM KCl, 90 mM MgCl2, 5 mM CaCl2, 5 mM HEPES, pH 7.4) and bath buffer (200 mM KCl, 90 mM MgCl2, 5 mM CaCl2, 5 mM HEPES, pH 7.4, 450 mM Sucrose). Excised inside-out patches from spheroplast membranes clamped at −20 mV membrane potential were treated with 5-second symmetric triangle pressure ramps of amplitudes from −50 to −290 mm Hg, using pipettes with bubble number of about 4.5, as previously described57. A high-speed pressure clamp system, HSPC-1 (ALA Scientific), was utilized in the experiments. Data were acquired with an Axopatch 200B amplifier and a Digidata 1440 digitizer (Molecular Devices) at 20 kHz, filtered at 5 kHz, and further analyzed with the pCLAMP 10.6 software suite (Molecular Devices). Unitary conductances of MscL channel variants were corrected for pipette access resistance.
Electronic supplementary material
Acknowledgements
We thank Troy Walton, Chinenye (Chinny) Idigo, Jens Kaiser and Jeff Abramson for enlightening discussions and the Gordon and Betty Moore Foundation and the Beckman Institute for their generous support of the Molecular Observatory at Caltech. X-ray crystallography data was collected at the Stanford Synchrotron Radiation Lightsource (SSRL), a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated by the United States Department of Energy, by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393) and the National Center for Research Resources, Biomedical Technology Program (P41RR001209). This research was funded in part by the National Institutes of Health National Institute of General Medical Sciences (5R01GM084211-07) and the Howard Hughes Medical Institute. N.H. was also independently funded by the Howard Hughes Medical Institute Gilliam Fellowship. Coordinates and structure factors have been deposited in the Protein Data Bank of the Research Collaboratory for Structural Bioinformatics, with PDBID 6CTD.
Author Contributions
N.H., G.M., E.H., D.C.R. conceptualized the approach and developed the experimental protocols. N.H. purified, crystallized and solved the crystal structure and with D.C.R. analyzed the structure. G.M. performed and with E.H. analyzed the electrophysiology experiments. N.H. wrote the initial manuscript draft, and N.H., G.M., E.H., D.C.R. revised and edited the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32536-6.
References
- 1.Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 2.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 3.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 4.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth IR, Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol. 2012;194:4802–4809. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukharev SI, Martinac B, Arshavsky VY, Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993;65:177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumann U, et al. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci USA. 2010;107:12664–12669. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards MD, et al. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 2012;6:272–281. doi: 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blount P, Sukharev SI, Moe PC, Nagle SK, Kung C. Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol Cell. 1996;87:1–8. doi: 10.1111/j.1768-322X.1996.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 11.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 12.Walton TA, Idigo CA, Herrera N, Rees DC. MscL: channeling membrane tension. Pflugers Arch. 2015;467:15–25. doi: 10.1007/s00424-014-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang LM, Blount P. Manipulating the permeation of charged compounds through the MscL nanovalve. Faseb J. 2011;25:428–434. doi: 10.1096/fj.10-170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doerner JF, Febvay S, Clapham DE. Controlled delivery of bioactive molecules into live cells using the bacterial mechanosensitive channel MscL. Nat Commun. 2012;3:990. doi: 10.1038/ncomms1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iscla I, Wray R, Wei S, Posner B, Blount P. Streptomycin potency is dependent on MscL channel expression. Nat Commun. 2014;5:4891. doi: 10.1038/ncomms5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soloperto Alessandro, Boccaccio Anna, Contestabile Andrea, Moroni Monica, Hallinan Grace I., Palazzolo Gemma, Chad John, Deinhardt Katrin, Carugo Dario, Difato Francesco. Mechano-sensitization of mammalian neuronal networks through expression of the bacterial large-conductance mechanosensitive ion channel. Journal of Cell Science. 2018;131(5):jcs210393. doi: 10.1242/jcs.210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 18.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Curr Top Membr. 2007;58:1–24. doi: 10.1016/S1063-5823(06)58001-9. [DOI] [Google Scholar]
- 19.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, et al. Mechanical coupling of the multiple structural elements of the large-conductance mechanosensitive channel during expansion. Proc Natl Acad Sci USA. 2015;112:10726–10731. doi: 10.1073/pnas.1503202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckstein O, Tai K, Sansom MS. Not ions alone: barriers to ion permeation in nanopores and channels. J Am Chem Soc. 2004;126:14694–14695. doi: 10.1021/ja045271e. [DOI] [PubMed] [Google Scholar]
- 22.Cruickshank CC, Minchin RF, Le Dain AC, Martinac B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys J. 1997;73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Single molecule FRET reveals pore size and opening mechanism of a mechano-sensitive ion channel. Elife. 2014;3:e01834. doi: 10.7554/eLife.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukharev S, Durell SR, Guy HR. Structural models of the MscL gating mechanism. Biophys J. 2001;81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deplazes E, Louhivuori M, Jayatilaka D, Marrink SJ, Corry B. Structural investigation of MscL gating using experimental data and coarse grained MD simulations. PLoS Comput Biol. 2012;8:e1002683. doi: 10.1371/journal.pcbi.1002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou X, Blount P, Hoffman RJ, Kung C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc Natl Acad Sci USA. 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer JA, Dougherty DA. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J Biol Chem. 2003;278:21076–21082. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- 28.Blount P, Sukharev SI, Schroeder MJ, Nagle SK, Kung C. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:11652–11657. doi: 10.1073/pnas.93.21.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anishkin A, et al. On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL. J Gen Physiol. 2003;121:227–244. doi: 10.1085/jgp.20028768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang LM, et al. Three routes to modulate the pore size of the MscL channel/nanovalve. ACS Nano. 2012;6:1134–1141. doi: 10.1021/nn203703j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walton TA, Rees DC. Structure and stability of the C-terminal helical bundle of the E. coli mechanosensitive channel of large conductance. Protein Sci. 2013;22:1592–1601. doi: 10.1002/pro.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274:761–765. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 33.Ando C, Liu N, Yoshimura K. A cytoplasmic helix is required for pentamer formation of the Escherichia coli MscL mechanosensitive channel. J Biochem. 2015;158:109–114. doi: 10.1093/jb/mvv019. [DOI] [PubMed] [Google Scholar]
- 34.Reading E, et al. The Effect of Detergent, Temperature, and Lipid on the Oligomeric State of MscL Constructs: Insights from Mass Spectrometry. Chem Biol. 2015;22:593–603. doi: 10.1016/j.chembiol.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorwart MR, Wray R, Brautigam CA, Jiang Y, Blount P. S. aureus MscL is a pentamer in vivo but of variable stoichiometries in vitro: implications for detergent-solubilized membrane proteins. PLoS Biol. 2010;8:e1000555. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi CS, Walton TA, Rees DC. OCAM: a new tool for studying the oligomeric diversity of MscL channels. Protein Sci. 2011;20:313–326. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iscla I, Wray R, Blount P. The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Protein Sci. 2011;20:1638–1642. doi: 10.1002/pro.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iscla I, Levin G, Wray R, Reynolds R, Blount P. Defining the physical gate of a mechanosensitive channel, MscL, by engineering metal-binding sites. Biophys J. 2004;87:3172–3180. doi: 10.1529/biophysj.104.049833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moe PC, Levin G, Blount P. Correlating a protein structure with function of a bacterial mechanosensitive channel. J Biol Chem. 2000;275:31121–31127. doi: 10.1074/jbc.M002971200. [DOI] [PubMed] [Google Scholar]
- 42.Smart OS, Breed J, Smith GR, Sansom MS. A novel method for structure-based prediction of ion channel conductance properties. Biophys J. 1997;72:1109–1126. doi: 10.1016/S0006-3495(97)78760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saint N, et al. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J Biol Chem. 1996;271:20676–20680. [PubMed] [Google Scholar]
- 44.Kreusch A, Schulz GE. Refined structure of the porin from Rhodopseudomonas blastica. Comparison with the porin from Rhodobacter capsulatus. J Mol Biol. 1994;243:891–905. doi: 10.1006/jmbi.1994.1690. [DOI] [PubMed] [Google Scholar]
- 45.Ujwal R, et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004;86:2883–2895. doi: 10.1016/S0006-3495(04)74340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–298. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukharev S, Betanzos M, Chiang CS, Guy HR. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 50.Koprowski P, et al. Cytoplasmic Domain of MscS Interacts with Cell Division Protein FtsZ: A Possible Non-Channel Function of the Mechanosensitive Channel in Escherichia Coli. PLoS One. 2015;10:e0127029. doi: 10.1371/journal.pone.0127029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox CD, et al. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat Commun. 2013;4:2137. doi: 10.1038/ncomms3137. [DOI] [PubMed] [Google Scholar]
- 52.Moe PC, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 53.Vagin AA, et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol Crystallogr. 2004;60:2184–2195. doi: 10.1107/S0907444904023510. [DOI] [PubMed] [Google Scholar]
- 54.Cowan SW, et al. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 55.Schmid B, Maveyraud L, Kromer M, Schulz GE. Porin mutants with new channel properties. Protein Sci. 1998;7:1603–1611. doi: 10.1002/pro.5560070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinac B, Rohde PR, Cranfield CG, Nomura T. Patch clamp electrophysiology for the study of bacterial ion channels in giant spheroplasts of E. coli. Methods Mol Biol. 2013;966:367–380. doi: 10.1007/978-1-62703-245-2_23. [DOI] [PubMed] [Google Scholar]
- 57.Schnorf M, Potrykus I, Neuhaus G. Microinjection technique: routine system for characterization of microcapillaries by bubble pressure measurement. Exp Cell Res. 1994;210:260–267. doi: 10.1006/excr.1994.1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.