Abstract
The aim of this study was an examination of 240 multifloral honey samples collected from Polish apiaries to determine Clostridium botulinum occurrence. Honey was collected from apiaries directly after the extraction process. Samples were inoculated by using the dilution and centrifugation method. Suspected isolates were examined by using mouse bioassay, polymerase chain reaction (PCR), and real-time PCR methods. C. botulinum type A and B strains were detected in 5 of 240 examined honey samples (2.1%). Bacterial strains were also detected that were phenotypically similar to C. botulinum but that did not exhibit the ability to produce botulinum toxins and did not show the presence of the botulinum cluster (ntnh and bont genes) or expression of the ntnh gene. The methods used in the examination, especially the expression analysis of ntnh gene, enabled specific analysis of suspected strains and could be used routinely in environmental isolate analyses of C. botulinum occurrence.
Keywords: Clostridium botulinum, Polish apiaries, honey, neurotoxins
Introduction
Clostridium botulinum is a bacterium commonly found in soil and aquatic environments. This species is divided into four physiological groups (I–IV) which produce botulinum neurotoxins (BoNTs) and into eight different serotypes (A–G and X) [3,6,11,12,28]. BoNT/X, isolated in 2017, is the first BoNT serotype identified by applying sequencing and bioinformatics approaches [28]. C. botulinum strains, being divided into four genetically diverse metabolic groups, have inter-group heterogeneity, which causes problems in the detection of this microorganism.
Foodborne botulism is a severe type of food poisoning caused by the ingestion of food containing potent neurotoxins formed during the growth of C. botulinum [12]. Infant botulism is a common form, to which children between 2 weeks old and 1 year old are most susceptible [19]. It differs from foodborne botulism which proceeds after ingestion of the ready-formed neurotoxin, while in infant botulism the immature infantile intestinal flora allows ingested spores to germinate, multiply, and produce BoNTs in the intestinal lumen. The first infant botulism case was reported in the USA in 1976 [20]. Subsequently, numerous studies have associated the occurrence of infant botulism with the consumption of honey [15,19,24]. Given such evidence, the Centers for Disease Control and Prevention have issued a special recommendation that honey should not be given to infants under the age of 1 year [24]. It is significant that honey contaminated with C. botulinum spores does not differ in taste, color, or smell from uncontaminated honey [1].
The aim of this study was to examine honey samples collected from Polish apiaries situated in all 16 provinces of Poland for the presence of C. botulinum. This is the first representative study on the occurrence of C. botulinum in honey collected from the entire area of Poland.
Materials and Methods
Samples
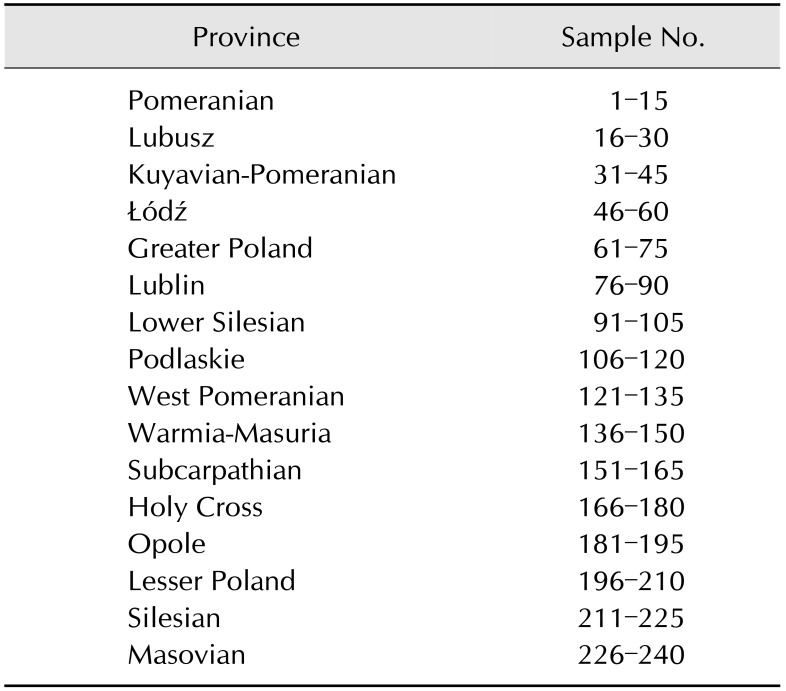
The study was carried out on 240 honey samples (1 sample = 1 apiary) from 16 provinces (15 samples per province) in Poland (Table 1). Honey was collected in 2015 and 2016, directly from apiaries after the extraction process. The analyses were conducted using C. botulinum reference stains from the National Collection of Type Cultures (NCTC) collection as controls: NCTC 887 (toxinotype A), NCTC 3815 (toxinotype B), NCTC 8548 (toxinotype C), NCTC 8265 (toxinotype D), NCTC 8266 (toxinotype E), and NCTC 10281 (toxinotype F).
Table 1. Province of origin and numbers of samples collected from each Polish province.
Culture process
The direct centrifugation method, previously described [13,19], was used for culturing C. botulinum. A mass of 10 g of each honey sample was diluted in 90 mL of sterile distilled water with 1% Tween 80, and the mixture stirred until the solution became homogeneous. Subsequently, centrifugation was conducted for 30 min at 9,000 × g in a 4K15 centrifuge (Sigma, Germany). The precipitates were transferred into 90 mL of tryptone peptone glucose yeast extract broth (TPGY; 50 g/L casein enzymic hydrolysate, 5 g/L peptic digest of animal tissue, 20 g/L yeast extract, 4 g/L dextrose, and 1 g/L sodium thioglycolate with a final pH of 7.0 ± 0.2 at 25℃). The inocula were pasteurized for 15 min at 70 ± 2℃ and then incubated under anaerobic conditions at 30 ± 1℃ for 7 days. After incubation, 1 mL of each liquid culture was transferred to tubes containing 10 mL of TPGY broth. Simultaneously, a few drops from each liquid culture were inoculated onto plates with Willis-Hobbs medium (10 g/L peptic digest of animal tissue, 10 g/L meat extract, 5 g/L sodium chloride, 12 g/L lactose, 0.032 g/L neutral red, and 10 g/L agar with a final pH of 7.0 ± 0.2 at 25℃) and fastidious anaerobe agar (FAA) medium (23 g/L peptone, 5 g/L sodium chloride, 1 g/L soluble starch, 0.4 g/L sodium bicarbonate, 1 g/L glucose, 1 g/L sodium pyruvate, 0.5 g/L L-cysteine HCl × H2O, 0.25 g/L sodium pyrophosphate, 1 g/L L-arginine, 0.5 g/L sodium succinate, 0.01 g/L haemin, 0.001 g/L vitamin K, 12 g/L agar with a final pH of 7.2 ± 0.2 at 25℃). The inoculated tubes and plates were incubated at 30 ± 1℃ for 48 h.
Strains considered to be C. botulinum that were isolated from Willis-Hobbs and FAA media were evaluated by examination of the surface, shape, size, and lipolytic and proteolytic features of the cultures. In order to evaluate BoNT production, colonies suspected of belonging to C. botulinum were inoculated into tubes with 10 mL of TPGY broth and incubated anaerobically for 48 h at 30 ± 1℃.
Nucleic acids preparation
DNA preparation
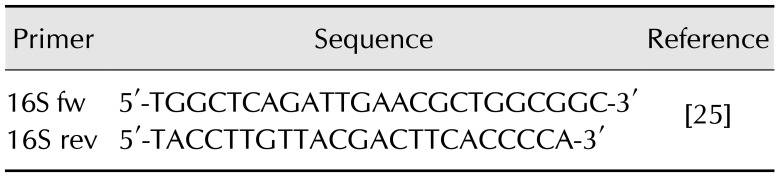
DNA was isolated from 1 mL of liquid culture and from several characteristic colonies obtained on agar plates by using the commercial Genomic Mini AX Bacteria kit (A&A Biotechnology, Poland) according to the manufacturer's instructions. The DNA isolated from suspected C. botulinum strains was subjected to amplification of the 16S rDNA gene according to the method described by Vaneechoutte et al. [25]. The isolated DNA from liquid culture and the suspected strains were examined to detect ntnh and bont genes by using polymerase chain reaction (PCR) and real-time PCR techniques.
RNA preparation
RNA was extracted from isolates suspected of being C. botulinum. Total RNA was prepared by using the commercial Total RNA Mini kit (A&A Biotechnology). At least 500 ng of total RNA were subjected to cDNA synthesis.
cDNA synthesis
cDNA was synthesized with a High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, USA). Before being subjected to real-time PCR, the cDNA was diluted 10 times to achieve a concentration of approximately 100 ng per reaction mixture.
Molecular methods
Amplification and Sequencing of 16S rDNA
For identification of 16S rDNA from unidentified anaerobic strains suspected of being C. botulinum, primers previously described by Vaneechoutte et al. [25] were used (Table 2). Reactions were performed in the volume of 25 µL with the following reagent constituents: 5 µL of DNA matrix, 2.5 µL 10× Taq buffer with KCl (Fermentas, Lithuania), 4 mM MgCl2, 200 µM dNTP, and 1.25 U per 25 µL Taq polymerase. The reaction was staged as follows: initial denaturation at 95℃ for 5 min, 35 cycles of denaturation at 95℃ for 45 sec, annealing at 55℃ for 45 sec, extension at 72℃ for 1 min, and a final extension at 72℃ for 10 min.
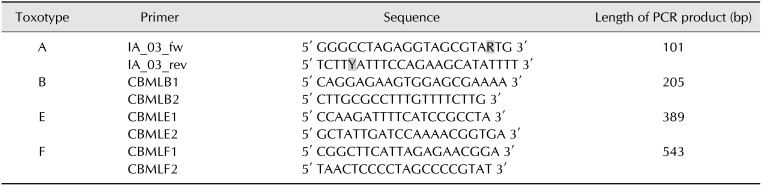
Table 2. Sequences of primers used in amplification of the conservative 16S rDNA region.
The length of the obtained product was about 1500 bp. Sequencing of the obtained amplicons was entrusted to Genomed (Poland). The obtained FASTA files were analyzed by using the BLAST (National Center for Biotechnology Information, NCBI) algorithm, and the results were compared and assigned to the sequences from the NCBI that had the highest score and identity.
Real-time PCR for ntnh and 16S rRNA housekeeping gene
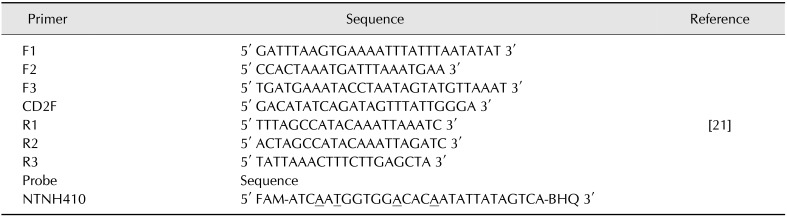
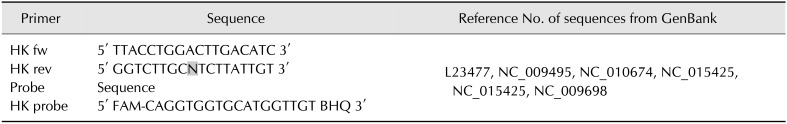
A set of 7 primers and a TaqMan probe (Table 3) were used for detection of the ntnh gene according to the method described by Raphael and Andreadis [21]. For expression analysis, a set of 2 primers and a TaqMan probe designed (Table 4) for C. botulinum sequences, and available from the NCBI database, was used additionally to detect the 16S rRNA housekeeping gene. The reactions for both genes were conducted with reagents comprising: 5 µL DNA or cDNA, 4 µL LightCycler TaqMan Master (Roche Diagnostics, Germany), 0.7 µM of each primer, and 0.24 µM TaqMan probe. Real-time PCR was performed by using a LightCycler 2.0 thermocycler with a temperature profile of 10 min at 95℃ for initial denaturation, 45 cycles of denaturation at 95℃ for 15 sec, annealing at 42℃ for 15 sec, and elongation at 55℃ for 1 min.
Table 3. Sequences of primers and the molecular probe used for real-time polymerase chain reaction for ntnh gene detection.
All locked nucleic acids are marked with underlines (A;T). FAM, carboxyfluorescein; BHQ, black hole quencher.
Table 4. Primers and molecular probe for housekeeping 16S rRNA gene detection in real-time polymerase chain reaction.
N = A/C/G/T. FAM, carboxyfluorescein; BHQ, black hole quencher.
Normalization of the expression changes was performed according to Schmittgen and Livak's protocol [22], on the assumption that reaction efficiency was close to 100%. The efficiency (E) was calculated on the basis of the slope value (m) obtained from the relationship between the cycle threshold (Ct) and dilutions of cDNA, according to the formula: E = 10(−1/m).
Calculation of ntnh gene expression
Relative expression was calculated according to the comparative method described by Schmittgen and Livak [22]. The calculations were undertaken by applying the following formula: fold change = 2−ΔΔCt, where ΔΔCt = [(Ct gene of interest − Ct internal control)sample A − (Ct gene of interest − Ct internal control)sample B]. The reference C. botulinum NCTC 887 strain (toxin type A) served as sample A and the isolates obtained from positive honey were used as sample B as shown in the following:
ΔΔCt = [(Ctntnh − Ct16S rRNA)C. botulinum NCTC 887 − (Ctntnh − Ct16S rRNA)C. botulinum isolated from honey]. All samples were tested three times, and, for further analyses, the mean value was used.
Multiplex PCR for bont/A, bont/B, bont/E, and bont/F genes
The bont/A, B, E, and F genes were detected according to the multiplex PCR (mPCR) method described by De Medici et al. [5] and by utilizing four pairs of primers (Table 5). The reaction was prepared in a volume of 25 µL with the following set of reagents: 5 µL of DNA, 0.3 µM of each primer, 2.5 µL of 10× Taq buffer with KCl (Thermo Fisher Scientific), 4 mM of MgCl2, 200 µM of dNTP, and 1.25 U per 25 µL of Taq polymerase (Thermo Fisher Scientific). Detection of products was carried out on agarose gel.
Table 5. Primers used in multiplex PCR method for detection of bont/A, B, E, F genes.
R = A and G; Y = C and T. PCR, polymerase chain reaction.
Gel electrophoresis was conducted on 2% agarose gel stained with SimplySafe (EURx, Poland) and was run in 1× TBE buffer (Thermo Fisher Scientific) for 1.5 h at 100 V. The reaction mixture in a 10 µL volume with 2 µL of loading buffer, 6× DNA Loading Dye (Thermo Fisher Scientific), were loaded into each well. The molecular weights of the obtained products were compared with the GeneRuler 100 bp DNA Ladder Mix (Thermo Fisher Scientific) molecular weight marker. Finally, PCR products were analyzed under a Chemi-Smart 3000 UV light transilluminator (Vilber-Lourmat, France).
Mouse bioassay (MBA)
In order to verify positive PCR results, an MBA was performed for isolates considered to be C. botulinum. The single experiment involved three laboratory mice and followed procedure described by Solomon and Lilly [23]. After centrifugation of liquid culture in TPGY broth, the supernatant was divided into three 0.2 mL portions. One portion was heated at 100 ± 2℃ for 10 min and, after cooling, administered intraperitoneally to one mouse. The other two portions were administered intraperitoneally into two mice, one of which had previously been seroneutralized by treatment with equine monovalent antitoxin to BoNT A (BoNT/A) and B (BoNT/B) (HPA, UK). All experiments on animals were conducted in an approved laboratory unit after obtaining permission from the II Local Ethical Committee in Lublin (Poland) (permission No. 5/2015).
Results
Culture characteristics
Eight bacterial strains with phenotypic features characteristic of C. botulinum were isolated from the examined samples. The obtained colonies exhibited the characteristic “pearl layer” and their precipitation zones indicated lipolytic properties. Proteolytic activity of all isolates (bright zones surrounding the colonies) on agar media was also observed (Table 6).
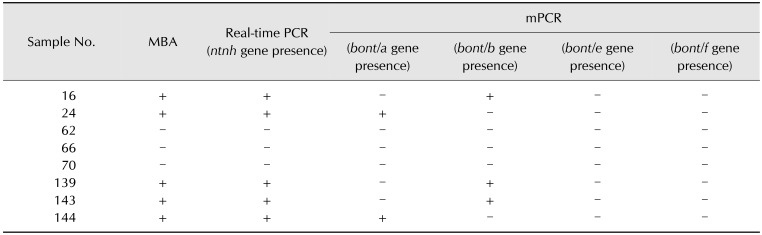
Table 6. Summary of culture, MBA, real-time PCR, and mPCR results obtained for suspected Clostridium botulinum isolates from examined honey samples.
Only samples from which characteristic colonies suspected of belonging to C. botulinum were isolated. MBA, mouse bioassay; PCR, polymerase chain reaction; mPCR, multiplex PCR.
Sequencing analysis of 16S rDNA
Analysis of 16S rDNA sequences revealed that 6 isolates among the 8 isolates phenotypically similar to C. botulinum had the highest scores and identities with C. botulinum in the BLAST search. These strains were isolated from samples isolated from Lubusz, Greater Poland, and the Warmia-Masuria provinces. The percentage identity of all 8 examined isolates with phenotypic features characteristic of C. botulinum ranged from 91% to 99% (Table 7).
Table 7. Sequencing analysis of isolates phenotypically similar to Clostridium botulinum.
Real-time PCR for ntnh
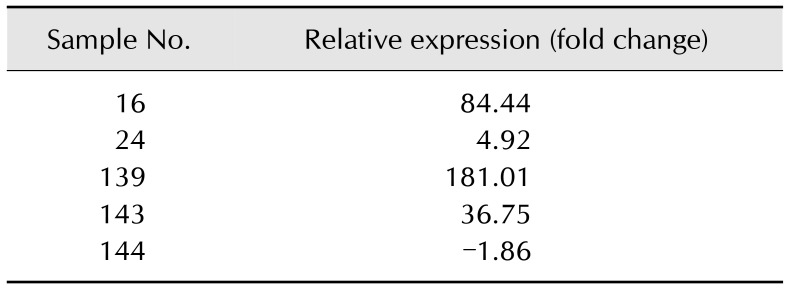
The ntnh gene was detected in only 5 samples (16, 24, 139, 143, and 144). Positive results were obtained for DNA extracted from liquid culture and from isolates (Table 6).
Multiplex PCR for detection of bont/A, bont/B, bont/E, and bont F genes
The occurrences of bont/A and bont/B strains were detected in 5 of the 240 examined honey samples (2.1%). Two of the detected strains were qualified as toxinotype A and three as toxinotype B (Table 6).
Expression analysis of ntnh gene
The efficiency of the real-time PCR was calculated at 92.89% for the ntnh gene and at 96.38% for the 16S rRNA housekeeping gene. Relative expression was assessed for the C. botulinum isolates and is presented as a fold change relative to ntnh gene expression from the C. botulinum NCTC 887 reference strain. Relative expression values are reported in Table 8.
Table 8. Relative expression of honey isolates.
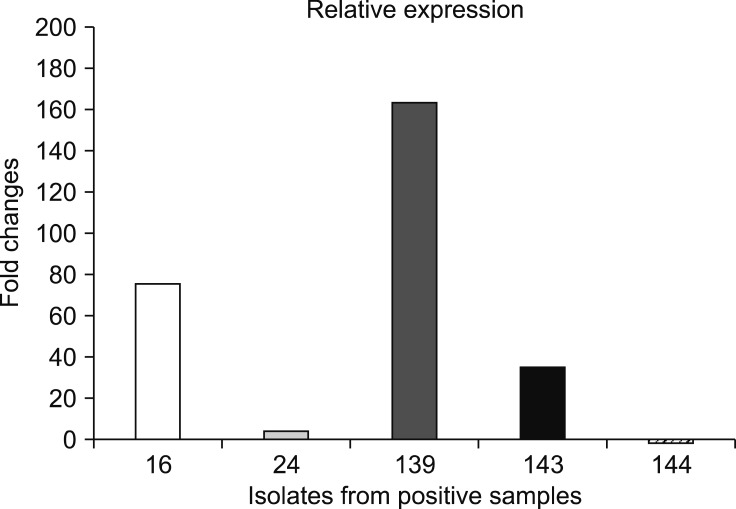
After data analysis, the highest expression level for a C. botulinum isolate was from sample 139 and was calculated at indicating a 181.01-fold change relative to the NCTC 887 reference strain. The lowest expression was calculated for an isolate from sample 144 and was equal to a −1.86-fold change relative to NCTC 887 (Fig. 1). Positive samples were collected from Lubusz, Greater Poland, and the Warmia-Masuria provinces.
Fig. 1. Relative expression of the ntnh gene from Clostridium botulinum isolates. Fold changes obtained after expression analysis of ntnh gene for isolates from samples 16, 24, 139, 143, and 144 in relation to the C. botulinum NCTC 887 reference strain. The highest expression level for C. botulinum isolate was from sample 139 and was calculated as a 181.01-fold change. The lowest expression was noticed for an isolate from sample 144 and equaled a −1.86-fold change.
MBA results
The MBA test proved the specificity of results that were obtained in the PCR and expression analyses. Positive results were obtained for only 5 of the samples included above.
Discussion
Since 1976, there have been over 1,500 cases of infant botulism reported in more than 15 countries worldwide [16]. Among the various potential sources of C. botulinum spores (soil, dust, etc.), honey is the only dietary source that has been linked to botulism through both laboratory and epidemiological studies [1,17,18].
The dose of C. botulinum spores that can cause infection in human infants is undetermined. In a review by Austin [2], the author stated that a minimum dose for infant botulism has not been established. Arnon et al. [1] estimated that 10 to 100 C. botulinum spores are able to cause an infection. The lowest minimum number of cells needed to cause botulism was obtained in a sample of honey from Canada, which contained 1 spore per gram of honey. These figures were calculated from honey samples involved in actual infant botulism cases [15] and are based on the exposure of human infants to spore-containing honey. Therefore even 1 spore per gram can pose a potential risk of infant botulism [7].
The occurrence of C. botulinum noticed in Polish apiaries (2%) is lower or similar to the C. botulinum presence reported in samples from most other countries. Nevas et al. [19] described contamination of 25.9% (29/112) of Danish samples by C. botulinum spores (1 sample positive for toxin type A and 28 for type B). Nevas et al. [19] also reported that C. botulinum occurrence in Finnish honey samples was 10.5% (20/190; 8 samples showing type A toxin and 12 showing type B). In Norway, the percentage of positive samples was determined to be 10.7% (12/112; 7 of type B, 4 of type E, and 1 of type F) [19]. The lowest level of contamination in Nordic countries was observed in Swedish samples where it was 2% (1 occurrence of toxin type E) [19]. In Japan, C. botulinum occurrence has been reported twice; according to Nakano and Sakaguchi [18], the percentage of positive samples was 30.6% (11/36), whereas Nakano et al. [17] observed the occurrence of C. botulinum in 8.5% of the examined samples. In Taiwan, C. botulinum presence was detected in 1.3% (2/152) of examined samples [10]. In Turkey, Küplülü et al. [13] described C. botulinum spore detection in 12.5% of honey samples. Whereas Gücükoğlu et al. [10] reported a 2.7% (4/150) prevalence of C. botulinum spores in honey collected from the Turkish Black Sea region. Midura [15] reported C. botulinum occurrence in 10.0% (9/90) of examined samples from the USA. In Kazakhstan, Mustafina et al. [16] noted C. botulinum occurrence in only 0.9% (1/110) of honey samples. Polish honey was examined by two independent scientific teams and a sample contamination level of 8.6% (6/70) from the Lublin and Subcarpathia provinces of Poland was described by Grenda et al. [8], whereas Wojtacka et al. [27] detected C. botulinum spores in 21.6% (22/102) of samples from small apiaries in an undetermined area in Poland. Wojtacka et al. [26] described a high prevalence of C. botulinum spores in Lithuanian honey sold directly from the apiary, 62.5% (30/48), which is the highest level described to date in related literature. The procedure described by Wojtacka et al. [26,27] comprised only one mPCR method, which, in our opinion, is inadequate to survey C. botulinum occurrence in honey samples. The data in relevant literature and our own experience indicate the possibility of false-positive results caused by silent genes or frequently noticed non-specific PCR products, especially with detection performed on agarose gel [9]. The level of naturally occurring C. botulinum spore contamination is estimated to be in the range of 10 to 1,000 spores per gram of honey [14].
The detection of C. botulinum spores is a complicated task because of the high heterogenicity of this pathogen and horizontal gene transfer during the isolation process. The genus Clostridium comprises strains that show similar biochemical features to C. botulinum but are not able to produce botulinum toxins. Because of their heterogenicity, C. botulinum strains are classified into four metabolic groups, and other microorganisms that are not considered able to produce botulinum toxins are also related to these groups (excluding some toxinogenic strains of Clostridium butyricum and Clostridium beijerinckii with the former having been reported as the causative agent in some infant botulism cases). Clostridium sporogenes is related to group I, C. beijerinckii and C. butyricum to group II, Clostridium novyi to group III, and Clostridium subterminale and Clostridium schirmacherense are related to group IV. In the honey samples of this study, we detected strains phenotypically similar to C. botulinum that did not show the presence of ntnh or bont genes. On the basis of 16S rDNA analysis, these strains were most related to C. botulinum and C. sporogenes [3,4]. This method used in this study enabled discrimination of suspected isolates only to the genus level. Among the 6 strains with the highest score and a C. botulinum identity, only 5 were classified to C. botulinum on the basis of real-time PCR, mPCR, and MBA analyses. The contamination level of C. botulinum spores could be dependent on the harvesting region of the honey samples and on hygienic aspects of the entire honey harvest process. According to Nevas et al. [19], the most influential factors on the presence of C. botulinum spores are: extractor size, wearing the same footwear outdoors and in the extraction room, the availability of hand-washing facilities in the extraction room, and the presence of C. botulinum in soil samples. The variability in phenotypic features among the strains of this pathogen, the silent bont genes, and the high probability of toxin gene loss in culture process (through the subsequent culture passages) beset C. botulinum detection with difficulties.
The set of methods used in this study enabled specific detection of C. botulinum and we recommend using them in routine analyses of honey samples for the occurrence of this pathogen. Simultaneous detection of ntnh and bont genes enables false positive results caused by non-specific products to be avoided. The common presence of the ntnh gene in all C. botulinum toxin types simplifies screening of suspected strains. In addition, expression analysis is easier because it is limited to only one gene. The presented set of methods could be used as a set of tools for supporting the laboratory diagnosis of botulism, thereby serving as an alternative to performing MBA for C. botulinum detection.
The number of Clostridia spores sufficient for infection is undetermined; however, even a single colony forming unit of C. botulinum could cause botulism symptoms in an infant. The obtained results have shown that risk assessment of the entire honey harvesting process should be undertaken in order to ensure the microbiological safety of the product, especially for infants and people with weakened immune systems.
Acknowledgments
This publication was funded by the KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal - Safe Food”, Polish Ministry of Science and Higher Education (resolution No. 05-1/KNOW2/2015).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Arnon SS, Midura TF, Damus K, Thompson B, Wood RM, Chin J. Honey and other environmental risk factors for infant botulism. J Pediatr. 1979;94:331–336. doi: 10.1016/s0022-3476(79)80863-x. [DOI] [PubMed] [Google Scholar]
- 2.Austin JW. Clostridium botulinum. In: Beuchat LR, Doyle MP, Montville TJ, editors. Food Microbiology: Fundamentals and Frontiers. 2nd ed. Washington: ASM Press; 2001. pp. 329–349. [Google Scholar]
- 3.Carter AT, Peck MW. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res Microbiol. 2015;166:303–317. doi: 10.1016/j.resmic.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins MD, East AK. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J Appl Microbiol. 1998;84:5–17. doi: 10.1046/j.1365-2672.1997.00313.x. [DOI] [PubMed] [Google Scholar]
- 5.De Medici D, Anniballi F, Wyatt GM, Lindström M, Messelhäusser U, Aldus CF, Delibato E, Korkeala H, Peck MW, Fenicia L. Multiplex PCR for detection of botulinum neurotoxin-producing clostridia in clinical, food, and environmental samples. Appl Environ Microbiol. 2009;75:6457–6461. doi: 10.1128/AEM.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin type H gene. J Infect Dis. 2014;209:192–202. doi: 10.1093/infdis/jit450. [DOI] [PubMed] [Google Scholar]
- 7.Glass K, Marshall K. Clostridium botulinum. In: Morris JG, Potter ME, editors. Foodborne Infections and Intoxications. 4th ed. Boston: Elsevier; 2013. pp. 371–387. [Google Scholar]
- 8.Grenda T, Grabczak M, Kwiatek K, Bober A. Prevalence of C. botulinum and C. perfringens spores in food products available on Polish market. J Vet Res. 2017;61:287–291. doi: 10.1515/jvetres-2017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grenda T, Kukier E, Kwiatek K. Methods and difficulties in detection of Clostridium botulinum and its toxins. Pol J Vet Sci. 2014;17:195–205. doi: 10.2478/pjvs-2014-0029. [DOI] [PubMed] [Google Scholar]
- 10.Gücükoğlu A, Terzi G, Çadirci Ö, Alişarli M, Kevenk O, Uyanik T. Detection of C. botulinum types in honey by mPCR. J Food Sci. 2014;79:M600–M603. doi: 10.1111/1750-3841.12402. [DOI] [PubMed] [Google Scholar]
- 11.Hatheway CL. Botulism: the present status of the disease. Curr Top Microbiol Immunol. 1995;195:55–75. doi: 10.1007/978-3-642-85173-5_3. [DOI] [PubMed] [Google Scholar]
- 12.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Küplülü Ö, Göncüoğlu M, Özdemir H, Koluman A. Incidence of Clostridium botulinum spores in honey in Turkey. Food Control. 2006;17:222–224. [Google Scholar]
- 14.Lindström M, Korkeala H. Laboratory diagnostics of botulism. Clin Microbiol Rev. 2006;19:298–314. doi: 10.1128/CMR.19.2.298-314.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Midura TF. Update: infant botulism. Clin Microbiol Rev. 1996;9:119–125. doi: 10.1128/cmr.9.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafina R, Maikanov B, Wiśniewski J, Tracz M, Anusz K, Grenda T, Kukier E, Goldsztejn M, Kwiatek K. Contamination of honey produced in the Republic of Kazakhstan with Clostridium botulinum. Bull Vet Inst Pulawy. 2015;59:241–246. [Google Scholar]
- 17.Nakano H, Okabe T, Hashimoto H, Sakaguchi G. Incidence of Clostridium botulinum in honey of various origins. Jpn J Med Sci Biol. 1990;43:183–195. doi: 10.7883/yoken1952.43.183. [DOI] [PubMed] [Google Scholar]
- 18.Nakano H, Sakaguchi G. An unusually heavy contamination of honey products by Clostridium botulinum type F and Bacillus alvei. FEMS Microbiol Lett. 1991;63:171–177. doi: 10.1016/0378-1097(91)90081-k. [DOI] [PubMed] [Google Scholar]
- 19.Nevas M, Lindström M, Hautamäki K, Puoskari S, Korkeala H. Prevalence and diversity of Clostridium botulinum types A, B, E and F in honey produced in the Nordic countries. Int J Food Microbiol. 2005;105:145–151. doi: 10.1016/j.ijfoodmicro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Pickett J, Berg B, Chaplin E, Brunstetter-Shafer MA. Syndrome of botulism in infancy: clinical and electrophysiologic study. N Engl J Med. 1976;295:770–772. doi: 10.1056/NEJM197609302951407. [DOI] [PubMed] [Google Scholar]
- 21.Raphael BH, Andreadis JD. Real-time PCR detection of the nontoxic nonhemagglutinin gene as a rapid screening method for bacterial isolates harboring the botulinum neurotoxin (A-G) gene complex. J Microbiol Methods. 2007;71:343–346. doi: 10.1016/j.mimet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Solomon HM, Lilly T., Jr . Clostridium botulinum. In: Merker RI, editor. Bacteriological Analytical Manual Online, Revision A. Maryland: Center for Food Safety and Applied Nutrition, Food and Drug Administration; 2001. [Google Scholar]
- 24.Tanzi MG, Gabay MP. Association between honey consumption and infant botulism. Pharmacotherapy. 2002;22:1479–1483. doi: 10.1592/phco.22.16.1479.33696. [DOI] [PubMed] [Google Scholar]
- 25.Vaneechoutte M, Cartwright CP, Williams EC, Jäger B, Tichy HV, De Baere T, De Rouck A, Verschraegen G. Evaluation of 16S rRNA gene restriction analysis for the identification of cultured organisms of clinically important Clostridium species. Anaerobe. 1996;2:249–256. [Google Scholar]
- 26.Wojtacka J, Wysok B, Kabašinškienė A, Wiszniewska-Łaszczych A, Gomółka-Pawlicka M, Szteyn J, Malakauskas M, Migowska-Calik A. Prevalence of Clostridium botulinum type A, B, E and F isolated from directly sold honey in Lithuania. J Agr Sci Tech. 2017;19:335–343. [Google Scholar]
- 27.Wojtacka J, Wysok B, Lipiński Z, Gomółka-Pawlicka M, Rybak-Chmielewska H, Wiszniewska-Łaszczych A. Clostridium botulinum spores found in honey from small apiaries in Poland. J Apic Sci. 2016;60:89–100. [Google Scholar]
- 28.Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, Henriksson L, Miyashita SI, Martínez-Carranza M, Dong M, Stenmark P. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017;8:14130. doi: 10.1038/ncomms14130. [DOI] [PMC free article] [PubMed] [Google Scholar]