Abstract
Aim
There are no effective, tolerable, and established medications for preventing delirium in critically ill patients admitted to the intensive care unit (ICU). We investigated whether suvorexant was effective in preventing ICU delirium.
Methods
This randomized controlled study evaluated 70 adult patients (age ≥20 years) admitted to the mixed medical ICU of the Tokyo Medical University Hospital (Tokyo, Japan) between May 2015 and February 2017. Patients were randomized using a sealed envelope method to receive either suvorexant (n = 34; 15 mg for elderly patients and 20 mg for younger adults) or conventional treatment (n = 36) for a 7‐day period. The primary outcome was delirium incidence based on the definition in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders.
Results
No significant between‐group differences were observed in the demographic or clinical characteristics. Kaplan−Meier estimates revealed that time to delirium onset was significantly longer in the suvorexant group than in the conventional group (P < 0.05).
Conclusion
Suvorexant might be effective in preventing delirium in ICU patients.
Keywords: Delirium, intensive care unit, prevention, suvorexant
Introduction
Delirium is a clinical syndrome of acute cerebral dysfunction characterized by three cardinal features: fluctuating mental status, inattention, and altered level of consciousness or disorganized thinking.1 Delirium develops with changes in environmental and physical conditions, with a prevalence of 3–56% among hospitalized patients.1, 2 The risk of delirium is elevated in elderly and physically weak individuals, such as patients in the intensive care unit (ICU) and those receiving mechanical ventilation.3 Patients in the ICU who develop delirium have prolonged ICU and hospital stays, with associated higher in‐hospital and long‐term mortality rates and a higher incidence of long‐term cognitive dysfunction.4 Therefore, the development of safe and effective treatments for preventing delirium in the ICU is important. However, there are no approved medications for the treatment of ICU delirium, and there is insufficient evidence to support the preventive effect of antipsychotic drugs on delirium, notwithstanding their limited therapeutic effects on delirium.5 Moreover, antipsychotic drugs are associated with severe side‐effects, such as cardiovascular events and an increased mortality risk.6
The pathophysiologic mechanisms underlying delirium, although still unclear, are related to imbalances in neurotransmitters modulating cognition, behavior, and mood. The average medical ICU patient has 11 or more risk factors for developing delirium, which can be divided into predisposing baseline factors like underlying patient characteristics and comorbidities and hospital‐related (precipitating) factors like acute illness, treatment, and ICU management.7
The pathophysiology of delirium is proposed to be related to sleep disturbances in critically ill patients. Sleep hygiene programs in ICUs improve sleep quality and quantity and can reduce delirium incidence and duration by >50%.8, 9 Attention disturbances, a core delirium symptom, are among the most important symptoms and a critical item in the diagnostic criteria for delirium based on the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM‐5).10, 11 Attention disturbances are caused by dysfunction in the ascending reticular activating system, which regulates arousal. Arousal maintenance is controlled by multiple neurotransmitters (acetylcholine, dopamine, serotonin, histamine, and noradrenaline) released from neurons in the ascending reticular activating system and brainstem.12, 13 In the hypothalamus, orexin plays an important role in awakening. Orexin‐containing neurons help integrate metabolism, stress response, and circadian rhythms, along with the governance of various neurotransmitters involved in the sleep/wake cycle. de Lecea et al.13 reported that orexin‐containing neurons were powerful orchestrators of various neurotransmitters involved in sleep/wake cycle dynamics. We hypothesize that sleep/wake rhythm improvement using the orexin receptor antagonist suvorexant leads to attention disturbance reduction and subsequent delirium prevention in ICU patients. The neuropeptide orexin is widely thought to be involved in sleep/wake cycle regulation.14 Suvorexant is an orexin receptor antagonist approved for the treatment of insomnia in the USA and Japan. Therefore, we aimed to determine whether suvorexant was a safe and effective drug for preventing delirium in ICU patients.
Methods
Participants
This study was carried out between May 2015 and February 2017 at the mixed medical ICU of the Tokyo Medical University Hospital (Tokyo, Japan). The study protocol was approved by the ethical committee at the Tokyo Medical University and written informed consent was obtained from all participants or their surrogate health‐care decision‐makers. Patients aged ≥20 years and admitted to ICU for <24 h were considered eligible. Patients were excluded if they had a life expectancy of <48 h or were unclear of consciousness or had baseline dementia, severe liver dysfunction, history of alcohol or other substance abuse, or had already received medications for delirium since their admission to the ICU (e.g., antipsychotics, antidepressants, and hypnotics).
Randomization and intervention
Participants were randomized to receive either suvorexant or conventional treatment using a random number table and a sealed envelope method. Patients in the conventional treatment group did not receive suvorexant but received trazodone if they experienced insomnia. The suvorexant group received daily doses of 20 mg (age <65 years) or 15 mg (age ≥65 years) suvorexant orally or through a nasogastric tube at 9:00 pm every day until the patient developed delirium or received treatment for seven consecutive days. As the study was undertaken as part of routine clinical practice, patients in both groups received 25 mg trazodone as needed for insomnia. Treatment with benzodiazepines was not permitted in either group because they could affect delirium development.15 In this study, only opioid (fentanyl) was selected as an analgesic for intubation patients. If sedation was required, then only dexmedetomidine was selected. Patients in both groups received treatment according to the ABCDEF bundle as a non‐pharmacological approach to delirium.16
Measurements
Nurses in the ICU were trained to evaluate patients for delirium development using the Intensive Care Delirium Screening Checklist (ICDSC).17 Data on patient characteristics were collected at admission, and each patient's physical condition was assessed using the Sequential Organ Failure Assessment (SOFA)18 and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores.19 Both SOFA and APACHE II scores were calculated for all patients. The baseline risk of delirium was evaluated using the Prediction of Delirium for ICU patients (PRE‐DELERIC) delirium risk score,20 which was based on data from blood tests and vital signs throughout the day, from the time of admission to registration for the study.
Outcomes
The primary outcome was delirium incidence in the ICU, based on the DSM‐5 definition of delirium. The trained nurses screened for delirium development using ICDSC at 10:00–11:00 am each morning; the ICDSC cut‐off score was defined as 1, corresponding to subsyndromal delirium, a precursor to delirium.21 Patients with an ICDSC score ≥1 were next evaluated by a board‐certified psychiatrist (KA) to confirm delirium diagnosis.11
Statistical analysis
The Mann–Whitney U‐test and χ2‐test or Fisher's exact test were used for comparing continuous and categorical variables, respectively. Kaplan–Meier curves were used to estimate delirium incidence. All data were analyzed using IBM SPSS software (version 24; IBM, Armonk, NY, USA); significance was set at P < 0.05. Delirium incidence rates of 3–56% during hospitalization2 and 32% in the ICU have been reported.22 In a randomized placebo‐controlled study for delirium prevention, delirium incidence in the ramelteon group was 3%, although one‐third of the subjects in that study were in an ICU, with the remaining two‐thirds in regular acute wards.23 Therefore, delirium incidence rates in the conventional treatment and suvorexant groups in our study were assumed to be 32% and 3% (the lower limit of the range), respectively. To detect a significant difference, we assumed a beta error of 20% and an alpha level of 5%.19 Thus, 35 patients were planned for recruitment per group.
Results
Figure 1 shows the CONSORT flow diagram of the study.24 During the study period, 1,603 patients were admitted to ICU. Of these, 1,533 were excluded because they failed to fulfill the inclusion criteria (n = 1,508), their duration of ICU stay was <48 h (n = 25), or they refused study participation (n = 25). Finally, 70 eligible ICU patients agreed to participate. After randomization, 36 and 34 patients were allocated to the conventional and suvorexant treatment groups, respectively. There were no significant between‐group differences in baseline demographics, clinical characteristics, admission diagnoses, PRE‐DELERIC delirium risk score, or severity of illness (Table 1).
Figure 1.
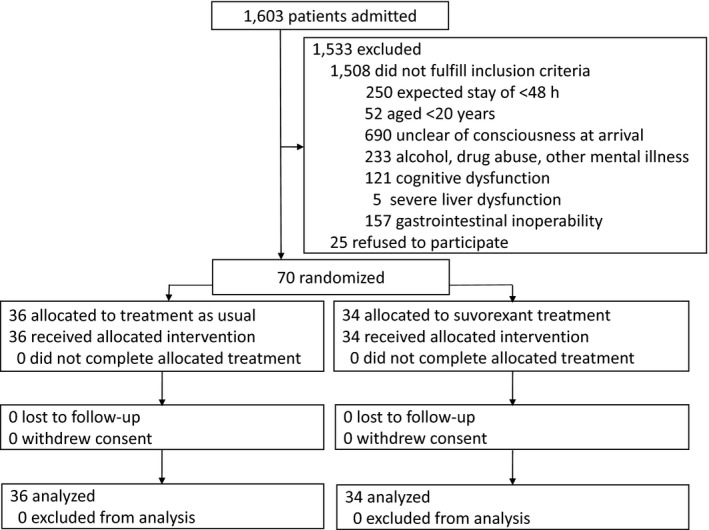
Study flow chart. Seventy adult patients treated in the intensive care unit were enrolled in the study and assigned to the suvorexant (n = 36) or conventional treatment (n = 34) group.
Table 1.
Baseline demographic and clinical characteristics of intensive care unit patients who received suvorexant or conventional treatment for delirium
| Suvorexant treatment group n = 34 | Conventional treatment group n = 36 | P‐value | |
|---|---|---|---|
| Age at enrollment (years) | 61.9 ± 19.6 | 61.6 ± 21.7 | 0.943 |
| Sex, male/female (%) | 27/7 (79.4) | 27/9 (75.0) | 0.778 |
| Hypertension, n | 10 | 11 | 1.000 |
| Diabetes mellitus, n | 4 | 7 | 0.515 |
| Smoker, n | 12 | 10 | 0.609 |
| Admission diagnosis | |||
| Trauma, n (%) | 21 (61.8) | 14 (38.9) | 0.093 |
| Respiratory failure, n (%) | 4 (11.8) | 4 (11.1) | 1.000 |
| Infection, n (%) | 6 (17.6) | 10 (27.8) | 0.398 |
| Heart disease, n (%) | 2 (5.9) | 5 (13.9) | 0.430 |
| Others, n (%) | 1 (2.9) | 3 (8.3) | 0.615 |
| SOFA score | 3.8 ± 3.5 | 4.2 ± 2.5 | 0.608 |
| APACHE II score | 10.6 ± 7.8 | 11.5 ± 7.2 | 0.609 |
| PRE‐DELERIC delirium risk score | 38.4 ± 28.7 | 40.8 ± 28.8 | 0.735 |
| Use of ventilator, n | 17 | 19 | 1.000 |
| Blood urea nitrogen, mmol/L | 8.7 ± 5.9 | 10.7 ± 8.1 | 0.668 |
Data are presented as mean ± standard deviation or number (%). Mann–Whitney U‐test was used to compare continuous variables between the two groups; χ2‐test or Fisher's exact test was used to compare categorical variables between the two groups. APACHE II, Acute Physiology and Chronic Health Evaluation II; PRE‐DELERIC, Prediction of Delirium for ICU patients; SOFA, Sequential Organ Failure Assessment.
No patients developed delirium before 9:00 pm on the day of admission. As a result, all patients in the suvorexant group were given suvorexant prior to the development of delirium. All patients were initially screened for delirium with ICDSC. Six of 34 (17.6%) and 17/36 (47.2%) patients developed subsyndromal delirium in the suvorexant and conventional treatment groups, respectively, indicating a significant between‐group difference in its incidence (P = 0.011). All patients with subsyndromal delirium were next evaluated for delirium based on the DSM‐5 criteria by the study psychiatrist. Delirium incidence tended to be lower in the suvorexant group (5/34; 14.7%) than in the conventional treatment group (12/36; 33.3%; P = 0.069); Kaplan–Meier estimates revealed that time to development of delirium was significantly longer in the former than in the latter (6.26 days, 95% confidence interval, 5.66–6.86 days versus 5.68 days, 95% confidence interval 4.96–6.41 days; log–rank test, P < 0.05; Fig. 2). However, there was no significant between‐group difference in delirium duration (1.6 ± 0.9 days in the suvorexant group versus 3.0 ± 2.3 days in the conventional treatment group; P = 0.236; Table 2).
Figure 2.
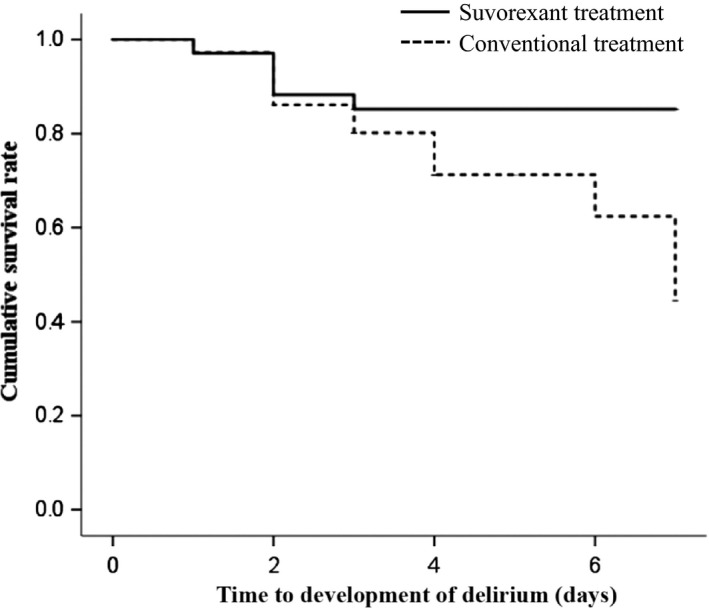
Kaplan–Meier survival curves for patients treated in the intensive care unit who received suvorexant or conventional therapy for delirium. Time to onset of delirium was significantly longer in the suvorexant group (6.26 days, 95% confidence interval, 5.66–6.86 days) than in the conventional treatment group (5.68 days, 95% confidence interval, 4.96–6.41 days; log–rank test, P < 0.05).
Table 2.
Clinical outcomes and use of medication during the study period in intensive care unit patients who received suvorexant or conventional treatment for delirium
| Suvorexant treatment group n = 34 | Conventional treatment group n = 36 | P‐value | |
|---|---|---|---|
| Incidence of subsyndromal delirium by ICDSC, n (%) | 6 (17.6) | 17 (47.2) | 0.011 |
| Incidence of delirium by DSM‐5, n (%) | 5 (14.7) | 12 (33.3) | 0.069 |
| Duration of delirium (days) | 1.6 ± 0.9 | 3.0 ± 2.3 | 0.236 |
| Intensive care unit stay (days) | 5.6 ± 1.6 | 5.1 ± 1.9 | 0.236 |
| Time on ventilation (days) | 2.1 ± 2.6 | 2.1 ± 2.7 | 1.000 |
| Adverse event potentially attributable to study drug (yes/no) | 0/34 (0) | 0/36 (0) | – |
| Dexmedetomidine dose (μg) | 270.6 ± 869.6 | 425.0 ± 1,054.9 | 0.345 |
| Opioid dose (mg) | 3.3 ± 5.7 | 2.8 ± 3.4 | 0.702 |
| Trazodone dose for insomnia (mg) | 2.9 ± 10.2 | 20.8 ± 51.6 | 0.048 |
Data are presented as mean ± standard deviation or frequencies (percent). Mann–Whitney U‐test was used to compare continuous variables between the two groups; χ2‐test or Fisher's exact test was used to compare categorical variables between the two groups. DSM‐5, Diagnostic and Statistical Manual of Mental Disorders, 5th Edition; ICDSC, Intensive Care Delirium Screening Checklist.
The trazodone dose, as needed for insomnia, was significantly lower in the suvorexant group than in the conventional treatment group, although no other significant differences in clinical outcomes or medication doses were observed. No adverse events potentially attributable to suvorexant were noted (Table 2).
Discussion
This, to the best of our knowledge, is the first study suggesting suvorexant's effectiveness in delirium prevention in ICU patients. The pharmacological mechanisms underlying wakefulness suppression by suvorexant, leading to a delayed delirium onset in these patients, remain unclear; further studies are needed to elucidate this issue. Nevertheless, suvorexant's unique profile and efficacy for treating sleep disturbances14 implicate its potential efficacy in preventing delirium in critically ill patients.
In this study, patients who regularly took hypnotics before hospitalization were excluded. Therefore, patients were divided into two groups on the basis of hypnotics used as follows: the suvorexant group (only suvorexant at 9:00 pm every day and trazodone, as needed, for insomnia) and the conventional treatment group (only trazodone, as needed, for insomnia). As a result, the use of trazodone in the conventional treatment group was more frequent, but the development of delirium was lower in the suvorexant group, indicating that delirium was prevented by the effect of suvorexant without the influence of the other hypnotic.
Antipsychotics are widely utilized to treat delirium in general clinical settings.5, 25 One study, however, did not show clear evidence that atypical antipsychotics could prevent delirium in ICU patients.26 Dexmedetomidine is associated with lower delirium prevalence and a shorter mechanical ventilation duration in ICU patients; however, it was not reported to prevent ICU delirium.27, 28
The two other sleep‐promoting agents used in ICU patients, melatonin and the synthetic melatonin receptor antagonist ramelteon, might prevent delirium by modulating melatonin's effects on the sleep/wake cycle and circadian rhythm.29, 30 In their study, Hatta et al.30 showed that delirium incidence in the ramelteon group was significantly lower than that in the placebo group (3% versus 32%). However, that study excluded patients with nasogastric tubes and those on mechanical ventilators as well as patients in the general ward and ICU. Conversely, we focused exclusively on ICU patients, with the majority of the patients in both groups receiving mechanical ventilation. Therefore, our results provide a better overview of the therapeutic effects of suvorexant on delirium in the ICU.
A recent study, very similar to the current study, examining the potential preventive effect of suvorexant on delirium23 found that suvorexant prevented delirium in the ICU and the regular acute ward. However, there are several differences in the characteristics of the two studies. The current study included more severely ill patients than the other study, which excluded patients requiring intubation and mechanical ventilation; furthermore, approximately half of the patients in that study were admitted to the regular acute ward and not the ICU. Conversely, our study included 36 (51.4%) patients who required intubation and mechanical ventilation; all 70 patients were admitted to the ICU. Therefore, the current study is more representative of actual ICU practices. The ICU pain, agitation, and delirium guidelines recommend routine screening for delirium based on the Confusion Assessment Method for ICU or ICDSC. We determined delirium using ICDSC and confirmed its diagnosis using the DSM‐5 criteria. This procedure is consistent with the recommendations of the ICU pain, agitation, and delirium guidelines. Albeit not a main outcome, subsyndromal delirium incidence was significantly different between the two groups in the current study, highlighting the preventive effect of suvorexant on subsyndromal delirium, another novel finding of this study.
Our study has several limitations. First, the single‐center design and relatively small sample size limit the generalizability of findings to all ICU patients. The assumed delirium incidence of 3% in the suvorexant group might have been overestimated during the sample size calculation because the actual incidence of delirium in the suvorexant group was 17.6%. In future studies, a larger sample size will be needed based on the results of this study to confirm the preventive effect of suvorexant for delirium. The mean times to delirium onset were 5 and 6 days in the conventional and suvorexant treatment groups, respectively, which were twice as long as 2–3 days reported in previous studies,3 indicating the possibility that the present study results are not generalizable to all ICU or critically ill patients. Second, the study design was associated with a risk of bias as a placebo group could not be used. Third, objective measurements such as actigraphy, polysomnography, and melatonin levels were not used for sleep and delirium evaluation. Fourth, there was no quantitative data on analgesics, such as Behavioral Pain Scale (BPS) or Critical‐Care Pain Observation Tool (CPOT), in this study. Therefore, the quantitative comparison of pain between the two groups was insufficient. Finally, there was deviation in patient population diagnosis between patients with trauma and those with infection, although not significant. Therefore, we examined risks of patients with trauma and those with infection between both groups. We compared PRE‐DELIRIC scores of target patients using Student's t‐test, but there was no significant difference between the two groups (PRE‐DELIRIC score of patients with infection: 68.0 ± 20.4 in the suvorexant group versus 50.8 ± 33.2 in the conventional treatment group; P = 0.22, PRE‐DELIRIC score of patients with trauma: 30.0 ± 26.8 in the suvorexant group versus 24.9 ± 21.4 in the conventional treatment group; P = 0.53).
Conclusions
Suvorexant might be a safe and effective drug for preventing delirium in adult ICU patients. Larger studies in more heterogeneous populations of ICU patients are needed to validate our findings.
Disclosure
Approval of the research protocol: The study protocol was approved by the ethical committee at the Tokyo Medical University (approval number: 2954).
Informed consent: All participants provided informed consent.
Registry and the registration no. of the trial: UMIN Clinical Trials Registry (UMIN‐CTR) identifier: UMIN 000016471 (registered February 7, 2015).
Animal studies: N/A.
Conflict of Interest: Dr. Yoshikazu Takaesu reports grants from Otsuka Pharmaceutical, Meiji Seika Pharma, MSD and Eisai, personal fees from Otsuka Pharmaceutical, Meiji Seika Pharma, Eli Lilly, Eisai, Mitubishi Tanabe Pharma, MSD, and Yoshitomi Pharmaceutical, outside the submitted work. Dr. Takeshi Inoue reports grants from Astellas, Otsuka Pharmaceutical, Dainippon Sumitomo Pharma, Eli Lilly, Eisai, Mitubishi Tanabe Pharma, Pfizer, Abb Vie GK, MSD, Yoshitomiyakuhin, Takeda Pharmaceutical, and Meiji Seika Pharma, personal fees from GlaxoSmithKline, Mochida Pharmaceutical, Asahi Kasei Pharma, Shionogi, Otsuka Pharmaceutical, Dainippon Sumitomo Pharma, Eli Lilly, Eisai, Mitubishi Tanabe Pharma, Pfizer, Abb Vie GK, MSD, Yoshitomiyakuhin, Takeda Pharmaceutical, and Meiji Seika Pharma, outside the submitted work. Dr. Yuichi Inoue reports grants from Takeda Pharmaceutical, Astellas, Philips Respironis, MSD, Eisai, Otsuka Pharmaceutical, and Koike Medical, and personal fees from Takeda Pharmaceutical, Astellas, Philips Respironis, MSD, Eisai, Otsuka Pharmaceutical, and Koike Medical, outside the submitted work. The other authors have no conflict of interest.
Funding Information
No funding information provided.
References
- 1. Inouye SK. Delirium in older persons. N. Engl. J. Med. 2006; 354: 1157–65. [DOI] [PubMed] [Google Scholar]
- 2. Michaud L, Bula C, Berney A et al Delirium: guidelines for general hospitals. J. Psychosom. Res. 2007; 62: 371–83. [DOI] [PubMed] [Google Scholar]
- 3. Ely EW, Inouye SK, Bernard GR et al Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA 2001; 286: 2703–10. [DOI] [PubMed] [Google Scholar]
- 4. Girard TD, Jackson JC, Pandharipande PP et al Delirium as a predictor of long‐term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010; 38: 1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell N, Boustani MA, Ayub A et al Pharmacological management of delirium in hospitalized adults – a systematic evidence review. J. Gen. Intern. Med. 2009; 24: 848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N. Engl. J. Med. 2009; 360: 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brummel NE, Girard TD. Preventing delirium in the intensive care unit. Crit. Care Clin. 2013; 29: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alway A, Halm MA, Shilhanek M, St Pierre J. Do earplugs and eye masks affect sleep and delirium outcomes in the critically ill? Am. J. Crit. Care 2013; 22: 357–60. [DOI] [PubMed] [Google Scholar]
- 9. Shaw R. Using music to promote sleep for hospitalized adults. Am. J. Crit. Care 2016; 25: 181–4. [DOI] [PubMed] [Google Scholar]
- 10. Gupta N, de Jonghe J, Schieveld J, Leonard M, Meagher D. Delirium phenomenology: what can we learn from the symptoms of delirium? J. Psychosom. Res. 2008; 65: 215–22. [DOI] [PubMed] [Google Scholar]
- 11. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn Arlington: American Psychiatric Association, 2013. [Google Scholar]
- 12. Siegel J. Brain mechanisms that control sleep and waking. Naturwissenschaften 2004; 91: 355–65. [DOI] [PubMed] [Google Scholar]
- 13. de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep‐to‐wake transitions. Front. Pharmacol. 2014; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther. Adv. Drug Saf. 2015; 6: 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaal IJ, Devlin JW, Hazelbag M et al Benzodiazepine‐associated delirium in critically ill adults. Intensive Care Med. 2015; 41: 2130–7. [DOI] [PubMed] [Google Scholar]
- 16. Barnes‐Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit. Care Med. 2017; 45: 171–8. [DOI] [PubMed] [Google Scholar]
- 17. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001; 27: 859–64. [DOI] [PubMed] [Google Scholar]
- 18. Vincent JL, Moreno R, Takala J et al The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22: 707–10. [DOI] [PubMed] [Google Scholar]
- 19. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit. Care Med. 1985; 13: 818–29. [PubMed] [Google Scholar]
- 20. van den Boogaard M, Pickkers P, Slooter AJ et al Development and validation of PRE‐DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ 2012; 344: e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007; 33: 1007–13. [DOI] [PubMed] [Google Scholar]
- 22. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007; 33: 66–73. [DOI] [PubMed] [Google Scholar]
- 23. Hatta K, Kishi Y, Wada K et al Preventive effects of suvorexant on delirium: a randomized placebo‐controlled trial. J. Clin. Psychiatry 2017; 78: e970–9. [DOI] [PubMed] [Google Scholar]
- 24. Cancer‐Pain.org [homepage on the internet]. New York: Association of Cancer Online Resources, Inc.; c2000–01 [updated May 2002; cited 9 Jul 2000]. Available from: http://www.cancer-pain.org/. [Google Scholar]
- 25. Devlin JW, Roberts RJ, Fong JJ et al Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Crit. Care Med. 2010; 38: 419–27. [DOI] [PubMed] [Google Scholar]
- 26. Girard TD, Pandharipande PP, Carson SS et al Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo‐controlled trial. Crit. Care Med. 2010; 38: 428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pandharipande PP, Pun BT, Herr DL et al Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298: 2644–53. [DOI] [PubMed] [Google Scholar]
- 28. Riker RR, Shehabi Y, Bokesch PM et al Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301: 489–99. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Aama T, Brymer C, Gutmanis I, Woolmore‐Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. Int. J. Geriatr. Psychiatry 2011; 26: 687–94. [DOI] [PubMed] [Google Scholar]
- 30. Hatta K, Kishi Y, Wada K et al Preventive effects of ramelteon on delirium: a randomized placebo‐controlled trial. JAMA Psychiatry 2014; 71: 397–403. [DOI] [PubMed] [Google Scholar]


