Abstract
Case
Several successful uses of extracorporeal membrane oxygenation (ECMO) for acute respiratory distress syndrome in patients with novel HIV/AIDS infection have been reported; however, the therapeutic keys have not always been discussed.
A 47‐year‐old man was admitted with progressive shortness of breath. He was in respiratory failure with a PaO2/FIO2 ratio of 110.8 requiring intubation. Chest computed tomography showed diffuse ground glass opacities. An HIV infection was suspected, and a diagnosis of acute respiratory distress syndrome was made. Based on clinical indications, treatment for Pneumocystis jirovecii pneumonia and concomitant bacterial infection was started.
Outcome
Despite broad‐spectrum antibiotics, the patient's oxygenation deteriorated, necessitating ECMO. After 19 days of ECMO therapy, the patient was successfully decannulated and was eventually discharged.
Conclusion
In acute respiratory distress syndrome in patients with HIV/AIDS refractory to treatment, ECMO should be considered. Post‐ECMO antiretroviral therapy could improve outcomes.
Keywords: Acute respiratory distress syndrome, HIV/AIDS, post‐ECMO ART, V‐V ECMO
Background
Although the frequency of extracorporeal membrane oxygenation (ECMO) use in respiratory failure is increasing, only half of such cases are discharged or transferred, and high survival rates have not yet been achieved.1 The indications for ECMO remain controversial, especially in patients who are immunosuppressed or have non‐recoverable comorbidities. There are several reports of successful ECMO use for severe hypoxia in patients with HIV/AIDS.2, 3, 4, 5, 6, 7, 8, 9, 10 However, key therapeutic issues, such as mechanical ventilation (MV) settings, indications for ECMO, and the timing of antiretroviral therapy (ART), have not always been discussed in these reports. A case of acute respiratory distress syndrome in a patient with novel HIV/AIDS, who was successfully treated with veno‐venous (V‐V) ECMO and discharged, is presented.
Case
A 47‐year‐old man with an unknown medical history was admitted to our tertiary medical center due to worsening shortness of breath. He had been in his usual state of health until he developed oral white plaques 3 months prior to admission. In the previous month, he had mild chest pain, productive cough, and a mild fever. On the day of admission, he was found lying down with severe shortness of breath. He presented with a respiratory rate of 42 breaths/min and unmeasurable O2 saturation, and was brought in by ambulance for severe respiratory failure. Physical examination showed coarse crackles over the right lung. Chest computed tomography (Fig. 1A) showed diffuse, bilateral, ground glass opacities. He was in severe respiratory failure with a PaO2/FIO2 ratio of 110.8 requiring intubation and MV.
Figure 1.
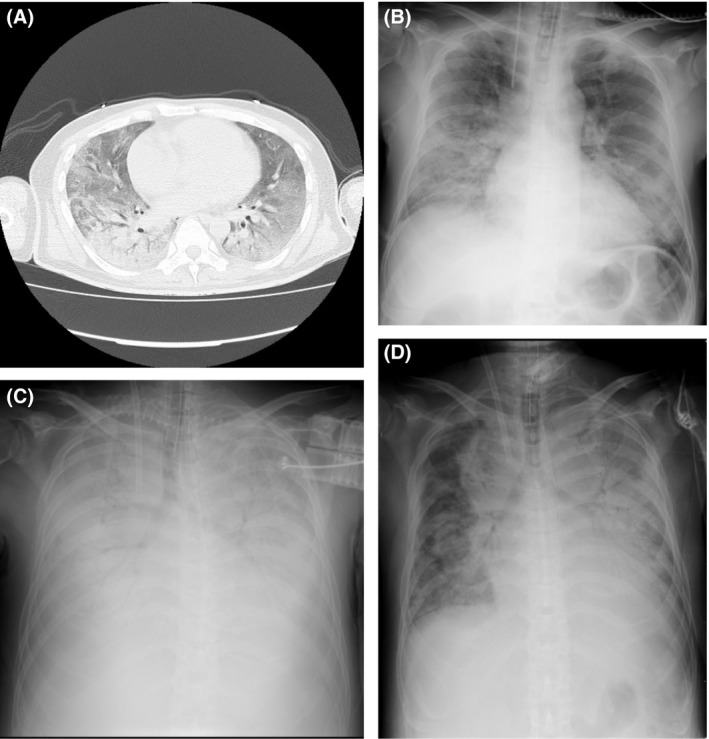
Chest computed tomography and X‐ray findings in a patient with HIV/AIDS and severe acute respiratory distress syndrome treated with veno‐venous extracorporeal membrane oxygenation. A, Initial chest computed tomography. B, Portable chest X‐ray on hospital day 4. C, Portable chest X‐ray on hospital day 11, showing prominent bilateral consolidation. D, Portable chest X‐ray on hospital day 17, showing decreased lung consolidation.
The diagnosis of HIV infection was suspected on admission because of positive screening for HIV antigen–antibody (CLIA test), and it was later confirmed by a CD4+ T‐cell count of 6/μL and an HIV viral load of 1.4 × 105 copies/mL. Simultaneously, Pneumocystis jirovecii pneumonia was highly suspected on admission based on clinical signs, diagnostic imaging, a lactate dehydrogenase level of 1,511 U/L, and an elevated β‐D‐glucan level of 25.9 pg/mL. His respiratory failure was so severe that we believed it was safer and more appropriate to start treatment for P. jirovecii pneumonia than to carry out bronchoalveolar lavage. Polymerase chain reaction test was not available. Although a definitive diagnosis of P. jirovecii pneumonia was not made, treatment with trimethoprim/sulfamethoxazole and prednisolone was started appropriately, and piperacillin/tazobactam and vancomycin were started to cover concomitant bacterial infections from admission. Sputum culture was negative for common pneumonia pathogens, urine Streptococcus pneumoniae antibody was negative, and blood cultures were negative; therefore, concomitant bacterial infection was considered unlikely, and piperacillin/tazobactam and vancomycin were discontinued after 4 days.
Four days after his admission, the patient developed progressive hypoxia requiring FIO2 0.5/positive end‐expiratory pressure (PEEP) 10 increasing to FIO2 0.7/PEEP 15 within 1 day and worsening consolidation on X‐ray (Fig. 1B). The Murray score was 3 points, and progressive respiratory failure was predicted. As he developed progressive symptoms despite appropriate care and had no prospect of improvement within 7 days, beyond which time ECMO use is associated with worse outcomes, and because there have been some case reports of successful ECMO use in cases of acute respiratory distress syndrome with novel HIV/AIDS, it was decided to initiate V‐V ECMO (Capiox; Terumo, Tokyo, Japan).
Conventional MV settings (FIO2 < 0.4 and plateau pressure < 25) were used (Table 1), maintaining SaO2 > 80%, hemoglobin > 12 g/dL, and platelets > 10 × 104/μL with transfusion based on protocols or Extracorporeal Life Support Organization (ELSO) guidelines.11, 12 The patient required two membrane exchanges, and after the second membrane replacement, surgical tracheostomy was carried out following heparin antagonization with protamine; by that time his oxygen demand decreased to FIO2 0.3/PEEP 10, and significant X‐ray improvement was noted (Fig. 1C,D). His course was further complicated by mediastinal emphysema before ECMO decannulation, which was managed supportively. He was successfully decannulated after 19 days of ECMO, because arterial blood gases were satisfactory for at least 8 hours with a blood flow of 1.5 L/min and a sweep gas of 0.5 L/min (parameters from a previous article).6
Table 1.
Mechanical ventilation (MV) settings in a patient with HIV/AIDS and severe acute respiratory distress syndrome treated with veno‐venous extracorporeal membrane oxygenation (ECMO)
| Hospital day | Mode | FIO2 | Vt (mL) or PIP (cmH2O) | PEEP (cmH2O) | RR (/min) |
|---|---|---|---|---|---|
| Day 1 | VCV | 0.50 | 420 mL | 8 | 12 |
| Day 2 | PCV | 0.45 | 15 | 8 | 12 |
| Day 3 | PCV | 0.40 | 15 | 10 | 8 |
| Day 4 | PCV | 0.50 | 15 | 10 | 8 |
| Day 5 | PCV | 0.70 | 18 | 15 | 8 |
| ECMO introduced | |||||
| Day 6 | PSV | 0.40 | 8 | ||
| Day 9 | PCV | 0.50 | 18 | 6 | 15 |
| Day 11 | PCV | 0.40 | 20 | 10 | 8 |
| Fixed MV settings and changed only as needed | |||||
| Day 23 | PCV | 0.40 | 25 | 8 | 20 |
| PCV | 0.60 | 25 | 8 | 20 | |
| Increased FIO2, then ECMO decannulated | |||||
PCV, pressure control ventilation; PEEP, positive end‐expiratory pressure; PIP, peak inspiratory pressure; PSV, pressure support ventilation; RR, respiratory rate; VCV, volume control ventilation; Vt, tidal volume.
Several microbial infections concurred during his hospital stay (Table 2). Detection of cytomegalovirus antigen in neutrophils came back positive and ganciclovir was started on hospital day 7. The patient's respiratory status worsened on ECMO and because atypical pneumonia pathogens had not been covered since admission, levofloxacin was added on hospital day 10 and a 14‐day course was completed. In addition, P. jirovecii pneumonia treatment was switched to pentamidine per recommendation from consultants followed by initiation of prophylactic treatment with azithromycin for mycobacterium avium complex on hospital day 21.
Table 2.
Antibiotic, antimycotic, and antiviral treatment during extracorporeal membrane oxygenation (ECMO) use in a patient with HIV/AIDS and severe acute respiratory distress syndrome
| Admission | Day 5 | Day 7 | Day 10 | Day 18 | Day 20 | Day 21 | Day 23 | |
|---|---|---|---|---|---|---|---|---|
| Bacterial pneumonia | ||||||||
| Piperacillin/tazobactam and vancomycin | Piperacillin/tazobactam and vancomycin | Levofloxacin | Levofloxacin | Levofloxacin | Levofloxacin | Levofloxacin | ||
| Novel HIV/AIDS | ||||||||
| Pneumocystis jirovecii pneumonia | SMX + TMP | SMX + TMP | SMX + TMP | SMX + TMP | Pentamidine | Pentamidine | Pentamidine | Pentamidine |
| CMV | Ganciclovir | Ganciclovir | Ganciclovir | Ganciclovir | ||||
| MAC | Azithromycin | Azithromycin | ||||||
| ECMO | ||||||||
| Initiated | Decannulated | |||||||
CMV, cytomegalovirus; MAC, mycobacterium avium complex; SMX, sulfamethoxazole; TMP, trimethoprim.
Thirteen days after decannulation, after the successful treatment regimen and to avoid reported immune reconstitution inflammatory syndrome (IRIS), ART was started. Subsequently, the patient was weaned off ventilator support. The patient was then moved to a regular ward and eventually discharged home ambulatory.
Discussion
The course of this patient highlighted some important clinical issues. The discharge rate of acute respiratory distress syndrome cases with HIV/AIDS treated with ECMO is reportedly better than that of general patients treated with V‐V ECMO (67% versus 58%, Table 3).1 Although there is no absolute contraindication to V‐V ECMO in the guidelines, treating immune‐compromised patients, including those with HIV/AIDS, with V‐V ECMO is sometimes considered controversial.11, 12 However, previous reports and the present case show that severe respiratory failure with HIV/AIDS can now be treated by V‐V ECMO support. In these cases, respiratory status should be closely monitored with evaluations including the Murray score to determine the indication for ECMO.
Table 3.
Review and comparison of previous cases with HIV/AIDS treated with extracorporeal membrane oxygenation (ECMO)
| Age (years) | Sex | New HIV diagnosis | Diagnosis | CD4 | HIV viral load (/mL) | Timing of ART initiation (pre‐, on‐, post‐ECMO) | PaO2 (mmHg)/FIO2 (%) | ECMO initiation (hospital day) | ECMO duration (days) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present case | 47 | M | Yes | HIV/AIDS | 6 cells/μL | 140,000 | Post | 54.9/50 | 5 | 19 | Survived to discharge |
| Goodman4 | 30 | F | Yes | HIV/AIDS, PCP | 13 cells/mL | 976,631 | Post | 50.1/100 | 3 | 7 | Survived to discharge |
| Simpson8 | 35 | M | Yes | HIV/AIDS, PCP | NA | 1,269,866 | NA | NA/NA | NA (7 days on MV) | 27 | Survived for 6 months |
| Ali10 | 26 | M | Yes | HIV/AIDS, PCP | 84 | 907,302 | Post | 200 (P/F ratio) | NA | 6 | Survived to discharge |
| Horikita9 | 23 | M | Yes | HIV/AIDS, PCP | 8.5 cells/μL | 550,000 | On | 48/100 | NA (ICU day 3) | 12, 14 (2nd course) | Survived to discharge |
| De Rosa6 | 24 | M | Yes | HIV/AIDS, PCP | 3 cells/mm3 | 50,728 | On | 100 (P/F ratio) | 12 (6 days on MV) | 24 | Died in hospital, s/p decannulation |
| Cawcutt7 | 45 | M | Yes | HIV/AIDS, PCP | 33 cells/mL | 113,000 | Pre | 59/60 | 5 | 57 | Died in hospital, s/p decannulation |
| Gutermann2 | 55 | M | Yes | HIV/AIDS, PCP | 9 cells/mL | 80,235 | Post | NA/NA | 4 | 4 | Survived to discharge |
| Goodman4 | 25 | M | Yes | HIV/AIDS, PCP | 36 cells/mL | 622,234 | Pre | 63.6/100 | 18 | 69 | Died on ECMO |
| Steppan3 | 39 | M | NA | HIV/AIDS, PCP | 69 cells/mL | 6,297 | Pre | NA/100 | 12 | 14 | Died on ECMO |
| De Rosa6 | 21 | F | No | HIV/AIDS, PCP | 2 cells/mm3 | 118,330 | Non‐compliant, restarted | 120 (P/F ratio) | 10 | 20 | Survived to discharge |
| Simpson8 | 40 | M | No | HIV, disseminated Kaposi's sarcoma | NA | 126,947 | NA | NA | NA (1 day on MV) | 28 | Died in ICU |
| Simpson8 | 20 | F | No | HIV, adenovirus | NA | Undetectable | NA | NA | NA (1 day on MV) | 5 | Survived for 6 months |
| 25 | M | Yes | HIV/AIDS, MDR bacterial PNA | 134 cells/μL | 2,220,000 | Pre | 80/90 | 27 (11 days on MV) | 56 | Survived to discharge | |
| De Rosa6 | 38 | F | No | HIV, HCV, Legionella pneumophila | 170 cells/mm3 | 500 | Compliant and continued | 90 (P/F ratio) | 2 | 13 | Survived to discharge |
ART, antiretroviral therapy; F, female; HCV, hepatitis C virus; ICU, intensive care unit; M, male; MDR, multidrug resistant; MV, mechanical ventilation; NA, not applicable; PCP, pneumocystis pneumonia; P/F, PaO2/FiO2; s/p, status post.
To our knowledge, there have been 14 reported cases of HIV/AIDS patients with progressive respiratory failure treated with ECMO. Eleven of these 14 cases had P. jirovecii pneumonia. As P. jirovecii pneumonia was highly suspected in the present case, we managed our patient with novel HIV/AIDS for P. jirovecii pneumonia. The focus in the present case was on early achievement of lung rest management by the early introduction of ECMO, which is a novel approach in these types of cases. Boonsarngsuk et al. concluded that PEEP on the 3rd day of ventilation management was related to higher mortality in P. jirovecii pneumonia,13 so we hypothesize that lung rest settings achieved by ECMO do have benefits. In the present case, the patient needed higher PEEP settings due to worsening oxygenation before ECMO introduction. In terms of timing of ECMO initiation, it seems ideal to start ECMO within 7 days from ventilator introduction based on the ELSO guideline12 and Respiratory ECMO Survival Prediction (RESP) score.14 In addition, as Goodman et al.4 reported, patients given ECMO early during their ventilator management course showed higher survival rates (Table 3).
In addition, post‐ECMO ART should be considered in HIV/AIDS patients with acute respiratory distress syndrome. All cases listed in Table 3 that received post‐ECMO ART survived. It was difficult to extract all the conditions related to the RESP score, but when age and time to starting ECMO are based on the RESP score, post‐ECMO ART achieved survival in all cases. In general, ART is not started in P. jirovecii pneumonia cases presenting with high P. jirovecii activity, because immune reconstitution induced by ART can worsen P. jirovecii pneumonia. Especially in severe P. jirovecii pneumonia requiring ECMO support, P. jirovecii activity is considered to be very high. Furthermore, as IRIS has been thought to cause deterioration in some cases, we believe it is more effective and safer not to start ART until patients recover to the point where they have sufficient spontaneous oxygenation and ventilation.
Conclusion
Veno‐venous ECMO achieved a favorable outcome in an HIV/AIDS patient with severe acute respiratory distress syndrome. Post‐ECMO ART is likely a key factor for success when treating severe respiratory infection in HIV/AIDS patients requiring ECMO support.
Disclosure
Approval of the research protocol: Ethical approval to report this case was not required.
Informed consent: Written, informed consent was obtained from the patient for publication of this case report and any accompanying images.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interests: None.
Acknowledgments
The authors are grateful to Professor Shiro Mishima from Tokyo Medical University of Emergency and Critical Care Medicine for his instruction and inspiring guidance.
Funding Information
No funding information provided.
References
- 1. elso.org [homepage on the internet]. ECLS Registry Report (January 2017). [cited 29 Jul 2017]. Available from: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx.
- 2. Gutermann H, van Roy B, Meersseman W, Meyns B, Herijgers P. Successful extracorporeal lung assistance for overwhelming pneumonia in a patient with undiagnosed full blown AIDS–a controversial therapy in HIV‐patients. Thorac. Cardiovasc. Surg. 2005; 53: 252–4. [DOI] [PubMed] [Google Scholar]
- 3. Steppan J, Sikazwe I. Extra‐corporeal membrane oxygenation in an adult with severe pneumocystis pneumonia [Abstract]. Baltimore (MD): American College of Physicians Meeting, 2009. [Google Scholar]
- 4. Goodman JJ, Goodman LF, Sarvepalli SK, Firstenberg MS, Lustberg ME, Bazan JA. Extracorporeal membrane oxygenation as adjunctive therapy for refractory hypoxemic respiratory failure in HIV‐positive patients with severe Pneumocystis jirovecii pneumonia. Clin. Pulm. Med. 2013; 20: 117–20. [Google Scholar]
- 5. Samalavicius R, Serpytis M, Ringaitiene D et al Successful use of extracorporeal membrane oxygenation in a human immunodeficiency virus infected patient with severe acute respiratory distress syndrome. AIDS Res. Ther. 2014; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Rosa FG, Fanelli V, Corcione S et al Extra Corporeal Membrane Oxygenation (ECMO) in three HIV‐positive patients with acute respiratory distress syndrome. BMC Anesthesiol. 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cawcutt K, Gallo De Moraes A, Lee SJ, Park JG, Schears GJ, Nemergut ME. The use of ECMO in HIV/AIDS with Pneumocystis jirovecii Pneumonia: a case report and review of the literature. ASAIO J. 2014; 60: 606–8. [DOI] [PubMed] [Google Scholar]
- 8. Simpson T, Haidari G, Barrett N et al Severe respiratory failure, extra‐corporeal membrane oxygenation and human immunodeficiency virus: a single centre case series. Conference: 14th European AIDS Conference, Brussels, Belgium. [cited 29 Jul 2017]. Available from: https://www.researchgate.net/publication/276887672_Severe_respiratory_failure_extracorporeal_membrane_oxygenation_and_human_immunodeficiency_virus_a_single_centre_case_series.
- 9. Horikita S, Sanui M, Fujimoto Y, Lefor AK. Successful repeat ECMO in a patient with AIDS and ARDS. BMJ Case Rep. 2017;. 10.1136/bcr-2017-219870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali HS, Hassan IF, George S. Extra corporeal membrane oxygenation to facilitate lung protective ventilation and prevent ventilator‐induced lung injury in severe pneumocystis pneumonia with pneumomediastinum: a case report and short literature review. BMC Pulm. Med. 2016; 16: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nippon Medical University Surgical ICU series. [cited 29 Jul 2017]. Available from: square.umin.ac.jp/jrcm/pdf/ecmo/ecmotext04.pdf.
- 12. elso.org . [homepage on the internet]. ELSO Guidelines General v 1.3. [cited 27 Jul 2017]. Available from: https://www.elso.org/Resources/Guidelines.aspx.
- 13. Boonsarngsuk V, Sirilak S, Kiatbonsri S. Acute respiratory failure due to Pneumocystis pneumonia: outcome and prognostic factors. Int. J. Infect. Dis. 2009; 13: 59–66. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Bailey M, Sheldrake J et al Predicting survival after ECMO for severe acute respiratory failure: the respiratory ECMO Survival Prediction (RESP)‐Score. Am. J. Respir. Crit. Care Med. 2014; 189: 1374–82. [DOI] [PubMed] [Google Scholar]


