Summary
Background
Adverse effects on reproductive function are a key concern in young women treated with chemotherapy for advanced Hodgkin's lymphoma. We aimed to identify risk factors for the extent of ovarian damage in women with Hodgkin's lymphoma treated with different chemotherapy regimens to inform accurate advice on options for fertility preservation.
Methods
We recruited female participants from the randomised phase 3 RATHL trial, aged 18–45 years, based on availability of participants at recruiting sites in the UK. The RATHL trial key inclusion criteria were histologically confirmed classic Hodgkin's lymphoma, stage IIB–IV or IIA with adverse features (bulky disease or more than two sites of involvement), no previous treatments, and a performance status of 0–3. As part of RATHL, participants were treated with two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) or AVD followed by an interim PET-CT scan. Participants who had negative interim scans (PET score of 1 to 3 according to the Lugano classification) were randomly assigned (1:1) by use of minimisation, stratified by interim PET score and study centre, to continue ABVD or AVD for four more cycles. Participants with positive scans (PET score of 4 or 5) were escalated to treatment with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP-14 or escalated BEACOPP) for four cycles. For the protocol-driven prospective cohort substudy, ovarian function was assessed before treatment, during chemotherapy, and then annually for 3 years by use of serum antimüllerian hormone and follicle-stimulating hormone measurements. The RATHL study is registered with ClinicalTrials.gov, number NCT00678327.
Findings
Between Dec 13, 2010, and Dec 19, 2012, 67 eligible participants were recruited for this prospective cohort study; 57 had received ABVD or AVD (ABVD-AVD group) and ten BEACOPP-14 or escalated BEACOPP (BEACOPP group). Follow-up was fixed at 3 years. Antimüllerian hormone concentrations decreased during both chemotherapy regimens. At 1 year after chemotherapy, antimüllerian hormone concentrations recovered to a median of 10·5 pmol/L (IQR 4·3–17·3) in the ABVD-AVD group, but little recovery was seen after BEACOPP (median 0·11 pmol/L [0·07–0·20]). Age also affected the extent of ovarian function recovery, with antimüllerian hormone recovery in participants aged 35 years or older in the ABVD-AVD group to 37% (SD 10) of their before treatment concentrations, compared with full recovery to 127% (SD 12) in those younger than 35 years (p<0·0001). Follicle-stimulating hormone recovery to less than 25 IU/L occurred for 95% of women younger than 35 years in the ABVD-AVD group by 2 years and was also dependent on age (hazard ratio 0·49, 95% CI 0·37–0·65; p<0·0001).
Interpretation
Reduced recovery of ovarian function observed in women older than 35 years treated with ABVD or AVD compared with younger women indicates that treatment could reduce their reproductive lifespan and supports discussion of fertility preservation before treatment. Women treated with BEACOPP should be informed of its potential high gonadotoxicity. These findings warrant further investigation in large, prospective studies with fertility and reproductive lifespan as outcomes.
Funding
Medical Research Foundation and Cancer Research UK.
Introduction
Hodgkin's lymphoma is predominantly a cancer of young adult life, occurring at a median age of 35 years. Systemic treatment for this condition has evolved from chemotherapy regimens based on alkylating agents, which had a high risk of infertility and premature ovarian insufficiency in women, to treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), which is the current treatment of choice for many adult patients with Hodgkin's lymphoma. ABVD results in a disease-free survival of 75%,1 and with less gonadotoxicity than previous treatments.2 Increasing evidence supports that treatment intensification could benefit patients who are at higher risk of relapse, and some clinicians advocate treatment with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP) for patients with advanced stage or high-risk early stage disease. However, female gonadal toxicity is high with dose-escalated BEACOPP resulting in amenorrhoea (a surrogate marker of premature ovarian insufficiency) in about 95% of patients older than 30 years.3
Research in context.
Evidence before this study
Chemotherapy regimens are associated with toxic effects on human ovaries, manifesting as premature ovarian insufficiency, infertility, and reduced reproductive lifespan. Therefore, accurate assessment of the ovarian toxicity of widely used chemotherapy regimens is needed. We searched PubMed for articles published in English between inception and March 12, 2018, using the terms “ovary”, “ovarian reserve”, “chemotherapy”, and “cancer”. We found little data from prospective studies that used current biomarkers of ovarian function. Both age and ovarian reserves before treatment are predictive of ovarian recovery after chemotherapy with some regimens, but their importance is unknown for treatments for patients with Hodgkin's lymphoma.
Added value of this study
We found that changes in antimüllerian hormone concentration could distinguish between the effects of treatment on ovarian function with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) or AVD, and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP) in patients with advanced Hodgkin's lymphoma, particularly from 3 years of follow-up after chemotherapy. Treatment with ABVD or AVD was confirmed to have little gonadotoxcity in female patients with Hodgkin's lymphoma who were younger than 35 years at diagnosis; however, in older patients (≥35 years) recovery of ovarian function was affected by age. We found that among older women baseline concentrations of antimüllerian hormone did not affect recovery, so reduced hormone recovery in older women was not due to the age-related decline in ovarian reserve. Little recovery in ovarian function was seen after treatment with BEACOPP in women of all ages, although a similar number of pregnancies was observed in the two treatment groups. Due to the small size of the cohorts analysed, these results warrant further confirmation.
Implication of all the available evidence
The absence of detectable ovarian damage after treatment with ABVD or AVD in younger women with Hodgkin's lymphoma is reassuring, although longer follow-up is needed in future studies for a full assessment of potential reproductive effects. Antimüllerian hormone is not a predictor of short-term fertility. The finding of reduced ovarian recovery in older women with Hodgkin's lymphoma treated with chemotherapy indicates that age-specific discussions and consideration of fertility preservation procedures should be considered before treatment. These considerations are of growing importance given the increasing age at which women are having children in developed countries.
Analysis of the effects of cancer therapies on the ovaries requires consideration of both the degree of loss of ovarian function during chemotherapy and changes in ovarian function thereafter. The degree of loss of the primordial follicle pool (the ovarian reserve) during chemotherapy is the most important effect since it determines the potential for any recovery of ovarian function and fertility, and subsequent age at menopause, although the degree of loss cannot be assessed in vivo. Additional effects of chemotherapy might be seen on the non-follicular components of the ovary, including the stroma and vasculature.4 Few prospective analyses have been done of ovarian function from the time of diagnosis and before chemotherapy.5, 6, 7, 8, 9 Most larger studies use amenorrhoea to indicate loss of ovarian function,3 and so are unable to differentiate between women who have largely intact remaining ovarian function and those who have lost most of their ovarian reserve and will soon become menopausal and infertile.2, 10 Although measurement of follicle-stimulating hormone concentration is central to the diagnosis of premature ovarian insufficiency, the hormone has substantial variation during the menstrual cycle, complicating its use to measure ovarian reserve in women with ovarian activity.11 Analysis of concentrations of serum antimüllerian hormone as an indirect biomarker of the ovarian reserve has become increasingly valuable for women with ovarian activity.12 Antimüllerian hormone concentrations decrease during chemotherapy in girls and women, with variable recovery after treatment reflecting the gonadotoxicity of each regimen.5, 6, 7, 8 In the past 5 years, refinements in assay methods have improved their sensitivity, and thus their ability to detect low circulating concentrations of antimüllerian hormone in women after chemotherapy, which were previously undetectable.13, 14 How the pretreatment ovarian reserve and the patient's age influence recovery of ovarian function after chemotherapy is unclear, with most data derived from women who have been treated for breast cancer.5, 9, 15, 16 In this study, we aimed to determine the effect of response-adapted chemotherapy regimens on ovarian function in adult women with Hodgkin's lymphoma, since they are both a younger population and often treated with less gonadotoxic drugs than women with breast cancer.
Methods
Study design and participants
In this prospective cohort study, participants were recruited from the RATHL trial, which used PET-CT to guide response-adapted chemotherapy, for patients with advanced Hodgkin's lymphoma (appendix p 1).17 Recruitment for RATHL was open in hospitals across the UK, Italy, Norway, Sweden, Denmark, Australia, and New Zealand from Aug 29, 2008, to Dec 21, 2012. RATHL inclusion criteria were histologically confirmed classic Hodgkin's lymphoma according to the WHO classification; age 18 years or older; stage IIB–IV or IIA with adverse features (such as bulky disease or more than two sites of involvement); no previous treatment with chemotherapy, radiotherapy, or investigational treatments for Hodgkin's lymphoma; performance status 0–3 (according to WHO criteria); adequate bone marrow function; creatinine less than 150% of the upper limit of normal (ULN); bilirubin concentration less than two times ULN; aminotransferase concentration less than 2·5 times ULN; and life expectancy of more than 3 months. The full eligibility criteria for the RATHL trial are available in the protocol.
For this prospective cohort substudy, women and aged 18–45 years (ie, premenopausal) were actively recruited from RATHL sites in the UK (appendix pp 3–5). The protocol for the substudy is available online.
This cohort substudy received centralised ethical committee approval that covered all sites (National Research Ethics Service, Southampton and South West Hampshire) and all participants gave written informed consent.
Procedures
In the RATHL study, after initial staging and a baseline PET-CT scan, participants were treated with two cycles of ABVD, intravenously, on days 1 and 15 of a 28 days cycle followed by an interim PET-CT scan (doses and schedule in the appendix [p 2]). Participants who had negative interim scans by central review (PET score of 1 to 3 per Lugano criteria) were randomly assigned (1:1) by the Cancer Research UK and University College London Cancer Trials Centre using minimisation, with stratification according to PET score (1, 2, or 3) and centre, to continue ABVD (intravenously, days 1 and 15) or to receive doxorubicin, vinblastine, and dacarbazine (AVD; intravenously, days 1 and 15) for four more cycles. Since randomisation was done centrally, concealment of assignment was not an issue. Participants with positive scans (PET score of 4 or 5) were escalated to either BEACOPP-14 for six cycles of 14 days or escalated BEACOPP for four cycles (doses and schedule in the appendix [p 2]). Neither patients nor study staff were masked to allocation.
Ovarian function was assessed by use of serum concentrations of antimüllerian hormone in participants enrolled into this substudy and follicle-stimulating hormone concentrations from all eligible participants (women and aged 18–45 years) from the RATHL trial. Luteinising hormone and oestradiol measurements were also taken. Oestradiol measurements are necessary for the identification of women with premature ovarian insufficiency.11
During the RATHL trial, blood samples were taken before any treatment, after two cycles of initial ABVD treatment, at the end of chemotherapy, and at 1, 2, and 3 years after chemotherapy. Samples taken after participants showed disease progression and required additional treatment were excluded from both analyses reported here. Sampling was not timed by the participant's stage of menstrual cycle. Follicle-stimulating hormone measurements were done locally as part of the RATHL trial; additional samples from participants enrolled in this substudy were taken to measure antimüllerian hormone, follicle-stimulating hormone, luteinising hormone, and oestradiol concentrations by use of Roche Elecsys assays (automated cobas e 411 analyser, Roche, Burgess Hill, UK). The substudy samples were sent by post to a central laboratory (MRC Centre for Reproductive Health, Edinburgh), where the serum was stored at −20°C until analysis. The antimüllerian hormone assay has a limit of detection of 0·07 pmol/L, and for all assays the interassay and intra-assay coefficients of variation were below 5%. A follicle-stimulating hormone threshold of more than 25 IU/L indicated probable premature ovarian insufficiency.11
After recovery from chemotherapy, antimüllerian hormone was additionally analysed in association with predictors from before treatment, specifically age and antimüllerian hormone concentration. Antimüllerian hormone recovery was calculated at 2 years after chemotherapy to allow full recovery of ovarian function but minimise any age-related changes. The study was not designed to assess fertility, and menstrual bleeding was not recorded since it does not help to distinguish between women with normal ovarian function and those with very depleted ovarian reserves.
Data on previous pregnancy or use of hormonal contraceptives were only collected from participants enrolled in the ovarian function substudy. Decisions regarding fertility preservation procedures were between the participant and the local centre, and were not recorded as part of the study.
Outcomes
The primary outcome of this study, specified as an exploratory outcome in the RATHL trial, was assessment of gonadal function. The prespecified outcomes in this substudy were analysis of acute toxic effects of each treatment regimen on the ovaries, and ovarian function after treatment (including progression to ovarian failure and recovery of ovarian function). Other prespecified outcomes were the association between fertility after treatment and ovarian function before treatment, degree of acute ovarian toxic effects, which will be reported separately.
Statistical analysis
As a secondary analysis of the RATHL trial, the prespecified exploratory analyses presented here are not powered; we aimed to recruit as many patients as possible while the RATHL trial was open. We hypothesised that antimüllerian hormone concentrations could show differences between the gonadotoxicity of the chemotherapy regimens in the RATHL trial, both during and after treatment, and that concentrations of antimüllerian hormone before treatment would be predictive of recovery of ovarian function after treatment. We analysed antimüllerian hormone and follicle-stimulating hormone concentrations in the ovarian subgroup after log transformation using ANOVA with Bonferroni's multiple comparisons test for changes during treatment and recovery. We calculated Pearson correlation coefficients and used multiple linear regression without transformation to analyse age and before treatment concentration of antimüllerian hormone against percentage hormone recovery (calculated as [concentration at 2 years÷concentration before treatment] x 100) and against concentration at 2 years. We used the Mann-Whitney U test to compare hormone concentrations between the treatment groups. We analysed proportions of participants using Fisher's exact test to determine the number of women who had a pregnancy after chemotherapy. We measured follicle-stimulating hormone recovery using Cox regression and Kaplan-Meier survival analysis. We measured time to recovery (follicle-stimulating hormone concentration of ≤25 IU/L) from the end of treatment until the first follicle-stimulating hormone measurement of 25 IU/L or less, or a reported pregnancy (whichever came first). Patients with follicle-stimulating hormone concentrations of more than 25 IU/L at baseline were excluded, and patients who had neither event were censored at the last reported follicle-stimulating hormone measurement. Any measurements recorded after disease progression were excluded from all analyses because patients would have been exposed to further treatment at this point. We tested the assumption of proportional hazards using Schoenfeld residuals, and when it did not hold we used restricted mean survival times with a 3 year cutoff. All p values are two-sided and a p value of less than 0·05 was considered statistically significant.
Data analyses were done in STATA (version 15.1) and Graphpad Prism 7. The RATHL trial is registered with ClinicalTrials.gov, number NCT00678327.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. RAA, AAK, and PWMJ had access to the raw data. The corresponding author had final responsibility for the decision to submit for publication.
Results
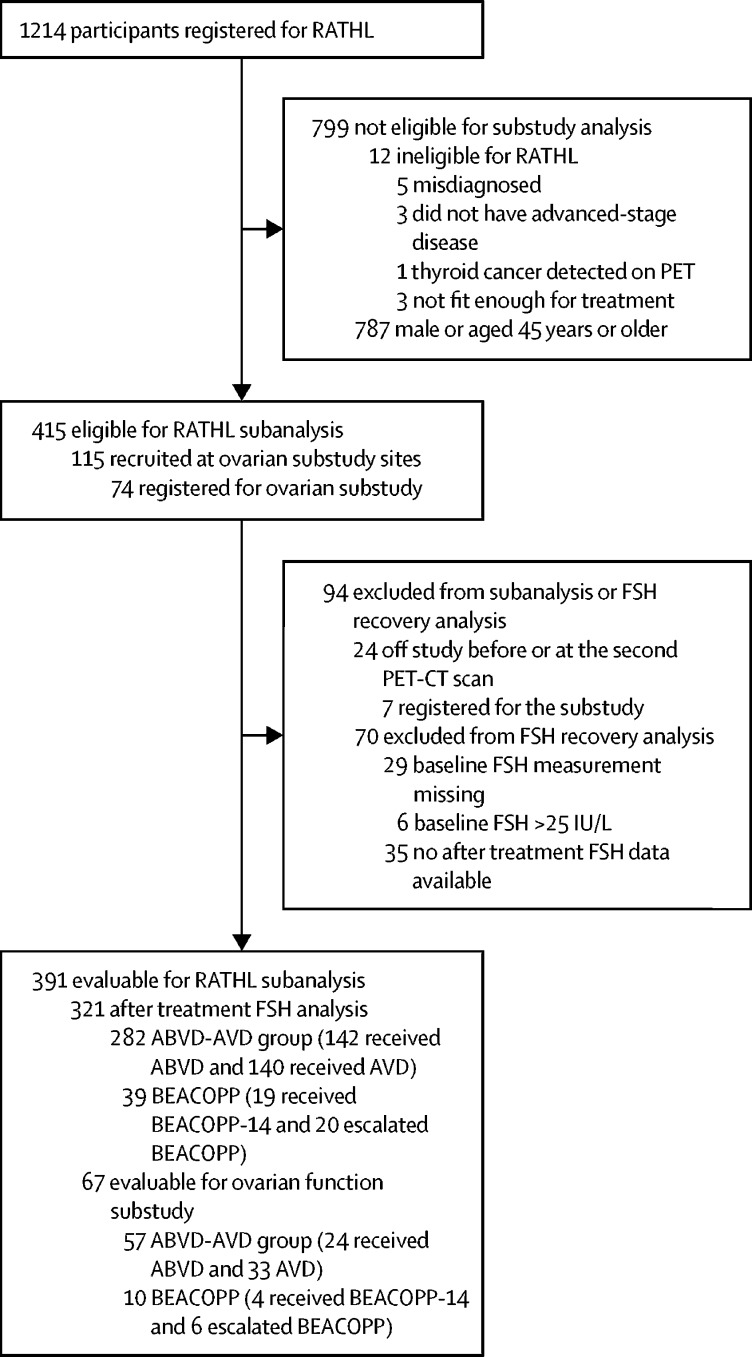
Between Dec 13, 2010, and Dec 19, 2012, participants from the RATHL trial were recruited for this ovarian function substudy (figure 1; appendix p 3). 115 (9%) of 1214 participants in the RATHL trial were eligible for the substudy, of whom 74 (69%) registered. Seven (10%) of 74 registered participants were excluded from the substudy and the main trial, six for scan protocol deviations, and one for psychological reasons, leaving 391 (94%) of 415 eligible (18–45 years) female RATHL participants, of whom 321 (82%) were evaluable for follicle-stimulating hormone analyses, and 67 (91%) were evaluable for substudy analyses. Of the 67 evaluable participants for the substudy analyses, 24 (36%) had been treated with ABVD, 33 (49%) with AVD, four (6%) with BEACOPP-14, and six (9%) with escalated BEACOPP. No difference in baseline hormone concentrations were found between participants treated with ABVD versus AVD, or BEACOPP-14 versus escalated BEACOPP (table); thus these subgroups were combined as ABVD-AVD (n=57) and BEACOPP (n=10), respectively, for subsequent analyses. The baseline characteristics of the participants who had follicle-stimulating hormone measurements from the RATHL trial, and the combined ABVD-AVD and BEACOPP subgroups evaluated in this substudy are in the table.
Figure 1.
Study profile
RATHL trial and ovarian function substudy participants who contributed to analysis. ABVD=doxorubicin, bleomycin, vinblastine, and dacarbazine. AVD=doxorubicin, vinblastine, and dacarbazine. BEACOPP=bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone. FSH=follicile-stimulating hormone.
Table.
Baseline characteristics
|
Ovarian function substudy cohorts |
RATHL trial cohorts |
||||||
|---|---|---|---|---|---|---|---|
| ABVD-AVD group (n=57) | BEACOPP group (n=10) | ABVD group (n=165) | AVD group (n=171) | BEACOPP-14 group (n=27) | Escalated BEACOPP group (n=28) | ||
| Age, years | 26 (18–44) | 31 (19–43) | 27 (18–44) | 28 (18–44) | 24 (18–44) | 35 (18–43) | |
| International prognostic index of 3 or more | 4 (7%) | 4 (40%) | 33 (20%) | 24 (14%) | 9 (33%) | 7 (25%) | |
| WHO performance status | |||||||
| 0 | 47 (82%) | 7 (70%) | 123 (75%) | 143 (84%) | 20 (74%) | 20 (74%)* | |
| 1 | 9 (16%) | 2 (20%) | 34 (21%) | 26 (15%) | 7 (26%) | 6 (22%)* | |
| 2 | 1 (2%) | 1 (10%) | 4 (2%) | 2 (1%) | 0 | 1 (4%) | |
| 3 | 0 | 0 | 4 (2%) | 0 | 0 | 0 | |
| Stage | 4 (2%) | ||||||
| II | 34 (60%) | 3 (30%) | 90 (55%) | 86 (50%) | 14 (52%) | 11 (39%) | |
| III | 9 (16%) | 1 (10%) | 37 (22%) | 46 (27%) | 2 (7%) | 7 (25%) | |
| IV | 14 (25%) | 6 (60%) | 38 (23%) | 39 (23%) | 11 (41%) | 10 (36%) | |
| Previous pregnancy | 16 (28%) | 7 (70%) | .. | .. | .. | .. | |
| Hormonal contraception at baseline | 17 (30%) | 3 (30%) | .. | .. | .. | .. | |
| Baseline antimüllerian hormone, pmol/L | 9·8 (5·9–18·1) | 6·8 (2·2–12·8) | .. | .. | .. | .. | |
| Baseline follicle-stimulating hormone concertation, IU/L | 4·6 (0·3–12) | 5·7 (1·9–21·8) | 4·4 (0·1–48·7) | 4·7 (0·1–50·7) | 5·7 (0·3–26·5) | 3·6 (0·1–35·7) | |
Data are median (range) or n (%) unless otherwise stated. RATHL trial cohort data are for women younger than 45 years included in this study. ABVD=doxorubicin, bleomycin, vinblastine, and dacarbazine. AVD=doxorubicin, vinblastine, and dacarbazine. BEACOPP= bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone.
One patient in this group had a missing performance status.
For nine participants in the substudy, data at post-baseline timepoints were not available because of disease progression and further treatment (six in the ABVD-AVD group, three in the BEACOPP group), and 13 blood samples were missing or taken at incorrect timepoints. Eight blood samples taken from eight women during pregnancy in the ovarian subgroup were excluded from analysis.
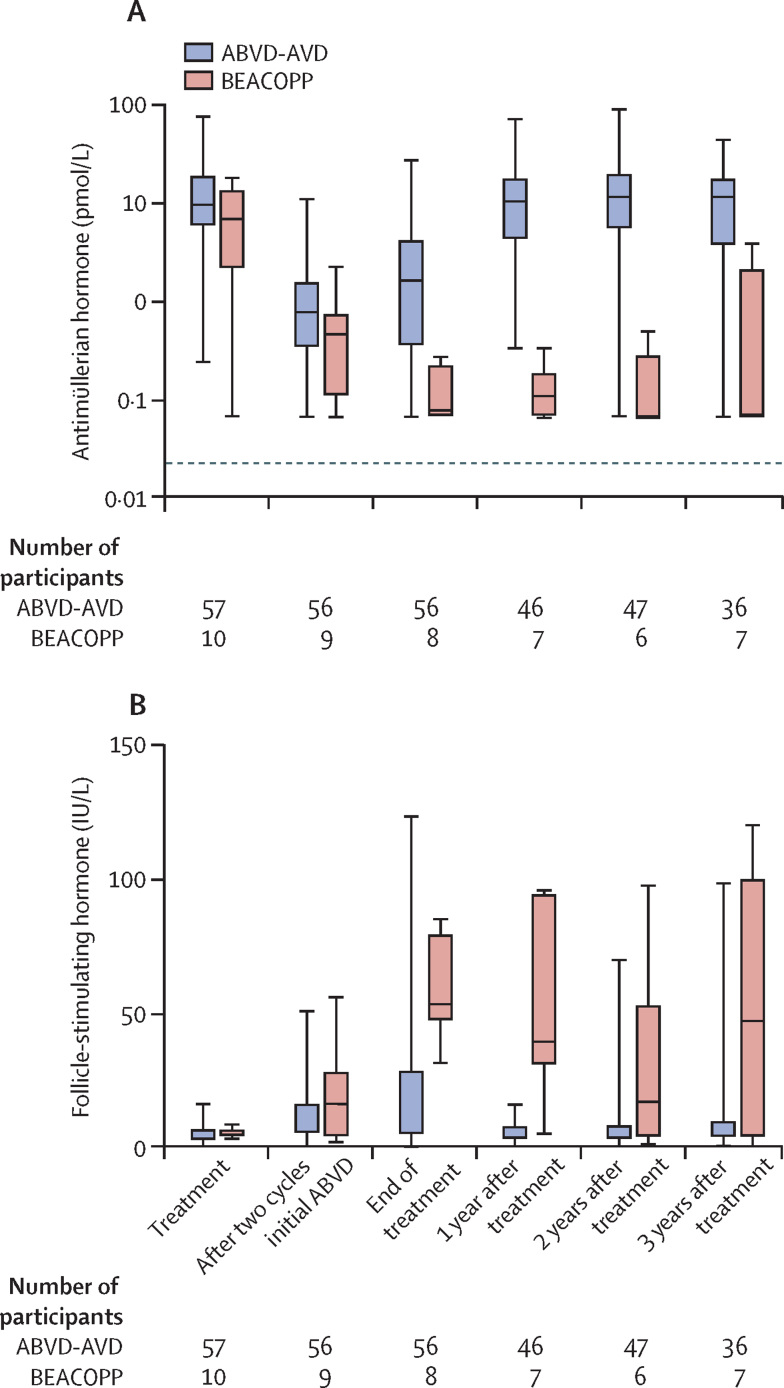
Antimüllerian hormone concentrations decreased in all participants during chemotherapy treatment with reciprocal increases in follicle-stimulating hormone concentrations (figure 2). In the ABVD-AVD group, concentrations of antimüllerian hormone decreased from a median of 9·8 pmol/L (IQR 5·9–18·1) before treatment to 1·7 pmol/L (IQR 0·4–4·3) at the end of treatment (p<0·0001), with a small rise between cycle two of initial ABVD treatment and the end of treatment (p<0·0001). After chemotherapy in the ABVD-AVD group, concentrations of antimüllerian hormone increased to 10·5 pmol/L (IQR 4·3–17·3) at 1 year, similar to concentrations before treatment, with no change at later timepoints (figure 2). Antimüllerian hormone was undetectable in two (4%) of 56 participants at the end of treatment with follicle-stimulating hormone concentration higher than 25 IU/L and low oestradiol concentrations indicating premature ovarian insufficiency. For one of these participants, antimüllerian hormone became detectable during recovery, and for the other, antimüllerian hormone showed a transient recovery at 1 year after treatment, becoming undetectable thereafter.
Figure 2.
Ovarian function biomarkers at prespecified timepoints per treatment group
(A) Concentrations of antimüllerian hormone and (B) follicle-stimulating hormone. All participants included in the substudy were analysed (ABVD-AVD group, n=57; BEACOPP group, n=10). Boxes are median and IQR, bars are ranges. Panel A is plotted on a log10 scale to clearly show the low concentrations seen in the BEACOPP group. ABVD=doxorubicin, bleomycin, vinblastine, and dacarbazine. AVD=doxorubicin, vinblastine, and dacarbazine. BEACOPP=bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone.
In the BEACOPP group, concentrations of antimüllerian hormone decreased from a median of 6·8 pmol/L (IQR 2·2–12·8) before treatment to 0·08 pmol/L (0·07–0·24) at the end of treatment (p<0·0001; figure 2). After treatment, by contrast with participants in the ABVD-AVD group, concentrations of antimüllerian hormone showed very little recovery (median 0·11 pmol/L [IQR 0·07–0·20] at 1 year) and were undetectable in five (71%) of seven participants at 3 years (figure 2A).
Follicle-stimulating hormone concentrations had generally reciprocal changes compared with concentrations of antimüllerian hormone and increased during treatment in both groups (figure 2B). Subsequently, in the ABVD-AVD group concentrations of follicle-stimulating hormone decreased to levels similar to before treatment at years 1 to 3 after treatment. However, in the BEACOPP group, follicle-stimulating hormone concentrations did not decrease in the follow-up period. Concentrations of luteinising hormone showed the same pattern of changes, increasing from before treatment to the end of treatment in both groups (both p<0·0001) and then recovering after treatment to before-treatment concentrations in the ABVD-AVD group, but remaining significantly increased after treatment in the BEACOPP group (data not shown).
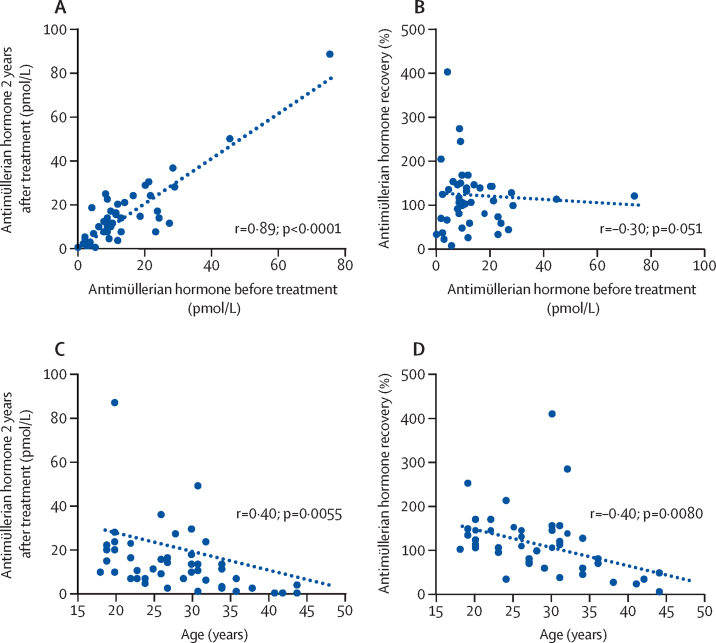
Both antimüllerian hormone concentration 2 years after treatment and follicle-stimulating hormone recovery data provide evidence of the effect of age on ovarian recovery in women treated with ABVD or AVD (Figure 3, Figure 4). We saw a correlation between the concentration of antimüllerian hormone before treatment and at 2 years after treatment (r=0·89; p<0·0001) but not with antimüllerian hormone recovery (r=–0·30; p=0·051). However, significant and similar negative correlations were seen between age and antimüllerian hormone concentrations at 2 years (r=–0·40; p=0·0055) and with antimüllerian hormone recovery (r=–0·40; p=0·0080). This negative effect of age on recovery was supported by mean concentrations of antimüllerian hormone at recovery, with participants younger than 35 years showing complete recovery at 127% (SD 12; median 10·0 pmol/L [IQR 7·5–21·1] before treatment vs 13·4 pmol/L [7·1–22·3] at 2 years), whereas concentrations in those aged 35 years and older had incomplete recovery (37% [SD 10]; ≤35 years, median 5·7 pmol/L [IQR 2·1–9·7] before treatment vs 1·4 pmol/L [0·2–4·2]) at 2 years; for comparison of age groups p<0·0001). Analysis of antimüllerian hormone concentrations before treatment grouped by whether they were below or above the overall median value (9·77 pmol/L [IQR 5·9–18·1]) showed that recovery was similar between the two groups (below 123% [SD 21] vs above 103% [SD 9]; p=0·85). Multiple linear regression analysis also confirmed that age had a significant effect on antimüllerian hormone recovery (β=–0·43; p=0·004), but that antimüllerian hormone concentrations before treatment did not have an effect on hormone recovery (β=–0·15, p=0·30). Thus, although overall concentrations of antimüllerian hormone after treatment seem to recover to before treatment concentrations, the degree of recovery is restricted by increasing age.
Figure 3.
Correlations of antimüllerian hormone recovery and before treatment hormone concentrations or age for the ABVD-AVD group
Panels show scatter plots of baseline hormone concentration versus concentration at 2 years (A) and versus percentage hormone recovery (B), and age versus hormone concentration at 2 years (C) and percentage hormone recovery (D). All patients in the ABVD-AVD group of the substudy included. Each dot shows antimüllerian hormone concentration or recovery; recovery was calculated for each participant as the concentration at 2 years after treatment as a proportion of the concentration before treatment. In the case of missing data, recovery could not be calculated. Dotted ine shows linear regression. ABVD=doxorubicin, bleomycin, vinblastine, and dacarbazine. AVD=doxorubicin, vinblastine, and dacarbazine.
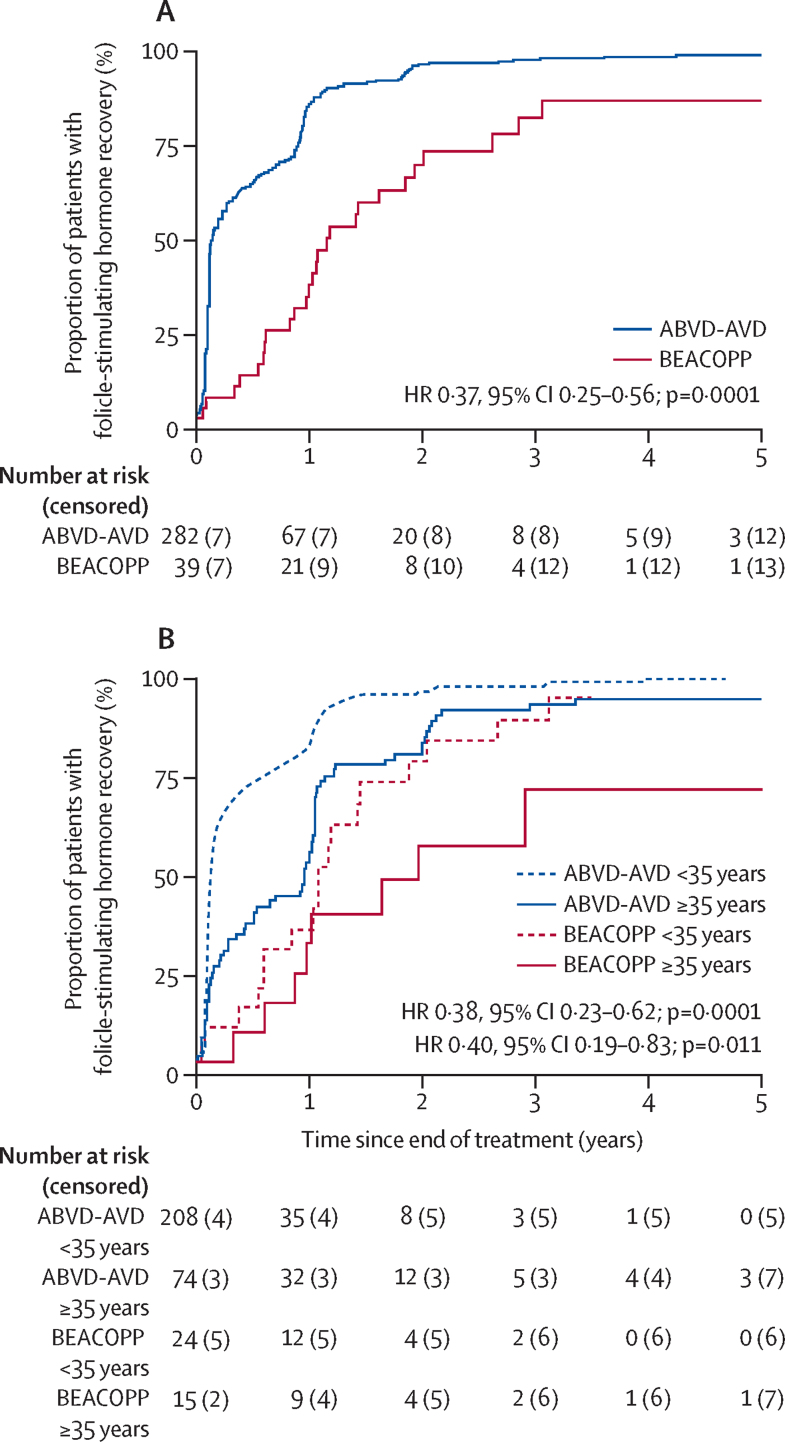
Figure 4.
Recovery of follicle-stimulating hormone concentrations after the end of treatment in evaluable patients from the RATHL trial
Kaplan-Meier curves shows recovery of follicle-stimulating hormone concentrations per treatment group (A) and by age group (B). Evaluable female participants (n=391) were divided by chemotherapy regimen received: ABVD or ABVD followed by AVD (ABVD-AVD group), and ABVD followed by BEACOPP-14 or escalated BEACOPP (BEACOPP group). ABVD=doxorubicin, bleomycin, vinblastine, and dacarbazine. AVD=doxorubicin, vinblastine, and dacarbazine. BEACOPP=bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone. HR=hazard ratio.
We also assessed the degree and timecourse of recovery of ovarian function, as indicated by normalisation of follicle-stimulating hormone concentrations, in the evaluable participants from the RATHL trial (figure 4). Follicle-stimulating hormone measurements after treatment were available from 321 women in the RATHL trial, with a median follow-up (censoring at recovery) of 59·3 months (IQR 41·9–60·9). Follicle-stimulating hormone concentration recovery to 25 IU/L or lower was seen in 270 (96%) of 282 of participants treated with ABVD or AVD, compared with 26 (67%) of 39 treated with BEACOPP-14 or escalated BEACOPP (hazard ratio [HR] 0·37, 95% CI 0·25–0·56; p=0·0001). The assumption of proportional hazards did not hold for this comparison so a better estimate is given by the restricted mean survival (recovery) times (areas under the curves up to 3 years), which are 208·9 days for ABVD or AVD and 529·9 days for BEACOPP (p<0·0001)—ie, patients treated with ABVD or AVD have a mean recovery time of 321·0 days (95% CI 205·7–436·4) less than those treated with BEACOPP. Kaplan-Meier estimates for participants treated with ABVD or AVD were 75% (95% CI 70–80) 1 year after treatment and 93% (89–95) 2 years after treatment, whereas the estimates for those treated with BEACOPP were 33% (20–52) 1 year after treatment and 69% (52–84) 2 years after treatment. The relative difference in ovarian function (between ABVD or AVD and BEACOPP) did not differ when analysed by age (<35 years, HR 0·38, 95% CI 0·23–0·62; p=0·0001 vs ≥35 years, HR 0·40, 0·19–0·83; p=0·011). This pattern was also found when analysed by use of restricted mean survival (recovery) times (<35 years, 154·6 days for ABVD or AVD and 446·5 days for BEACOPP [difference: 291·9 days, 95% CI 163·4–420·4; p<0·0001] vs ≥35 years, 361·5 days for for ABVD or AVD and 663·4 days for BEACOPP [difference 301·9 days, 95% CI 94·1–509·7; p=0·005]). However, age seemed to affect time to recovery among these participants, with a smaller proportion of those aged 35 years or older showing recovery at both 1 year (41 [50%]) and 2 years (64 [79%]) after treatment than those younger than 35 years did (176 [79%] at 1 year and 211 [95%] at 2 years after treatment; HR 0·49, 95% CI 0·37–0·65; p<0·0001). Thus, the Kaplan-Meier estimates for follicle-stimulating hormone concentration recovery for those younger than 35 years treated with ABVD or AVD were 83% (95% CI 77–88) at 1 year and 96% (93–98) at 2 years compared with estimates for those aged 35 years and older of 54% (43–66) at 1 year and 83% (73–91) at 2 years. The proportions were similar between the age groups at 3 years (<35 years 98% [95–99] vs ≥35 years 93% [85–97]). We saw no difference in follicle-stimulating hormone recovery between participants treated with escalated BEACOPP or BEACOPP-14 for all ages (HR 0·72, 95% CI 0·33–1·56).
In the RATHL cohort, 64 (16%) of 391 participants were recorded as having 81 pregnancies. Median follow-up for the 91 patients was 52 months (IQR 42–64). 57 (17%) participants in the ABVD-AVD group had 71 pregnancies and seven (13%) participants in the BEACOPP group had ten pregnancies. These proportions were not substantially different. In the subgroup cohort, antimüllerian hormone measurements were available within the follow-up period for six women who became pregnant in the ABVD-AVD group and two in the BEACOPP group. At 1 year after chemotherapy, concentrations of antimüllerian hormone ranged from 0·74 pmol/L to 26·9 pmol/L in those who became pregnant in the ABVD-AVD group, and were 0·19 pmol/L and 0·20 pmol/L in those in the BEACOPP group. Two women, one in each treatment group, had undetectable antimüllerian hormone, high follicle-stimulating hormone, and low oestradiol concentrations after the recorded pregnancy, consistent with development of premature ovarian insufficiency within 3 years of chemotherapy treatment.
Adverse event data were reported in detail previously,17 but they did not include any data on ovarian function or fertility. The main study17 found an excess of grade 3–4 toxic effects from BEACOPP regimens, specifically febrile neutropenia and thrombocytopenia.
Discussion
In this prospective cohort study, we have shown that antimüllerian hormone concentrations decrease in women with Hodgkin's lymphoma who are treated with ABVD, AVD, or BEACOPP (BEACOPP-14 or escalated BEACOPP), and although antimüllerian hormone concentrations recover to baseline concentrations after treatment with ABVD or AVD, little recovery is seen after treatment with BEACOPP. However, we found that recovery of ovarian function after treatment with ABVD or AVD is dependent on age, with full recovery of antimüllerian hormone seen in participants younger than 35 years, but not in women aged 35 years or older. We found that recovery of follicle-stimulating hormone after these two chemotherapy regimens also differed in both speed and extent. Follicle-stimulating hormone recovery after treatment with ABVD or AVD was slower in women aged 35 years or older than in those younger than 35 years, and the extent of recovery was much lower for those treated with either BEACOPP regimen, supporting the increased ovarian toxicity of this drug combination. The greater toxicity to other organ systems is also reflected in the higher incidence of febrile neutropenia and thrombocytopenia with BEACOPP.
The potential adverse effect of chemotherapy on ovarian function in women with Hodgkin's lymphoma is of major concern, affecting fertility, sexual and bone health, and cardiovascular risk. Potential loss of fertility is a key concern of young women treated for cancer18 and, when appropriate, options for fertility preservation have been established; thus, the accurate assessment of the effect of different chemotherapy regimens is of substantial importance. Assessment of ovarian function after treatment via menstrual function and traditional biomarkers such as follicle-stimulating hormone are not of value in detecting ovarian damage that has resulted in incomplete loss of ovarian function. Measurement of concentrations of antimüllerian hormone has emerged as a reliable biomarker to detect partial loss of ovarian function in women after cancer therapy, with the additional advantages of showing little variation across the menstrual cycle.12 Although most data are from women after treatment for breast cancer and childhood cancer,5, 7, 8 we have shown the merit of antimüllerian hormone measurements in the assessment of ovarian function in adult women with Hodgkin's lymphoma. Specifically, younger women treated with ABVD or AVD showed full initial recovery of antimüllerian hormone after chemotherapy, consistent with previous data2 that indicate that this regimen has little effect on age at menopause, but reduced recovery was seen in women aged 35 years or older at diagnosis. By contrast, treatment with BEACOPP resulted in noticeable and irreversible decreases in antimüllerian hormone concentrations, so low in many women that the hormone was undetectable after treatment even with the highly sensitive assay we used. However, the number of participants we analysed was small, which should inform interpretation of tests of significance. Participants received either escalated BEACOPP or BEACOPP-14, a dose-intense version that appears to be similarly gonadotoxic to baseline BEACOPP.3 Our data support this similarity, although the confidence intervals in our estimates were wide.
Antimüllerian hormone concentrations decreased quickly during treatment with both regimens, consistent with both treatments resulting in loss of the growing follicles that are the source of antimüllerian hormone. After the initial decrease, a small partial recovery in antimüllerian hormone was seen after two cycles in women treated with ABVD or AVD during later treatment cycles. This finding is consistent with increased activation of early follicles during chemotherapy resulting from reduced inhibition of growth initiation, which is proposed to be part of the mechanism for chemotherapy-induced premature ovarian insufficiency. This mechanism has been shown for high toxicity, cyclophosphamide-based regimens,19 but has not previously been identified for low toxicity regimens such as ABVD or AVD.
The degree of recovery of antimüllerian hormone after treatment with ABVD or AVD in young women could indicate a low toxic effect on the non-growing primordial follicle pool, which constitutes the true ovarian reserve and is the basis for after treatment fertility and the duration of the female reproductive lifespan. Under physiological conditions, antimüllerian hormone concentrations reflect the size of the primordial follicle population, although this population size can be perturbed under circumstances such as a new diagnosis of lymphoma.20 However, the 3-year follow-up period in this study is likely to have been sufficient for full recovery of ovarian function and therefore restoration of physiological associations between antimüllerian hormone and the ovarian reserve. We saw a prominent effect of age on recovery, with substantially lower recovery in women aged 35 years or older than in those younger than 35 years, independent of their antimüllerian hormone concentration before treatment. This finding indicates an effect either of age alone or of age in addition to the effects of ABVD or AVD that result in a substantial decrease in the size of the growing follicle population, and by implication of the non-growing population, after treatment. Chemotherapy has been shown to affect both the stroma and vasculature of the human ovary, causing fibrosis and hyalinisation of small vessels with loss of primordial follicles within areas of damage.4 Changes in ovarian stromal function with age include fibrosis and other hallmarks of chronic inflammation, such as the presence of macrophages,21 and can impair follicle development.22 These changes could be reversed by gonadotropin suppression,22 indicating that the increases in concentrations of luteinising hormone and follicle-stimulating hormone during chemotherapy shown here could be of aetiological importance, and the pre-existing more fibrotic stroma in older women could be more susceptible to chemotherapy-induced changes than the stroma in younger women, even with so-called low gonadotoxicity regimens such as ABVD or AVD. These age-associated effects of chemotherapy could have therapeutic implications and be part of the mechanism of action of gonadotropin-releasing hormone agonists to reduce the risk of premature ovarian insufficiency after chemotherapy for breast cancer,23, 24 although this effect has not been confirmed for women treated for lymphoma.25
The effect of age on recovery of ovarian function after both regimens, and the different toxic effects of each regimen on the ovaries, is also supported by our analysis of follicle-stimulating hormone recovery in the whole RATHL study cohort. In this analysis, follicle-stimulating hormone was dichotomised at a value consistent with premature ovarian insufficiency.11 In addition to confirming the effect of age on recovery from treatment with ABVD or AVD, our analysis showed clear differences in both the speed and extent of recovery between regimens, supporting the antimüllerian hormone data. Follicle-stimulating hormone concentrations are affected to a much greater extent than antimüllerian hormone concentrations by changes across the menstrual cycle and use of hormonal contraception, but our analysis shows measurement of follicle-stimulating hormone concentrations is of value in large cohorts in which more detailed characterisation is difficult.
Previous analyses in women with breast cancer have shown that receiving treatment at a young age and high concentrations of antimüllerian hormone before treatment are predictive of recovery of ovarian function after chemotherapy, albeit with variable relative contributions.5, 9, 14, 15 Women with breast cancer are generally older (approximate mean age 40 years5, 9, 14, 15) than those with Hodgkin's lymphoma and treatment involves more gonadotoxic alkylating agent-based regimens, resulting in a high probability of premature loss of ovarian function, particularly in women older than 40 years. These differences emphasise the need for defined populations and treatment regimens to analyse the toxic effects of chemotherapeutic regimens on the ovaries and, particularly, recovery.
The data in this study support the paucity of the value of current ovarian reserve markers for the prediction of fertility in the short term, because very low concentrations of antimüllerian hormone did not preclude chances of pregnancy. The lack of predictive value of antimüllerian hormone measurements for short term fertility has been shown in prospective cohorts of women mostly in their twenties and thirties,26, 27 and in women after cancer treatment.28 This study was not designed to assess chances of pregnancy after treatment, and indeed current clinical advice is that women should not attempt to conceive after chemotherapy for around 1–2 years—ie, most of the duration of follow-up in this study. We do not know how many women in each group attempted to conceive, but a similar proportion of pregnancies were seen in women in the BEACOPP group as in the ABVD-AVD group within the substudy. Although these results show that a low concentrations of antimüllerian hormone do not preclude pregnancy in the short term, they probably do indicate a reduced interval to menopause and thus a shortened duration of opportunity to achieve pregnancy in the longer term.29 Thus, a low concentration of antimüllerian hormone after recovery from chemotherapy could identify women who should not unduly postpone pregnancy, and inform individualised discussion; longer follow-up studies are needed to assess this interpretation.
Our study had several limitations. The study was not designed to assess fertility, which would require longer follow-up and a design that incorporates intention to conceive. The antimüllerian hormone analysis is restricted by the size of the dataset, reflecting the short period for which recruitment was open for the substudy. We also acknowledge the limitations of using follicle-stimulating hormone measurements in isolation without a more robust evidence of premature ovarian insufficiency, which was not possible in the main RATHL trial, and data on hormonal contraceptive use during and after treatment were not available.
In conclusion, the results of this secondary analysis of the RATHL trial indicate the value of antimüllerian hormone as a biomarker of toxic effects of chemotherapy on the ovaries during and after different chemotherapy regimens for advanced Hodgkin's lymphoma. We provide additional evidence that treatment with ABVD or AVD has no detectable effect on gonadal function in young women, although increasing age does restrict ovarian recovery; confirmation in larger studies is needed to confirm and define this interpretation more precisely, and determine its mechanism. By contrast, BEACOPP shows substantial gonadotoxicity in women of all ages, although some patients might have sufficient ovarian function after treatment to achieve pregnancy. Concentrations of antimüllerian hormone after treatment could be of use in advising women treated for Hodgkin's lymphoma of their probable reproductive lifespan after treatment.
Acknowledgments
Acknowledgments
The RATHL trial was funded by Cancer Research UK (reference CRUK/07/033), and the ovarian substudy by the Medical Research Foundation (reference 509909). Part of this work was done in the MRC Centre for Reproductive Health, University of Edinburgh, UK, which is funded by MRC Centre grant MR/N022556/1. We thank Roche Diagnostics for the supply of assay reagents, A Forbes Howie for hormone assays, and all investigators in RATHL for their contributions to patient recruitment and management.
Contributors
RAA and PWMJ designed the study; collected, analysed, and interpreted data; contributed to manuscript writing; and approved the manuscript before submission. RR collected and analysed data and approved the manuscript before submission. AAK and LC-H collected, analysed, and interpreted data, contributed to manuscript writing, and approved the manuscript before submission. PP managed the study, collected data, gave constructive comments on the manuscript, and approved the manuscript before submission. LS and TR collected data, gave constructive comments on the manuscript, and approved the manuscript before submission. CH collected data via patient recruitment, and gave constructive comments on the manuscript. NK, DWM, PM, and FMS collected data, contributed to manuscript writing, and approved the manuscript before submission. CR recruited patients, collected data, contributed to manuscript writing, and approved the manuscript before submission.
Declaration of interests
RAA reports non-financial support from Roche diagnostics, and a grant from the Medical Research Foundation. All other authors declare no competing interests.
Supplementary Material
References
- 1.Hoskin PJ, Lowry L, Horwich A. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin's lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–5396. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow AJ, Cooke R, Bates A. Risk of premature menopause after treatment for Hodgkin's lymphoma. J Natl Cancer Inst. 2014;106:dju207. doi: 10.1093/jnci/dju207. [DOI] [PubMed] [Google Scholar]
- 3.Behringer K, Breuer K, Reineke T. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Dor J, Kaufman B. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22:1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RA, Cameron DA. Pretreatment serum anti-müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 6.Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280–285. doi: 10.1016/j.rbmo.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.van Beek RD, van den Heuvel-Eibrink MM, Laven JS. Anti-müllerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–3874. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 8.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–2067. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 9.Su HC, Haunschild C, Chung K. Prechemotherapy antimüllerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer. 2014;120:3691–3698. doi: 10.1002/cncr.28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge AH, Ruddy KJ, Gelber S. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Eurpoean Society for Human Reproduction and Embryology Guideline Group on POI ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 12.Jayasinghe YL, Wallace WHB, Anderson RA. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev Endocrinol Metab. 2018;13:125–136. doi: 10.1080/17446651.2018.1455498. [DOI] [PubMed] [Google Scholar]
- 13.Decanter C, Peigne M, Mailliez A. Toward a better follow-up of ovarian recovery in young women after chemotherapy with a hypersensitive antimüllerian hormone assay. Fertil Steril. 2014;102:483–487. doi: 10.1016/j.fertnstert.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RA, Mansi J, Coleman RE, Adamson DJA, Leonard RCF. The utility of anti-müllerian hormone in the diagnosis and prediction of loss of ovarian function following chemotherapy for early breast cancer. Eur J Cancer. 2017;87:58–64. doi: 10.1016/j.ejca.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnabei A, Strigari L, Marchetti P. Predicting ovarian activity in women affected by early breast cancer: a meta-analysis-based nomogram. Oncologist. 2015;20:1111–1118. doi: 10.1634/theoncologist.2015-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dezellus A, Barriere P, Campone M. Prospective evaluation of serum anti-müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer. 2017;79:72–80. doi: 10.1016/j.ejca.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Johnson P, Federico M, Kirkwood A. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116:215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- 19.Kalich-Philosoph L, Roness H, Carmely A. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 20.Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet. 2016;33:657–662. doi: 10.1007/s10815-016-0689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briley SM, Jasti S, McCracken JM. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152:245–260. doi: 10.1530/REP-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umehara T, Kawai T, Kawashima I. The acceleration of reproductive aging in Nrg1flox/flox;Cyp19-Cre female mice. Aging Cell. 2017;16:1288–1299. doi: 10.1111/acel.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard RCF, Adamson DJA, Bertelli G. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017;28:1811–1816. doi: 10.1093/annonc/mdx184. [DOI] [PubMed] [Google Scholar]
- 24.Lambertini M, Moore HCF, Leonard RCF. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. 2018;36:1981–1990. doi: 10.1200/JCO.2018.78.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demeestere I, Brice P, Peccatori FA. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016;34:2568–2574. doi: 10.1200/JCO.2015.65.8864. [DOI] [PubMed] [Google Scholar]
- 26.Hagen CP, Vestergaard S, Juul A. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril. 2012;98:1602–1608. doi: 10.1016/j.fertnstert.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Steiner AZ, Pritchard D, Stanczyk FZ. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318:1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamy AS, Porcher R, Eskenazi S. Anti-müllerian hormone in breast cancer patients treated with chemotherapy: a retrospective evaluation of subsequent pregnancies. Reprod Biomed Online. 2016;32:299–307. doi: 10.1016/j.rbmo.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Depmann M, Broer SL, van der Schouw YT. Can we predict age at natural menopause using ovarian reserve tests or mother's age at menopause? A systematic literature review. Menopause. 2015;23:224–232. doi: 10.1097/GME.0000000000000509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.