Abstract
Objective: To examine the association between brain natriuretic peptide (BNP) gene single nucleotide polymorphisms (SNPs) and chronic obstructive pulmonary disease (COPD) and COPD with pulmonary hypertension (PH) and to analyze its mechanism. Methods: The genotypes of BNP at the rs198389, rs6668352, and rs198388 loci in 339 patients with COPD (205 in the COPD/PH− group and 134 in the COPD/PH+ group) and 125 healthy subjects were detected by PCR/Sanger sequencing. The serum levels of BNP, fibrinogen (Fbg), and Apelin were measured in all subjects by ELISA. Results: The BNP rs198389 locus G allele, rs6668352 locus A allele, and 198388 locus T allele were high risk factors for COPD (P<0.001). Logistics regression analysis showed that BNP rs198389 locus G allele, rs6668352 locus A allele, and rs198388 locus T allele were high risk factors for PH in COPD patients (all P<0.001). The levels of the serum BNP and Fbg protein in the control group, COPD/PH− group, and COPD/PH+ group increased successively, and the expression levels of Apelin protein decreased successively (all P<0.001). The BNP and Fbg protein levels in the wild-type, heterozygote, and mutant homozygote in BNP rs198389, rs6668352, and rs198388 loci increased successively, and the serum Apelin protein levels decreased successively (all P<0.001). Conclusion: The polymorphisms of BNP at the rs198389, rs6668352, and rs198388 loci are associated with the occurrence of COPD and COPD with PH, and the occurrence may be related to the abnormal expression level of BNP, Fbg, and Apelin protein in the serum.
Keywords: Brain natriuretic peptide, Chronic obstructive pulmonary disease, Pulmonary hypertension, Single nucleotide polymorphism
Chronic obstructive pulmonary disease (COPD) is a common chronic disease, and its incidence has gradually increased recently [1]. Pulmonary hypertension (PH) is a common complication of COPD, which seriously affects the prognosis and quality of life of patients [2]. Previous studies show that the occurrence of COPD is related to factors such as the living environment, air quality, smoking history, and genetic factors [3]. There are few studies on the correlation between COPD and COPD with PH and genetic factors.
Brain natriuretic peptide (BNP) is a functional peptide synthesized by cardiomyocytes, and its function is mainly to regulate the physiological balance of blood pressure and blood flow [4]. Studies show that BNP plays an important role in the diagnosis of heart failure, hypertension, and cardiopulmonary diseases [5,6]. For example, Chen et al. [7] showed that N-terminal hormone BNP (NT-proBNP) has a certain value in the rapid diagnosis of patients requiring hospitalization for AECOPD. At present, many single nucleotide polymorphism (SNP) loci of the BNP gene are associated with hypertension and chronic heart failure. A study by Zhang et al. [8] showed that the BNP gene SNP loci rs198389 and rs198388 are associated with the genetic susceptibility to congenital heart disease. Poreba et al. [9] showed that the SNP at the rs198389 site of the BNP gene is associated with the development of atherosclerotic lesions in the renal artery. In addition, Fox et al. [10] studies showed that the rs198389, rs6668352, and rs198388 SNP sites of the BNP gene were associated with ventricular dysfunction (VnD) after primary coronary bypass surgery. However, there are few studies on the association between gene polymorphisms of the BNP genes rs198389, rs6668352, and rs198388 and the occurrences of COPD and COPD with PH. The associations between SNPs in the BNP genes, including rs198389, rs6668352, and rs198388, and COPD and COPD with PH were analyzed. This article focuses on this issue. Apelin is a small molecule polypeptide, and studies show that Apelin can be used as an effective indicator of COPD associated with a diagnosis of PH [11]. Fibrinogen (Fbg) is a protein that is present in plasma. The level of plasma Fbg is positively related to the severity of COPD and thus can be used to reflect the severity of COPD [12].
Methods
General information
A total of 339 patients with COPD from August 2014 to August 2017 were enrolled in the present study; according to whether they were complicated with PH, they were subdivided into a COPD/PH− group (205 cases) and a COPD/PH+ group (134 cases). The diagnostic criteria of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [] {13}. The diagnosis of PH is based on the 2015 guideline for the diagnosis and treatment of PH in ESC/ERS [14], excluding patients with bronchial asthma, tuberculosis, lung cancer, bronchiectasis, interstitial fibrosis, and other respiratory diseases as well as patients with hypertension, malignancy, and connective tissue disease. A total of 125 healthy subjects [forced expiratory volume in one second as a percentage of the predicted (FEV1%) ≥80% and forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ≥70%] without chronic bronchitis and emphysema were enrolled as the control group. The study was approved by our medical ethics committee, and all the participants signed an informed consent (approval number: 201407013).
Clinical data collection
Clinical data from all subjects were collected, including their age, sex, course of disease, body mass index (BMI), and smoking index (number of cigarettes per year). The blood gas parameters of the COPD/PH patients were measured with a blood gas analyzer, and the lung function of the subjects was measured with a Vmax pulmonary function meter, including the FEV1 as a percentage of the predicted value, the FVC, the arterial partial pressure PaO2, and the arterial carbon dioxide partial pressure PaCO2.
Methods of collecting and treating blood
From all subjects, we collected 5 ml of fasting venous blood, of which 3 ml was used to separate the serum after coagulation. The expressions of the BNP (Ek-Bioscience, CAT#: EK-H11285), Fbg (Elabscience, CAT#: E-EL-M0498c), and Apelin (Elabscience, CAT#: E-EL-R2413c) proteins in the serum were detected by ELISA. After anticoagulation by adding EDTA-K2, the remaining 2 ml of blood was subjected to the QIAamp DNA Blood Mini Kit (Qiagen, 51106, German) to extract the genomic DNA. All experimental operations were performed strictly in accordance with the kit instructions.
Genotyping of the BNP gene rs198389, rs6668352, and rs198388 loci
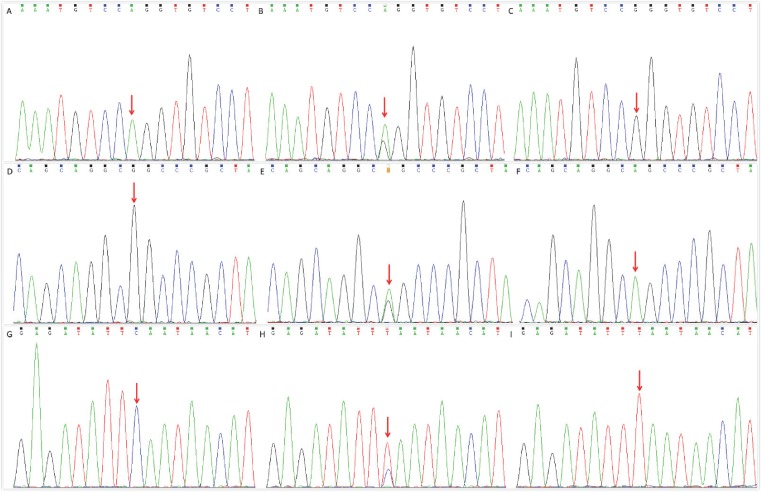
Using the extracted gDNA as a template, the target DNA fragment was amplified by PCR. The amplified primers at each locus and the reaction system and conditions of the PCR amplification reaction are shown in Table 1. After PCR, the PCR product was purified by a DNA Gel Extraction Kit (Beyotime, CAT#:0056), and the sequence of the target DNA was detected by Sanger sequencing. The results are shown in Figure 1.
Table 1. PCR amplification information of the BNP gene rs198389, rs6668352, and rs198388 loci.
| SNP loci | Primer sequence (5′ to 3′) | PCR mix | PCR procedure |
|---|---|---|---|
| rs198389 | Forward primer: AGACACAGACAAGTCCCCGT; Reverse primer: GAAAGCGCCAACCTAGGACA |
ddH2O: 13.8 μl; 10× PCR buffer: 2 μl; 10 mM dNTP mix: 1 μl; Primer Forward: 1 μl; Primer Reverse: 1 μl; Taq DNA Polymerase: 0.25 μl; genomic DNA: 1 μl | 95°C, 5 min (94°C, 45 s; 58°C, 45 s; 72°C, 30 s) 30 cycles; 72°C, 10 min |
| rs6668352 | Forward primer: TGTCACTCACTGGGTACAGC; Reverse primer: ACCTGCGAAGGAGCCAAATG |
||
| rs198388 | Forward primer: TTCTCCCAAGTGCCTCAAGT; Reverse primer: AGGTAGCAGGCTTTCTTTTCT |
Figure 1. The sequencing results of the PCR product of the BNP gene.
A, B, and C were wild-type (AA-type), heterozygous (AG-type), and homozygous mutants (GG-type) of the BNP gene rs198389 locus, respectively. D, E, and F were wild-type (GG type), heterozygous (GA type), and homozygous mutants (AA type) of the BNP gene rs6668352 locus, respectively. G, H, and I were wild-type (CC type), heterozygous (CT type), and mutant homozygous (TT type) of BNP gene rs198388 locus, respectively.
Statistical analysis
The statistical analysis of the data was performed using SPSS 20.0 software (SPSS Inc., Chicago). The continuous and non-parametric data are represented by (±s), and the categorical variables are represented by [n(%)]. The Pearson chi-square test was used to compare the classification variables. The average values of the continuous variables of the normal distribution were compared by one-way analysis of variance (ANOVA). The Mann–Whitney U test was used to compare the skewed distributions of the continuous variables. A non-parametric statistical analysis between the two groups was performed using a t-test. The χ2 test was used to detect whether the genotype frequencies were consistent with the Hardy–Weinberg equilibrium. The odds ratio (OR) and the 95% confidence interval (CI) were used to analyze the correlations between the genotypes and COPD and COPD with PH. A multivariate logistic regression analysis was used to correct the factors such as the age, gender, BMI, and smoking index. P<0.05 indicated that the difference was statistically significant.
Results
Comparison of the clinical parameters
The clinical parameters of the subjects in the control group, COPD/PH− group, and COPD/PH+ group are shown in Table 2. There were no significant differences in the clinical parameters, including the age, gender, BMI, and smoking index, amongst the three groups (P>0.05). There was no significant difference in the course of disease between the COPD/PH− group and the COPD/PH+ group (P>0.05). The PaCO2 of the subjects in the control group, COPD/PH− group, and COPD/PH+ group increased successively, while the FEV1/predicted value, FEV1/FVC, FVC, and PaO2 decreased successively, and the differences were statistically significant (P<0.05).
Table 2. Comparison of the general clinical data amongst the three groups.
| Index | Control group (n=125) | COPD/PH− group (n=205) | COPD/PH+ group (n=134) | P-value |
|---|---|---|---|---|
| Age (years) | 63.8 ± 5.3 (49, 76) | 64.2 ± 4.8 (45, 77) | 64.5 ± 5.2 (45, 77) | 0.597 |
| Gender [male, n (%)] | 68 (54.4%) | 112 (54.6%) | 81 (60.4%) | 0.291 |
| Course (years) | – | 16.1 ± 5.8 (2, 27) | 16.4 ± 5.5 (5, 27) | 0.635 |
| BMI (kg/m2) | 22.8 ± 2.4 (18.9, 26.1) | 22.5 ± 2.4 (16.7, 27.5) | 22.4 ± 2.5 (17.8, 27.3) | 0.490 |
| Smoking (pack/year) | 31.6 ± 3.7 (24, 38) | 32.4 ± 4.1 (23, 39) | 32.0 ± 4.1 (23, 39) | 0.221 |
| FEV1/predicted value (%) | 80.8 ± 12.4 (53, 96) | 43.5 ± 4.7 (32, 63) | 43.6 ± 5.1 (30, 65) | <0.001 |
| FEV1/FVC (%) | 73.5 ± 13.4 (56, 86) | 55.6 ± 11.4 (43, 72) | 53.5 ± 12.1 (40, 76) | <0.001 |
| FVC (%) | 82.1 ± 20.4 (65, 97) | 49.2 ± 17.5 (32, 61) | 40.5 ± 16.7 (26, 58) | <0.001 |
| PaO2 (mmHg) | 88.0 ± 6.1 (76.5, 98.5) | 62.6 ± 8.3 (54.2, 73.6) | 54.5 ± 4.0 (51.2, 59.7) | <0.001 |
| PaCO2 (mmHg) | 44.2 ± 4.2 (36.8, 52.3) | 60.8 ± 7.6 (51.2, 66.7) | 66.4 ± 7.6 (58.4, 72.4) | <0.001 |
| SpO2 decreased by 3% during exercise | 27 (21.6%) | 123 (48.3%) | 105 (85.1%) | <0.001 |
| D-dimer (μg/l) | 307.5 ± 205.4 (102.5, 495.4) | 498.4 ± 207.6 (208.6, 603.7) | 651.7 ± 325.6 (422.8, 982.7) | <0.001 |
| 6MWT/m | 413.8 ± 45.3 (375.8, 462.1) | 312.6 ± 39.8 (264.4, 345.7) | 250.5 ± 36.5 (214.5, 295.6) | <0.001 |
| DLCO/% | 88.5 ± 12.3 (70.5, 95.8) | 56.5 ± 7.8 (44.8, 72.3) | 55.7 ± 7.6 (45.4, 69.8) | <0.001 |
| mPAP/mmHg | 20.1 ± 2.5 (17.9, 23.1) | 23.5 ± 2.7 (19.2, 24.9) | 36.7 ± 5.7 (31.6, 43.8) | <0.001 |
| Pulmonary vascular resistance (WU) | 1.1 ± 0.3 (0.9, 1.4) | 2.4 ± 0.5 (2.2, 2.7) | 4.6 ± 0.7 (4.2, 4.9) | <0.001 |
| Cardiac output (l/min) | 5.9 ± 1.1 (5.1, 6.8) | 6.5 ± 1.2 (5.7, 7.6) | 6.1 ± 1.4 (5.5, 7.7) | 0.764 |
| Cardiac index (l/min/m2) | 2.5 ± 0.9 (2.0, 3.9) | 3.7 ± 1.4 (2.9, 4.9) | 3.4 ± 1.6 (2.7, 5.3) | 0.217 |
| GFR (ml/min × 1.73 m2) | 87.6 ± 4.1 (48.4, 102.3) | 78.5 ± 3.6 (45.5, 99.6) | 62.5 ± 3.1 (37.1, 81.4) | 0.007 |
| RBF (ml/min × 1.73 m2) | 1054.6 ± 65.2 (706.9, 135.4) | 851.2 ± 42.3 (760.1, 991.3) | 653.8 ± 33.9 (583.4, 701.4) | <0.001 |
FVC (minimum, maximum). Abbreviations: GFR, glomerular filtration rate; 6MWT, 6 min walking tests; RBF, renal blood flow.
The correlations between the SNPs of the BNP gene, including the rs198389, rs6668352, and rs198388 loci, and COPD
The genotype distributions of the BNP gene SNP loci rs198389, rs6668352, and rs198388 of the subjects in the three groups are shown in Table 3. The percentage of the rs198389 site homozygous mutation of the BNP gene in the COPD group was significantly higher than that of the control group (adjusted OR = 1.265, 95% CI = 1.100–1.407, P=0.001), and the risk of COPD in the G allele carriers increased significantly (adjusted OR = 1.165, 95% CI = 1.076–1.254, P<0.001). The percentage of the rs6668352 site homozygous mutation of the BNP gene in the COPD group was significantly higher than that in the control group (adjusted OR = 1.327, 95% CI = 1.158–1.463, P<0.001), and the risk of COPD in the A allele carriers increased significantly (adjusted OR = 1.199, 95% CI = 1.108–1.288, P<0.001). The ratio of the rs198388 site homozygous mutation of the BNP gene in the COPD group was significantly higher than that of the control group (adjusted OR = 1.261, 95% CI = 1.091–1.396, P = 0.002), and the risk of COPD in the T allele carriers increased significantly (adjusted OR = 1.145, 95% CI = 1.055–1.232, P<0.001).
Table 3. Genotypic distributions of the rs198389, rs6668352, and rs198388 loci in the BNP gene in the control group and the COPD group.
| SNPs | Control group (n=125) | COPD group (n=339) | P-value | OR (95% CI) | P-value* | OR* (95% CI) |
|---|---|---|---|---|---|---|
| rs198389 genotype | ||||||
| AA | 69 (55.2%) | 141 (41.6%) | 1.00 | |||
| AG | 40 (32.0%) | 108 (31.9%) | 0.238 | 1.321 (0.811–2.157) | 0.287 | 1.087 (0.937–1.247) |
| GG | 16 (12.8%) | 90 (26.5%) | 0.005 | 2.753 (1.450–5.282) | 0.001 | 1.265 (1.100–1.407) |
| Allele | ||||||
| A | 178 (71.2%) | 390 (57.5%) | 1.00 | |||
| G | 72 (28.8%) | 288 (42.5%) | <0.001 | 1.826 (1.319–2.529) | <0.001 | 1.165 (1.076–1.254) |
| rs6668352 genotype | ||||||
| GG | 71 (56.8%) | 137 (40.4%) | 1.00 | |||
| GA | 41 (32.8%) | 112 (33.0%) | 0.136 | 1.416 (0.873–2.299) | 0.169 | 1.111 (0.958–1.276) |
| AA | 13 (10.4%) | 90 (26.5%) | <0.001 | 3.588 (1.804–7.248) | <0.001 | 1.327 (1.158–1.463) |
| Allele | ||||||
| G | 183 (73.2%) | 386 (56.9%) | 1.00 | |||
| A | 67 (26.8%) | 292 (43.1%) | <0.001 | 2.066 (1.485–2.878) | <0.001 | 1.199 (1.108–1.288) |
| rs198388 genotype | ||||||
| CC | 68 (54.4%) | 147 (43.4%) | 1.00 | |||
| CT | 45 (36.0%) | 117 (34.5%) | 0.419 | 1.203 (0.750–1.931) | 0.488 | 1.056 (0.916–1.208) |
| TT | 12 (9.6%) | 75 (22.1%) | 0.001 | 2.891 (1.414–6.017) | 0.002 | 1.261 (1.091–1.396) |
| Allele | ||||||
| C | 181 (72.4%) | 411 (60.6%) | 1.00 | |||
| T | 69 (27.6%) | 267 (39.4%) | <0.001 | 1.704 (1.226–2.371) | 0.001 | 1.145 (1.055–1.232) |
Corrected according to age, sex, BMI, smoking index, and other clinical parameters.
The correlation between the rs198389, rs6668352, and rs198388 loci SNP in the BNP gene and COPD complicated with PH
The genotypic distributions of the rs198389, rs6668352, and rs198388 loci in the BNP gene in the COPD/PH− and COPD/PH+ groups are shown in Table 4. The proportion of homozygous mutations in the rs198389, rs6668352, and rs198388 loci in the COPD/PH+ group was significantly higher than in the COPD/PH− group (P<0.001). The results of the logistic regression analysis showed that the G allele at the rs198389 locus, the A allele at the rs6668352 locus, and the T allele at the rs198388 locus of BNP were high risk factors for the COPD patients complicated with PH (adjusted OR = 2.426, 95% CI = 1.992–2.955, P<0.001; adjusted OR = 2.257, 95% CI = 1.853–2.747, P<0.001; and adjusted OR = 1.842, 95% CI = 1.524–2.219, P<0.001, respectively).
Table 4. Genotypic distributions of the rs198389, rs6668352, and rs198388 loci in the BNP gene in the COPD/PH− group and COPD/PH+ group.
| SNPs | COPD/PH− group (n=205) | COPD/PH+ group (n=134) | P-value | OR (95% CI) | P-value* | OR* (95% CI) |
|---|---|---|---|---|---|---|
| rs198389 genotype | ||||||
| AA | 105 (51.2%) | 36 (26.9%) | 1.00 | |||
| AG | 84 (41.0%) | 24 (17.9%) | 0.545 | 0.833 (0.442–1.566) | 0.649 | 0.870 (0.532–1.403) |
| GG | 16 (7.8%) | 74 (55.2%) | <0.001 | 13.490 (6.661–27.666) | <0.001 | 3.220 (2.444–4.094) |
| Allele | ||||||
| A | 294 (71.7%) | 96 (35.8%) | 1.00 | |||
| G | 116 (28.3%) | 172 (64.2%) | <0.001 | 4.541 (3.223–6.403) | <0.001 | 2.426 (1.992–2.955) |
| rs6668352 genotype | ||||||
| GG | 106 (51.7%) | 31 (23.1%) | 1.00 | |||
| GA | 75 (36.6%) | 37 (27.6%) | 0.067 | 1.687 (0.927–3.074) | 0.091 | 1.460 (0.946–2.258) |
| AA | 24 (11.7%) | 66 (49.3%) | <0.001 | 9.403 (4.871–18.302) | <0.001 | 3.241 (2.342–4.426) |
| Allele | ||||||
| G | 287 (70.0%) | 99 (36.9%) | 1.00 | |||
| A | 123 (30.0%) | 169 (63.1%) | <0.001 | 3.983 (2.838–5.594) | <0.001 | 2.257 (1.853–2.747) |
| rs198388 genotype | ||||||
| CC | 100 (48.8%) | 47 (35.1%) | 1.00 | |||
| CT | 89 (43.4%) | 28 (20.9%) | 0.150 | 0.669 (0.373–1.199) | 0.193 | 0.748 (0.485–1.139) |
| TT | 16 (7.8%) | 59 (44.0%) | <0.001 | 7.846 (3.908–15.928) | <0.001 | 2.460 (1.890–3.063) |
| Allele | ||||||
| C | 289 (70.5%) | 122 (45.5%) | 1.00 | |||
| T | 121 (29.5%) | 146 (54.5%) | <0.001 | 2.858 (2.048–3.991) | <0.001 | 1.842 (1.524–2.219) |
Corrected according to age, sex, BMI, smoking index, and other clinical parameters.
Analysis of serum BNP, Fbg, and Apelin protein expression
The results of the ELISA detection of serum BNP, Fbg, and Apelin protein expression in the subjects are shown in Table 5. The expressions of BNP and Fbg protein in the control group, COPD/PH− group and COPD/PH+ group increased sequentially (P<0.001), while the expression levels of the Apelin protein in the control group, COPD/PH− group and COPD/PH + group decreased sequentially (P<0.001).
Table 5. Comparison of the plasma BNP, Fbg, and Apelin content in the three groups ( ± s).
| Plasma parameters | Control group (n=125) | COPD/PH− group (n=205) | COPD/PH+ group (n=134) | P-value |
|---|---|---|---|---|
| BNP (ng/l) | 28.1 ± 9.8 | 158.6 ± 23.0 | 220.2 ± 18.9 | <0.001 |
| Fbg (g/l) | 2.5 ± 0.5 | 4.2 ± 0.5 | 5.4 ± 0.8 | <0.001 |
| Apelin (ng/l) | 90.5 ± 8.4 | 43.9 ± 4.6 | 29.4 ± 6.2 | <0.001 |
The correlation between the rs198389, rs6668352, and rs198388 loci SNPs in the BNP gene and serum protein content of BNP, Fbg, and Apelin
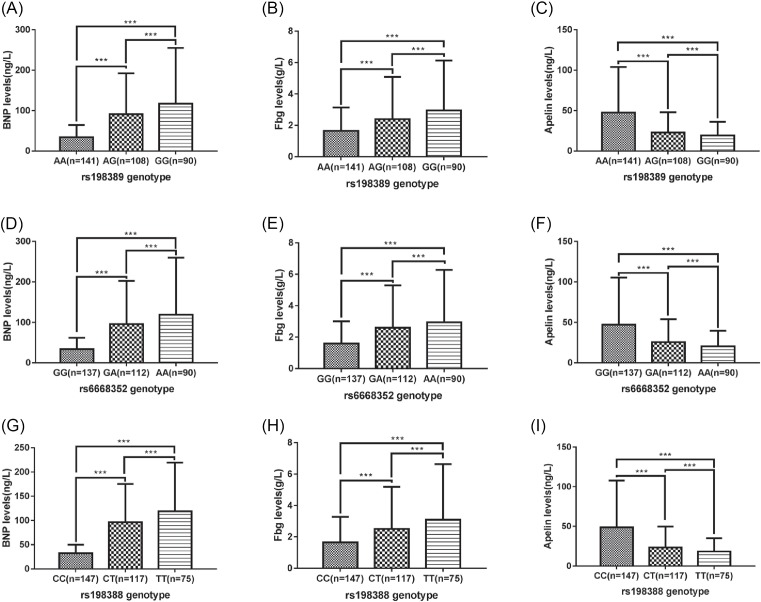
The BNP, Fbg, and Apelin protein expressions in the subjects for each genotype of the BNP gene for the loci rs198389, rs6668352, and rs198388 are shown in Figure 2. According to the results, the content of BNP and Fbg protein in the serum of the heterozygote and mutant homozygote at the BNP gene rs198389, rs6668352, rs198388 loci increased successively, while the content of serum Apelin protein decreased successively (P<0.001).
Figure 2. Comparison of plasma BNP, Fbg, and Apelin protein content in subjects with each genotype of BNP gene rs198389, rs6668352, and rs198388 loci.
***P<0.001.
Correlation between BNP gene rs198389, rs6668352, rs198388 locus SNP and serum IL-6, IL-8 levels
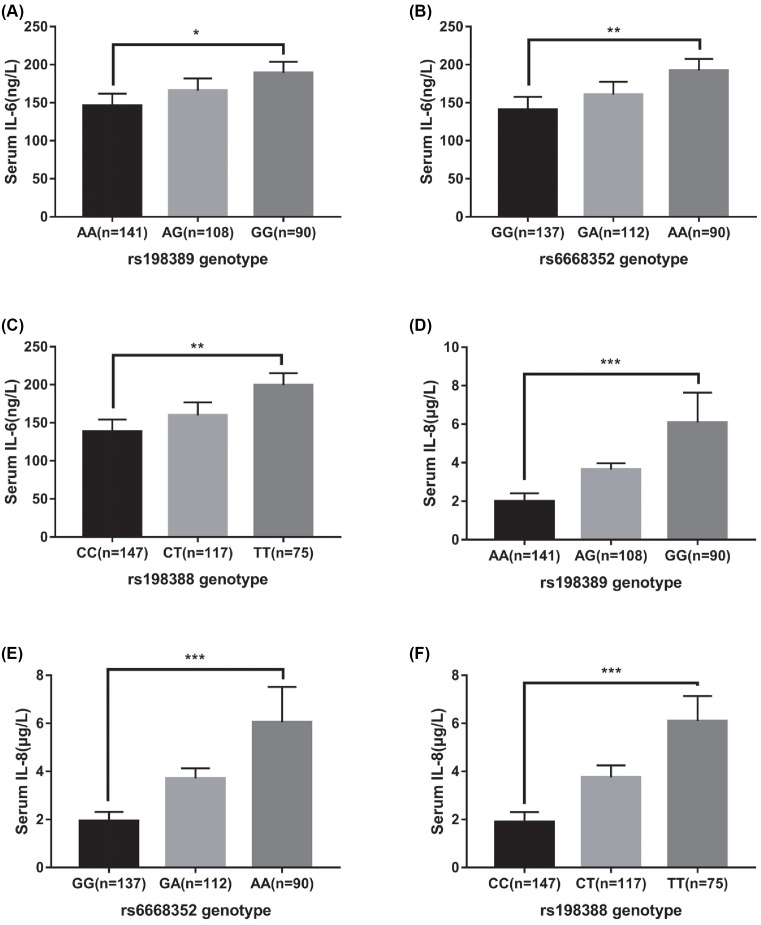
The levels of IL-6 and IL-8 in serum were detected by ELISA, as shown in Figure 3. The results showed that the serum levels of IL-6 and IL-8 in wild-type, heterozygote, and homozygote of BNP gene rs198389, rs6668352, and rs198388 were increased in turn (P<0.05).
Figure 3. Comparison of plasma IL-6, IL-8 expression levels in subjects with genotypes of the BNP gene rs198389, rs6668352, and rs198388 loci.
***P<0.001; **P<0.01, *P<0.05.
Data availability statement
All data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Discussion
PH often accompanies patients with advanced COPD; the pulmonary vascular endothelial cells and smooth muscle cells in these patients are abnormal, and they have pulmonary vascular remodeling and right heart failure, which can cause death in severe cases [15]. The occurrence of COPD is associated with lung airway inflammation in patients, and airway restriction is also one of the clinical features of COPD [16]. Fbg is a blood coagulation factor and one of the lung airway inflammation markers [17]. Studies show that serum Fbg content reflects the severity of COPD, and in patients with exacerbated COPD, it is significantly higher [18]. The results of the present study showed that the serum Fbg content in COPD/PH+ patients was significantly higher than in COPD/PH− patients [(5.4±0.8) g/l vs. (4.2±0.5) g/l], which was in agreement with the results by Ilisie et al. [18]. Furthermore, a comparison of the expression of serum Fbg protein in the subjects with different genotypes showed that the Fbg protein content of the wild types of the BNP gene rs198389, rs6668352, and rs198388 loci is lower than heterozygotes, and that of heterozygotes is lower than that of mutant homozygotes (all P<0.05), indicating that mutations in the rs198389, rs6668352, and rs198388 loci of the BNP gene lead to an increased expression of Fbg in the serum and increased lung airway inflammation in the mutant gene carriers. We speculated that this may be one of the causes of PH in the patients with COPD.
Apelin is a small molecule polypeptide that is an endogenous ligand for the orphan G protein-coupled receptor APJ. It has many biological effects, such as lowering blood pressure, enhancing myocardial contractility, regulating immunity, and regulating water and salt balances [19–21]. A study showed that Apelin could reduce myocardial damage in the PH rat model induced by monocrotaline and improve their right ventricle function [22]. Clinical studies show that the serum levels of Apelin in patients with PH are reduced, and an exogenous supplementation of it improves the clinical symptoms of PH to a certain extent and increases the cardiac output of patients with PH [23]. Recently, a serum Apelin test was used as a very important way to diagnose the severity of PH [24]. The results of the present study showed that the serum Apelin in patients with COPD is significantly lower than that in the control group, and the serum Apelin in the COPD/PH+ group is lower than that in the COPD/PH− group, indicating that, with the worsening of COPD in patients, the expression of Apelin in the serum gradually decreases, which is consistent with the findings by Andersen et al. In addition, the present study analyzed the correlation between BNP gene SNPs and serum Apelin expression levels. The results showed that the content of Apelin protein in the serum of the heterozygote and mutant homozygote at the BNP gene rs198389, rs6668352, and rs198388 loci decreased successively. In addition, the present study analyzed the correlation between the BNP gene SNPs and the serum Apelin content, which showed that the serum Apelin protein in the wild types of the BNP gene rs198389, rs6668352, and rs198388 loci is higher than that of heterozygotes, and that of heterozygotes is higher than that of mutant homozygotes (all P<0.05), indicating that mutations at the rs198389, rs6668352, and rs198388 sites of BNP resulted in a decrease in serum Apelin protein, which may be one of the causes of the exacerbations of COPD, and thus, it might be used as a potential therapeutic target for COPD with PH.
BNP is located on human chromosome 1, which contains three exons and two introns encoding the BNP prohormone precursor [25]. The BNP prohormone precursor is synthesized in cardiac myocytes and is then processed under shear stress and secreted into the plasma to regulate blood pressure and blood flow to maintain homeostasis [26]. Studies show that plasma BNP levels may be associated with postoperative low cardiac output syndrome in children with congenital heart disease. Approximately 90% of PH patients who undergo congenital heart disease surgery have a preoperative plasma BNP higher than 125.5 pg/ml, leading to an increased risk of low cardiac output syndrome [27]. Studies show that an elevated BNP level is associated with heart failure and that the detection of the BNP content is expected to be used for clinical heart failure screening [28]. Left ventricular systolic dysfunction (LVSD) and cardiac decompensation are usually accompanied by AECOPD, and studies have shown that NT-proBNP can be used as a diagnostic marker for LVSD in acute exacerbation of COPD [29]. The results of the present study showed that the serum BNP level in patients with COPD was significantly higher than that in the control group. With the exacerbation of COPD patients, the serum BNP expression level gradually increased, which was consistent with the results by Inoue et al. [30]. At the same time, the present study also analyzed the correlation between the BNP gene SNPs and serum BNP expression levels, which showed that the content of the BNP protein in the serum of the wild types at the BNP gene rs198389, rs6668352, and rs198388 loci is lower than that of heterozygotes and that of heterozygotes is lower than that of mutant homozygotes (all P<0.001), indicating that mutations in the BNP gene at the rs198389, rs6668352, and rs198388 SNPs resulted in the elevation of BNP and might be one of the causes of COPD and COPD with PH.
In addition, we also analyzed the correlation between the polymorphisms of the BNP rs198389, rs6668352, rs198388 loci and the levels of IL-6 and IL-8 in serum. The results showed that the levels of IL-6 and IL-8 in the BNP rs198389, rs6668352, and rs198388 mutants were higher than those in the wild type. COPD usually activates inflammatory cells to release inflammatory mediators, such as IL-6 and IL-8, and IL-6 can induce inflammation by secreting cytokines such as IgG, IgA, and IgE through promoting cell maturation [31]. IL-8 is an endogenous chemokine of inflammatory cells, whose main role is to chemotactic neutrophils, elevate the intracellular concentration of Ca2+, resulting in increased histamine release in peripheral blood and trigger inflammatory reaction [32]. The elevated levels of IL-6 and IL-8 in the serum of subjects with BNP gene rs198389, rs6668352, and rs198388 mutations in the present study indicate that these SNPs are involved in the development of inflammatory response and may contribute to COPD. Ghobadi et al. [33] showed that the levels of serum IL-6 and pro-BNP increased with the severity of COPD, and the results were consistent with the results of the present study.
The present study also had some limitations. First, the present study did not analyze the impacts of different ethnic groups on gene polymorphisms at the rs198389, rs6668352, and rs198388 loci of the BNP gene and did not distinguish amongst different ethnic groups when enrolling the subjects. Second, in order to understand the effect of the BNP polymorphisms on the natriuretic peptide pathway, other natriuretic peptides, including atrial natriuretic peptide and C-type natriuretic peptide, should also be included in the study.
Conclusion
Gene polymorphisms of the BNP gene, including rs198389, rs6668352, and rs198388, were associated with COPD and COPD with PH. Mutant individuals were more susceptible to COPD and prone to PH. The mechanism may be related to the high expression of BNP and Fbg protein in the serum and the abnormally low expression of the Apelin protein.
Abbreviations
- AECOPD
acute exacerbation of chronic obstructive pulmonary disease
- BMI
body mass index
- BNP
brain natriuretic peptide
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- Fbg
fibrinogen
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- IgA/IgE/IgG
immunoglobulins A, E and G
- IL
Interleukin
- LVSD
left ventricular systolic dysfunction
- NT-proBNP
N-terminal hormone brain natriuretic peptide
- OR
odds ratio
- PH
pulmonary hypertension
- SNP
single nucleotide polymorphism
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
Guangjun Jin participated in the study design, data collection, analysis of data, and drafted the manuscript. Zhu Chen participated in the study design, data collection, and analysis of data. Jiancheng Zhang, Jia Song, and Jun Shi participated in the data collection and analysis of data. Bingzhi Zhou carried out the study design, the analysis and interpretation of data, and preparation of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Zhejiang Provincial Medicine Health Science and Technology Program [grant number 2018KY560 (to Guangjun Jin)].
References
- 1.Lopez-Campos J.L. and Tan W. Soriano J.B. (2016) Global burden of COPD. Respirology 21, 14–23 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 2.Samareh Fekri M., et al. (2018) Prevalence and predictors associated with severe pulmonary hypertension in COPD. Am. J. Emerg. Med. 36, 277–280 10.1016/j.ajem.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 3.Liu S., et al. (2015) Prevalence and risk factors for COPD in greenhouse farmers: a large, cross-sectional survey of 5,880 farmers from northeast China. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 2097–2108 10.2147/COPD.S79264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishimoto I., et al. (2009) Natriuretic peptide signaling via guanylyl cyclase (GC)-A: an endogenous protective mechanism of the heart. Curr. Cardiol. Rev. 5, 45–51 10.2174/157340309787048068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto I., et al. (2016) Impact of B-type natriuretic peptide (BNP) on development of atrial fibrillation in people with Type 2 diabetes. Diabet. Med. 33, 1118–1124 10.1111/dme.12856 [DOI] [PubMed] [Google Scholar]
- 6.Ndumele C.E., et al. (2016) N-terminal pro-brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 133, 631–638 10.1161/CIRCULATIONAHA.115.017298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y.R., et al. (2017) C-reactive protein and N-terminal prohormone brain natriuretic peptide as biomarkers in acute exacerbations of COPD leading to hospitalizations. PLoS One 12, e0174063 10.1371/journal.pone.0174063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q., et al. (2015) Correlation between rs198388 and rs198389 polymorphismsin brainnatriuretic peptide (NPPB) gene and susceptibility to congenital heart diseases in a Chinese population. Int. J. Clin. Exp. Med. 8, 19162–19166 [PMC free article] [PubMed] [Google Scholar]
- 9.Poreba R., et al. (2009) SNP rs198389 (T-381 C) polymorphism in the B-type natriuretic peptide gene promoter in patients with atherosclerotic renovascular hypertension. Pol. Arch. Med. Wewn. 119, 219–224 [PubMed] [Google Scholar]
- 10.Fox A.A., et al. (2009) Natriuretic peptide system gene variants are associated with ventricular dysfunction after coronary artery bypass grafting. Anesthesiology 110, 738–747 10.1097/ALN.0b013e31819c7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madamanchi C., et al. (2014) Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 176, 611–617 10.1016/j.ijcard.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polatli M., et al. (2008) Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J. Thromb. Thrombolysis 26, 97–102 10.1007/s11239-007-0073-1 [DOI] [PubMed] [Google Scholar]
- 13.Pauwels RA., Buist AS., Calverley PM., Jenkins CR., Hurd SS. (2001) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 163, 1256–76 [DOI] [PubMed] [Google Scholar]
- 14.Lau E.M., et al. (2015) The 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: a practical chronicle of progress. Eur. Respir. J. 46, 879–882 10.1183/13993003.01177-2015 [DOI] [PubMed] [Google Scholar]
- 15.Kurashima K., et al. (2015) Lobe-based computed tomography assessment of airway diameter, airway or vessel number, and emphysema extent in relation to the clinical outcomes of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 1027–1033 10.2147/COPD.S81748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana S., et al. (2014) Clinical characteristics and airway inflammation profile of COPD persistent sputum producers. Respir. Med. 108, 1761–1770 10.1016/j.rmed.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Leavy O. (2013) Asthma and allergy: a fibrinogen root to airway inflammation. Nat. Rev. Immunol. 13, 704 10.1038/nri3538 [DOI] [PubMed] [Google Scholar]
- 18.Ilisie M., et al. (2014) Fibrinogen and CRP biomarkers in patients with exacerbation of COPD group C and D. Eur. Respir. J. 44 (suppl 58), Abstract P3996 [Google Scholar]
- 19.Boucher J., et al. (2005) Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 10.1210/en.2004-1427 [DOI] [PubMed] [Google Scholar]
- 20.Tatemoto K., et al. (2001) The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99, 87–92 10.1016/S0167-0115(01)00236-1 [DOI] [PubMed] [Google Scholar]
- 21.Lee D.K., et al. (2000) Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 74, 34–41 10.1046/j.1471-4159.2000.0740034.x [DOI] [PubMed] [Google Scholar]
- 22.Falcao-Pires I., et al. (2009) Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 296, H2007–H2014 10.1152/ajpheart.00089.2009 [DOI] [PubMed] [Google Scholar]
- 23.Brash L., et al. (2015) Apelin improves cardiac output in patients with pulmonary arterial hypertension. Eur. Respir. J. 46 (suppl 59) [Google Scholar]
- 24.Andersen C.U., et al. (2011) Apelin and pulmonary hypertension. Pulm. Circ. 1, 334–346 10.4103/2045-8932.87299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudoh T., et al. (1988) A new natriuretic peptide in porcine brain. Nature 332, 78–81 10.1038/332078a0 [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz Z., et al. (2014) Relationship between fluid status as assessed by bioimpedance analysis and NT-pro BNP, blood pressure and left ventricular mass index in hemodialysis patients. Clin. Ter. 165, e52–e58 [DOI] [PubMed] [Google Scholar]
- 27.Baysal A., et al. (2014) The predictive value of plasma B-type natriuretic peptide levels on outcome in children with pulmonary hypertension undergoing congenital heart surgery. Rev. Bras. Anestesiol. 64, 326–334 10.1016/j.bjan.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 28.Silver M.A. and Pisano C. (2003) High incidence of elevated B-type natriuretic peptide levels and risk factors for heart failure in an unselected at-risk population (stage A): implications for heart failure screening programs. Congest. Heart Fail. 9, 127–132 10.1111/j.1527-5299.2003.02589.x [DOI] [PubMed] [Google Scholar]
- 29.Andrijevic I., et al. (2018) N-terminal prohormone of brain natriuretic peptide (NT-proBNP) as a diagnostic biomarker of left ventricular systolic dysfunction in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Lung 196, 583–590 10.1007/s00408-018-0137-3 [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y., et al. (2009) High plasma brain natriuretic peptide levels in stable COPD without pulmonary hypertension or cor pulmonale. Intern. Med. 48, 503–512 10.2169/internalmedicine.48.1701 [DOI] [PubMed] [Google Scholar]
- 31.Renata B., et al. (2016) Inflammatory markers (IL-6, suPAR) and arterial stiffness parameters in COPD patients. 48, PA3670 [Google Scholar]
- 32.Anzalone G., et al. (2016) IL-17A induces chromatin remodeling promoting IL-8 release in bronchial epithelial cells: effect of tiotropium. Life Sci. 152, 107–116 10.1016/j.lfs.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 33.Ghobadi H., et al. (2017) The correlation of serum brain natriuretic peptide and interleukin-6 with quality of life using the chronic obstructive pulmonary disease assessment test. Med. Princ. Pract. 26, 509–515 10.1159/000484900 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.