Abstract
Background
Dual regimen with dolutegravir plus cobicistat-boosted darunavir (DTG/DRV/c) is reasonable alternative option for patients with existing resistance and/or intolerance to nucleoside reverse transcriptase inhibitors (NRTIs).
Material and Methods
All patients who switched to DTG/DRV/c among treatment-experienced patients with human immunodeficiency virus (HIV) in a tertiary university hospital were selected. We analyzed the effectiveness, safety, and tolerability based on serial laboratory data and clinical findings. The primary endpoint was defined as the proportion of patients with plasma HIV RNA below 50 copies/mL at week 48 after switch. Secondary endpoints included evaluation of safety and tolerability.
Results
Thirty-one patients were retrospectively analyzed. The main reasons for the change to DTG/DRV/c were treatment failure in 13 patients (41.9%), simplification in 12 patients (38.7%), and adverse drug reaction in 6 patients (19.4%). Among the 13 patients who switched owing to treatment failure, the proportion of patients in whom the viral loads were suppressed to less than 50 copies/mL increased from 0% at baseline to 45% at 4 weeks, 50% at 12 weeks, 50% at 24 weeks, and 66.7% at 48 weeks. HIV virus levels decreased and CD4+ T cell counts increased during the follow-up period. In non-treatment failure patients (18 patients), the levels of viral suppression and CD4+ T cells were maintained. There were no significant differences in renal function, liver function, glucose levels, and lipid profile before and after regimen changes. The tolerability was very good: 30 patients (96.8%) tolerated the drugs well and only 1 patient discontinued owing to no improvement in renal insufficiency. Two patients (6.4%) in treatment failure group failed to reach viral suppression.
Conclusion
The use of DTG/DRV/c in HIV treatment-experienced patients appears to be a very good regimen for switch therapy that is effective and well tolerated, without significant adverse drug reaction.
Keywords: Dolutegravir, Darunavir, Cobicistat, Human immunodeficiency virus
Introduction
‘Induction and maintenance therapy’ involves taking full, highly active antiretroviral therapy (HAART) during the early induction period and “simplifying” the drug in the maintenance period for at least 6 months after the virus is suppressed. In order to overcome high pill burden, drug toxicity, and drug-drug interaction, the simplification of a combination of drugs to mono or dual regimens during maintenance period has been attempted since late 2000 [1,2]. Several studies have been conducted on monotherapy with dolutegravir (DTG), darunavir (DRV), or atazanavir (ATV); however, a sufficiently effective and stable treatment has not yet been established [3,4,5,6]. A review article for ritonavir-boosted protease inhibitor (PI/r) monotherapy showed that these monotherapies were inferior to HAART [3]. A retrospective study revealed that DRV/r monotherapy was effective in virologically suppressed Human immunodeficiency virus (HIV)-infected patients for 12 months. However, this study included just a small number of 31 patients [4]. The 96-week analysis result of MODAT study (efficacy of ATV/r monotherapy as maintenance in patients with viral suppression) showed inferior efficacy of ATV/r monotherapy compared with ATV/r based triple therapy. The 48-week of MODAT study showed inferior to triple therapy therefore the Data and Safety Monitoring Board (DSMB) recommended stopping study [5]. A phase 2 randomized non-inferiority trial (DOMONO study) with 24 weeks follow-up showed that virological failure of patients switched to DTG monotherapy and led to DTG resistance [6].
Dual regimen combinations have been considered as maintenance therapy for simplification [7,8,9,10,11,12,13,14,15,16,17,18]. Dual therapy with a favorable outcome has been reported in treatment-experienced patients without previous virological failure; LAMIDOL study (a trial evaluating maintenance therapy with lamivudine and DTG in HIV-1 infected patients virologically suppressed with triple HAART), SWORD study (regimen switch to DTG plus rilpivirine from current antiretroviral regimen in HIV-1 infected and virologically suppressed adults) and DUALIS study (dual therapy with boosted DRV plus DTG) [7,8,9,10].
The selection of drug with high resistance barriers is necessary to achieve successful treatment in treatment failure or experienced patient because of the high drug resistance was seen. In several studies, the rate of drug resistance in treatment failure or experienced patients has varied from 42% to 61%; number of patients with drug resistance/number of patients with treatment failure; 33/65 [19], 27/63 [20], and 219/359 [21].
Therefore, regimens based on DTG with high resistance barriers are attractive for use in treatment failure or experienced patients [22]. Nucleoside reverse transcriptase inhibitors (NRTIs) have been shown to cause many adverse drug reactions when taken over a long period of time. Therefore, NRTI-free combinations are preferred for the maintenance regimen. The DTG/DRV was considered as a combination of DTG plus PIs with high resistance barrier and few adverse drug reactions.
In this study, we analyzed treatment-experienced patients who were switched to DTG/DRV/c owing to reasons such as effectiveness, safety, or tolerability based on serial laboratory data and clinical findings before and after the regimen change.
Materials and Methods
1. Patient characteristics
All patients switched to a combination of DTG/DRV/c (DTG 50 mg plus DRV 800 mg and cobicistat 150 mg co-formulate once daily or twice daily) from among the HIV-1 treatment-experienced patients with more than two drug changes treated at the Kyungpook National University Hospital, a tertiary hospital in Daegu, Korea. Patients were included between 2016 and December 2017, and the data were analyzed retrospectively. Patients for whom the regimen was switched at a previous hospital, and those who were not present at the time of the study were excluded. The included patients were divided into two groups based on the reason for the switch of regimen: a treatment failure group (failure group) and a non-failure group. In general, HIV treatment failure is defined when the number of plasma HIV ribonucleic acid (RNA) load is above 200 copies/mL after 6 months of ART. However, in this study, patients with treatment failure were defined as those who had a clinical need to change their regimen because the HIV RNA virus was not sufficiently suppressed to below 50 copies/mL. Patients with treatment experience of more than 2 years were included.
The non-failure group included patients who changed their regimen because of adverse drug reaction or for simplification. Patients with adverse drug reaction were defined as those who experienced adverse effects during the previous regimen and then changed to the study drug, as recorded on the electronic charts. Patients switching for simplification were defined as those without evidence of treatment failure and no adverse drug reaction during a previous regimen.
2. Methods
The baseline characteristics of the patients included the duration of HIV-1 infection, age, sex, and other infectious diseases and underlying diseases, such as diabetes and cardiovascular disease. Other infectious diseases included hepatitis B virus (HBV), hepatitis C virus (HCV), and tuberculosis (TB) infection. The HIV RNA and CD4+ T cell counts of these two groups were analyzed to determine effectiveness of the DTG/DRV/c combination before and after the regimen change. HIV RNA copies/mL levels were converted to log10 copies/mL for comparison. The viral state of RNA copies was defined as undetectable for <50 copies/mL.
In the failure group, the changes in HIV RNA viral load and CD4+ T cell count were compared. In the non-failure group, we observed whether the viral suppression of HIV RNA below 50 copies/mL and stable CD4+ T cell counts were maintained while on the studied regimen. To evaluate the effectiveness of this combination, primary endpoints were defined as patients with HIV viral load below 50 copies/mL at 48 weeks after switch.
We analyzed patient complaints, adverse effects recorded by the physician on the chart during follow-up periods, and laboratory changes to evaluate the safety and tolerability, as secondary endpoints. Physicians routinely asked about adverse effects that include skin rash, gastrointestinal symptoms such as diarrhea and abdominal pain, and psychologic symptoms such as insomnia, and mood changes [23]. In addition, the medical staff questioned whether there were uncomfortable subjective symptoms with an open question. Laboratory findings included renal function (estimated glomerular filtration rate; eGFR), liver function (aspartate transaminase [AST] and alanine transaminase [ALT] levels), and metabolic state (glucose, low-density lipoprotein [LDL], high-density lipoprotein [HDL], and triglyceride [TG] levels). Finally, we identified whether the patients continued or discontinued medication during the study period and confirmed the reason for discontinuation. The period of observation for efficacy and safety was the same.
3. Statistical analysis
The statistical analysis was performed by using a paired t-test for comparison of the parameters before and after drug change; analyses were computed by using R statistics ver. 3.1. The Kaplan-Meier estimator was used to estimate the drug tolerability from data, with 0.2 P-value threshold entering multiple regression.
4. Ethics statement
This study protocol was undertaken in accordance with the principles outlined in the Declaration of Helsinki and approved by the Institutional Review Board of Kyungpook National University Medical Center (IRB file No. 2018-02-027).
Results
Initially, 33 patients were initially included; two patients were subsequently excluded. One patient had a regimen change at another hospital and one patient was not followed-up during the study period. Overall, 31 patients were analyzed, comprising 28 men (90.3%) and 3 women (9.7%) with a mean age of 47.9 ± 8.9 years. Three patients (9.7%) had hepatitis B, 2 patients (6.5%) had hepatitis C, and 11 patients (35.5%) had tuberculosis. Four patients (12.9%) had diabetes mellitus as an underlying disease; however, no patients were diagnosed with cardiovascular disease, autoimmune disease, malignancy, or renal disease. The mean duration of HIV infection was 13.7 ± 6.3 years, and the mean number of drug changes was 4.9 ± 2.0. The duration of follow-up was 44.8 ± 13.5 weeks after treatment with DTG/DRV/c. The main reasons for the change to the study regimen were treatment failure, 13 patients (41.9%); simplification, 12 patients (38.7%); and adverse drug reaction, 6 patients (19.4%). The main characteristics and outcomes of the patients are summarized in Tables 1 and 2. Three patients took these medicines twice daily, and the remaining 28 patients (90%) took these once daily. One in three patients took once daily, and at week 24 the virus decreased from 34,162 copies/mL to 52 copies/mL before the start of the dose. However, at 48 weeks, the virus level was increased to 128 copies/mL and the dose was increased to twice daily. The virus was not detected after increasing the dose. One patient had Guillain–Barré syndrome due to cytomegalovirus and the other patient had severe peritonitis due to non-tuberculous mycobacteria. Therefore, they started taking twice daily dose from the first change.
Table 1. The baseline characteristics and outcomes of the patients.
| Variables | Failure group (n = 13) | Non-failure group (n = 18) | Total (n = 31) | |
|---|---|---|---|---|
| Male sex (%) | 12 (92.3%) | 16 (88.9%) | 28 (90.3%) | |
| Age (yr) | 44.8 ± 8.8 | 50.2 ± 8.6 | 47.9 ± 8.9 | |
| Duration with HIV-1 infection (yr) | 14.4 ± 5.4 | 13.3 ± 7.0 | 13.7 ± 6.3 | |
| Prior lines of medication change | 4.8 ± 1.9 | 5.0 ± 2.3 | 4.9 ± 2.1 | |
| Co-infection with HBV (%) | 1 (7.7%) | 2 (11.1%) | 3 (9.7%) | |
| Co-infection with HCV (%) | 1 (7.7%) | 1 (5.6%) | 2 (6.5%) | |
| Co-infection with TB (%) | 5 (38.5%) | 6 (33.3%) | 11 (35.5%) | |
| Reasons for changing regimen | ||||
| Treatment failure | 13 (100%) | 0 (0.0%) | 13 (41.9%) | |
| Simplification | 0 (0.0%) | 6 (33.3%) | 6 (19.4%) | |
| Adverse drug reaction | 0 (0.0%) | 12 (66.7%) | 12 (38.7%) | |
| Mean changes in HIV RNA titer and CD4+ T cell counts | ||||
| RNA (log10 copies/mL) at week 0 | 4.5 (4.3–4.8) | undetectable | ||
| RNA (log10 copies/mL) at week 4 | 1.8 (1.5–2.5) | undetectable | ||
| RNA (log10 copies/mL) at week 12 | 1.7 (1.5–2.2) | undetectable | ||
| RNA (log10 copies/mL) at week 24 | 1.7 (1.5–2.4) | undetectable | ||
| RNA (log10 copies/mL) at week 48 | 1.9 (1.6–2.1) | undetectable | ||
| CD4+ T cell (/mm3) at week 0 | 148.0 (99.0–266.0) | 427.0 (355.0–549.0) | ||
| CD4+ T cell (/mm3) at week 4 | 195.0 (176.0–311.0) | 356.5 (353.0–540.0) | ||
| CD4+ T cell (/mm3) at week 12 | 164.0 (101.0–251.0) | 431.0 (331.5–488.5) | ||
| CD4+ T cell (/mm3) at week 24 | 165.5 (153.0–303.0) | 864.0 (669.0–1059.0) | ||
| CD4+ T cell (/mm3) at week 48 | 376.5 (353.0–400.0) | 447.5 (407.0–507.0) | ||
HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; TB, tuberculosis; RNA, ribonucleic acid.
Table 2. Laboratory findings before and after regimen changes.
| Variablesa | Failure group (n = 13) | Non-failure group (n = 18) | Total (n = 31) |
|---|---|---|---|
| Initial eGFR (mL/min/BSA) | 107.9 ± 33.6 | 108.4 ± 29.6 | 108.2 ± 30.8 |
| Last eGFR (mL/min/BSA) | 77.9 ± 17.0 | 103.1 ± 35.5 | 88.5 ± 28.8 |
| Initial AST (U/L) | 26.9 ± 13.2 | 34.2 ± 22.3 | 30.8 ± 18.7 |
| Last AST (U/L) | 25.3 ± 18.9 | 23.2 ± 8.1 | 24.4 ± 15.2 |
| Initial ALT (U/L) | 33.1 ± 13.0 | 32.9 ± 18.6 | 33.0 ± 15.9 |
| Last ALT (U/L) | 26.7 ± 20.8 | 24.2 ± 19.5 | 25.6 ± 20.0 |
| Initial glucose (mg/dL) | 144.3 ± 85.8 | 87.9 ± 16.9 | 117.4 ± 68.1 |
| Last glucose (mg/dL) | 121.5 ± 55.1 | 98.5 ± 16.8 | 92.3 ± 45.2 |
| Initial LDL (mg/dL) | 87.1 ± 56.9 | 98.7 ± 27.1 | 92.3 ± 45.2 |
| Last LDL (mg/dL) | 116.3 ± 60.3 | 103.0 ± 39.4 | 110.7 ± 52.2 |
| Initial HDL (mg/dL) | 52.8 ± 19.1 | 47.9 ± 16.5 | 49.3 ± 13.6 |
| Last HDL (mg/dL) | 47.9 ± 11.3 | 51.2 ± 16.6 | 49.3 ± 13.6 |
| Initial TG (mg/dL) | 329.4 ± 369.4 | 145.8 ± 69.4 | 242.0 ± 281.4 |
| Last TG (mg/dL) | 268.6 ± 223.1 | 143.0 ± 87.0 | 215.9 ± 187.6 |
| Initial hTG (no) | 3 (27.3%) | 7 (70.0%) | 10 (47.6%) |
| Initial hTG (yes) | 8 (72.7%) | 3 (30.0%) | 11 (52.4%) |
| Last hTG (no) | 4 (22.2%) | 10 (76.9%) | 14 (45.2%) |
| Last hTG (yes) | 14 (77.8%) | 3 (23.1%) | 17 (54.8%) |
aVariables are expressed as mean ± standard deviation.
eGFR, estimated glomerular filtration rate; BSA, body surface area; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; hTG (hypertriglyceridemia), TG >150 mg/dL.
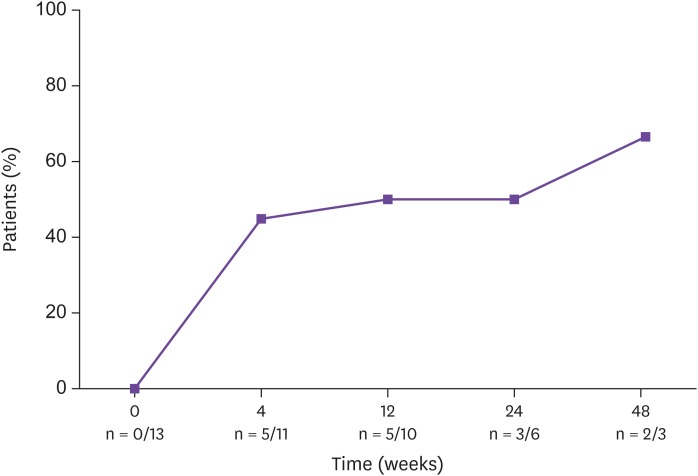
Of the 13 patients in the treatment failure group, the rate of patients in whom the suppression was less than 50 copies/mL increased from 0% (0 /13 patients) at the start of treatment to 45% (5 patients/11 patients) at 4 weeks, 50% (5/10 patients) at 12 weeks, 50% (3/6 patients) at 24 weeks, and 66.7% (2/3 patients) at 48 weeks (Fig. 1). In this group, genotypic resistance tests to HIV drugs was determined in 11 patients before the regimen change. Three patients did not achieve the results because the virus was not amplified, and four patients were found not to have drug resistance. Based on the tests, four patients were found to have 3TC, RPV, emtricitabine, didanosine, abacavir, etravirine, efavirenz, and nevirapine resistance. Of these four patients with resistance, three were resistant to the drug before the change and one patient did not have resistance to the drug combination before use.
Figure 1. Percentage of patients with HIV-1 RNA counts less than 50 copies/mL in the failure group.
HIV, human immunodeficiency virus; RNA, ribonucleic acid; n, number of the patients.
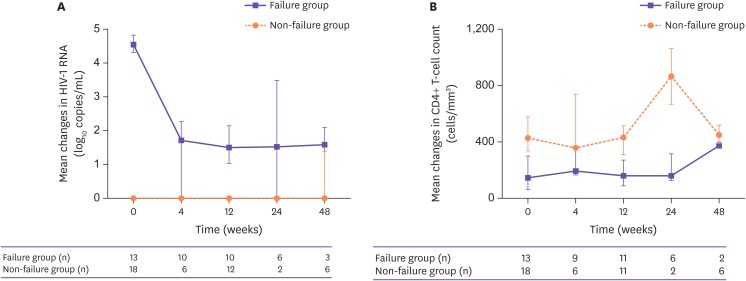
The HIV virus load was reduced from 4.5 (4.3–4.8) at 0 week to 1.8 (1.5–2.5) at 4 weeks, 1.7 (1.5–2.2) at 12 weeks, 1.7 (1.5–2.4) at 24 weeks and 1.9 (1.6–2.1) log10 copies/mL at 48 weeks in the treatment failure group. The CD4+ T cell counts increased (Table 1 and Fig. 2).
Figure 2. Mean changes in HIV RNA titer (A) and CD4+ T cell count (B). In the failure group (purple line), suppression of the HIV RNA titer decrease is shown and CD4+ T cell count increased. In the non-failure group (orange line), virus suppression and high CD4+ T cell levels are maintained.
HIV, human immunodeficiency virus; RNA, ribonucleic acid.
In the 18 patients in the non-failure group who changed medication for simplification or owing to adverse drug reaction, viral suppression was maintained at less than 50 copies for all periods. The average number of CD4+ T cells was 408 cells/mm3 and well maintained at over 200 cells/mm3 (Table 1 and Fig. 2).
During the follow-up period, 30 of the 31 patients continued the DTG/DRV/c combination therapy without adverse drug reaction that required a regimen change. However, one patient was changed from the study regimen to other medications by a HIV care physician owing to persistent renal insufficiency after regimen change. Patients complained of itching, acne, weight loss, constipation, and general weakness during the period of medication; however, the adverse drug reaction were of grade 1 (mild). The tolerability of this combination was very good, because thirty patients complied well with the drug regimen; however, one patient had no improvement in renal function. None of the patients complained of neuropsychiatric adverse drug reaction, such as depression, suicidality, and sleep disturbance (Fig. 3). Although we could not check the medical records at other hospitals, there were no psychiatric and neurological records at our hospital during the study period.
Figure 3. Drug tolerability can be used to determine drug adherence (by Kaplan-Meier plot with 0.2 P-value threshold entering multiple regression). Tolerability is determined by whether each group continued taking this regimen without dropout during the follow-up period.
Two patients (6.4%) had failed treatment with more than 50 copies/mL of RNA, which suggested poor compliance in these patients.
The laboratory parameters were analyzed for renal function, liver function, and blood and lipid abnormalities before and after the regimen change to evaluate adverse drug reaction. There was no significant adverse reaction when comparing the blood test at the time of drug change and the last follow-up blood test after the change.
Discussion
Previous DTG/3TC studies showed that in all patients who achieved virological suppression, the RNA was maintained at a stable state after the regimen change [11,12]. However, this study did not include patients with treatment failure. Physicians must still consider the problems that could arise from the continued use of an NRTI (3TC). In previous DTG/RPV studies, effective viral suppression was observed in patients who switched owing to treatment failure [13,14,15,16]. The outcome was favorable in the DTG/RPV study. Although RPV had fewer adverse drug reactions than the first-generation non-nucleoside reverse transcriptase inhibitors (NNRTIs), there are still associated neuropsychiatric problems and hepatic complications.
Thus, a DTG/DRV regimen, boosted with pharmacokinetic enhancers, has been studied. Thus far, 2 studies have examined the treatment boosted with ritonavir. The DUALIS study with 320 patients with HIV with sustained virological suppression was initiated in 2015 and reported interim pharmacokinetic results in 2017. Data from this pharmacokinetic study demonstrated adequate steady-state drug concentrations and no significant change in liver and kidney functions. This study is ongoing on a large scale as a prospective, multicenter study, but it is limited to drug modifications in patients with well-suppressed viruses [10]. The TIVISTA study (tivicay plus prezista observational cohort), which began in 2016 and was reported in 2017, included 130 patients with duration of 48 weeks of those, 30% were patients with treatment failure. Studies have shown effective viral suppression in patients with treatment failure [17,18]. In both studies, there were no significant adverse drug reactions such as those seen [10,17,18].
However, in these DTG/DRV/boosted studies, DRV was boosted with ritonavir, and thus the pill burden was still high. The choice of the effective drug combination for the treatment of HIV is also important, but the simplification and low pill burden of drugs for simplification is a key factor in compliance. Currently, a single tablet with DRV boosted with cobicistat (DRV/c) was commercialized as a fixed-dose single tablet (DRV 800 mg and cobicistat 150 mg), which makes it possible to further simplify drug administration [24]. The deNUC study (DRV/cobicistat and DTG to maintain virologic suppression and reduce NRTI-associated toxicity) started in 2015, but the results have not been published. This study was also conducted in patients with HIV who were virologically suppressed. In a poster presented at the recent International AIDS Society Conference on HIV Science (IAS 2017) in Paris, it was reported that DTG/DRV/c was effective [25]. This study retrospectively analyzed 44 patients, including patients with failed therapy. In the poster, patients were followed-up for 24 weeks and there were no data on the adverse drug reaction.
If the drug modification aims to achieve simplification in patients with viral suppression, the viral suppression should be maintained after the change. An important point in drug change is that the virus should be suppressed, even if it is changed in patients who have failed treatment. A combination with high resistance barriers is required in patients with poor compliance and treatment failure. PI plus integrase strand transfer inhibitors (INSTI), with a high resistance barrier, is a good candidate regimen for switch therapy. DTG/DRV/r combination studies have been published recently [10,17,18]. Given the approval of DRV co-formulated with cobicistat, we attempted a DTG/DRV/c regimen to further reduce the pill burden of drug and reduce the adverse drug reaction of ritonavir, such as diarrhea. The pill burden of this regimen is low, and therefore patients feel that it is easy to take in a clinical setting. One of the most important aspects of treatment is to allow the patient to successfully take the medicine. Therefore, it may be advantageous in patients who have poor compliance owing to the inconvenience in taking medication.
In this study, the boost drug of the patient who stopped the DTG/DRV/c was changed from cobicistat to ritonavir because of no improvement in eGFR and creatinine. This patient was previously switched to DTG/DRV/c with a gradually mild decrease in eGFR while taking previous ART medication. After switching to ritonavir, the patient's eGFR remained at approximately 50–55 mL/min/BSA and mild proteinuria persisted. The reduction of eGFR in patients may be related to nephropathy with hypertension as an underlying disease in the patient. Indeed, DTG and cobicistat inhibit the renal transporters without any renal damage and could cause eGFR reduction.
In the TIVISTA study, TG decreased during the 48-week observation period. However, the proportion of hypertriglyceridemia increased after drug change in this study. The mean TG value of the patients was high even before the drug change. The elevated TG values were the result of tests performed without fasting in clinical settings. In addition, patients with hypertriglyceridemia did not show any particular symptoms. It was difficult to determine the high mean TG value of the patients, and this was probably related to the diet rather than the adverse drug reaction of the drugs.
This study has several limitations. At the time of the study, INSTIs were not included in the genotypic HIV resistance test in our hospital and resistance to DTG was not confirmed in the treatment failure group. Although two patients who did not achieve viral suppression missed the resistance testing results for INSTIs, the failure was at least suggested to be related with poor adherence to treatment. Because of the retrospective nature of this study, only some adverse drug reactions were described by the physician in the chart. Although the exact adverse drug reactions were not analyzed, no serious adverse drug reactions were determined, because they were not recorded on the chart, and no specific findings were reported after the blood tests. And most of all, we have two HIV counseling nurses in our hospital. HIV patients being traced at our clinic have regular contact with them and adequate counseling on medication and patient status through these people. We therefore feel confident that there were no serious side effects that were not recorded on the chart and unknown to the medical staff. In addition, RNA and CD4+ counts were not routinely followed up and small number of patients were included at each week for evaluation. Lastly, because the definition of treatment failure was based on 50 copies/mL, there was difficult to distinguish from other temporary viremia such as blips.
Despite the small number of patients and retrospective design, this study can be considered a basis for the selection of drugs in clinical practice before the formal reports of other DTG/DRV/c studies are published. However, we consider that this study provides meaningful information, because data about effectiveness, safety, and tolerability of a switch to a simple dual regimen in treatment-experienced HIV patients are rare.
In conclusion, the combination of DTG/DRV/c in HIV-treatment experienced patients appears to achieve successful viral suppression in the treatment failure group and to exert viral suppression in patients who are in need of simplification and want to avoid adverse drug reaction caused by a previous drug. The tolerability of this regimen in the real practice setting appears to be very good, with no significant adverse drug reaction.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58:1297–1307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgos J, Crespo M, Falcó V, Curran A, Navarro J, Imaz A, Domingo P, Podzamczer D, Mateo MG, Villar S, Van den Eynde E, Ribera E, Pahissa A. Simplification to dual antiretroviral therapy including a ritonavir-boosted protease inhibitor in treatment-experienced HIV-1-infected patients. J Antimicrob Chemother. 2012;67:2479–2486. doi: 10.1093/jac/dks227. [DOI] [PubMed] [Google Scholar]
- 3.Bierman WF, van Agtmael MA, Nijhuis M, Danner SA, Boucher CA. HIV monotherapy with ritonavir-boosted protease inhibitors: a systematic review. AIDS. 2009;23:279–291. doi: 10.1097/QAD.0b013e32831c54e5. [DOI] [PubMed] [Google Scholar]
- 4.Seang S, Schneider L, Nguyen T, Lê MP, Soulie C, Calin R, Caby F, Valantin MA, Tubiana R, Assoumou L, Marcelin AG, Peytavin G, Katlama C. Darunavir/ritonavir monotherapy at a low dose (600/100 mg/day) in HIV-1-infected individuals with suppressed HIV viraemia. J Antimicrob Chemother. 2017;73:490–493. doi: 10.1093/jac/dkx417. [DOI] [PubMed] [Google Scholar]
- 5.Spagnuolo V, Galli L, Bigoloni A, Nozza S, Monforte Ad, Antinori A, Di Biagio A, Rusconi S, Guaraldi G, Di Giambenedetto S, Lazzarin A, Castagna A. Atazanavir/ritonavir monotherapy as maintenance strategy in HIV-1 treated subjects with viral suppression: 96-week analysis results of the MODAT study. J Int AIDS Soc. 2014;17(4 Suppl 3):19806. doi: 10.7448/IAS.17.4.19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijting I, Rokx C, Boucher C, van Kampen J, Pas S, de Vries-Sluijs T, Schurink C, Bax H, Derksen M, Andrinopoulou ER, van der Ende M, van Gorp E, Nouwen J, Verbon A, Bierman W, Rijnders B. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV. 2017;4:e547–e554. doi: 10.1016/S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 7.Yendewa GA, Salata RA. Hot news: ready for HIV dual therapy? - new data from international HIV/AIDS society 2017. AIDS Rev. 2017;19:167–172. [PubMed] [Google Scholar]
- 8.Soriano V, Peña JM. A new HIV paradigm: dual antiretroviral regimens as maintenance therapy. AIDS Rev. 2017;19:113–114. [PubMed] [Google Scholar]
- 9.Soriano V, Fernandez-Montero JV, Benitez-Gutierrez L, Mendoza C, Arias A, Barreiro P, Peña JM, Labarga P. Dual antiretroviral therapy for HIV infection. Expert Opin Drug Saf. 2017;16:923–932. doi: 10.1080/14740338.2017.1343300. [DOI] [PubMed] [Google Scholar]
- 10.Spinner CD, Kummerle T, Krznaric I, Degen O, Schwerdtfeger C, Zink A, Wolf E, Klinker HHF, Boesecke C. Pharmacokinetics of once-daily dolutegravir and ritonavir-boosted darunavir in HIV patients: the DUALIS study. J Antimicrob Chemother. 2017;72:2679–2681. doi: 10.1093/jac/dkx105. [DOI] [PubMed] [Google Scholar]
- 11.Borghetti A, Baldin G, Ciccullo A, Gagliardini R, D'Avino A, Mondi A, Ciccarelli N, Lamonica S, Fanti I, Trecarichi E, Fabbiani M, Cauda R, De Luca A, Di Giambenedetto S. Virological control and metabolic improvement in HIV-infected, virologically suppressed patients switching to lamivudine/dolutegravir dual therapy. J Antimicrob Chemother. 2016;71:2359–2361. doi: 10.1093/jac/dkw147. [DOI] [PubMed] [Google Scholar]
- 12.Maggiolo F, Gulminetti R, Pagnucco L, Digaetano M, Benatti S, Valenti D, Callegaro A, Ripamonti D, Mussini C. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17:215. doi: 10.1186/s12879-017-2311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capetti AF, Sterrantino G, Cossu MV, Orofino G, Barbarini G, De Socio GV, Di Giambenedetto S, Di Biagio A, Celesia BM, Argenteri B, Rizzardini G. Switch to dolutegravir plus rilpivirine dual therapy in cART-experienced subjects: an observational cohort. PLoS One. 2016;11:e0164753. doi: 10.1371/journal.pone.0164753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gantner P, Cuzin L, Allavena C, Cabie A, Pugliese P, Valantin MA, Bani-Sadr F, Joly V, Ferry T, Poizot-Martin I, Garraffo R, Peytavin G, Fafi-Kremer S, Rey D Dat'AIDS study group. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med. 2017;18:704–708. doi: 10.1111/hiv.12506. [DOI] [PubMed] [Google Scholar]
- 15.Revuelta-Herrero JL, Chamorro-de-Vega E, Rodríguez-González CG, Alonso R, Herranz-Alonso A, Sanjurjo-Sáez M. Effectiveness, safety, and costs of a treatment switch to dolutegravir plus rilpivirine dual therapy in treatment-experienced HIV patients. Ann Pharmacother. 2018;52:11–18. doi: 10.1177/1060028017728294. [DOI] [PubMed] [Google Scholar]
- 16.Capetti AF, Cossu MV, Paladini L, Rizzardini G. Dolutegravir plus rilpivirine dual therapy in treating HIV-1 infection. Expert Opin Pharmacother. 2018;19:65–77. doi: 10.1080/14656566.2017.1417984. [DOI] [PubMed] [Google Scholar]
- 17.Capetti AF, Sterrantino G, Cossu MV, Cenderello G, Cattelan AM, De Socio GV, Rusconi S, Riccardi N, Baldin GM, Cima S, Niero FP, Rizzardini G, Sasset L. Salvage therapy or simplification of salvage regimens with dolutegravir plus ritonavir-boosted darunavir dual therapy in highly cART-experienced subjects: an Italian cohort. Antivir Ther. 2017;22:257–262. doi: 10.3851/IMP3095. [DOI] [PubMed] [Google Scholar]
- 18.Capetti AF, Cossu MV, Orofino G, Sterrantino G, Cenderello G, De Socio GV, Cattelan AM, Soria A, Rusconi S, Riccardi N, Baldin GM, Niero FP, Barbarini G, Rizzardini G. A dual regimen of ritonavir/darunavir plus dolutegravir for rescue or simplification of rescue therapy: 48 weeks' observational data. BMC Infect Dis. 2017;17:658. doi: 10.1186/s12879-017-2755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo Z, Liang S, Sun X, Bussell S, Yan J, Kan W, Leng X, Liao L, Ruan Y, Shao Y, Xing H. Drug resistance and virological failure among HIV-infected patients after a decade of antiretroviral treatment expansion in eight provinces of China. PLoS One. 2016;11:e0166661. doi: 10.1371/journal.pone.0166661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan W, Teng T, Liang S, Ma Y, Tang H, Zuohela T, Sun G, He C, Wall KM, Marconi VC, Liao L, Leng X, Liu P, Ruan Y, Xing H, Shao Y. Predictors of HIV virological failure and drug resistance in Chinese patients after 48 months of antiretroviral treatment, 2008-2012: a prospective cohort study. BMJ Open. 2017;7:e016012. doi: 10.1136/bmjopen-2017-016012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai HC, Chen IT, Wu KS, Tseng YT, Sy CL, Chen JK, Lee SS, Chen YS. High rate of HIV-1 drug resistance in treatment failure patients in Taiwan, 2009-2014. Infect Drug Resist. 2017;10:343–352. doi: 10.2147/IDR.S146584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubavu C, Prazuck T, Niang M, Buret J, Mille C, Guinard J, Avettand-Fenoel V, Hocqueloux L. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046–1050. doi: 10.1093/jac/dkv430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MJ, Kim SW, Chang HH, Kim Y, Jin S, Jung H, Park JH, Kim S, Lee JM. Comparison of antiretroviral regimens: adverse effects and tolerability failure that cause regimen switching. Infect Chemother. 2015;47:231–238. doi: 10.3947/ic.2015.47.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capetti A, Cossu MV, Rizzardini G. Darunavir/cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. Expert Opin Pharmacother. 2015;16:2689–2702. doi: 10.1517/14656566.2015.1109632. [DOI] [PubMed] [Google Scholar]
- 25.De La Fuente S, Gutierrez A, Gomez A, Díaz-de Santiago A, Anula Á, Pintos I, Roque F, Sanz J, Ángel-Moreno A. Dual therapy with dolutegravir plus darunavir/cobicistat as salvage therapy regimen. results at 24 weeks; Poster session presented at: International AIDS Society 2017; 2017 July 23-26; France, Paris. [Google Scholar]