Abstract
Background
Endogenous endophthalmitis (EE) is a fulminant ocular disease. This study was conducted to explore frequent pathogens and significant prognostic factors associated with poor ocular outcomes.
Materials and Methods
A retrospective analysis was performed in a tertiary hospital in Korea. Thirty-nine patients, treated between January 2000 and June 2017, were eligible for the analysis. Ocular outcomes were classified as follows: 1) no light perception (NLP), 2) light perception (LP), 3) hand motion (HM), 4) counting fingers (CF), and 5) 20/200 or better. Logistic regression and decision tree analyses were used to identify risk factors that were associated with poor outcomes.
Results
Pathogens were identified in 23 (58.9%) samples from blood, liver aspirate, and/or vitreous humor. Klebsiella pneumoniae was the most frequent organism (12/39, 30.8%), followed by Candida species (3/39, 8.3%). The most common combined infection was liver abscess (16/39, 41.0%). Acute pyelonephritis occurred in 30.8% of cases (12/39). Final ocular outcomes were as follows: 35.9% (14/39) NLP, 15.4% (6/39) LP, 15.4% (6/39) HM, 7.7% (3/39) CF, and 25.6% (10/39) 20/200 or better. K. pneumoniae was a poor prognostic factor in univariate (odds ratio [OR], 13.3; 95% confidence interval [CI], 2.1-130.9) and multivariate (OR, 17.5; 95% CI, 2.1-398.8) regression analyses (NLP and LP vs. HM, CF, and 20/200 or better). Other factors did not reach statistical difference. Decision tree analysis identified K. pneumoniae as a node that divided ocular outcomes (P = 0.017).
Conclusions
In conclusion, K. pneumoniae is the most frequent causative pathogen of EE. Considering the poor prognosis and rapid progression of K. pneumoniae EE, physicians should test for K. pneumoniae EE in patients who experience acute systemic infections with ocular signs and symptoms.
Keywords: Endogenous, Endophthalmitis, Klebsiella pneumoniae, Ocular outcome
Introduction
Endogenous endophthalmitis (EE) is a devastating disease that consists of any inflammation of the internal ocular space [1,2]. EE occurs when organisms disseminate through the bloodstream and invade the eye via the blood ocular barrier [3]. Notably, EE is less common than exogenous endophthalmitis [2]. A recent study found that EE comprises only 2–17% of all cases of endophthalmitis [2]. Nevertheless, EE is a sight-threatening emergency, and its risk factors are often multifactorial [4]. Diabetes, intravenous drug use, malignancy, renal and genitourinary infections, indwelling catheters, and orthopedic surgeries are well established risk factors for EE [4,5]. Diabetes and liver cirrhosis were common underlying diseases of EE patients in one retrospective Korean study [6]. Patients typically exhibit EE symptoms in relation to systemic infections; however, in some cases, they simply present with ocular symptoms [7].
There have been extensive literature reviews, as well as prospective and retrospective studies, regarding the microbial etiology and prognostic factors of EE. Therefore, physicians are keenly aware of the significance of EE as a fatal complication of sepsis. Most studies of EE are performed by ophthalmologists and usually focus on ophthalmic prognostic factors of visual outcome, including early vitrectomy [6,8,9], initial visual acuity (VA) [10], unilateral ocular involvement [3], rapid onset of ocular symptoms [3] and hypopyon [3]. Several studies in Korea have investigated the visual prognosis of EE. Initial good VA [6,11,12], fungi [12,13], Gram-positive cocci [6], and vitrectomy [6,13] are favorable prognostic factors of visual outcome. Gram-negative bacteria [11] are a poor visual prognostic factor. These previous studies rarely focused on specific bacterial pathogens for visual prognosis.
Since there has been little improvement in the treatment outcomes of EE for many years [2,14], there is a need for investigation of changes in the causative pathogens and prognostic factors. We aimed to investigate the profiles of causative microorganisms in cases of EE, as well as identify significant prognostic factors for poor visual outcomes in EE.
Materials and Methods
1. Subjects
A retrospective study was conducted in a tertiary medical center in Korea. We included patients who were diagnosed with EE from January 2000 through June 2017. All EE cases were reviewed from the electronic medical database of the hospital.
Patients with EE were admitted directly to the Department of Internal Medicine with a manifestation of extraocular infection or to the Department of Ophthalmology with a manifestation of ocular symptoms. All patients admitted to internal medicine department with a manifestation of extraocular symptoms complained of ocular problems including blurred or altered vision and ocular pain or injection at the time of admission or during hospitalization. We directly referred these patients to ophthalmologists to evaluate EE. Moreover, we routinely follow up patients with bacteremia after discharge. We could possibly find any abnormal ocular signs or symptoms. Considering that the disease course of EE is rapidly progressing and most of the patients with EE experience clinical ocular symptoms and signs, we could hardly miss diagnosis of EE. Consequently, we think all patients with symptomatic ocular problems of EE were included in our study during the consecutive study period. Endophthalmitis was defined as a purulent inflammation of the intraocular fluids (vitreous and aqueous) usually due to infection. Diagnosis of endophthalmitis was made by ophthalmologists. The keynote feature of EE is significant involvement of the vitreous humor identified through dilated funduscopic examination. EE was defined as endophthalmitis without a history of ocular trauma, intraocular surgery, or ocular procedure.
We reviewed the records of patients with clinical ocular symptoms and signs. Specifically, we reviewed the findings from the ophthalmologists and the systemic infection signs and pathogens isolated from blood, intraocular aspirates, and other pus cultures.
Patients with recent ocular trauma or a history of intraocular surgery within 1 year of presentation were excluded. Of the 39 patients ultimately included in this study, seven patients had a history of intraocular surgery; however, no surgeries had been performed within 1 year of presentation. Two patients had ocular trauma history. However, those instances of trauma had not occurred within 1 year prior to hospital admission, and there was no evidence of exogenous endophthalmitis.
2. Variables for the prognosis
Variables that represent prognostic factors for poor outcome were analyzed, including time to diagnosis after symptom onset [15], time to systemic antibiotics after symptom onset, time to the surgical procedure after symptom onset [11,12], predisposing medical conditions (e.g., age, and underlying host diseases), other sites of involvement in the infection, pathogens in cultures (from blood, vitreous material obtained during the operation, cerebrospinal fluid (CSF), and/or pus aspirate), and levels of C-reactive protein (CRP) (Table 1).
Table 1. Descriptive statistics for the ocular outcome of endogenous endophthalmitis.
| Total of 39 EE patients (23 culture positive cases and 16 culture negative cases) | |||||
|---|---|---|---|---|---|
| Variables | Good ocular outcomea N = 19 (%) | Poor ocular outcomeb N = 20 (%) | Total N = 39 (%) | P-value | |
| Gender | 1.000 | ||||
| Female | 6 (31.6) | 6 (30.0) | 12 (30.8) | ||
| Male | 13 (68.4) | 14 (70.0) | 27 (69.2) | ||
| Age | 0.250 | ||||
| ≤60 years | 13 (68.4) | 9 (45.0) | 22 (56.4) | ||
| >60 years | 6 (31.6) | 11 (55.0) | 17 (43.6) | ||
| Predisposing disease | 1.000 | ||||
| Yes | 16 (84.2) | 17 (85.0) | 33 (84.6) | ||
| No | 3 (15.8) | 3 (15.0) | 6 (15.4) | ||
| Diabetes mellitus | 1.000 | ||||
| Yes | 6 (31.6) | 7 (35.0) | 13 (33.3) | ||
| No | 13 (68.4) | 13 (65.0) | 26 (66.7) | ||
| Malignancy | 0.308 | ||||
| Yes | 4 (21.1) | 1 (5.0) | 5 (12.8) | ||
| No | 15 (78.9) | 19 (95.0) | 34 (87.2) | ||
| Indwelling catheter | 1.000 | ||||
| Yes | 2 (10.5) | 2 (10.0) | 4 (10.3) | ||
| No | 17 (89.5) | 18 (90.0) | 35 (89.7) | ||
| Liver abscess | 0.399 | ||||
| Yes | 6 (31.6) | 10 (50.0) | 16 (41.0) | ||
| No | 13 (68.4) | 10 (50.0) | 23 (59.0) | ||
| Acute pyelonephritis | 0.350 | ||||
| Yes | 4 (21.1) | 8 (40.0) | 12 (30.8) | ||
| No | 15 (78.9) | 12 (60.0) | 27 (69.2) | ||
| Klebsiella as pathogen | 0.020 | ||||
| No | 17 (89.5) | 10 (50.0) | 27 (69.2) | ||
| Yes | 2 (10.5) | 10 (50.0) | 12 (30.8) | ||
| Pathogens in culture | 0.646 | ||||
| No growth | 9 (47.4) | 7 (35.0) | 16 (41.0) | ||
| Growth | 10 (52.6) | 13 (65.0) | 23 (59.0) | ||
| Diagnosis after symptom onset | 0.129 | ||||
| ≤3 days | 13 (72.2) | 8 (42.1) | 21 (56.8) | ||
| >3 days | 5 (27.8) | 11 (57.9) | 16 (43.2) | ||
| Antibiotic treatment after symptom onset | 0.259 | ||||
| ≤4 days | 14 (82.4) | 10 (58.8) | 24 (70.6) | ||
| >4 days | 3 (17.6) | 7 (41.2) | 10 (29.4) | ||
| Surgical procedurec after symptom onset | 0.990 | ||||
| ≤5 days | 10 (71.4) | 11 (64.7) | 21 (67.7) | ||
| >5 days | 4 (28.6) | 6 (35.3) | 10 (32.3) | ||
| C-reactive protein (mg/dL) | 0.539 | ||||
| ≤10 | 5 (33.3) | 3 (17.6) | 8 (25.0) | ||
| >10 | 10 (66.7) | 14 (82.4) | 24 (75.0) | ||
EE, endogenous endophthalmitis.
aHand motion, counting fingers, and 20/200 or better.
bNo light perception and light perception.
cIntravitreal antibiotics or pars plana vitrectomy.
We did not consider variables such as initial visual acuity, unilateral ocular involvement, rapid onset of ocular symptoms, or hypopyon as prognostic factors of EE, since they have already been described in many previous studies. We considered predisposing medical conditions, extraocular site of infection, and pathogens as important risk or prognostic factors of EE.
3. Microbiological diagnosis
All microbiological analyses were performed at the hospital's microbiology laboratory. Microbiological diagnosis was made according to culture positivity and antimicrobial susceptibility. A culture was defined as positive if there was growth of the organism in more than one media, or if there was a confluence of growth on solid media at the inoculation site [16]. For ocular microbial analysis, samples were taken from the anterior chamber, aqueous humor, and vitreous humor through routine clinical practice. Vitreous humor sampling was done in 69.2% (27/39) of patients who underwent either intravitreal antibiotics or vitrectomy. After vitreous sampling, patients were routinely treated with intravitreal antibiotics (e.g., ceftazidime plus vancomycin).
4. Ophthalmologic evaluation
Ophthalmologic evaluation was performed by ophthalmologists and included measurement of visual acuity, as well as assessment of the presence of ocular pain, floaters, photophobia, ocular discharge, and hypopyon. VA were determined using Snellen charts. Clinical features, such as initial VA, VA at discharge, and final VA at the end of follow-up, were evaluated. Vitrectomy was performed if the ocular condition began to deteriorate, according to the clinical discretion of the attending ophthalmologists.
5. Outcome accessed
Visual outcomes were classified as follows: 1) no light perception (NLP), 2) light perception (LP), 3) hand motion (HM), 4) counting fingers (CF), and 5) 20/200 or better, as measured by ophthalmologists. We defined HM, CF, and 20/200 or better as good ocular outcomes, whereas NLP and LP were defined as poor ocular outcomes, for the purpose of statistical analyses.
6. Statistical analysis
Statistical significance was set at P < 0.05; R statistics ver. 3.2.1 was used for the statistical analysis. Univariate and multivariate logistic regression analyses were performed to determine the odds ratios (OR) of ocular outcome of EE with 95% confidence intervals (CI). Decision tree analysis was used to identify significant prognostic factors that were associated with poor outcomes of EE. Classification tree analysis was then applied to our categorical outcome. A Chi-squared automatic interaction decision algorithm was chosen; therefore Chi-statistics were used to select optimal dividing nodes.
7. Ethics statement
The Institutional Review Board of Kyungpook National University Hospital approved the study protocol (No. 2018-02-032). All clinical investigations were conducted in accordance with the guidelines of the 2008 Declaration of Helsinki.
Results
1. Demographics
A total of 39 patients, who presented to our hospital with EE over an 18-year period, were included in this study. All patients were hospitalized in the Departments of Internal Medicine or Ophthalmology. The median age of the patients was 59.0 [Interquartile range (IQR) 55.0; 70.0] years. They were predominantly male (n = 27/39, 69.2%). The patients underwent surgical interventions after the time of admission. The median follow-up period was 587 [IQR 189; 1947] days. The shortest follow-up period was 15 days, and the final visual outcome for this patient was 20/200. Therefore, there was only one visit after discharge, and then a follow-up loss occurred. Final VA examined by ophthalmologists during the follow-up period before death or follow-up loss was regarded as a final visual outcome.
2. Clinical features
Median days from symptom onset to diagnosis was 3.0 [IQR 1.0; 4.0] days. Delayed diagnosis was defined as more than 3 days after symptom onset to diagnosis of EE [15,17]. Delayed diagnosis did not reach statistical significance as a factor influencing the visual outcomes of EE (Table 2). Median days from symptom onset to antibiotic treatment was 4.0 [IQR 2.0; 5.0] days. Delayed antibiotic treatment was defined as more than 4 days after symptom onset to antibiotic treatment [2,11,12]. Median days from symptom onset to surgical treatment was 5.0 [IQR 2.5; 7.5] days. Delayed surgical treatment was defined as more than 5 days after symptom onset to surgical treatment [2,11,12]. There was no statistically significant relation between VA and time to treatment (Table 2). Fever was the most frequent systemic clinical finding. The mortality rate in this study was 5% (n = 2/39), and these patients had liver abscess coinfections.
Table 2. Univariate and multivariate logistic regression analysis for poor ocular outcome of endogenous endophthalmitis.
| Total of 23 EE patients (23 culture positive cases only) | |||||
|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | |||
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Gender | 0.253 | ||||
| Female | reference | ||||
| Male | 0.25 (0.01–2.12) | ||||
| Age | 0.511 | 0.728 | |||
| ≤60 years | reference | reference | |||
| >60 years | 1.75 (0.33–9.93) | 0.63 (0.03–7.37) | |||
| Predisposing disease | 0.706 | ||||
| Yes | 0.61 (0.03–7.43) | ||||
| No | reference | ||||
| Diabetes mellitus | 0.673 | 0.659 | |||
| Yes | 1.46 (0.26–9.31) | 1.68 (0.18–21.24) | |||
| No | reference | reference | |||
| Malignancy | 0.401 | ||||
| Yes | 0.33 (0.01–4.05) | ||||
| No | reference | ||||
| Indwelling catheter | 0.995 | ||||
| Yes | No results | ||||
| No | reference | ||||
| Liver abscess | 0.511 | ||||
| Yes | 1.75 (0.33–9.93) | ||||
| No | reference | ||||
| Acute pyelonephritis | 0.434 | ||||
| Yes | 2.00 (0.36–12.71) | ||||
| No | reference | ||||
| Klebsiella as pathogen | 0.012 | 0.021 | |||
| No | reference | reference | |||
| Yes | 13.33 (2.07–130.86) | 17.53 (2.09–398.84) | |||
| Pathogens in culture | 0.533 | ||||
| No growth | reference | ||||
| Growth | No results | ||||
| Diagnosis of EE after symptom onset | 0.286 | ||||
| ≤3 days | reference | ||||
| >3 days | 2.86 (0.45–24.73) | ||||
| Antibiotic treatment after symptom onset | 0.697 | ||||
| ≤4 days | reference | ||||
| >4 days | 1.50 (0.20–13.86) | ||||
| Surgical procedure after symptom onset | 0.960 | ||||
| ≤5 days | reference | ||||
| >5 days | 0.95 (0.14–6.73) | ||||
| C-reactive protein (mg/dL) | 0.382 | ||||
| ≤10 | reference | ||||
| >10 | 2.50 (0.32–23.57) | ||||
EE, endogenous endophthalmitis. OR, odds ratio; CI, confidence interval.
Common presenting ocular complaints consisted of decreased vision, injected eye, ocular pain, floaters, photophobia, and ocular discharge. Hypopyon was found in 17.9% of cases (n = 7/39). Six patients exhibited bilateral EE, whereas 33 exhibited uniocular EE. Right eyes were more commonly affected (n = 22/39, 56.4%); 11 patients exclusively exhibited left eye involvement. VA before symptom onset for EE was not accessible within the medical records. Twenty-nine of 39 patients (74.4%) initially presented with VA that was beyond the range of the Snellen chart, with seven patients (17.9%) having VA worse than light perception in either eye.
3. Risk factors
A total of six different risk factors were identified among the 39 EE patients. Six patients had no risk factors. Three patients had two risk factors (Table 3), including diabetes mellitus (33.3%), liver cirrhosis (15.4%), malignancy (12.8%), recent invasive surgery (12.8%), indwelling catheter (10.3%), and renal failure (7.7%). Five patients exhibited malignancy; two of these patients had undergone recent invasive surgery. Recent invasive surgery was defined as operations relating to non-ocular disease performed within 1 year before onset of EE. One patient had undergone a pylorus-preserving pancreatoduodenectomy; the other had undergone a prostatectomy. Notably, none of these five patients were currently undergoing immunosuppressive therapy. Immunosuppressive therapy was defined as a cytotoxic chemotherapy, immune-modulating medication within 6 weeks before admission. Indwelling catheter referred to a Foley catheter, central venous catheter or percutaneous drainage catheter. No patient had a history of organ transplantation or immune-modulating medications. Further, there were no human immunodeficiency virus (HIV) patients. Six patients (15.4%) exhibited no underlying medical conditions. Underlying medical conditions included any disease that may cause an immunosuppressive state such as malignancy, HIV, liver, renal, heart and lung diseases, diabetes mellitus and any disease resulting in immunosuppressive therapy. There was no statistically significant relationship between the underlying medical conditions and the clinical outcome in cases of EE.
Table 3. Frequencies of known risk factors of endogenous endophthalmitis in this study.
| Risk factors | Numbers (%) |
|---|---|
| Diabetes mellitus | 13 (33.3) |
| Liver cirrhosis | 6 (15.4) |
| Malignancy | 5 (12.8) |
| Recent invasive surgery | 5 (12.8) |
| Indwelling catheter | 4 (10.3) |
| Renal failure | 3 (7.7) |
| No risk factor found | 6 (15.4) |
4. Pathogens
Pathogens were identified from patient samples (blood and/or ocular samples, cerebrospinal fluid, or pus) in 23 of 39 patients (60%) (Table 4). Blood cultures of 29 patients were performed at the time of EE diagnosis. Of 29 blood cultures that were performed, 14 positive blood cultures indicated the presence of bacteremia. Of a total of 23 culture-positive cases, six exhibited positive growth from vitreous samples and were identified as pathogens. Notably, more cases exhibited positive cultures from blood samples (n = 14) than from vitreous samples.
Table 4. Pathogens identified from cultures of endogenous endophthalmitis.
| Organisms | Numbers (%) |
|---|---|
| Klebsiella pneumoniae | 12 (30.8) |
| Candida albicans | 3 (8.3) |
| Enterococcus faecalis | 2 (5.6) |
| Staphylococcus aureus | 2 (5.6) |
| Viridians Streptococcus | 1 (2.8) |
| Streptococcus constellatus | 1 (2.8) |
| Fusarium spp. | 1 (2.8) |
| Providencia rettgeri | 1 (2.8) |
| No growth of pathogens | 16 (41.0) |
| Total | 39 (100) |
Cultures from blood, urine, cerebrospinal fluid, vitreous humor and pus.
Table 4 shows microorganisms that were identified from cultures of EE patients. In this study, EE was predominantly caused by Gram-negative bacteria (13 of 39 patients, 33.3%; Table 3). Fungi were identified as the causative agent in 10.3% of all cases (Table 4). Gram-positive bacteria were the causative agent in 15.4% of cases (Table 4). No anaerobes or mycobacteria were identified in patient samples. A total of 16 cases demonstrated no identifiable microbial growth in culture. In these 16 cases, there were nine positive cultures with normal skin flora, and we did not consider those as pathogens of EE. Causative pathogens were isolated from blood culture (14 patients), vitreous culture (six patients), pus culture (10 patients), and CSF culture (one patient). Of a total of 12 K. pneumoniae cases, there was only a single positive blood culture result that demonstrated the same sensitivity pattern as the organism that was cultured from the eye. A total of seven patients yielded pus cultures of an identical pathogen of blood or eye aspirates from extraocular infection sites (Table 5). Six of these cultures were grown from liver abscesses, and one was grown from a gluteus muscle abscess.
Table 5. Combined infections identified in imaging studies or aspiration cultures.
| Imaging findings | Total numbers (%) | Aspiration culture proven numbers (Pathogen same as blood or eye aspirate culture) | Numbers of any other infection combined |
|---|---|---|---|
| Liver abscess | 16 | 9 (6) | 5 |
| Acute pyelonephritis | 12 | 0 | 7 |
| Cholangitis | 4 | 0 | 2 |
| Pneumonia | 2 | 0 | 1 |
| Septic lung | 2 | 0 | 0 |
| Infective endocarditis | 2 | 0 | 1 |
| Septic arthritis | 2 | 0 | 0 |
| Meningitis | 1 | 1 | 0 |
| Muscle (gluteus) abscess | 1 | 1 (1) | 0 |
| Pelvic abscess | 1 | 0 | 0 |
| Prostate abscess | 1 | 0 | 0 |
| None | 3 | 0 | 0 |
5. Treatment
Intravitreal antibiotic therapy, in addition to systemic antibiotic therapy, was performed in 24 of 39 patients. Pars plana vitrectomy was performed in 14 of 39 patients who showed no response to initial systemic or intravitreal antibiotic therapy. Median period from symptom onset to vitrectomy was 5 [IQR 4.0; 8.0] days. Some patients could not be treated with surgical interventions because of severe sepsis. Of those managed with vitrectomy, the visual outcomes were as follows: five patients exhibited NLP, two exhibited LP, four exhibited HM, one exhibited FC, and two exhibited 20/200. Of the 14 patients who underwent pars plana vitrectomy, only two patients had improved visual outcome after vitrectomy. One patient progressed from HM to 20/200, the other from FC to 20/200. The remaining patients had no interval change of outcome or worse outcome following vitrectomy. Therefore, pars plana vitrectomy was not a good prognostic factor for ocular outcome (OR, 1.30; 95% CI, 0.34-5.03, P = 0.700 in univariate logistic regression).
6. Visual outcomes
According to our categorization method, the visual outcomes were poor in 20 out of 39 patients. Final ocular outcomes were as follows: 35.9% (n = 14/39) exhibited NLP, 15.4% (n = 6/39) exhibited LP, 15.4% (n = 6/39) exhibited HM, 7.7% (n = 3/39) exhibited FC, and 25.6% (n = 10/39) exhibited 20/200 or better (Table 6). The final outcome of VA, as assessed by Snellen chart, ranged from NLP to 20/200 or better.
Table 6. Final ocular outcomes of endogenous endophthalmitis.
| Ocular outcome | Numbers (%) |
|---|---|
| No light perception | 14 (35.9) |
| Light perception | 6 (15.4) |
| Hand motion | 6 (15.4) |
| Counting fingers | 3 (7.7) |
| 20/200 or better | 10 (25.6) |
| Total | 39 (100) |
7. Combined infections
An extraocular focus of infection was detected in 33 of 39 patients (Table 5). All associated infection sites were confirmed by imaging studies or aspiration cultures. The most common co-infection was liver abscess (n = 16/39), followed by acute pyelonephritis (12/39), cholangitis (4/39), endocarditis (2/39), pneumonia (2/39), septic lung (2/39), septic arthritis (2/39) and (equal incidence) gluteus muscle abscess, pelvic abscess, meningitis and prostate abscess (each 1/39). Eight patients showed two different extraocular infections sites. Combined extraocular infections were liver abscess, acute pyelonephritis, cholangitis, pneumonia, and infective endocarditis. We performed orbital CT scans or brain MRIs in seven patients and examined cerebrospinal fluid from four patients for central nervous system evaluation. One meningitis case was confirmed.
8. Logistic regression and decision tree analysis
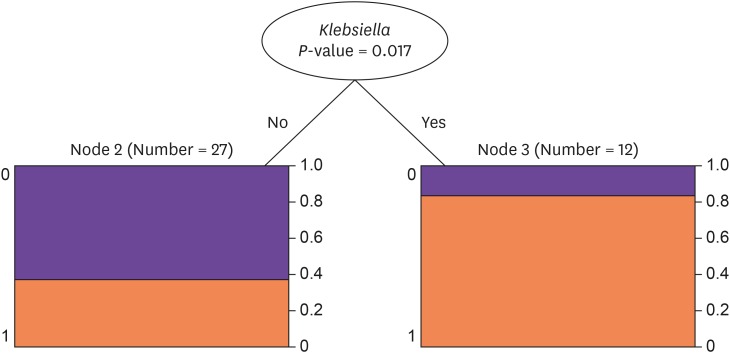
In our univariate and multivariate regression analyses, the presence of K. pneumoniae was a statistically significant independent factor associated with poor ocular outcomes of EE in a total of 39 patients, including culture negative cases (OR, 8.50; 95% CI, 1.79-63.11, P = 0.014 in univariate and OR, 7.20; 95% CI, 1.43-55.16, P = 0.027 in multivariate). K. pneumoniae remained a statistically significant risk factor in 23 culture positive only cases (OR, 13.3; 95% CI, 2.1-130.9, P = 0.012 in univariate and OR, 17.5; 95% CI, 2.1-398.8, P = 0.021 in multivariate; Table 2). Other factors did not reach statistical significance. The same results were found in our decision tree analysis, where K. pneumoniae was a significant dividing node for the prediction of ocular outcome (P = 0.017; Fig. 1).
Figure 1. Decision tree analysis for the outcome of endogenous endophthalmitis.
Discussion
This study revealed a statistically significant relationship between K. pneumoniae as a pathogen and poor visual outcomes, in both logistic regression and decision tree analyses. K. pneumoniae was also the most common pathogen in our EE cases, comprising 30.8% of pathogens in our study.
During our study period, a total of 137 endophthalmitis patients were admitted to our hospital. EE accounted for 28.5% (n = 39/137) of total endophthalmitis cases. This was a relatively large proportion of EE compared to previous studies, which range from 2 to 17% [2]. This might relate to characteristics of our hospital. Our hospital is a major tertiary hospital in this province that admits patients with serious diseases including bacteremia. Therefore, we postulate that as more patients with bacteremia are admitted, more patients could possibly have EE.
K. pneumoniae was the only significant visual prognostic factor in our study. Additionally, it was the most frequent pathogen in our EE study. Klebsiella spp. was present in 90% of endogenous bacterial endophthalmitis cases in Asia [7,14,18,19,20,21,22,23]. Some recent Korean retrospective studies have also shown that K. pneumoniae is the most common pathogen of EE, similar to previous Asian studies [6,11,13]. To our knowledge, there have been few studies in which K. pneumoniae was analyzed as a prognostic variable for ocular outcome [24]. Many studies described K. pneumoniae as a possible pathogen of EE; however, they do not identify it as an ocular prognostic factor with statistical analysis. Generally, pathogens such as Gram-positive or negative bacteria or fungi have been analyzed as prognostic factors of EE. Gram-negative bacteria are known to cause a worse visual outcome than Gram-positive bacteria or fungi [6,11]. Some previous studies have revealed that fungi are a good prognostic factor for ocular outcomes [25,26,27,28]. There were four fungal EE patients in our study; all exhibited good ocular outcomes (one with HM, three with 20/200 or better). These findings are similar to those of previous studies; however, the small sample size could not justify them.
Diabetes mellitus was the most prevalent underlying risk factor in our study. This result was consistent with several other studies [2,3,5]. Diabetes seemed to be a poor ocular prognostic factor of EE in our study. However, this effect was not statistically significant (P = 0.690). It is possible that diabetes was associated with bacteremia itself and then bacteremia caused EE.
The most common co-infection in our study was liver abscess (n = 16/39, 41.0%). There were 66.7% of K. pneumoniae EE patients whose co-infection sites were liver abscesses. However, liver abscess was not a significant prognostic factor for ocular outcome in EE. We suspect that the liver abscess itself could respond to the systemic antibiotics and surgical procedure; however, the bacterial characteristics associated with the ocular tropism of K. pneumoniae might contribute to the ocular outcome. The mortality rate of EE is known to be 4–5% [2,5]. All cases of mortality in our study arose from K. pneumoniae EE with liver abscess, thus yielding a 5% mortality rate. This implies that K. pneumoniae is a fulminant risk factor for mortality, as well as prognostic factor for ocular outcome.
Other common risk factors in previous studies such as malignancy and indwelling catheters were presented as good prognostic factors in our univariate logistic regression. These results were opposite those of previous studies [4,5]. We did not regard either of these factors as clinically meaningful results in our study given that they were not statistically significant. Acute pyelonephritis was a poor prognostic factor that was not statistically significant in univariate logistic regression (P = 0.206). There were a total of 12 acute pyelonephritis cases in our study, and the urine culture results were all negative.
It might be suspected that an early diagnosis of EE could enable physicians to make a good prognosis. We noted that delayed diagnosis and treatment have been major risk factors of EE in previous studies [2,11,17]. In our study, however, delayed diagnosis was not a statistically significant predictive variable for visual outcomes in EE. Further, delayed appropriate antibiotic and surgical treatment after symptom onset did not reach statistical significance as poor prognostic factors. Patients with EE were admitted to the hospital with initially poor VA. We also suspect that there are difficulties in identifying the intensity of the disease, which result in an inability to overcome a poor outcome because of the overwhelming speed of this fulminant disease.
In this study, of all 23 culture-positive cases, there were six positive culture results from vitreous samples, which were identified as pathogens. These were proportionally fewer than in previous studies, which reported that up to 56-74% of vitreous cultures were positive in cases of EE [2,29]. This may be related to the small sample size and the time to collect vitreous cultures after prompt administration of systemic antibiotics. Approximately half of the all means of positive culture results in our study were from blood cultures. There were 14 positive blood cultures out of 29 total blood cultures, which comprised a relatively smaller proportion compared with previous studies. Positive blood cultures were obtained from 61 of 65 patients (94%) in one review article [2]. Blood cultures were more likely to be positive than vitreous cultures; thus, blood culture was the most common means to confirm the diagnosis of EE [2,14,30,31]. There were 16 (41%) culture negative EE in our analysis. Of these 16 patients, nine had good ocular outcomes and seven had poor ocular outcomes. A previous retrospective study demonstrated that culture positive EE showed a poor visual result (VA of less than 20/400) [32].
Intravitreal injection is a standard treatment for infectious endophthalmitis, which can achieve a greater intraocular concentration than any other method of antibiotic administration [33]. Intravitreal antibiotics more strongly contribute to the preservation of VA [2,4]. There were 24 cases of intravitreal antibiotic injections and 14 cases of vitrectomy performed. Neither of these treatments showed a significantly decreased OR, which was consistent with the results of previous studies (OR of intravitreal antibiotics, 1.02; 95% CI, 0.24-4.41, P = 0.983 and OR of vitrectomy, 1.30; 95% CI, 0.34-5.03, P = 0.700 in univariate logistic regression). Because of the drastic progression of this disease, surgical treatment could not be a prognostic factor for a good ocular outcome.
This study has a few limitations. Because of the lack of follow-up medical records, this study could only describe the final VA as an outcome. The visual outcome can be calculated from differences between pre-treatment VA and post-treatment VA, categorized as follows: improved, no interval change, deteriorated. However, other reports also use the latest VA as a final outcome because of the lack of VA data before EE.
In this study, patients without ocular symptoms and signs were not evaluated for EE. Since it is known that most of the patients with EE experience clinical ocular symptoms and signs, we think that the possibility of EE in bacteremic patients without ocular problems is very low. A prospective study has reported that ocular lesion (Roth's spots, cotton wool exudates, retinal hemorrhages, and chorioretinal and vitreous inflammation not attributable to other causes) related to bacteremia were found in 12% of patients who were mostly asymptomatic. Those were mainly caused by fungi and anaerobic bacteria but not by gram negative pathogen [34]. However, there were still some limitations that asymptomatic EE with bacteremia were not included in this study, although those were few cases.
Nevertheless, we analyzed EE patient cases in a tertiary hospital over 18 years, which is a relatively long collection period for a single center. K. pneumoniae was the most predictive factor for ocular outcome of EE in both multivariate logistic regression and decision tree analyses in this study.
Of 12 K. pneumoniae EE patients in our study, 10 patients (83.3%) had poor ocular outcomes (NLP and LP). These were similar results as in previous studies; thus, there has been little improvement of treatment outcomes. K. pneumoniae infection is not a rare infection in patients hospitalized in department of internal medicine. Therefore, we suggest immediate and careful attention should be given to K. pneumoniae EE cases, considering the poor prognosis.
In conclusion, K. pneumoniae is the most frequent pathogen of EE. Considering the poor prognosis and rapid progression of K. pneumoniae EE, physicians should be aware of and test for K. pneumoniae EE in patients who experience acute systemic infections with ocular symptoms and signs.
We hope that these findings will assist physicians with the care and management of patients with this infection from the perspective of ocular outcomes as well as sepsis.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001;12:464–470. doi: 10.1097/00055735-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 3.Ang M, Jap A, Chee SP. Prognostic factors and outcomes in endogenous Klebsiella pneumoniae endophthalmitis. Am J Ophthalmol. 2011;151:338–344.e2. doi: 10.1016/j.ajo.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Connell PP, O'Neill EC, Fabinyi D, Islam FM, Buttery R, McCombe M, Essex RW, Roufail E, Clark B, Chiu D, Campbell W, Allen P. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond) 2011;25:66–72. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014;59:627–635. doi: 10.1016/j.survophthal.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Um T, Joe SG, Hwang JU, Kim JG, Yoon YH, Lee JY. Changes in the clinical features and prognostic factors of endogenous endophthalmitis: fifteen years of clinical experience in Korea. Retina. 2012;32:977–984. doi: 10.1097/IAE.0b013e318228e312. [DOI] [PubMed] [Google Scholar]
- 7.Durand ML, Heier JS. Endophthalmitis. Curr Clin Top Infect Dis. 2000;20:271–297. [PubMed] [Google Scholar]
- 8.Yoon YH, Lee SU, Sohn JH, Lee SE. Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis. Retina. 2003;23:366–370. doi: 10.1097/00006982-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Ishii K, Hiraoka T, Kaji Y, Sakata N, Motoyama Y, Oshika T. Successful treatment of endogenous Klebsiella pneumoniae endophthalmitis: a case report. Int Ophthalmol. 2011;31:29–31. doi: 10.1007/s10792-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 10.Sallam A, Taylor SR, Khan A, McCluskey P, Lynn WA, Manku K, Pacheco PA, Lightman S. Factors determining visual outcome in endogenous Candida endophthalmitis. Retina. 2012;32:1129–1134. doi: 10.1097/IAE.0b013e31822d3a34. [DOI] [PubMed] [Google Scholar]
- 11.Lim HW, Shin JW, Cho HY, Kim HK, Kang SW, Song SJ, Yu HG, Oh JR, Kim JS, Moon SW. Endogenous endophthalmitis in the Korean population: a six-year retrospective study. Retina. 2014;34:592–602. doi: 10.1097/IAE.0b013e3182a2e705. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JH, Cho NC. Prognostic factors in patients with endogenous endophthalmitis. J Korean Ophthalmol Soc. 2009;50:858–863. [Google Scholar]
- 13.Cho H, Shin YU, Siegel NH, Yu HG, Sobrin L, Patel A, Durand ML, Miller JW, Husain D. Endogenous endophthalmitis in the American and Korean population: an 8-year retrospective study. Ocul Immunol Inflamm. 2018;26:496–503. doi: 10.1080/09273948.2016.1195000. [DOI] [PubMed] [Google Scholar]
- 14.Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107:1483–1491. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 15.Takebayashi H, Mizota A, Tanaka M. Relation between stage of endogenous fungal endophthalmitis and prognosis. Graefes Arch Clin Exp Ophthalmol. 2006;244:816–820. doi: 10.1007/s00417-005-0182-5. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmus KR, Specter S, Wilhelmus KR. Laboratory diagnosis of ocular infections: American Society for Microbiology. 1994. [Google Scholar]
- 17.Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986;31:81–101. doi: 10.1016/0039-6257(86)90076-7. [DOI] [PubMed] [Google Scholar]
- 18.Chang FY, Chou MY, Fan RL, Shaio MF. A clinical study of Klebsiella liver abscess. Taiwan Yi Xue Hui Za Zhi. 1988;87:282–287. [PubMed] [Google Scholar]
- 19.Chee SP, Ang CL. Endogenous Klebsiella endophthalmitis--a case series. Ann Acad Med Singapore. 1995;24:473–478. [PubMed] [Google Scholar]
- 20.Chiu CT, Lin DY, Liaw YF. Metastatic septic endophthalmitis in pyogenic liver abscess. J Clin Gastroenterol. 1988;10:524–527. doi: 10.1097/00004836-198810000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Chen CY, Chen FH, Zimmerman RA, Hsiao HS. Septic metastatic endophthalmitis from Klebsiella pneumoniae liver abscess: CT and MR imaging characteristics--report of three cases. Radiology. 1998;207:411–416. doi: 10.1148/radiology.207.2.9577489. [DOI] [PubMed] [Google Scholar]
- 22.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. [PubMed] [Google Scholar]
- 23.Wang FD, Wang LS, Liu YC, Liu CY, Lin CL, Wong WW. Successful treatment of metastatic endophthalmitis. Case reports. Ophthalmologica. 1989;198:124–128. doi: 10.1159/000309973. [DOI] [PubMed] [Google Scholar]
- 24.Sridhar J, Flynn HW, Jr, Kuriyan AE, Dubovy S, Miller D. Endophthalmitis caused by Klebsiella species. Retina. 2014;34:1875–1881. doi: 10.1097/IAE.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiedler V, Scott IU, Flynn HW, Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137:725–731. doi: 10.1016/j.ajo.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Essman TF, Flynn HW, Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28:185–194. [PubMed] [Google Scholar]
- 27.Ness T, Pelz K, Hansen LL. Endogenous endophthalmitis: microorganisms, disposition and prognosis. Acta Ophthalmol Scand. 2007;85:852–856. doi: 10.1111/j.1600-0420.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 28.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003;82:97–105. doi: 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 30.Liao HR, Lee HW, Leu HS, Lin BJ, Juang CJ. Endogenous Klebsiella pneumoniae endophthalmitis in diabetic patients. Can J Ophthalmol. 1992;27:143–147. [PubMed] [Google Scholar]
- 31.Okada AA, Johnson RP, Liles WC, D'Amico DJ, Baker AS. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994;101:832–838. [PubMed] [Google Scholar]
- 32.Bohigian GM, Olk RJ. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986;101:332–341. doi: 10.1016/0002-9394(86)90829-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen KJ, Hwang YS, Wang NK, Chao AN. Endogenous Klebsiella pneumoniae endophthalmitis with renal abscess: Report of two cases. Int J Infect Dis. 2010;14:e429–e432. doi: 10.1016/j.ijid.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Bouza E, Cobo-Soriano R, Rodriguez-Creixems M, Munoz P, Suarez-Leoz M, Cortes C. A prospective search for ocular lesions in hospitalized patients with significant bacteremia. Clin Infect Dis. 2000;30:306–312. doi: 10.1086/313648. [DOI] [PubMed] [Google Scholar]