Abstract
Since the mid 1980s, the prevalence of liver abscess caused by hypervirulent Klebsiella pneumoniae strain has increased in Asia, particularly in Taiwan and Korea. This strain is mostly K1 or K2 serotype, and has hypercapsular and hypermucoid phenotypes. Most infections are community acquired, and patients rarely have a hepatobiliary disease prior to infection. Clinical manifestations are characterized by fever and high C-reactive protein, and metastatic infections, such as septic emboli in the lung and endophthalmitis and meningitis are frequently observed. Antibiotic resistance is rare. Antibiotic treatment and abscess drainage are needed, and early diagnosis and treatment of endophthalmitis is also important.
Keywords: Liver Abscess, Klebsiella pneumoniae, C-reactive protein
Introduction
In the mid-1980s, investigators in Taiwan first noted a distinctive syndrome of monomicrobial Klebsiella pneumoniae pyogenic liver abscess in individuals who were often diabetic but had no biliary tract disorders [1,2,3]. Subsequently, community-acquired K. pneumoniae liver abscess has become a major health problem in parts of Asia, accounting for 80% of all cases of pyogenic liver abscess in Taiwan and Korea, and has been reported sporadically elsewhere in Asia, North America, Europe, and Australia [4,5,6].
Infections are primarily caused by hypermucoid strains of K. pneumoniae of the capsular K1 (or occasionally K2) serotype. The mortality rate is approximately 5%, which is not much different than non-K. pneumoniae liver abscess [5], but metastatic infections, such as endophthalmitis and meningitis, have been reported in approximately 10-16% of cases [7].
Route of hepatic invasion
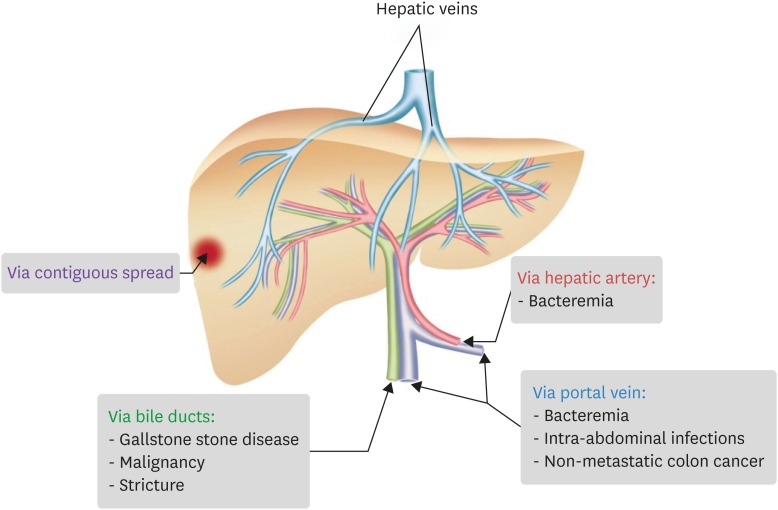
Pyogenic liver abscess occurs whenever the initial inflammatory response fails to clear an infectious insult to the liver. Pyogenic liver abscesses are usually classified by presumed route of hepatic invasion: (1) biliary tree, (2) portal vein, (3) hepatic artery, and (4) direct extension from contiguous focus of infection (Fig. 1) [8]. The most common etiologies are unknown cryptogenic (primary) origin and infection by biliary route [9,10]. Biliary infections are associated mostly with stricture subsequent to gallstone disease, malignancy, or hepaticojejunostomy, which is believed to involve bacterial proliferation in the bile duct that causes ascending cholangitis and invasion of the liver parenchyma [11,12]. Portal venous route may originate from an abdominal infection, where appendicitis, diverticulitis, colorectal cancer, and inflammatory bowel disease may be the cause [7]. Hepatic arterial route usually involves bacteremia caused by Staphylococcus aureus, leading to secondary liver abscess [7].
Figure 1. Routes of Infection (adapted from reference 8).
Compared to non-K. pneumoniae liver abscess, K. pneumoniae liver abscess rarely have biliary or portal vein route of infection involving hepatobiliary disease, history of hepatobiliary surgery, history of intra-abdominal trauma or surgery, or malignancy [4,13]. An animal study using a mouse model reported that K. pneumoniae crosses the intestinal barrier to cause liver abscess [14].
Epidemiology and risk factor
The cause in most patients was community-acquired infections, being reported predominantly in Asian countries, including Taiwan and Korea, while even the 2 case series reported in the US showed that the ethnic origin of half of the patients was Asian [5]. Studies have yet to identify any host genetic factors that can explain such high prevalence among Asians. Previous studies reported that the prevalence of K. pneumoniae in healthy adults in Asian countries was up to 75%, and that 23% of typeable stains in Taiwan were K1 or K2 serotype [15]. In a Korean study, K. pneumoniae was isolated from 248 of 1,175 stool samples (21.1%) and 23% (57/248) of K. pneumoniae isolates were K1 serotype [16]. In contrast, a European study with small sample size reported that the prevalence of K. pneumoniae in fecal samples was only about 10-19% [17,18]. While there is the limitation that the environmental reservoir of K. pneumoniae has not been identified, it is believed that an association between incidence of liver abscess and high prevalence of virulent K. pneumoniae strains among Asians does exist. In other words, it is suspected that after gastrointestinal colonization of K. pneumoniae through environmental exposure or fecal to oral transmission in Asian, the bacteria can cross the intestinal barrier to invade the liver.
Diabetes mellitus (DM) is believed to be one of the risk factors of K. pneumoniae liver abscess [13]. Compared to 5-33% of non-K. pneumoniae liver abscess patients having DM, up to 63% of the patients in the Taiwanese case series were reported to have DM [3,19,20]. Although the definitive mechanism associated with this has not been identified, but it has been reported that poor glycemic control may impair neutrophil phagocytosis of K1 and K2 capsular serotypes [21]. In patients with endophthalmitis, it has been reported to be associated with poor visual outcome [22]. In addition, others have reported that incidence of K. pneumoniae liver abscess is associated with history of antibiotics use, such as ampicillin and amoxicillin, within 30 days. In an accompanying animal study, ampicillin administration predisposed K. pneumoniae-colonized mice to increased liver abscess formation [23].
Virulence factors
“Classical” K. pneumoniae generally causes nosocomial acquired pneumonia, urinary tract infection, or bacteremia in immunocompromised patients, such as those with DM or malignancy [24]. Hypervirulent strains that have become more common around Asia in recent times have community origin and can often cause infection in even healthy and immunosufficient people [24]. Moreover, they characteristically cause pyogenic liver abscess and have hematogenous metastatic spread. Antibiotic resistance is a phenomenon associated primarily with classical K. pneumoniae. Although there have been reports of hypervirulent K. pneumoniae strains that carry extended-spectrum beta-lactamases (ESBLs) or carbapenemases, it is a rare occurrence [25]. An overview of the difference between classical and hypervirulent K. pneumoniae strain can be found in Table 1.
Table 1. Characteristics of classical and hypervirulent Klebsiella pneumoniae strains.
| Parameter | Characteristics for strain type | |
|---|---|---|
| Classical | Hypervirulent | |
| Common types of infection | Pneumonia, UTI, bacteremia | Pyogenic liver abscess, bacteremia, pneumonia, necrotizing fasciitis, myositis, meningitis, endophthalmitis |
| Susceptible population | Immunosuppressed (diabetics, patients with malignancies) | Diabetics, healthy people |
| Capsule type | Capsule serotypes K1-K78 | Hypercapsular serotype K1 (93%) or K2 |
| Siderophores (% of strains expressing siderophore) | Enterobactin (100), yersiniabactin (17–46), salmochelin (2–4), aerobactin (6) | Enterobactin (100), yersiniabactin (90), salmochelin (>90), aerobactin (93–100) |
| Primary acquired infection type | Nosocomial | Community acquired |
| Geographic concentration | Worldwide | Primarily Taiwan, Korea, and Southeast Asia |
| Frequency of reports of antibiotic resistance | Frequent (ESBL and carbapenemase producing) | Infrequent |
UTI, urinary tract infections; ESBL, extended-spectrum beta-lactamases.
To date, there are four major classes of virulence factors that have been well characterized in K. pneumoniae. These virulence factors consist of capsule, including the production of hypercapsule in hypervirulent strains; lipopolysaccharide (LPS); siderophores; and fimbriae, also known as pili. Differences between classical and hypervirulent strains that have been identified in studies to date include serotype K1 or K2 serotype being hypercapsular and greater expression of siderophores, such as yersiniabactin, salmochelin, and aerobactin [24].
Capsule is an extracellular polysaccharide matrix that envelops the bacteria. Both classical capsule and hypervirulent hypercapsule are made up of strain-specific polysaccharides termed K antigen (i.e., K1 and K2, up through K78). The genes needed for the production of capsule are located on a chromosome operon, cps. K antigens have been traditionally assigned by using serologic methods [26]. Recently however, K-antigen typing is often performed by sequencing of the wzi locus [27]. This locus is present in all capsular types of K. pneumoniae, and different wzi locus sequences are strongly associated with specific K antigens. Classical K. pneumoniae strainis produce a capsule that can be of any of the serotypes K1 to K78. Hypervirulent strains make a hypercapsule, which amplifies the production of capsular material, resulting in a relatively larger capsule, and are predominantly of the K1 serotype, while the remaining strains are of serotype K2. One study characterizing hypervirulent K. pneumoniae strains isolated from 4 different continents found that these strains were almost exclusively K1 strains (93%), while the remaining minority were K2 strains [28]. The hypercapsule phenotype showed enhanced resistance to a variety of humoral defenses, including complement killing and phagocytosis by human neutrophils and macrophages compared to a number of classical strains [29].
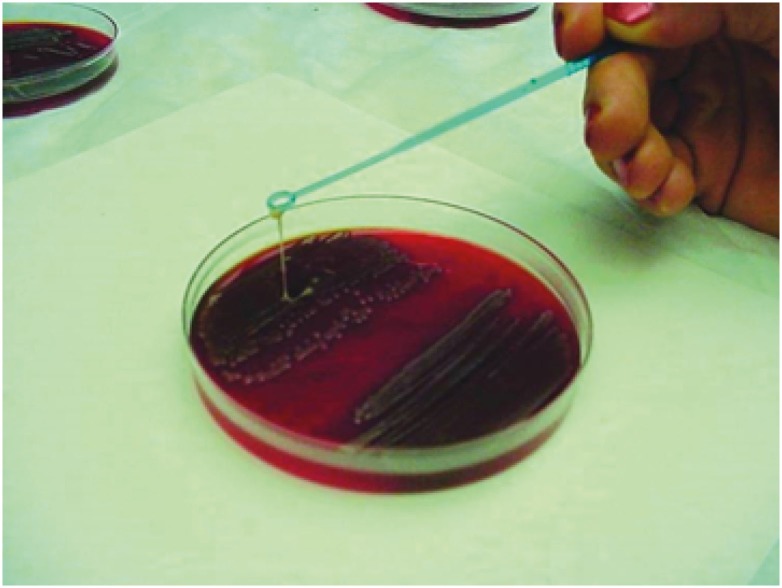
Hypervirulent K. pneumoniae shows a mucoid phenotype and the genes associated with this include regulator of mucoid phenotype A (rmpA) and rmpA2, mucoviscosity-associated gene A (magA), which increases capsule production. When colonies of mucoid phenotype were touched with a loop and the loop lifted vertically from the surface of the agar plate, mucoid isolates adhered to the loop as it was lifted from the plate (Fig. 2) [30]. In fact, 55 to 100% of hypervirulent K. pneumoniae strains express at least one copy of rmpA or rmpA2, compared to 7-20% of non-hypervirulent K. pneumoniae strains [31]. Gene magA is actually specific to K1 stains.
Figure 2. Mucoid phenotype of Klebsiella pneumoniae (adapted from reference 30).
K. pneumoniae, like many other bacterial pathogens, must employ tactics to acquire iron from the host in order to survive and propagate during mammalian infection. The predominant tactic used by many pathogens, including K. pneumoniae, to acquire iron is through the secretion of siderophores, which are molecules that possess a higher affinity for iron than host transport proteins do. Siderophores can steal iron from host iron-chelating proteins or scavenge it from the environment [32]. Several siderophores are expressed in K. pneumoniae, including enterobactin, yersiniabactin, salmochelin, and aerobactin. Enterobactin expression is almost ubiquitous among both classical and hypervirulent K. pneumoniae strains and is therefore considered to be the primary iron uptake system utilized by K. pneumoniae. In contrast, expression levels of yersiniabactin, salmochelin, and aerobactin are much higher in hypervirulent K. pneumoniae strain than in classical strain. Yersiniabactin has been observed in only 18% of classical but 90% of hyperevirulent K. pneumoniae clinical isolates [24]. Salmochelin is present in only about 2 to 4% of nosocomial K. pneumoniae strains but is much more prevalent in hypervirulnet K. pneumoniae strains, with one study reporting its presence in 90% of hypervirulent K. pneumoniae strains associated with pyogenic liver abscess. Aerobactin is found in only about 6% of classical strains, yet is present in 93 to 100% of hypervirulent K. pneumoniae isolates. The presence of aerobactin is always associated with a hypercapsule, although not all hypercapsulated strains possess this siderophore [24].
Clinical manifestation and diagnosis
The most common clinical manifestations in patients with K pneumoniae liver abscesses are fever, chills, and abdominal pain [19,20,33,34]. Leukocytosis, thrombocytopenia, increased concentrations of C-reactive protein and glucose in blood, and abnormal results of liver function tests were common. The mean value of C-reactive protein level that tends to be elevated in most patients is ≥20 [34].
Diagnosis of pyogenic liver abscess requires abdominal imaging study, such as ultrasonography or computed tomography (CT). Since it has been reported that imaging by ultrasonography offers poorer sensitivity than abdominal CT (85.8% vs. 100%), abdominal CT is recommended for diagnosis, whenever possible [35]. In the CT findings, K. pneumoniae liver abscesses were more likely than non-K. pneumoniae liver abscesses to show a single abscess, unilobar involvement, solid rather than cystic appearance, multilocular rather than unilocular septation, and greater association with thrombophlebitis of portal or hepatic vein [36]. Pigtail drainage of abscess during diagnosis is often difficult due to the solid appearance and multilocular characteristics of the abscesses. Thrombophlebitis of portal or hepatic vein was associated with hematogenous septic complications [36,37].
Mortality rate was about 5%, which did not differ much from that of non-K. pneumoniae liver abscesses [5]. Meanwhile, metastatic infections, such as endophthalmitis or meningitis, was reported in approximately 10-16% of cases, with the lungs, eyes, and central nervous system being the common sites of such infections [5]. Presence of metastatic infection is associated with higher frequency of intensive care unit (ICU) admission and in-hospital mortality [13]. The frequency of meningitis was higher in Taiwan than in Korea [5]. Endophthalmitis tends to be more prevalent during winter, and if the initial vision is counting fingers level and below, it may be associated with poor visual outcome [22]. It has been reported that Gram negative bacilli account for most cases of endogenous endophthalmitis in Asia, while K. pneumoniae has been reported to account for up to 60% [38,39,40]. Diabetes is a significant risk factor for the development of endogenous endophthalmitis and poor visual outcome in patients with K. pneumoniae liver abscess [22].
Management
Clinical isolates are characteristically highly drug sensitive and cephalosporin are the antibiotic mainstay of treatment for K. pneumoniae abscess [5]. A third-generation cephalosporin is preferable to a first-generation cephalosporin [41]. Many favor use of first-generation cephalosporins given their relative low cost and apparent efficacy with respect to rates of mortality, metastatic infection and complications [34]. However, others have reported higher metastatic infection rates among patients treated with cefazolin compared with those treated with a second- or third-generation cephalosporin [41]. Because ESBL-producing K. pneumoniae has been detected very rarely in patient with liver abscesses, antibiotics such as ampicillin-sulbactam, aztreonam, and a quinolone can also be used. Although aminoglycoside penetrate abscess cavities poorly, clinicians often add an aminoglycoside. In theory they may eradicate bloodstream organisms early in their course of infection, potentially decreasing risk for metastatic complications. However, this benefit is unproven and may be outweighed by the toxicity of aminoglycosides. Parenteral antibiotics are usually treated for 2 to 3 weeks, and a 4- to 6-week total course is completed with oral agents. Longer courses of treatment may be warranted for patients requiring subsequent drainage procedures or with persistent radiographic evidence of abscess. Abscess cavities usually resolve completely after therapy, but occasionally they persist despite prolonged courses of antibiotics. In such cases, patients should be observed closely. Recurrent symptoms such as fever or abdominal pain should prompt repeat imaging and possible reaspiration.
Adequate drainage of abscess is recommended for better clinical response. As mentioned above, K. pneumoniae abscess may not be amenable to immediate drainage, due to frequent multilocular and solid appearances. Two options are to postpone drainage and monitor closely until the abscess has matured and can then be drained or to insert the drain and retain the tubing so that the abscess can drain once it becomes liquefied. In a report, aggressive hepatic resection resulted in a better outcome than did conventional percutaneous drainage for those with more severe diseases [42].
Acute bacterial endophthalmitis is a medical emergency because delay in giving appropriate therapy may lead to irreversible loss of vision. Most patients with endophthalmitis present with acute decrease in vision and eye pain as the chief complaints. However, since patients whose conditions are severe enough to warrant ICU admission may not be able to express such symptoms, such patients should be checked for red eye, conjunctivitis, and hypopyon, followed by ophthalmic consultation. Automatic ophthalmic consultation may be considered for cases involving K. pneumoniae liver abscess. The prognosis for patients with endophthalmitis caused by K. pneumoniae is very poor; more than 85% of patients had a severe visual deficit. Prognosis for visual recovery is improved if a diagnosis is made early and the patient is given early antibiotic treatment [43,44]. In addition to systemic antibiotics, intravitreal antibiotics and vitrectomy are usually required [33,43,44].
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–1559. [PubMed] [Google Scholar]
- 2.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. [PubMed] [Google Scholar]
- 3.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Wann SR, Lin HH. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 4.Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ, Eom JS, Kim JS, Choi YH, Lee JS, Chung MH, Kim YS, Lee H, Lee MS, Park CK Korean Study Group for Liver Abscess. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007;54:578–583. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 6.Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol. 2007;56:593–597. doi: 10.1099/jmm.0.46964-0. [DOI] [PubMed] [Google Scholar]
- 7.Sifri CD, Madoff LC. Infections of the Liver and Biliary System (Liver Abscess, Cholangitis, Cholecystitis) In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier Saunders; 2015. p. 960. [Google Scholar]
- 8.Mavilia MG, Molina M, Wu GY. The evolving nature of hepatic abscess: a review. J Clin Transl Hepatol. 2016;4:158–168. doi: 10.14218/JCTH.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen PS, Schønheyder HC. Pyogenic hepatic abscess. A 10-year population-based retrospective study. APMIS. 1998;106:396–402. doi: 10.1111/j.1699-0463.1998.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 10.Pang TC, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: an audit of 10 years' experience. World J Gastroenterol. 2011;17:1622–1630. doi: 10.3748/wjg.v17.i12.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AbdelRafee A, El-Shobari M, Askar W, Sultan AM, El Nakeeb A. Long-term follow-up of 120 patients after hepaticojejunostomy for treatment of post-cholecystectomy bile duct injuries: a retrospective cohort study. Int J Surg. 2015;18:205–210. doi: 10.1016/j.ijsu.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Peng YC, Lin CL, Sung FC. Risk of pyogenic liver abscess and endoscopic sphincterotomy: a population-based cohort study. BMJ Open. 2018;8:e018818. doi: 10.1136/bmjopen-2017-018818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian Y, Wong CC, Lai S, Chen H, He X, Sun L, Wu J, Zhou J, Yu J, Liu W, Zhou D, Si J. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. 2016;6:38587. doi: 10.1038/srep38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun. 2009;77:2657–2671. doi: 10.1128/IAI.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YT, Siu LK, Lin JC, Chen TL, Tseng CP, Yeh KM, Chang FY, Fung CP. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung DR, Lee H, Park MH, Jung SI, Chang HH, Kim YS, Son JS, Moon C, Kwon KT, Ryu SY, Shin SY, Ko KS, Kang CI, Peck KR, Song JH. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31:481–486. doi: 10.1007/s10096-011-1334-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith GW, Blackwell CC, Nuki G. Faecal flora in spondyloarthropathy. Br J Rheumatol. 1997;36:850–854. doi: 10.1093/rheumatology/36.8.850. [DOI] [PubMed] [Google Scholar]
- 18.Thom BT. Klebsiella in faeces. Lancet. 1970;2:1033. doi: 10.1016/s0140-6736(70)92845-x. [DOI] [PubMed] [Google Scholar]
- 19.Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37:176–184. [PubMed] [Google Scholar]
- 20.Chan KS, Yu WL, Tsai CL, Cheng KC, Hou CC, Lee MC, Tan CK. Pyogenic liver abscess caused by Klebsiella pneumoniae: analysis of the clinical characteristics and outcomes of 84 patients. Chin Med J (Engl) 2007;120:136–139. [PubMed] [Google Scholar]
- 21.Lin JC, Siu LK, Fung CP, Tsou HH, Wang JJ, Chen CT, Wang SC, Chang FY. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91:3084–3087. doi: 10.1210/jc.2005-2749. [DOI] [PubMed] [Google Scholar]
- 22.Sheu SJ, Kung YH, Wu TT, Chang FP, Horng YH. Risk factors for endogenous endophthalmitis secondary to Klebsiella pneumoniae liver abscess: 20-year experience in Southern Taiwan. Retina. 2011;31:2026–2031. doi: 10.1097/IAE.0b013e31820d3f9e. [DOI] [PubMed] [Google Scholar]
- 23.Lin YT, Liu CJ, Yeh YC, Chen TJ, Fung CP. Ampicillin and amoxicillin use and the risk of Klebsiella pneumoniae liver abscess in Taiwan. J Infect Dis. 2013;208:211–217. doi: 10.1093/infdis/jit157. [DOI] [PubMed] [Google Scholar]
- 24.Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the dffense with a strong defense. Microbiol Mol Biol Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su SC, Siu LK, Ma L, Yeh KM, Fung CP, Lin JC, Chang FY. Community-acquired liver abscess caused by serotype K1 Klebsiella pneumoniae with CTX-M-15-type extended-spectrum beta-lactamase. Antimicrob Agents Chemother. 2008;52:804–805. doi: 10.1128/AAC.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, Tsai FC, Keynan Y, Wang JT. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol. 2008;46:2231–2240. doi: 10.1128/JCM.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decré D. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51:4073–4078. doi: 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, Andersen PS, Driebe EM, Keim P, Krogfelt KA. Mapping the evolution of hypervirulent Klebsiella pneumoniae . MBio. 2015;6:e00630. doi: 10.1128/mBio.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, Wang JJ, Huang LY, Siu LK. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6:1191–1198. doi: 10.1016/j.micinf.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ International Klebseilla Study Group. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CR, Lin TL, Chen YC, Chou HC, Wang JT. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology. 2011;157:3446–3457. doi: 10.1099/mic.0.050336-0. [DOI] [PubMed] [Google Scholar]
- 32.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SC, Wu WY, Yeh CH, Lai KC, Cheng KS, Jeng LB, Wang PH, Lin DB, Chen CC, Lee MC, Bell WR. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am J Med Sci. 2007;334:97–105. doi: 10.1097/MAJ.0b013e31812f59c7. [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Chen YS, Tsai HC, Wann SR, Lin HH, Huang CK, Liu YC. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47:642–650. doi: 10.1086/590932. [DOI] [PubMed] [Google Scholar]
- 35.Lin AC, Yeh DY, Hsu YH, Wu CC, Chang H, Jang TN, Huang CH. Diagnosis of pyogenic liver abscess by abdominal ultrasonography in the emergency department. Emerg Med J. 2009;26:273–275. doi: 10.1136/emj.2007.049254. [DOI] [PubMed] [Google Scholar]
- 36.Alsaif HS, Venkatesh SK, Chan DS, Archuleta S. CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae . Radiology. 2011;260:129–138. doi: 10.1148/radiol.11101876. [DOI] [PubMed] [Google Scholar]
- 37.Maffiolo C, Novellas S, Chevallier P, Brunner P, Mourou MY, Bruneton JN. Thrombophlebitis of the hepatic veins: complication of a Klebsiella liver abscess. Clin Imaging. 2006;30:63–65. doi: 10.1016/j.clinimag.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 2003;82:97–105. doi: 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 40.Jung H, Kim SW, Chang HH, Lee SA, Kim Y, Hwang S, Kim SJ, Lee JM. Analysis of Klebsiella as a prognostic factor of ocular outcomes in endogenous endophthalmitis with decision tree analysis. Infect Chemother. 2018;50:238–251. doi: 10.3947/ic.2018.50.3.238. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng HP, Siu LK, Chang FY. Extended-spectrum cephalosporin compared to cefazolin for treatment of Klebsiella pneumoniae-caused liver abscess. Antimicrob Agents Chemother. 2003;47:2088–2092. doi: 10.1128/AAC.47.7.2088-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh HF, Chen TW, Yu CY, Wang NC, Chu HC, Shih ML, Yu JC, Hsieh CB. Aggressive hepatic resection for patients with pyogenic liver abscess and APACHE II score > or =15. Am J Surg. 2008;196:346–350. doi: 10.1016/j.amjsurg.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Chee SP, Ang CL. Endogenous Klebsiella endophthalmitis--a case series. Ann Acad Med Singapore. 1995;24:473–478. [PubMed] [Google Scholar]
- 44.Chou FF, Kou HK. Endogenous endophthalmitis associated with pyogenic hepatic abscess. J Am Coll Surg. 1996;182:33–36. [PubMed] [Google Scholar]