Abstract
Background: Cochleotoxicity following the treatment with platinum-based chemotherapy is well documented. The potential for vestibulotoxicity is still unclear. This scoping review examined the extent of current research literature, summarized research findings and identified research gaps regarding vestibular-related adverse effects associated with platinum-based chemotherapy in survivors of cancer.
Methods: Inclusion criteria followed the PICO principles: Participants, adult, and pediatric cancer patients of any cancer type; Intervention, platinum-based chemotherapy (such as cisplatin, carboplatin, and oxaliplatin); Control, none or any; Outcomes, vestibular-related adverse effects. English language articles published since 1978 were retrieved. Seventy-five eligible studies were identified from a systematic literature search, and relevant data were charted, collated, and summarized.
Results: Testing for vestibulotoxicity predominately featured functional evaluation of the horizontal semicircular canal using the caloric and rotational tests. The rate of abnormal vestibular function test results after chemotherapy administration varied from 0 to 50%. The results of objective testing did not always correspond to patient symptoms. There is tentative support for patients with pre-existing loss of vestibular function to be more likely to experience vestibular toxicity after dosing with cisplatin.
Conclusions: A number of studies reported significant evidence of vestibular toxicities associated with platinum-based chemotherapy, especially cisplatin. This scoping review emphasizes that vestibular toxicity needs more attention and comprehensive evaluation. Specifically, studies that analyse cumulative dose of platinum-based chemotherapy, affected sites of lesion in vestibular end organs, and the correlation and temporal patterns of cochlear and vestibular toxicity are needed.
Keywords: vestibulotoxicity, vestibular, adverse effect, platinum-based chemotherapy, cancer
Introduction
More than 15.5 million cancer survivors are living in the United States alone, and this number is set to increase to 20 million by 2026 because of advances in early detection, effective treatment, and the aging and growth of the population (1). As a consequence of these increased survival rates, more attention is being given to improvements in long-term effects, health-related quality of life, and follow up care after cancer treatment (2).
Platinum-based chemotherapy is an effective antineoplastic intervention that is used for a variety of human malignancies including testicular, ovarian, bladder, head and neck, and non-small cell lung cancer (3). Irreversible hearing loss following the treatment with platinum-based drugs, especially cisplatin (which causes permanent hearing loss of variable degree in 40–80% of treated patients) is well documented (4). Long-term monitoring of cochleotoxic effects of platinum-based chemotherapy is therefore advised (5) and implemented in clinical practice (6). There is also some evidence that cisplatin has long-term retention in the cochlea (7). Given that the auditory and vestibular organs of the inner ear share the same blood, nerve and fluid supplies, this finding has potential implications for functions of both compartments (8). However, there are some differences in physiology and function between the cochlear and vestibular end organs, and these may mediate the impact of toxicity associated with platinum compounds. Specific areas of interest include the role of the stria vascularis in the cochlea (9), as no analogous structure is present in the vestibular labyrinth, and the possible role of transporters such as megalin (10). There is a strong potential for cochlear toxicity to be accompanied by vestibular toxicity in patients receiving platinum-based chemotherapy. However, clinical reports of inner ear toxicity are limited largely to auditory symptoms.
The vestibular organs of the inner ear play an important role in the complex and dynamic human balance system (11). The peripheral vestibular system in the inner ear consists of five sensory organs: three semicircular canals (horizontal, anterior, and posterior) and two otolith organs (saccule and utricle) on each side (11). The integration occurs at the central nervous system giving efferent fibers to vestibulo-ocular, and vestibulo-spinal pathways (11). No clinical test can directly measure the inner ear function of the vestibular periphery. Instead, inferences must be made about vestibular function on the basis of the performance of downstream processes especially the vestibulo-ocular reflex (VOR). In the wider context, the balance system covers the spatial orientation of the whole body, which is maintained by the integration of visual, somatosensory, and vestibular inputs to the central nervous system (11). While the vestibular function refers to the health of the vestibular portion of the inner ear, balance function is not restricted to a vestibular component alone.
The potential for vestibular toxicity is still unclear, though clinical data suggests it is problematic (12, 13). There are a number of potential explanations for why vestibulotoxicity from platinum-based chemotherapy is less frequently described than auditory symptoms. First, some of the clinical signs and patient-reported symptoms may be underappreciated by clinicians. Notably, drug-induced vestibular loss may affect both ears symmetrically and gradually resulting in insidious disequilibrium, postural imbalance, and oscillopsia (illusion of movement of the visible world during head movement). These symptoms are less likely to undergo clinical assessment, compared with the sudden onset of spinning vertigo such as benign paroxysmal positional vertigo (BPPV), vestibular neuritis and Meniere's disease (14, 15). Secondly, vestibular organ dysfunction may be masked by central compensation or substitution by vision and proprioception, obscuring vestibular damage compared with the more noticeable cochlear damage (16). Thirdly, platinum-based chemotherapy agents are used in combination with several other drugs, and some of these may have ototoxic properties such as aminoglycoside antibiotics (17) and loop diuretics (18). Thus, the specific attribution of vestibulotoxicity to platinum-based compounds may be obscured. Finally, non-specific symptoms of imbalance may be attributed to underlying cancer diseases and general deconditioning of patients during and after treatment such as dehydration, nausea and vomiting, chronic fatigue, and anemia.
Vestibular dysfunction can have a considerable impact on quality of life (19) and substantial economic burden on individuals and society (20). Recent evidence suggests that balance problems such as falls and mobility disability in survivors of cancer are more common than amongst the general population (21–23). This is of great importance as falling is a leading cause of morbidity and mortality in the community population (24). Therefore, there is a need to increase awareness of balance problems in this vulnerable group of cancer patients in order to provide accurate prevention and intervention measures (25).
While systematic review methodology seeks to collate all evidence in order to address a specific research question, scoping review methodology aims to map the key concepts underpinning a research area and the main sources and types of evidence available (26). A scoping review can be undertaken as a stand-alone project in its own right, especially where an area has not previously been reviewed in a comprehensive manner (26). This scoping review examined the extent of current research literature on vestibular-related adverse effects associated with platinum-based chemotherapy in survivors of cancer to summarize research findings and identify research gaps.
Method
The method of this scoping review is largely based on the steps of the framework proposed by Arksey and O'Malley (26): (1) identify relevant studies, (2) select studies, (3) chart the data, (4) collate, summarize and report the results, and (5) consult clinical experts. Study details were registered on PROSPERO (CRD42017083576).
Identify relevant studies
The following databases were searched: Medline, EMBASE, TOXLINE, IPA (International Pharmaceutical Abstracts), Science Citation Index-Expanded, ProQuest Dissertations & Theses A&I, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), and International Clinical Trials Registry Platform (ICTRP). Gray literature was also considered through the Database of Adverse Event Notifications [Australia], Drug Safety Update [UK], European Public Assessment Reports via European Medicines Agency [Europe], and Medwatch [USA]. The steps followed the Cochrane Handbook (27) and the Cochrane Methodological Expectations of Cochrane Intervention Reviews (MECIR) (28) for conducting the search, the PRISMA guideline (29) for reporting the search, and the PRESS guideline for peer-reviewing the search strategies (30). The search strategy followed recommendations for optimizing the search syntax to identify adverse effects (31–34). Standardized terms and keywords were combined in the search for concepts of platinum-based chemotherapy and vestibular toxicity. Keywords were collected through expert opinions, literature reviews, controlled vocabulary (Medical Subject Headings = MeSH and Excerpta Medica Tree = EMTREE), and reviewing preliminary search results. Filters for electronic databases were applied where possible to retrieve articles in the English language, with human participants, and time of publication since 1978 which is the time that cisplatin, the first drug in the platinum-based chemotherapy group, was approved by Food and Drug Administration (FDA) for human use (3). We limited our search to the English language because of resource constraints. The search strategies are reported in Appendix 1.
Study selection
Two screening steps were undertaken independently by two authors. The first step checked that each title and abstract was within the scope of research question. Examples of “out of scope” decisions were where there was no mention of platinum-based chemotherapy, in vitro or in vivo studies, or review articles or conference papers. The second step of full text screening considered eligible records that contained data pertinent to the subject of the review, specifically vestibular-related symptoms and/or test results in adults and/or pediatric cancer patients undergoing platinum-based chemotherapy (such as cisplatin, carboplatin, and oxaliplatin) in any cancer types (either alone or in combination with other treatments such as radiotherapy or surgery). Eligible studies were randomized controlled trials (RCTs), non-randomized controlled trials, observational studies, cross-sectional studies, cohort studies, case control studies, case series, and case reports. Discrepancies were resolved through discussion and a third reviewer was consulted whenever a consensus was not reached. Literature saturation was further accomplished by additional hand searching of the reference lists of all included studies and also those of the excluded review articles.
Charting the data
Pre-specified data items included year, study design, participant demographics and characteristics, sample size, cancer treatment intervention, drug dose, and patient evaluation. These data items provided useful information about the scope and details of each record, enabling the authors to look for common themes and to identify possible gaps in the literature. Multiple reports pertaining to a single study were treated as one, but data extraction considered information presented in all records. Data from each included study was independently extracted by two clinical experts on the team (PP, an otorhinolaryngologist and DB, an audiologist). Discrepancies were identified and resolved through discussion.
Collating, summarizing and reporting the results
For the purpose of understanding key concepts in this literature, thematic analysis was conducted following article review to summarize the literature into themes by the two members of the research team (PP and DB). The authors independently reviewed the charted final data set and identified themes, and then met to discuss possible thematic structures, using the criteria that themes should be important clinical aspects and should adequately represent all of the records. Specific themes identified were objective tests of vestibular and/or balance function, patients' symptoms, physical examination, associated factors, and general considerations. The authors then grouped all studies according to these themes. The content of individual records does not necessarily fall exclusively in one theme or another; hence, the records could contribute to more than one theme. Research findings were summarized and research gaps were identified.
Clinical expert consultation
Two clinical experts on the team (AK, an otorhinolaryngologist and PMP, an oncologist) reviewed the themes and supporting evidence of the results.
Results
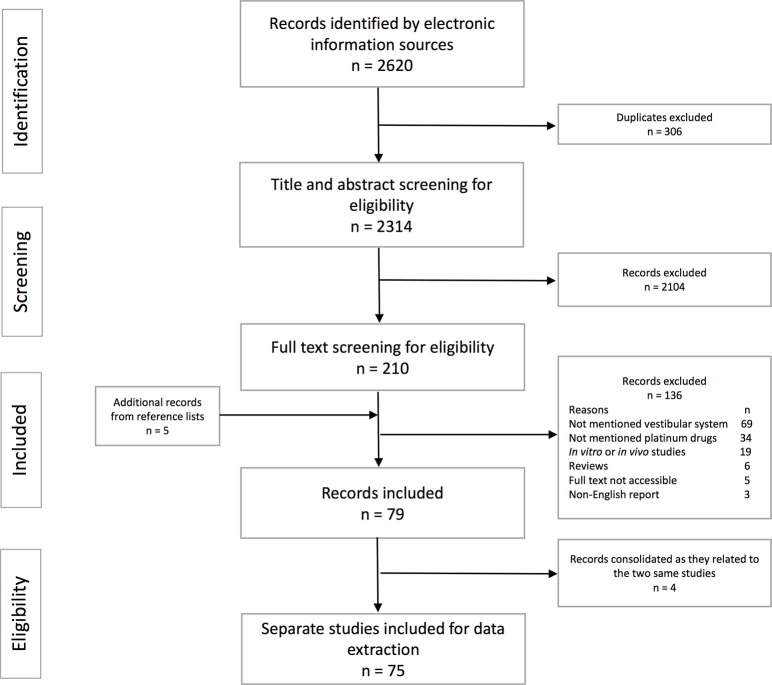
A summary of the study selection processes with the reasons for exclusion is represented in Figure 1. A total of 2,620 records were retrieved from the electronic databases and five additional articles were found by manually searching reference lists of those records included in the full text screening. Most records were excluded because they did not mention vestibular side effects, did not mention platinum-based chemotherapy, or were in vitro or in vivo studies. Overall, 75 individual studies were included for data extraction. The sample size of the study participants varied from 1 to 952. The age reported ranged from 11 to 83 years. Study patients had a variety of cancer sites including head and neck cancer, testicular cancer, gynecologic cancer, pulmonary cancer, breast cancer, brain cancer, and other sites. Patients also received concurrent cranial irradiation which may involve areas of inner ear organs in brain, and head and neck cancers (12, 35–38). Platinum-based medication reported in the included studies were cisplatin, carboplatin, oxaliplatin, and non-specified platinum compounds.
Figure 1.
Flow chart of stages of the study selection process.
Thematic analysis
From the data extraction and thematic analysis, five themes were defined to determine areas of interest for an overview of platinum-based vestibulotoxicity: (1) objective tests of vestibular and/or balance function, (2) patients' symptoms, (3) physical examination, (4) associated factors such as dosage, pre-existing vestibular loss, and accompanying cochleotoxicity, and (5) general considerations. Results are presented according to these themes.
Objective tests of vestibular and/or balance function (N = 10)
Ten individual studies reported outcomes using one or more objective vestibular or balance function tests (Table 1). Of these, there were multiple publications based on the same study; one reporting a prospective before and after study, plus three corresponding case reports (12, 36–38), and another reporting the same prospective before and after study in two publications (42, 43). Other studies were four prospective before and after studies (13, 39–41), and four cross-sectional studies (35, 44–46). Almost all of these reports focused on toxicities from cisplatin. From these 10 studies, several objective techniques to evaluate vestibular or balance function were reported.
Table 1.
Summary data of studies that reported objective test results.
| Study no. | First author | Sample size | Population | Platinum-based drug and dosage | Vestibular function test | Balance function test | Patients' symptoms | Physical examination | Concurrent symptoms |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Schaefer et al. (12, 36) |
24 | Adult, head and neck cancer | Cisplatin 100–800 mg/m2 | 4.2% had bilateral decreased caloric response before chemotherapy then absent response after the treatment. | No data | 3% had transient dizziness. One patient reported that the symptoms became more problematic in the dark. |
No data | 3 patients with vestibular symptoms had concurrent sensorineural hearing loss. |
| 2 | Black et al. (39) | 16 | Adult, head and neck cancer | Cisplatin 100–800 mg | 31.2% had abnormal rotational test results after completing chemotherapy treatment. 18.8% had new onset or worsening of rotational test results. 12.5% had transient reduction of vestibular function detected by rotational tests. |
18.8% had abnormal postural test after completing chemotherapy treatment. | No data | No data | No data |
| 3 | Hartwig et al. (40) | 74 | Adult, testicular and gynecologic cancer | Cisplatin 130–590 mg/m2 | 4.1% had transient abnormal caloric and rotational test | No data | 0% | Bedside postural tests, Romberg's test, gait tests, stepping test, and optokinetic test remained normal in all patients | 4.1% had hearing loss (Different patients to those with transient vestibular loss). |
| 4 | Kobayashi et al. (41) | 10 | Adult and padiatric, uterus, larynx, orbit, bladder, and bone cancer | Cisplatin 80–550 mg | 50% had abnormal caloric test results.20% had abnormal rotational test results. | 54.5% had abnormal body sway test. | 30% complained of unsteadiness. | 70% had spontaneous nystagmus. 60% had positional nystagmus. All patients had normal optokinetic test results. |
40% had hearing loss. 50% had tinnitus. |
| 5 | Kitsigianis et al. (42, 43) | 9 | Adult, testicular and pulmonary cancer | Cisplatin 360–800 mg/m2 | Vestibular autorotation test (VAT) showed decreased VOR gain and increased phase lag. | No data | 0% | No data | No data |
| 6 | Myers et al. (13) | 34 | Adult, head and neck cancer | Cisplatin 100–600 mg/m2 | 2.9% had abnormal caloric test results after the treatment. 20.6% had abnormal results rotational test after the treatment. 8.8% had transient reduction in caloric test results. 36.5% had transient VOR gain reduction in rotational test. |
No data | 0% complained of vertigo or imbalance | No data | 58.3% had hearing loss 66.7% had tinnitus |
| 7 | Waissbluth et al. (35) | 12 | Pediatric, brain and liver cancer | Cisplatin 100–800 mg/m2Carboplatin 1,000–2,800 mg/m2 | 25% had decreased VOR gain and one of these had overt saccade detected by video head impulse test (vHIT) | No data | 41.7% reported recurrent vertigo 16.7% referred disequilibrium |
25% had inability to walk in tandem gait 16.6% had gaze-evoked nystagmus |
No data |
| 8 | Camet et al. (44) | 50 | Pediatric, brain and other cancer | Cisplatin and carboplatin | No data | 6% had abnormal score of Modified Clinical Test of Sensory Interaction on Balance (CTSIB-M) | 42% had abnormal score of Pediatric Vestibular Symptom Questionnaire (PVSQ) | 28% had abnormal dynamic visual acuity test | No data |
| 9 | Miaskowski et al. (45) | 623 | Adult, breast, colon, lung, ovarian, and other cancer | Platinum compound | No data | Patients with chemotherapy-induced neuropathy had significant worse score on Time Up and Go (TUG) test and the Fullerton Advanced Balance (FAB) test | No data | No data | Patients with neurotoxicites are defined as those who had all toxicities of hearing loss, tinnitus, and neuropathy. |
| 10 | Miaskowski et al. (46) | 195 | Adult, breast, colon, lung, ovarian, and other cancer | Platinum compound | No data | Patients with neurotoxicities side effects had significant worse score on Time Up and Go (TUG) test and the Fullerton Advanced Balance (FAB) test | 64% of patients who had chemotherapy-induced neurotoxicities and 14% of patients without those toxicities reported trouble with balance. | No data | Patients with neurotoxicites are defined as those who had all toxicities of hearing loss, tinnitus, and neuropathy. |
Objective tests for vestibular function included the caloric test (12, 13, 36, 40, 41), rotational tests (13, 39–41), vestibular autorotation test (VAT) (42, 43), and horizontal video head impulse test (vHIT) (35). Details are given in Table 2. All of these tests detect the horizontal VOR abnormalities. Most studies reported a single vestibular function test such as the caloric test (12, 36), VAT (42, 43), and vHIT (35). Three studies evaluated both caloric and rotational tests (13, 40, 41). The rate of abnormal findings detected by the caloric test after chemotherapy administration varied between 0% (40), 2.9% (13), 4.2% (12, 36), and 50.0% (41). The rate of abnormal findings detected by the rotational test after chemotherapy administration ranged between 0% (40), 20.0% (41), 20.6% (13), and 31.2% (39). Abnormal vestibular function, as detected by the vHIT, was reported in 25.0% of survivors (35). Transient reduction of vestibular function on testing was reported in three studies (13, 39, 40). Bilateral vestibular hypofunction was reported in studies using the caloric test (12, 36) and vHIT (35), whilst some studies did not report which side had the abnormal caloric responses (13, 41). The rotational test and VAT were useful in detecting bilateral vestibular impairment, but the relative contribution could not be discriminated from the results (13, 39, 40, 42, 43).
Table 2.
Examples of objective tests of vestibular function.
| Test | Pathway of testing | Procedure | Advantages | Limitations |
|---|---|---|---|---|
| Caloric test | VOR via horizontal SCC at low frequency stimulus (0.002–0.004 Hertz) | Cold and warm water or air are irrigated into the external auditory canal. Nystagmus is detected and the two sides are compared. |
|
|
| Rotational chair test | VOR via horizontal SCC at low-mid frequency stimulus (0.01–0.7 Hertz) | Patient sits in a computerized chair and wears video goggles. Eye movements are recorded during rotation of the chair. |
|
|
| Active head rotation test (Autorotation test) | VOR via horizontal SCC at mid frequency stimulus (0.5–6 Hertz) | Eye movements are recorded during active head movement in synchrony with audio clicks of different frequencies. |
|
|
| Video head impulse test (vHIT) | VOR via all six SCC at high frequency stimulus (4–7 Hertz) | Patient sits on a chair and is instructed to stare at a target on the wall. Equipment to record head movements and video goggles are placed. The quick and unpredictable head turn is carried out by the tester. |
|
|
| Vestibular Evoked Myogenic Potentials (VEMPs) 1. oVEMP (Ocular) 2. cVEMP (Cervical) |
Otolith organs
|
Loud sound is presented and muscle activation, either at neck or ocular muscles, is detected through the surface electrodes. |
|
|
Source (47).
Objective tests for balance function included the postural test (39, 41), the Time Up and Go (TUG) test (45, 46), the Fullerton Advanced Balance (FAB) scale (45, 46), and the Modified Clinical Test of Sensory Interaction on Balance (CTSIB-M) (44). Details are given in Table 3. Two of these studies also assessed vestibular dysfunction (39, 41). The abnormal rate of the postural test after completion of chemotherapy treatment was 18.8% (39), and 54.5% (41). Cancer survivors with chemotherapy-induced neuropathy (CIN) had significantly worse scores on both the Time Up and Go (TUG) test and the Fullerton Advanced Balance (FAB) scale (45, 46). Three out of fifty patients (6%) who underwent cancer treatment during their childhood had an abnormal score of CTSIB-M when long-term effects were tested more than 10 years afterwards (44).
Table 3.
Examples of objective tests of balance function.
| Test | Pathway of testing | Procedure | Advantages | Limitations |
|---|---|---|---|---|
| Postural test (Posturography) |
|
Patient is instructed to stand in various conditions such as on a fixed or moving force plate with eyes open or closed. |
|
|
| Modified Clinical Test of Sensory Interaction on Balance (CTSIB-M) |
|
Patient is instructed to stand without shoes with feet together and arm crossed for up to 30 seconds in various conditions: solid surface or on foam with eyes open or closed. Time to complete the task is recorded. |
|
|
| Time Up and Go (TUG) test | Functional body balance by testing performance-based activities | Patient is instructed to stand from an armed chair, walk 10 feet, turn, and return to a seated position. Time to complete the task is recorded. |
|
|
| Fullerton Advanced Balance (FAB) scale | Functional body balance by testing performance-based activities | Patient is instructed to do 10 tasks comprised of standing with feet together and eyes closed, reaching forward to retrieve an object held at shoulder height with an outstretched arm, turning 360 degrees to the right and left, stepping up onto and over a 6-inch bench, tandem walking, standing on one leg, standing on foam with eyes closed, two-footed jumping, walking with head turns, and performing reactive postural control. The ability to complete the tasks is scored. |
|
|
Source (47).
The testing interval varied amongst studies. Some studies reported testing before each subsequent dose (12, 36, 40, 42, 43), one study tested at variably different time points (39), and some studies did not specify the interval of testing (13, 41). Cross-sectional studies assessed long-term side effects with no availability of baseline comparison (35, 44–46).
Patients' symptoms (N = 74)
Seventy-four studies mentioned some forms of patient-reported symptoms relating to possible vestibular or balance problems. It is difficult to reliably distinguish vestibular from balance dysfunction based on the symptoms alone (8). As a result, symptoms are reported collectively. Terms used by study authors to describe vestibular side effects are often used interchangeably and nonspecifically, for instance, “dizziness” includes “disequilibrium,” “lightheadedness,” and “vertigo” according to the Common Terminology Criteria for Adverse Events (CTCAE) (48). None of the included reports explicitly clarified the definition of these terms which may lead to some degree of ambiguity.
Most studies did not report on the methods used for patient symptom evaluation such as spontaneous reports, patient checklists, interviews or reports by the clinicians. The only study that used a standardized patient-reported outcome questionnaire was the study by Camet (44), which reported 21 out of fifty pediatric cancer survivors (42%) with an abnormal score on the Pediatric Vestibular Symptom Questionnaire (PVSQ).
Regarding the results of those studies with objective vestibular function tests, the rate of reported vestibular symptoms varied from 0% (13, 40, 42, 43) to 41.7% (35). Whilst some vestibular impairment was detected by an objective test, patients did not report any subjective sensation of vestibular disorder (13, 40, 42, 43). Vestibular symptoms reported were transient dizziness (12, 36), unsteadiness (41), and vertigo (35). One patient had compensated well for his vestibular loss, except in the dark (36). Patients' symptoms were not reported in one study despite a high rate of abnormal vestibular test results (39).
Many examples were found of studies where patients complained of symptoms that were potentially due to vestibular side effects, but without verification by objective measurement. Studies in cancer treatment involving multiple medications including platinum-based drugs have reported non-specific balance symptoms such as dizziness, vertigo, ataxia, balance problems, and gait disturbance. A recent multi-center study of 952 testicular cancer survivors receiving cisplatin-based chemotherapy stated 9.3% overall new cases of dizziness, vertigo or balance problems (49).
Physical examination (N = 4)
Physical examinations were reported in four studies (35, 40, 41, 44). In the study by Kobayashi et al. (41), there were reports of spontaneous nystagmus (nystagmus in resting position without any stimulus) in seven out of 10 patients (70%) and positional nystagmus (nystagmus in a specific head position) in 6 out of 10 patients (60%). All three patients that complained of dizziness had spontaneous nystagmus and two of them had positional nystagmus. However, not all patients who had spontaneous nystagmus complained of dizziness. The optokinetic tests, Romberg's tests, gait tests and stepping tests remained normal in all 74 patients (40). The rate of abnormal results of dynamic visual acuity (DVA) as a vestibular screening test was 28% (44). Three out of twelve patients (25%) diagnosed with brain cancer had an inability to walk in tandem gait and two of them (16.6%) had gaze-evoked nystagmus (35).
Associated factors (N = 8)
Some associated factors of vestibulotoxicity mentioned in the literature were dosage, pre-existing vestibular dysfunction, and accompanying cochleotoxicity. Vestibular function was reported to have declined with increasing dose of cisplatin within each patient (39, 42, 43). However, the data reviewed did not allow the identification of specific cumulative dose thresholds associated with vestibular or balance impact. There is some evidence to suggest that subjects with pre-existing loss of vestibular function are more likely to get cisplatin vestibular toxicity. Two of five patients (40%) with prior abnormal vestibular function had additional vestibular loss after the cessation of the treatment, whilst one out of nine patients (11.1%) with prior normal vestibular function developed vestibular toxicity after chemotherapy (39). The only report of the histological verification showed severe loss of hair cells in peripheral vestibular sensory organs, including a crystalline spherical concretion composed of calcium carbonate in the left posterior semicircular duct, which is compatible with a positive finding of benign paroxysmal positional vertigo (BPPV) (37, 38). This could be either the underlying cause of dizziness or an incidental finding.
Vestibular toxicity defined by an abnormal vestibular function test was accompanied by cochlear toxicity (either hearing impairment or tinnitus) in three studies (12, 35, 36, 41), whilst no hearing loss was found in three patients with reduced caloric and rotatory response in one report (40), and no hearing status was stated in the other studies (13, 39, 42, 43). None of these studies stated the temporal correlation of vestibular and cochlear symptoms.
General considerations
By pooling together details of the data items, some of the general considerations about development of relevant literature, and treatment modalities for cancer emerge from the literature.
Development of relevant literature
Some preliminary studies were conducted in the 1980s followed by few studies after that. Whilst the number of publications has increased since the 2000s, the majority of these mentioned symptoms that were possibly due to vestibular side effects. A few studies explored vestibular-related long-term effects of chemotherapy recently indicating a growing awareness and interest from the research community of vestibular toxicity of platinum-based chemotherapy.
Treatment modalities for cancer
Treatment options are determined by cancer sites and staging. Multimodality treatment and combination of cytotoxic medications are common for cancer treatment. Patients also received concurrent cranial irradiation, which may involve areas of inner ear organs especially in brain, and head and neck cancers (12, 35–38). Vincristine, etoposide and bleomycin have been co-administered for cancer treatment (42, 43). One of three symptomatic patients also received an aminoglycoside antibiotic, amikacin (35), which is a known vestibulotoxic medication (17). Moreover, cisplatin, carboplatin, and oxaliplatin may have different degrees of vestibulotoxicity due to drug components, pharmacodynamics, and pharmacokinetics (50).
Discussion
This scoping review explored issues related to the vestibular effects of platinum-based chemotherapy in order to identify established knowledge and research gaps which warrant further research.
Conclusions based on established knowledge
Evaluation of vestibulotoxicity in the existing literature included objective tests, findings of physical examination, and patient reported symptoms. Tests for evaluation of vestibulotoxicity in previous publications have been largely focused on low to mid frequencies of horizontal semicircular canal testing, utilizing the caloric test, rotational tests, and the VAT. VHIT, a relatively new technique, was used to test function of the horizontal semicircular canal at higher frequency stimulation in one recent study (35). The existing information of vestibular toxicity in the literature examined only the horizontal semicircular canal which is one of five peripheral vestibular sensory organs. This may be due to the feasibility of testing all potentially affected organs, or the fact that sensory cells in the semicircular canals are more sensitive to toxic medication than those in the otolith organs (51).
Vestibular function loss may not be recognized until the patient loses other cues from vision and somatosensory such as when walking in the dark or develops concomitant peripheral neuropathy. Balance function tests such as posturography, testing overall balance system function in various conditions, can provide additional information on balance in real life and dynamic situations. Subclinical vestibular toxicity is a concern because its effect may only be evident in patients with other impaired sensory inputs, previous vestibular problems, or it may lead to earlier onset of age-related vestibular impairment (52).
The distinction between “objective tests of clinical signs” that can be measured by the clinicians and “subjective clinical symptoms” that can only be reported by the patients should be emphasized. The existing literature showed that objective test findings and patient symptoms did not always correspond to one another. Multiple studies reported abnormal vestibular function tests in asymptomatic patients (13, 40, 42, 43), therefore it is quite clear that clinicians cannot rely solely on symptoms to detect vestibular toxicity. It is not surprising that most patients did not have intense symptoms due to the potential bilateral symmetrical insidious nature of ototoxic medication. Symptoms such as vertigo, dizziness, headache, nausea, double vision, photophobia, ataxia, and light-headedness have previously been reported in patients with bilateral vestibular impairment (53, 54). Balance symptoms such as dizziness and unsteadiness are associated with vestibular dysfunction; however, the relationship between vestibulotoxicity and non-specific balance symptoms may not always be clear.
Dizziness and other balance problems can be difficult to describe for the patient and to categorize by the physician. Hence, clarification of the terminology used is important for the interpretation. These non-specific symptoms of balance problems might automatically be associated with cancer diseases and general deconditioning of patients. Comorbid factors such as peripheral neuropathy, fatigue, and anemia, including brain pathology in brain cancer patients can cause some elements of postural unsteadiness in cancer patients. Clinical symptoms are often underappreciated by both patients and clinicians. For example, vestibular toxicity may be a subtle complaint presenting as nausea, vomiting, or dizziness, which are not uncommon in cancer patients receiving chemotherapy.
Known risk factors of cochlear ototoxicity are aging, the cumulative dose of chemotherapy, poor renal function, and co-administration of other ototoxic medications (55). Possible potential risk factors of vestibular toxicity identified in the literature are cumulative dose and pre-existing vestibular loss. Decreased vestibular function occurred as cumulative dose of cisplatin increased, which supports the assumption of dose-related response (39, 42, 43). There is some evidence to tentatively support that patients with pre-existing loss of vestibular function are prone to cisplatin vestibular toxicity, for instance, the additional loss of vestibular impairment also occurred in patients with previous abnormal vestibular function (12, 36, 39).
Knowledge gaps
The rate of abnormal vestibular function test findings associated with platinum-based chemotherapy in the existing literature varied from 0 to 50% after chemotherapy treatment and 4.3–36.5% during chemotherapy pathway. These should be viewed as preliminary reports due to variable methodology, limited reported information and the relatively small number of patients. Therefore, the incidence and prevalence of vestibular abnormality after platinum-based chemotherapy warrant further research.
None of the studies evaluated vestibular function using vHIT to test all six semicircular canals, nor vestibular evoked myogenic potentials (VEMPs) to test otolith organs. A single test may not be a good indicator of the true vestibular function and findings from a comprehensive vestibular test battery have not been yet reported. Limitations of vestibular and balance function tests are summarized in Tables 2, 3. The current review gleaned sparse information and details on the procedure of the physical examination. Baseline testing is required to evaluate prior vestibular impairment and to detect subtle changes. Based on the existent information, data on affected sites of lesions in vestibular end organs, and the impact on higher frequency function associated with vestibulotoxicity in clinical studies is still lacking.
Most of the included reports did not mention the methods used for patient symptom evaluation. The approaches utilized in detecting or monitoring adverse effects, such as spontaneous reporting, patient checklist, questionnaire, diary, systematic survey of patients or report by the investigators, are known to have an influence on the frequencies of adverse effects identified (56). For example, passive monitoring based on spontaneous reports might yield lower rates of adverse events, while active surveillance using specific questioning could find a higher rate.
The association between cochlear impairment and vestibular toxicity is still unknown. Cochlear function deficit is more frequently described in the literature (4), potentially because it is more common, or it is recognized earlier and before vestibular impairment, symptoms of which tend to occur after complete bilateral loss or asymmetrical loss (14).
BPPV is common in the general population, including subjects receiving ototoxic drugs (57). BPPV presenting in subjects receiving ototoxic drugs may complicate the clinical identification of ototoxicity and obscure the clinical decision. Nevertheless, the possible role of ototoxicity in the pathophysiology of BPPV was not clear from the literature.
A combination of treatments is common in cancer treatment so evaluating the relationship between platinum-based chemotherapy and vestibulotoxicity may not be straightforward. Vestibulotoxic medications such as aminoglycoside antibiotics (17) are sometimes used to cure infections in cancer patients. Although most chemotherapeutic agents are not classified as ototoxic medication, vincristine (58) and bleomycin (59) have been shown to cause cochlear hair cell damage in in vivo studies. It has also been postulated that platinum-based chemotherapy and radiotherapy have a combination of effects on cochleotoxicity (60, 61). Currently, there is limited information not only on platinum-based chemotherapy but also other treatment modalities and their combinations which should be taken into account in data interpretation.
Limitations of the study
At present, there are methodological limitations in the published literature about vestibular effects of ototoxic medications. Some limitations of the current review are that a significant portion of included studies reported limited information, and only publications in the English language were included; thus, language bias may have occurred.
Conclusion
A number of studies reported significant evidence of cisplatin vestibular toxicities with objective tests, although not always corroborated by patient symptoms. Multiple studies also reported non-specific imbalance symptoms which are possible complaints of vestibular dysfunction; however, the nature of these symptoms is unclear without any objective test. There was very limited clinical research data to date on the vestibular side effects of platinum-based chemotherapy in terms of incidence, evaluation and impact on health-related quality of life of the patients. The data of physical examination was reported in only a few studies and was not comprehensive. The current evidence was based solely on the horizontal semicircular canal evaluation using the caloric tests or rotational tests; therefore, the information of vertical semicircular canals and otolith organs is still lacking.
In conclusion, this scoping review summarizes the current research findings that vestibular toxicity needs more attention and emphasizes the need for future high-quality studies in this field. Comprehensive evaluation of vestibular toxicities, identifying risk factors such as cumulative dose, pre-existing abnormal vestibular function and aging, including the correlation with cochlear toxicity and peripheral neuropathy warrant further research. Another challenge tends toward which test would be the most sensitive and appropriate for early detection and monitoring changes before, during and after platinum-based chemotherapy treatment.
Author contributions
PP conducted the review and wrote the manuscript. DB conducted the review and helped revise the manuscript. JT, SP, RG, PMP, DH, and AK conducted the study selection and helped revise the manuscript.
Data availability statement
All datasets for this study are included in the manuscript and the Supplementary Files.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Farhad Shokraneh, a medical information specialist at the University of Nottingham, for his assistance in developing the search strategy and conducting the electronic search.
Footnotes
Funding. This work was funded by the Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand and the National Institute for Health Research (NIHR). DH is an NIHR Senior Investigator.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00363/full#supplementary-material
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. (2016) 66:271–89. 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro CL, Jacobsen PB, Henderson T, Hurria A, Nekhlyudov L, Ng A, et al. ReCAP: ASCO core curriculum for cancer survivorship education. J Oncol Pract. (2016) 12:145, e08-17. 10.1200/JOP.2015.009449 [DOI] [PubMed] [Google Scholar]
- 3.Kelland L. resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer (2007) 7:573–84. 10.1038/nrc2167 [DOI] [PubMed] [Google Scholar]
- 4.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol. (2016) 34:2712–20. 10.1200/JCO.2016.66.8822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Association AS-L-H. Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA (1994) 36(Suppl. 12):11–9. [Google Scholar]
- 6.Brooks B, Knight K. Ototoxicity monitoring in children treated with platinum chemotherapy. Int J Audiol. (2017) 10.1080/14992027.2017.1355570. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. (2017) 8:1654. 10.1038/s41467-017-01837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flint PW, Haughey BH, Robbins KT, Thomas JR, Niparko JK, Lund VJ, et al. Cummings Otolaryngology - Head and Neck Surgery E-Book. Philadelphia, PA: Elsevier Health Sciences; (2014). [Google Scholar]
- 9.Gauvin DV, Yoder J, Zimmermann ZJ, Tapp R. Ototoxicity: the radical drum beat and rhythm of cochlear hair cell life and death. Int J Toxicol. (2018) 37:195–206. 10.1177/1091581818761128 [DOI] [PubMed] [Google Scholar]
- 10.Mukherjea D, Rybak LP. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics (2011) 12:1039–50. 10.2217/pgs.11.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronstein AM. Multisensory integration in balance control. Handbook Clin Neurol. (2016) 137:57–66. 10.1016/B978-0-444-63437-5.00004-2 [DOI] [PubMed] [Google Scholar]
- 12.Schaefer SD, Post JD, Close LG, Wright CG. Ototoxicity of low- and moderate-dose cisplatin. Cancer (1985) 56:1934–9. [DOI] [PubMed] [Google Scholar]
- 13.Myers SF, Blakley BW, Schwan S. Is cis-platinum vestibulotoxic? Otolaryngol Head Neck Surgery (1993) 108:322–8. [DOI] [PubMed] [Google Scholar]
- 14.Lucieer F, Vonk P, Guinand N, Stokroos R, Kingma H, van de Berg R. Bilateral vestibular hypofunction: insights in etiologies, clinical subtypes, and diagnostics. Front Neurol. (2016) 7:26. 10.3389/fneur.2016.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Berg R, van Tilburg M, Kingma H. Bilateral vestibular hypofunction: challenges in establishing the diagnosis in adults. ORL (2015) 77:197–218. 10.1159/000433549 [DOI] [PubMed] [Google Scholar]
- 16.Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist's best friend. J Neurol. (2016) 263(Suppl. 1):S54–64. 10.1007/s00415-015-7903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hecke R, Van Rompaey V, Wuyts FL, Leyssens L, Maes L. Systemic Aminoglycosides-induced vestibulotoxicity in humans. Ear Hear. (2017) 38:653–62. 10.1097/AUD.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 18.Ding D, Liu H, Qi W, Jiang H, Li Y, Wu X, et al. Ototoxic effects and mechanisms of loop diuretics. J Otol. (2016) 11:145–56. 10.1016/j.joto.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC. Bilateral vestibular deficiency: quality of life and economic implications. JAMA Otolaryngol Head Neck Surgery. (2014) 140:527–34. 10.1001/jamaoto.2014.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinand N, Boselie F, Guyot JP, Kingma H. Quality of life of patients with bilateral vestibulopathy. Ann Otol Rhinol Laryngol. (2012) 121:471–7. 10.1177/000348941212100708 [DOI] [PubMed] [Google Scholar]
- 21.Wildes TM, Dua P, Fowler SA, Miller JP, Carpenter CR, Avidan MS, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. (2015) 6:70–83. 10.1016/j.jgo.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprod L, Mohile SG, Fan L, Janelsins MC, Peppone LJ, Chandwani KD, et al. Physical activity participation and functional limitations in geriatric cancer survivors. J Clin Oncol. (2012) 30(15 suppl.):9009 10.1200/jco.2012.30.15_suppl.9009 [DOI] [Google Scholar]
- 23.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. (2011) 29:1458–64. 10.1200/JCO.2010.31.6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged >/ = 65 Years - United States, 2014. MMWR Morb Mort Wkly Rep. (2016) 65:993–8. 10.15585/mmwr.mm6537a2 [DOI] [PubMed] [Google Scholar]
- 25.Guerard EJ, Deal AM, Williams GR, Jolly TA, Nyrop KA, Muss HB. Falls in older adults with cancer: evaluation by oncology providers. J Oncol Pract. (2015) 11:470–4. 10.1200/JOP.2014.003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 27.Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. West Sussex: The Cochrane Collaboration; (2011). [Google Scholar]
- 28.Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. London: Cochrane; (2016). [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. (2016) 75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 31.Golder S, Loke YK. The contribution of different information sources for adverse effects data. Int J Technol Assess Health Care (2012) 28:133–7. 10.1017/S0266462312000128 [DOI] [PubMed] [Google Scholar]
- 32.Golder S, Loke YK, Bland M. Unpublished data can be of value in systematic reviews of adverse effects: methodological overview. J Clin Epidemiol. (2010) 63:1071–81. 10.1016/j.jclinepi.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Golder S, McIntosh HM, Duffy S, Glanville J. Developing efficient search strategies to identify reports of adverse effects in MEDLINE and EMBASE. Health Inform Libraries J. (2006) 23:3–12. 10.1111/j.1471-1842.2006.00634.x [DOI] [PubMed] [Google Scholar]
- 34.Loke YK, Golder SP, Vandenbroucke JP. Comprehensive evaluations of the adverse effects of drugs: importance of appropriate study selection and data sources. Therapeut Adv Drug Safety. (2011) 2:59–68. 10.1177/2042098611401129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waissbluth S, Chuang A, Del Valle A, Cordova M. Long term platinum-induced ototoxicity in pediatric patients. Int J Pediatric Otorhinol. (2018) 107:75–9. 10.1016/j.ijporl.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 36.Schaefer SD, Wright CG, Post JD, Frenkel EP. Cis-platinum vestibular toxicity. Cancer (1981) 47:857–9. [DOI] [PubMed] [Google Scholar]
- 37.Wright CG, Rouse RC, Zajic GH, Schaefer SD, Hubbard DG, Barnard LA. A calcareous concretion in the posterior semicircular duct of a human labyrinth. Am J Otolaryngol. (1982) 3:196–201. 10.1016/S0196-0709(82)80054-9 [DOI] [PubMed] [Google Scholar]
- 38.Wright CG, Schaefer SD. Inner ear histopathology in patients treated with cis-platinum. Laryngoscope (1982) 92:1408–13. [DOI] [PubMed] [Google Scholar]
- 39.Black FO, Myers EN, Schramm VL, Johnson J, Sigler B, Thearle PB, et al. Cisplatin vestibular ototoxicity: preliminary report. Laryngoscope (1982) 92:1363–8. 10.1288/00005537-198212000-00003 [DOI] [PubMed] [Google Scholar]
- 40.Hartwig S, Pettersson U, Stahle J. cis-Diamminedichloroplatinum: a cytostatic with an ototoxic effect. ORL (1983) 45:257–61. 10.1159/000275652 [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Ohashi N, Watanabe Y, Mizukoshi K. Clinical features of cisplatin vestibulotoxicity and hearing loss. ORL (1987) 49:67–72. 10.1159/000275909 [DOI] [PubMed] [Google Scholar]
- 42.Kitsigianis GA, O'Leary DP, Davis LL. Active head-movement analysis of cisplatin-induced vestibulotoxicity. Otolaryngol Head Neck Surg. (1988) 98:82–7. 10.1177/019459988809800114 [DOI] [PubMed] [Google Scholar]
- 43.Kitsigianis GA, O'Leary DP, Davis LL. Vestibular autorotation testing of cisplatin chemotherapy patients. Adv Oto-Rhino-Laryngol. (1988) 42:250–3. 10.1159/000416117 [DOI] [PubMed] [Google Scholar]
- 44.Camet ML, Hayashi SS, Sinks BC, Henry J, Gettinger K, Hite A, et al. Determining the prevalence of vestibular screening failures in pediatric cancer patients whose therapies include radiation to the head/neck and platin-based therapies: a pilot study. Pediatric Blood Cancer. (2018) 65:e26992. 10.1002/pbc.26992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. (2017) 54:204–18 e2. 10.1016/j.jpainsymman.2016.12.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miaskowski C, Mastick J, Paul SM, Abrams G, Cheung S, Sabes JH, et al. Impact of chemotherapy-induced neurotoxicities on adult cancer survivors' symptom burden and quality of life. J Cancer Surv Res Pract. (2018) 12:234–45. 10.1007/s11764-017-0662-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobson GP, Shephard NT. Balance Function Assessment and Management. 2nd ed. San Diego, CA: Plural Publishing Incorporated; (2014). [Google Scholar]
- 48.National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS; (2009). [Google Scholar]
- 49.Fung C, Sesso HD, Williams AM, Kerns SL, Monahan P, Abu Zaid M, et al. Multi-institutional assessment of adverse health outcomes among North American testicular cancer survivors after modern cisplatin-based chemotherapy. J Clin Oncol. (2017) 35:1211–22. 10.1200/JCO.2016.70.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding D, Allman BL, Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anatomical Rec. (2012) 295:1851–67. 10.1002/ar.22577 [DOI] [PubMed] [Google Scholar]
- 51.Nakayama M, Riggs LC, Matz GJ. Quantitative study of vestibulotoxicity induced by gentamicin or cisplatin in the guinea pig. Laryngoscope (1996) 106(2 Pt 1):162–7. 10.1097/00005537-199602000-00011 [DOI] [PubMed] [Google Scholar]
- 52.Rogers C. Presbyastasis: a multifactorial cause of balance problems in the elderly. South Af Family Pract. (2010) 52:431–4. 10.1080/20786204.2010.10874018 [DOI] [Google Scholar]
- 53.Rinne T, Bronstein AM, Rudge P, Gresty MA, Luxon LM. Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol. (1998) 245:314–21. 10.1007/s004150050225 [DOI] [PubMed] [Google Scholar]
- 54.Zingler VC, Weintz E, Jahn K, Huppert D, Cnyrim C, Brandt T, et al. Causative factors, epidemiology, and follow-up of bilateral vestibulopathy. Ann N Y Acad Sci. (2009) 1164:505–8. 10.1111/j.1749-6632.2009.03765.x [DOI] [PubMed] [Google Scholar]
- 55.Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. (2016) 2016:1809394. 10.1155/2016/1809394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waller PC. Measuring the frequency of adverse drug reactions. Br J Clin Pharmacol. (1992) 33:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Black FO, Pesznecker SC, Homer L, Stallings V. Benign paroxysmal positional nystagmus in hospitalized subjects receiving ototoxic medications. Otology Neurotol. (2004) 25:353–8. 10.1097/00129492-200405000-00025 [DOI] [PubMed] [Google Scholar]
- 58.Hirose Y, Simon JA, Ou HC. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J Assoc Res Otolaryngol. (2011) 12:719–28. 10.1007/s10162-011-0278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dal I, Edsmyr F, Stahle J. Bleomycin therapy and ototoxicity. Acta Oto-laryngol. (1973) 75:323–4. 10.3109/00016487309139733 [DOI] [PubMed] [Google Scholar]
- 60.Wei Y, Zhou T, Zhu J, Zhang Y, Sun M, Ding X, et al. Long-term outcome of sensorineural hearing loss in nasopharyngeal carcinoma patients: comparison between treatment with radiotherapy alone and chemoradiotherapy. Cell Biochem Biophys. (2014) 69:433–7. 10.1007/s12013-014-9814-x [DOI] [PubMed] [Google Scholar]
- 61.Dell'Aringa AH, Isaac ML, Arruda GV, Esteves MC, Dell'aringa AR, Junior JL, et al. Audiological findings in patients treated with radio- and concomitant chemotherapy for head and neck tumors. Radiation Oncol. (2009) 4:53. 10.1186/1748-717X-4-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets for this study are included in the manuscript and the Supplementary Files.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.