Abstract
IMPORTANCE
Treatment of oropharyngeal squamous cell carcinoma (OPSCC) presents unique challenges and can be associated with significant morbidity. Transoral robotic surgery (TORS) has emerged as a treatment modality for OPSCC, but data comparing outcomes between patients treated with TORS-based therapy and nonsurgical therapy are limited.
OBJECTIVE
To compare survival and gastrostomy prevalence between patients with OPSCC treated with TORS-based therapy and those treated with nonsurgical therapy.
DESIGN, SETTING, AND PARTICIPANTS
This retrospective matched-cohort study identified patients with OPSCC treated at the University of Washington and University of Minnesota tertiary care medical centers from January 1, 2005, to December 31, 2013. Each patient treated with TORS-based therapy was matched by stage with as many as 3 patients treated with nonsurgical therapy. Final follow-up was completed on April 1, 2015.
MAIN OUTCOMES AND MEASURES
Disease-free survival, overall survival, and gastrostomy tube prevalence.
RESULTS
One hundred twenty-seven patients met the study criteria (113 men [89.0%];14 women [11.0%]; median [interquartile range] age, 57 [52–63] years); 39 patients who underwent TORS were matched to 88 patients who underwent nonsurgical therapy. Compared with the nonsurgical group, more patients had p16-positive tumors in the TORS group (30 of 31 [96.8%] vs 30 of 37 [81.1%] among patients with known p16 status). No statistically significant difference in survival between treatment groups was found in multivariable analysis (disease-free survival hazard ratio, 0.22; 95% CI, 0.04–1.36; P = .10). Patients who received TORS-based therapy had lower gastrostomy tube prevalence after treatment (13 of 39 [33.3%] vs 74 of 88 [84.1%]) for a univariable relative risk of 0.43 (95% CI, 0.27–0.67; P < .001) and a multivariable relative risk of 0.43 (95% CI, 0.27–0.68; P < .001). Gastrostomy prevalence decreased by time after treatment for both groups (TORS group:3 of 34 [9%] at 3 months to 1 of 33 [3%] at 12 months; nonsurgical group: 37 of 82 [45%] at3 months to 7 of 66 [11%] at 12 months).
CONCLUSIONS AND RELEVANCE
Patients undergoing TORS for OPSCC have statistically indistinguishable survival but lower gastrostomy prevalence compared with patients undergoing nonsurgical therapy for stage-matched OPSCC. TORS offers promise for improved swallowing function in patients with OPSCC.
Complete surgical resection of oropharyngeal squamous cell carcinoma (OPSCC) traditionally presents unique challenges. In particular, the intimate involvement of oropharyngeal neoplasms with functionally vital tissues has led to significant morbidity after open surgical treatment.1 As a result, radiotherapy (RT) or chemoradio-therapy (CRT) have been used increasingly as primary treatments for OPSCC.2 Transoral robotic surgery (TORS) has emerged as a viable treatment option for OPSCC. The TORS system offers ready access to the oropharynx without external incisions for precise resections with minimal disruption of adjacent structures.3 Studies have shown high survival rates,4–6 low complication rates,7 good swallowing function,4–6,8 and excellent quality-of-life (QOL)8 outcomes after TORS.
Although evidence demonstrates that nonsurgical treatment options are associated with improved swallowing and QOL compared with traditional, open surgery,9,10 these out comes seem to be worse compared with those for TORS.11–13 Studies13,14 have compared unmatched patients with OPSCC undergoing TORS and nonsurgical therapy with results suggesting better swallowing-related outcomes in patients undergoing TORS. A study examining QOL among patients with OPSCC matched on 10 factors (including age, sex, tumor stage, and tumor human papillomavirus [HPV] status) showed that patients who had undergone transoral surgery, such as TORS, had significantly higher scores in the swallowing domain at 1 year after treatment in univariable analysis (multivariable models were not generated in the study).11
A phase 2 randomized study15 comparing TORS and RT/CRT in patients with localized OPSCC has been initiated. However, because results from such studies will not be available in the near future, we conducted a matched retrospective cohort study comparing TORS and RT/CRT. We matched patients on the basis of T stage to increase the likelihood that the 2 treatment groups would be more similar with respect to non-treatment factors that affect survival and swallowing outcomes compared with an unmatched design. We aimed to compare survival and gastrostomy prevalence between patients with OPSCC treated with TORS-based therapy and stage-matched patients treated nonsurgically.
Methods
Study Sample and Data Collection
Patients were eligible if they were diagnosed as having a first, previously untreated, biopsy-proven OPSCC from January 1, 2005, to December 31, 2013, and did not have distant meta-static disease at diagnosis. All patients were recruited from the University of Washington Medical Center (UWMC) and University of Minnesota Medical Center (UMMC), both of which are tertiary care academic medical centers. The study was approved by the institutional review boards of the institutions, which approved waivers of informed consent.
Patients were eligible to be included in the nonsurgical group if they received RT or CRT to the primary site as the initial treatment for their OPSCC. Given the introduction of TORS at UWMC inMarch2010, patients receiving nonsurgical therapy at this site were included if they were treated from January 1, 2005, to February 28,2010;patients treated nonsurgically from March1,2010, at UWMC were not included in an attempt to decrease selection bias. TORS was not offered at UMMC during the study period, so patients treated nonsurgically at this site were eligible during the entire period. Potential patients in the nonsurgical group were identified if they were seen within the departments of Otolaryngology–Headand Neck Surgery at either institution and had diagnosis codes 141, 145, 146, and 149 (corresponding to OPSCC) from the International Classification of Diseases, Ninth Revision. Patients in the TORS group were identified through an existing UWMC database containing patients who had undergone TORS. Thus, all patients who underwent TORS were seen at UWMC and patients treated nonsurgically were seen at UWMC or UMMC. Most, but not all, patients received RT or CRT at the 2 participating institutions. A minority of patients received RT or CRT at other regional centers. Patients’electronic medical records were thenreviewed to ensure that theymet criteria for inclusion.
Two hundred one patients met criteria for the study. Each patient who underwent TORS was matched to as many as 3 patients in the nonsurgical group with the same T stage to maximize statistical power, leaving 39 and 88 patients in the TORS and nonsurgical groups, respectively. Because all of the patients in the TORS group in this study were from a single institution, some of the matched groups (33 of 39) included patients from different institutions.
Demographic information, comorbidity, substance use history, tumor characteristics (including p16 immunohistochemistry as a surrogate for tumor HPV status), treatment modalities, gastrostomy prevalence, and survival were abstracted from the electronic medical record in a uniform fashion for all patients in the study. Comorbidity was assessed using the Charlson Comorbidity Index.16 Disease-free survival (DFS) was calculated from the date of treatment completion to the date of biopsy-proven recurrence or death, last follow-up, or April 1, 2015, whichever was earliest. Overall survival (OS) was calculated from the date of treatment completion to the date of death, last follow-up, or April 1, 2015, whichever was earliest.
Statistical Analysis
Power calculations were performed using gastrostomy prevalence from the literature (5% in the TORS group4 and 35% in the nonsurgical group17,18). With the α value set at .05 and the β value set at .90, a 1:3 ratio would require 26 patients in the TORS group and 78 in the nonsurgical group.
Univariable survival was compared between groups graphically using Kaplan-Meier survival curves and statistically using the log-rank test. Cox proportional hazards regression models were fit to perform multivariable survival analysis. Poisson logistic regression with robust error variances was used to compare the risk for gastrostomy tube placement between the 2 treatment groups. Multivariable models included age, sex, smoking status, tumor p16 status, tumor N stage, and treatment group. Two-sided P values were calculated, and the threshold for statistical significance was set at P < .05. All statistical analyses were conducted using STATA software (version 11.1 Intercooled; StataCorp).
Results
Study Sample Characteristics
One hundred twenty-seven patients (113 men [89.0%]; 14 women [11.0%]; median [interquartile range] age, 57 [52–63] years) met the study criteria and were matched for inclusion (Figure 1). Characteristics of the matched patients by institution are shown in eTable 1 in the Supplement. Additional patient characteristics, tumor data, follow-up, and treatment are shown in Table 1 for each treatment group. Patients in the TORS group were more likely to be nonsmokers and to have fewer comorbidities. We found a higher proportion of p16-positive tumors in the TORS group compared with the nonsurgical group (30 of 31 [96.8%] vs 30 of 37 [81.1%]; P = .06) when only including patients with known p16 status. Most of the patients in the nonsurgical group received CRT (82 [93.2%]), whereas the remainder (6 [6.8%]) received RT only. Of the patients treated with TORS, 35 (89.7%) received adjuvant treatment, but the median (interquartile range) radiation dose was lower compared with that in the nonsurgical group (60 [60–63] vs 70 [70–70] Gy). Only 11 patients (28.2%) who underwent TORS received adjuvant CRT.
Figure 1. Flowchart of Retrospective Matched Cohort Study of Patients With Oropharyngeal Squamous Cell Carcinoma (OPSCC).
TORS indicates transoral robotic surgery.
Table 1.
Characteristics of Patients With OPSCC by Treatment Group
| Characteristic | Treatment Group, No. (%) of Patientsa | |
|---|---|---|
| Nonsurgical (n = 88) | TORS (n = 39) | |
| Age, median (IQR), y | 57 (53–64) | 58 (51–63) |
| Male sex | 76 (86.4) | 37 (94.9) |
| Smoking | ||
| Never | 24 (27.3) | 22 (56.4) |
| Former | 34 (38.6) | 16 (41.0) |
| Current | 28 (31.8) | 1 (2.6) |
| Unknown | 2 (2.3) | 0 |
| Charlson Comorbidity Indexb | ||
| 0 | 53 (60.2) | 28 (71.8) |
| 1 | 15 (17.0) | 9 (23.1) |
| ≥2 | 20 (22.7) | 2 (5.1) |
| Tumor subsite | ||
| Base of tongue | 37 (42.0) | 23 (59.0) |
| Tonsil | 47 (53.4) | 16 (41.0) |
| Other | 4 (4.5) | 0 |
| Tumor p16 status | ||
| Negative | 7 (8.0) | 1 (2.6) |
| Positive | 30 (34.1) | 30 (76.9) |
| Unknown | 51 (58.0) | 8 (20.5) |
| Tumor T stage | ||
| 1 | 22 (25.0) | 16 (41.0) |
| 2 | 51 (58.0) | 18 (46.2) |
| 3 | 15 (17.0) | 5 (12.8) |
| 4 | 0 | 0 |
| Tumor N stage | ||
| 0 | 7 (8.0) | 5 (12.8) |
| 1 | 8 (9.1) | 7 (17.9) |
| 2 | 64 (72.7) | 24 (61.5) |
| 3 | 9 (10.2) | 3 (7.7) |
| Follow-up, median (IQR), mo | 18 (12–24) | 24 (18–27) |
| Treatment | ||
| Neck dissection | 13 (14.8) | 34 (87.2) |
| Radiotherapy | 88 (100) | 35 (89.7) |
| Radiation dose, median (IQR), Gy | 70 (70–70) | 60 (60–63) |
| Chemoradiotherapy | 82 (93.1) | 11 (28.2) |
Abbreviations: OPSCC, oropharyngeal squamous cell carcinoma; TORS, transoral robotic surgery.
Percentages have been rounded and may not total 100.
Scores range from 0 to 35, with higher numbers indicating more comorbidities.
Survival
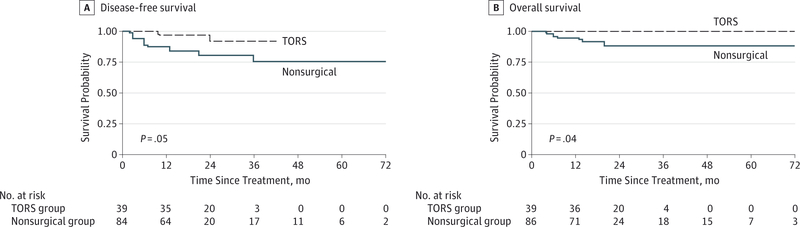
Median (interquartile range) follow-up among the matched patients with OPSCC was 18 (12–27) months. Nine recurrences and 8 deaths occurred in the nonsurgical group compared with 2 recurrences and no deaths in the TORS group. The TORS group had better DFS and OS (Figure 2). A multivariable Cox proportional hazards regression model was fit (eTable 2 in the Supplement), showing a clinically meaningful inverse association between TORS and mortality during follow-up (HR for the multivariable DFS in the full model, 0.22; 95% CI, 0.04–1.36). The wide CI demonstrates that the estimate of survival benefit with TORS is not very precise or informative. Multivariable models for OS are not shown because too few events occurred for detailed statistical analysis. In univariable analyses limited to patients with known p16 status, TORS was associated with better survival, although it was not statistically significant (HR for univariable DFS, 0.61; 95% CI, 0.07–5.55). Among patients who had p16-positive tumors, survival was similar between the TORS and nonsurgical groups (HR for uni-variable DFS, 0.98; 95% CI, 0.72–13.51).
Figure 2. Kaplan-Meier Survival Curves Showing Disease-Free and Overall Survival.
Patients with oropharyngeal squamous cell carcinoma are matched by T stage and compared by treatment group. P values are calculated by univariable log-rank test. TORS indicates transoral robotic surgery.
Gastrostomy Prevalence
Seventy-four patients (84.1%) in the nonsurgical group and 13 (33.3%) patients in the TORS group had a gastrostomy tube in place at some point after treatment; this difference was statistically significant in the univariable analysis (relative risk [RR], 0.43; 95% CI, 0.27–0.67) and the multivariable analysis (RR for full multivariable model, 0.43; 95% CI, 0.27–0.68) (multivariable models are shown in Table 2). Among patients in the nonsurgical group, gastrostomy prevalence was similar between the 2 institutions (29 of 35 [83%] at UWMC and 45 of 53 [85%] at UMMC). We found a decreasing difference in gastrostomy tube prevalence between groups over time after treatment (3 of 34 [9%] vs 37 of 82 [45%] at 3 months; 1 of 35 [3%] vs 18 of 72 [25%] at 6 months; 1 of 33 [3%] vs 7 of 66 [11%] at 12 months). The difference in gastrostomy prevalence between patients who underwent TORS and nonsurgical treatment was statistically significant at 3 and 6 months, but not at 12 months (Figure 3).
Table 2.
Multivariable Models Showing RR of Gastrostomy Prevalence in Patients With OPSCC by Time Since Treatment
| Multivariable Model Covariatea | Time Since Treatment, mo | |||||||
|---|---|---|---|---|---|---|---|---|
| Any | 3 | 6 | 12 | |||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| p16 | 0.41 (0.26–0.64) | <.001 | 0.19 (0.06–0.57) | .003 | 0.11 (0.02–0.81) | .03 | 0.19 (0.02–1.45) | .11 |
| N stage | 0.44 (0.28–0.69) | <.001 | 0.20 (0.07–0.63) | .005 | 0.12 (0.02–0.91) | .04 | 0.30 (0.04–2.01) | .22 |
| p16 and N stage | 0.42 (0.27–0.66) | <.001 | 0.20 (0.07–0.61) | .004 | 0.12 (0.02–0.87) | .04 | 0.19 (0.02–1.47) | .11 |
| N stage and smoking | 0.45 (0.28–0.71) | .001 | 0.25 (0.08–0.74) | .01 | 0.1 (0.02–0.89) | .04 | 0.36 (0.05–2.73) | .32 |
| p16, N stage, and smoking | 0.43 (0.27–0.68) | <.001 | 0.25 (0.08–0.74) | .01 | 0.12 (0.02–0.86) | .04 | 0.22 (0.03–1.87) | .17 |
| N stage, smoking, age, and sex | 0.45 (0.28–0.70) | .001 | 0.26 (0.09–0.77) | .02 | 0.13 (0.02–0.93) | .04 | 0.39 (0.06–2.73) | .34 |
| p16, N stage, smoking, age, and sex (full model) | 0.43 (0.27–0.68) | <.001 | 0.26 (0.09–0.79) | .02 | 0.12 (0.02–0.92) | .04 | 0.24 (0.03–1.68) | .15 |
Abbreviations: OPSCC, oropharyngeal squamous cell carcinoma; RR, relative risk.
The RRs, 95% CIs, and P values reflect the association between transoral robotic surgery (vs nonsurgical therapy) and gastrostomy prevalence in the multivariable models. Patients were matched by T stage.
Figure 3. Gastrostomy Tube Prevalence Over Time.
Patients with oropharyngeal squamous cell carcinoma are matched by T stage and compared by treatment group (88 patients in the nonsurgical treatment group and 39 in the transoral robotic surgery [TORS] group). RR indicates relative risk.
Discussion
The incidence, epidemiology, and management of OPSCC are rapidly changing with the increasing role of HPV in these malignant neoplasms and the use of TORS for localized OPSCC. Patients with HPV-associated OPSCC are younger, are less likely to smoke or drink, and have better survival outcomes compared with patients with HPV-negative OPSCC.19 Although concurrent CRT is effective in the treatment of OPSCC, 43% of patients who receive it have late severe toxic effects, the most common of which is pharyngeal dysfunction.20 This finding underscores the importance of optimizing the patients’ swallowing function and QOL. Our previous study21 showed that patients with HPV-associated cancers treated before the introduction of TORS had a steeper decline from their pretreat ment QOL to the immediate posttreatment QOL and worse immediate posttreatment QOL compared with patients with HPV-negative cancers. Prior work22 has shown that the presence of a gastrostomy tube strongly influences QOL, likely reflecting the importance of swallowing function on head and neck cancer–specific QOL.
The introduction of TORS has allowed for surgical resection of OPSCC without the functional adverse effects of open surgical approaches to the oropharynx. TORS has been studied largely in single-arm case series, revealing excellent onco-logic and functional outcomes. However, not all patients with OPSCC are candidates for TORS, and TORS is most often used in patients with local (especially small T stage) disease.3,15
The present study aimed to compare outcomes between patients with OPSCC undergoing TORS or nonsurgical treatment. Because T stage is one of the most important factors for determining whether patients are candidates for TORS and has a strong clinical correlation to swallowing function, wematched the patients in the 2 treatment groups on this factor. This strategy confers an advantage to the present study because it helps to increase the chance that the comparison groups are similar. Although we found other important differences between the 2 groups (eg, smoking status and tumor p16 status), we adjusted for these important characteristics in the multivariable analysis. We found that the 2 treatment groups had comparable survival, but those who underwent TORS had lower gastrostomy prevalence, especially in the early posttreatment period.
Many patients in the TORS group received adjuvant RT (89.7%). Only 28.2% required trimodality therapy (TORS followed by adjuvant CRT), which is less than the percentage of patients requiring trimodality therapy in some prior studies (43%−62%).7,8,23,24 The relatively low frequency of trimodality therapy in our study population may partially explain the lower gastrostomy prevalence in the TORS group compared with the nonsurgical group, because the use of CRT is known to have adverse effects on swallowing function.20 Although fewer patients undergoing TORS in our study received trimodality therapy compared with some prior studies, their survival was not inferior to that of patients undergoing RT/CRT in this matched analysis.
During the study period, HPV status was not used to alter therapy. Nonetheless, HPV status remained an important potential confounder in this study, and we included p16 measured by immunohistochemistry in the multivariable models for this reason. Patients undergoing TORS more often had p16-associated tumors (76.9% vs 34.1%), although p16 status was unknown in 8 patients in the TORS group (20.5%) and 51 patients in the nonsurgical group (58.0%). The percentage of patients with unknown p16 status and the difference in the percentage of patients with unknown p16 status between the 2 groups are sources of potential bias and important limitations of this study. Among the subset of patients with known tumor p16 status, however, the prevalence of p16 positivity was not as dramatically different between the 2 groups (96.8% in the TORS group vs 81.1% in the nonsurgical group). To address these limitations, a stratified survival analysis was performed using p16 status, although our sample size was greatly reduced and only univariable analysis was performed. Survival was better in the TORS group in the overall analysis, although not significantly; however, it was similar between treatment groups when the analysis was limited to patients with p16-positive tumors. Therefore, the favorable survival status seen in the patients undergoing TORS may well have been due to the effect of p16 status rather than the treatment modality.
Additional important limitations should be considered when interpreting the results of this study. The patients undergoing TORS were from a single study institution, which introduces the opportunity for bias in the results. Other differences present between the 2 treatment groups included less comorbidity and more nonsmokers in the TORS group. Some of these differences could reflect changes over time in the patient population with OPSCC, but also that the patients selected for TORS are inherently different from those selected for nonsurgical treatment. To reduce bias from this source, the patients in each group were drawn from nonoverlapping periods. However, this strategy does not completely eliminate selection bias and increases the chances that changes in the epidemiology or management of OPSCC over time (eg, increasing incidence of HPV-associated OPSCC) could affect the results. Another important limitation is that p16 status, an important confounder, was unequally measured between groups, as noted above. That p16 status was more often unknown in the nonsurgical group may be the result of nonoverlapping periods, because p16 testing has become more common.
Gastrostomy prevalence was the primary outcome of this study. Gastrostomy tube prevalence does not necessarily imply gastrostomy tube dependence, because these tubes are often placed prophylactically in anticipation of RT or CRT. However, we found that gastrostomy prevalence differed between treatment groups beyond the time of treatment (ie, for at least 6 months after completion of therapy), which suggests a difference in swallowing function after completion of therapy between both groups. Thus, another potential advantage of TORS is that prophylactic gastrostomy tube placement can be avoided.
Prior work has suggested that patients undergoing TORS have favorable survival and good swallowing function but has not compared these outcomes with those of T stage–matched patients undergoing nonsurgical treatment. de Almeida et al25 performed a systematic review and pooled analysis of OPSCC and focused on survival and adverse events. They found that survival was similar between patients who received TORS-based therapy and those who received intensity-modulated RT–based therapy. Each study used for the pooled analysis only contained patients from 1 of these 2 treatment groups, and no matching was performed. Two additional studies13,14 that com pared unmatched patients with OPSCC who underwent TORS-based therapy and nonsurgical therapy have shown improved swallowing-related outcomes in the former group. Chen et al11 studied QOL in patients with OPSCC who received transoral surgery (TORS or transoral laser microsurgery) or CRT. Matching was performed on a number of factors, including T and N stages. The investigators found that the QOL was similar between the 2 groups at 1 year after treatment. However, the scores in the swallowing domain were significantly better in the transoral surgery group. Chen et al11 found that gastrostomy dependence was 10% in the CRT group and 3% in the transoral surgery group at 1 year, almost identical to the level of gastrostomy prevalence in the present study. However, multivariable analyses were not performed in their study, and no survival data were reported. The lower gastrostomy prevalence seen in the present study and the study by Chen et al11 is related to less frequent need for chemotherapy and/or lower doses of radiation when patients were treated with transoral modalities such as TORS. Thirty-four of 39 patients (87.1%) treated with TORS in our study also underwent neck dissection, which suggests that any potential detrimental effect of neck dissection on swallowing function is less than that seen with nonsurgical therapy.
We found that patients undergoing TORS had clinically meaningful improved DFS and OS, even after adjusting for important factors, although this difference was not statistically significant. The absence of a statistical difference in the multivariable survival analysis most likely reflects the small study population and the resultant low statistical power to detect statistical differences when adjusting for multiple factors simultaneously. Given the multiple limitations discussed previously, our interpretation is that survival with TORS-based therapy is comparable to survival with nonsurgical therapy. This study also demonstrates statistically lower gastrostomy tube prevalence among patients who undergo TORS after adjustment for confounders in the multivariable analysis. The difference in gastrostomy prevalence between the patients undergoing TORS and nonsurgical therapy was greatest early after completing treatment. Because swallowing is an important determinant of QOL in this patient population, the use of TORS could be an effective modality to provide patients undergoing OPSCC with improved QOL.
Conclusions
This study provides evidence among T stage–matched patients that the use of TORS-based therapy for OPSCC could yield improvements in swallowing while offering survival outcomes that are comparable to those with nonsurgical therapy. Continued research that evaluates OPSCC treatment algorithms, such as deintensification of adjuvant therapy and patient selection for TORS, will provide further information about optimizing functional outcomes while preserving survival for patients with these malignant neoplasms.
Supplementary Material
Key Points.
Question Are survival and gastrostomy prevalence different between patients treated with transoral robotic surgery (TORS) vs nonsurgical therapy for oropharyngeal squamous cell carcinoma (OPSCC)?
Findings In this matched-cohort study, 39 patients with OPSCC undergoing TORS had statistically indistinguishable survival but lower gastrostomy prevalence compared with 88 patients with stage-matched OPSCC receiving nonsurgical therapy.
Meaning When TORS is an option for the treatment of OPSCC, it is associated with survival at least comparable to that of nonsurgical therapy and offers promise of improved swallowing function for patients with OPSCC.
Acknowledgments
Funding/Support: This study was supported by grant 5T32DC000018-30 from the National Institutes of Health and grant RSG TBG-123653 from the American Cancer Society.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaotolaryngology.com
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1.White H, Ford S, Bush B, et al. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches [published correction appears in JAMA Otolaryngol Head Neck Surg 2013;139(12):1290]. JAMA Otolaryngol Head Neck Surg. 2013;139(8):773–778. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002; 94(11):2967–2980. [DOI] [PubMed] [Google Scholar]
- 3.Dowthwaite SA, Franklin JH, Palma DA, Fung K, Yoo J, Nichols AC. The role of transoral robotic surgery in the management of oropharyngeal cancer: a review of the literature. ISRN Oncol. 2012; 2012:945162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87(3):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YM, Byeon HK, Chung HP, Choi EC, Kim SH. Comparison study of transoral robotic surgery and radical open surgery for hypopharyngeal cancer. Acta Otolaryngol. 2013;133(6):641–648. [DOI] [PubMed] [Google Scholar]
- 6.Park YM, Kim WS, Byeon HK, Lee SY, Kim SH. Oncological and functional outcomes of transoral robotic surgery for oropharyngeal cancer. Br J Oral Maxillofac Surg. 2013;51(5):408–412. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein GS, O’Malley BW Jr, Magnuson JS,et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122(8):1701–1707. [DOI] [PubMed] [Google Scholar]
- 8.Dziegielewski PT, Teknos TN, Durmus K, et al. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139(11): 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Deiry M, Funk GF, Nalwa S, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131(10):879–885. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114(8):1362–1367. [DOI] [PubMed] [Google Scholar]
- 11.Chen AM, Daly ME, Luu Q, Donald PJ, Farwell DG. Comparison of functional outcomes and quality of life between transoral surgery and definitive chemoradiotherapy for oropharyngeal cancer. Head Neck. 2015;37(3):381–385. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida JR, Villanueva NL, Moskowitz AJ,et al. Preferences and utilities for health states after treatment for oropharyngeal cancer: transoral robotic surgery versus definitive (chemo) radiotherapy. Head Neck. 2014;36(7):923–933. [DOI] [PubMed] [Google Scholar]
- 13.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668–1674. [DOI] [PubMed] [Google Scholar]
- 14.More YI, Tsue TT, Girod DA, et al. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. JAMA Otolaryngol Head Neck Surg. 2013; 139(1):43–48. [DOI] [PubMed] [Google Scholar]
- 15.Nichols AC, Yoo J, Hammond JA, et al. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs trans-oral robotic surgery (ORATOR)—study protocol for a randomized phase II trial. BMC Cancer. 2013;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 17.Shiley SG, Hargunani CA, Skoner JM, Holland JM, Wax MK. Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg. 2006;134(3): 455–459. [DOI] [PubMed] [Google Scholar]
- 18.Huang K, Xia P, Chuang C, et al. Intensity-modulated chemoradiation for treatment of stage III and IV oropharyngeal carcinoma: the University of California–San Francisco experience. Cancer. 2008;113(3):497–507. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. [DOI] [PubMed] [Google Scholar]
- 20.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Méndez E, Yueh B, et al. Human papillomavirus–positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg. 2012;146(5):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Deiry MW, Futran ND, McDowell JA, Weymuller EA Jr, Yueh B. Influences and predictors of long-term quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg. 2009; 135(4):380–384. [DOI] [PubMed] [Google Scholar]
- 23.Hurtuk AM, Marcinow A, Agrawal A, Old M, Teknos TN, Ozer E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg. 2012;146(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stucken CL, de Almeida JR, Sikora AG, Tong CCL, Genden EM. Impact of human papillomavirus and smoking on survival outcomes after transoral robotic surgery. Head Neck. 2016;38(3):380–386. [DOI] [PubMed] [Google Scholar]
- 25.de Almeida JR, Byrd JK, Wu R, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer:a systematic review. Laryngoscope. 2014;124(9): 2096–2102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.