See Kleen and Kirsch (doi:10.1093/awx178) for a scientific commentary on this article.
Cognitive impairments are common in epilepsy. Using intracranial EEG in 67 subjects, Ung et al. show that interictal epileptiform discharges (IEDs) outside the seizure onset zone affect memory encoding more than those within the seizure onset zone. IEDs are not benign, but instead disrupt functioning of underlying tissue.
Keywords: intracranial EEG, interictal spikes, epileptiform discharges, memory, cognition
Abstract
See Kleen and Kirsch (doi:10.1093/awx178) for a scientific commentary on this article.
Cognitive deficits are common among epilepsy patients. In these patients, interictal epileptiform discharges, also termed spikes, are seen routinely on electroencephalography and believed to be associated with transient cognitive impairments. In this study, we investigated the effect of spikes on memory encoding and retrieval, taking into account the spatial distribution of spikes in relation to the seizure onset zone as well as anatomical regions of the brain. Sixty-seven patients with medication refractory epilepsy undergoing continuous intracranial electroencephalography monitoring engaged in a delayed free recall task to test short-term memory. In this task, subjects were asked to memorize and recall lists of common nouns. We quantified the effect of each spike on the probability of successful recall using a generalized logistic mixed model. We found that in patients with left lateralized seizure onset zones, spikes outside the seizure onset zone impacted memory encoding, whereas those within the seizure onset zone did not. In addition, spikes in the left inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus, and fusiform gyrus during memory encoding reduced odds of recall by as much as 15% per spike. Spikes also reduced the odds of word retrieval, an effect that was stronger with spikes outside of the seizure onset zone. These results suggest that seizure onset regions are dysfunctional at baseline, and support the idea that interictal spikes disrupt cognitive processes related to the underlying tissue.
Introduction
Epilepsy is a chronic seizure disorder that affects over 65 million people worldwide (Ngugi et al., 2010). In these patients, cognitive and memory deficits are common complaints, some of which are not captured by traditional neuropsychological tests (Blake et al., 2000; Wang et al., 2011). Characteristic patterns recorded on EEG such as interictal spikes, rhythmic bursts, wave discharges, and focal slowing are not always accompanied by overt clinical symptoms but can still be detrimental to the patient’s psychosocial functioning and quality of life (Holmes and Lenck-Santini, 2006; Hoppe et al., 2007). Investigation of these electrographic patterns relative to cognition date back to 1939 (Schwab, 1939) and has since been associated with impairments in cognition and memory (Rausch et al., 1978; Aarts et al., 1984; Holmes and Lenck-Santini, 2006; Kleen et al., 2010, 2013; Gelinas et al., 2016). Elucidating the relationship between these electrophysiological patterns and their functional consequences is a crucial step in improving treatment and quality of life for patients with epilepsy.
Parameters for functional assessment of patients with epilepsy involve neuropsychological tests that explore cognitive, behavioural, linguistic or motor impairment. These tests provide information on a global level such as whether a person can safely live by themselves, return back to work or school, or drive. While important for patient management, these measures are limited in their ability to assess the anatomical location of abnormalities as well as the correlation with EEG phenomena such as seizures and interictal spikes. For these reasons, a more precise measure of cognitive function is necessary to understand the mechanisms underlying the prominent memory and cognitive complaints that plague a significant portion of patients with epilepsy. Here, we use a controlled memory task during intracranial EEG monitoring to evaluate the impact of interictal spikes on memory processes with high spatial and temporal resolution.
Intracranial EEG (iEEG) recordings provide more precise recordings of brain activity than non-invasive EEG. These data provide an opportunity to study the electrophysiological correlates of a wide range of cognitive processes with greater detail (Rausch et al., 1978; Lachaux et al., 2012). For example, iEEG has been used to study the neural basis of human cognition using non-epileptic regions of the brain as models for normal healthy brain and as controls. Typical electrophysiological patterns are consistently seen across various performance tasks. In studies of memory, there is evidence that electrophysiological and haemodynamic changes occur during encoding, termed the subsequent memory effect (Paller and Wagner, 2002; Sederberg et al., 2003). High frequency oscillatory activity of neurons has been associated with specific cortical network states and has been used to quantify the electrophysiological mechanisms of memory formation and recall. Gamma oscillatory activity, for example, exhibits anatomical, temporal and functional specificity (Jacobs and Kahana, 2010). Early studies showed that increases in gamma activity in the hippocampus and the left temporal and frontal cortices is associated with successful memory formation, consistent with previous functional MRI studies (Sederberg et al., 2007). Therefore the magnitude of these oscillations during encoding indicates that synchrony in widespread networks of cortical regions can serve as a predictor for successful memory recall (Sederberg et al., 2003).
In this study, we focus on interictal epileptiform discharges, also termed spikes, recorded on iEEG. Interictal spikes are highly correlated with the presence of epilepsy, though their representation with respect to regions of structural and functional abnormalities, influence on patient behaviour and physiology, and relationship to ictal activity has not yet been well defined (Spencer et al., 2008). EEG-functional MRI studies have confirmed that interictal spikes may be separated into different populations with some resulting in modifications of metabolism well beyond the clinically identified epileptic focus (Kobayashi et al., 2006). This suggests that spiking activity in the epileptic foci may be deleterious for a larger section of the brain (beyond the foci) and may interrupt normal electrophysiology and functioning, a concept introduced as ‘nociferous cortex’ by Penfield and Jasper (1954). Past studies have suggested a negative correlation of spiking with global neuropsychological tests of memory and intelligence (Rausch et al., 1978; Aarts et al., 1984) and a specific effect of hippocampal spikes on cognition (Kleen et al., 2010, 2013; Gelinas et al., 2016). In an independent study recently performed by the Restoring Active Memory collaborative research group, inferior temporal, middle temporal, and parietal spikes were shown to negatively impact memory processes (Horak et al., 2016). We aim to more specifically quantify the effect of spikes on a whole brain level and with respect to the seizure onset zone (SOZ).
Improper memory encoding and recall in correlation with interictal spiking places emphasis on targeted therapy for cognitive dysfunction and potential improvement in the localization of epileptic foci (Blake et al., 2000). As spikes are helpful in localization of the SOZ only in some patients (Marsh et al., 2010; Goncharova et al., 2013), understanding the differences between populations of spikes may better aid onset localization. We hypothesize that regional interictal spiking will have a detrimental impact on cognition by disrupting the subsequent memory effect, suggesting that altering epileptic networks to reduce spiking in normal brain may positively impact cognition. Furthermore, regions underlying the SOZ are believed to be damaged from not only the initial insult but also from recurrent seizures, leading to neuronal loss and cognitive decline (Sutula et al., 2003). Quantifying the effect of spikes on cognition relative to the SOZ may help elucidate the function (or dysfunction) of the involved tissue.
The overarching hypotheses that were tested during this study are (i) interictal spikes disrupt memory processes; (ii) there is an observable functional anatomy in which these spikes lie; and (iii) epileptiform activity outside of the seizure onset zone disrupt memory processes whereas spikes within the seizure onset zone are not deleterious to memory. We address these hypotheses using automated spike detection in iEEG during a delayed free recall task and identify the brain regions implicated in incorrect recall.
Materials and methods
Participants
Sixty-seven patients with drug resistant epilepsy at the Hospital of the University of Pennsylvania (n = 16) and Thomas Jefferson University Hospital (n = 51) were included in this study and comprised 45 males and 22 females, with an average age of 35.55 [range 15–57, standard deviation (SD) = 12.17]. Verbal intelligence quotient (IQ) was determined using the Wechsler Adult Intelligence Scale. Additional subject information is given in Supplementary Table 1. A total of 6144 intracranial electrodes were implanted across all patients during the clinical monitoring period and included both depth and subdural electrodes. Medications were reduced and or withdrawn during the monitoring period. Cognitive testing (described below) was not performed after a seizure until the patient was back to prior baseline neurologic status, which varied for each subject depending upon the seizure semiology, post-ictal symptoms, and abortive medications given during a seizure (e.g. lorazepam). Typically, cognitive testing was performed a minimum of 3 h after a generalized tonic clonic seizure and 1 h after a partial seizure. All participants provided informed consent with procedures approved by the Institutional Review Board at the University of Pennsylvania and Thomas Jefferson University Hospital.
Delayed free recall task
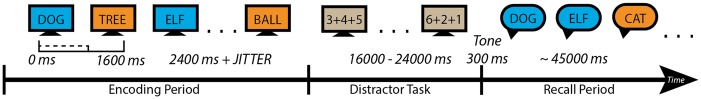
A delayed free recall episodic memory task was used to investigate memory encoding and recall. For each trial, subjects were asked to memorize a word list composed of 15 common high frequency nouns, chosen at random and without replacement from a pool of high-frequency English nouns (http://memory.psych.upenn.edu/WordPools), followed by a distractor task that consisted of simple arithmetic problems (Sederberg et al., 2003; Burke et al., 2013). After a tone, subjects engaged in free recall, vocalizing presented words in any order. A schematic of one word list/trial is provided in Fig. 1. Each subject received 12–60 such word lists to encode and recall in each session, the number of trials per subject as well as the number of sessions depended on the subject’s interest and availability for testing.
Figure 1.
Delayed Free Recall Task schematic. Blue indicates correctly recalled words and orange indicates incorrectly recalled words. Subjects were presented 15 words during the encoding period, followed by a distractor task that consisted of simple arithmetic problems. After a tone, subjects engaged in free recall, vocalizing presented words in any order. Subjects were presented with 12–60 word lists in each session.
A computer program presented stimuli and recorded subject responses. Each trial began with a plus sign to alert the subject to an upcoming presentation of words. The plus sign appeared for 1600 ms followed by an 800–1200 ms blank interval. Each word was then presented for 1600 ms followed by an 800–1200 ms blank interstimulus interval. Subjects were asked to read each word aloud or to themselves to ensure attentiveness. Following the presentation of a list of words, a short distracter period was introduced between the encoding and recall periods to reduce the recency effect often observed for free recall tasks (Murdock, 1962). During this distracter period subjects were asked to solve a series of simple arithmetic problems composed of A + B + C, where A, B, and C consisted of randomly selected one-digit positive integers. Participants responded by typing their answer onto the keyboard, with feedback provided through a high-pitched tone for correct answers and a low-pitched tone for incorrect answers. Each distracter period lasted for 1600–2000 ms and was followed by a 300 ms tone concurrent with a row of asterisks that signalled the start of the recall period. The recall period lasted for ∼4500 ms during which subjects were asked to recall words in any order presented during the encoding period and their vocal responses were recorded. Words that were presented during the encoding period and retrieved during the recall period were considered correctly encoded and correctly recalled. Furthermore, words from prior encoding periods that were retrieved during a recall period were considered incorrectly recalled.
The computer sent a pulse to an unused recording channel in order to synchronize the behavioural events during the memory task with the electrophysiological recordings. The time stamps associated with these pulses were used to annotate the iEEG recordings. Annotations and iEEG recordings were converted to the Multiscale Electrophysiology Format (Brinkmann et al., 2009) and uploaded to IEEG.org for analysis.
Preprocessing and removal of artefact channels
Sampling rates varied from 400 to 2000 Hz. Artefact channels were identified by calculating the line length feature across each channel and those that differ by more than four times the mean across all channels were removed (Esteller et al., 2001). This process is intended to remove grossly artefactual channels.
Spike detection
Despite complex methods in the literature, automatic spike detection has proved to be a difficult task to perfect. Many detectors suffer from a high false positive rate, as some artefacts have spike-like morphology (Wilson and Emerson, 2002) and performance varies from dataset to dataset. Another reason for this difficulty is reflected in the poor inter-rater reliability even among experts, which may range from 41–80% (Gaspard et al., 2014). However, automatic spike detection is a reasonable solution for our analysis because (i) we adopt a highly sensitive algorithm; (ii) our statistical analysis compares two groups that will be equally affected by false positives; and (iii) it provides an objective and quantifiable detection method. Thus, false positive detections are expected to be equally distributed within each individual between word presentations and any differences in means will be investigated.
An algorithm published by Janca et al. (2014) was used to automatically detect spikes for all patients. This algorithm applies a signal envelope to identify spikes by modelling background activity and determining transient outliers. Briefly, signals were downsampled to 200 Hz before 10–60 Hz bandpass and 60 Hz notch filters were applied. For each channel, the signal envelope was calculated with the absolute value of the Hilbert transform. Moving windows of 5 s with 4-s overlap were used to model a log-normal statistical distribution of the signal envelope. A threshold of κ1 [Mode + Median] was used for the initial detection of spikes, where κ1 = 3.65, determined empirically through cross-validation by the original authors (Janca et al., 2014). Following initial detections and to improve our positive predictive rate, we added a spatial filter to identify spikes across multiple channels. Any spikes within 200 ms on more than one channel were combined and treated as one spike. A subset of candidate spikes was randomly selected across all patients and validated by a board certified epileptologist (K.D.) to ensure adequate performance, specifically positive predictive value.
The encoding phase was defined to be the period after a word stimulus prior to the subsequent word stimulus. The number of spikes during this period was extracted for all words. Spikes were also categorized into anatomical locations determined by the Talairach coordinates of the electrodes following co-registration of post-implant CT to pre-implant T1-weighted MRI. Spikes that occurred in multiple brain regions or bilaterally were considered independently for each region.
While the delayed free recall task is most suited to investigate the encoding period, we also conducted an analysis of the retrieval period based on methods from prior literature (Burke et al., 2014). The recall phase was defined to be a 1-s period prior to a successful word recall. This is compared to a baseline period during unsuccessful recall, which is defined as a 1-s period in free recall trials in the same subject and session that are at minimum 3000 ms away from any vocal utterance. This 1-s period is time-matched to beginning of each recall session. Spikes were extracted from all periods.
Statistical analysis
A linear model was used to test the effect of subject level variables (age, sex, and verbal IQ) versus mean recall rate and mean spike rate across all patients. Welch’s t-test was used to determine differences in mean recall rates and spikes per electrode between left and right lateralized patients. To model the effect of spikes on correct versus incorrect recall on a word-by-word basis, we use a generalized linear mixed model (GLMM) with a logit link function. The GLMM was fit to predict successful recall with the serial order of word presentation, age, verbal IQ, and the number of spikes in the encoding period as fixed effects (Equation 1). To determine the effect of spikes on retrieval, a similar GLMM was fit for spikes within the retrieval period (Supplemental Table 4). A region of interest analysis for the encoding period was conducted by varying the spike count to correspond with each Brodmann area (BA).
| (1) |
where , denotes the probability of successful recall for word i of subject j, session k, and are random effects for differences in mean recall rate as well as intersession (k) variability, respectively for each subject.
A random effect was included for each subject nested by session. This allows us to model variability between subjects (with different baseline recall rates) as well as variability between different sessions within a subject. A logit link function permits us to model a binary outcome (recalled versus not recalled) and to interpret the estimated coefficients probabilistically. The fixed effects represent the mean effect across all subjects after removal of intersubject and intersession variability. To confirm the need for a mixed, nested model, a likelihood ratio test was used to test model fit before and after sequential addition of random effects and covariates (Supplementary Tables 2 and 4). Effect sizes (odds ratios, OR) and confidence intervals (CI) are reported with significance by Wald statistics when testing multiple parameters. Likelihood ratios are reported when testing single parameters (regional analysis). Further information about the model is provided in the Supplementary material.
Seizure onset zone
We also sought to determine the effect of spikes on identified SOZs. For this analysis, 57 patients with clinically localized SOZ were included. The clinical localization process varied for each patient and was directed towards identification of the seizure aetiology and onset region(s). This process typically includes video monitoring and patient reports to localize clinical semiology and correlate findings from imaging as well as electrophysiology. The final SOZ is determined through expert consensus. To determine the effect of spikes relative to the SOZ, channels were first divided into seizure onset and non-seizure onset channels. Since spikes may occur across multiple channels, if any channel within the SOZ was involved, the spike was categorized as a seizure onset spike. Similarly, spikes completely outside the SOZ were classified as non-seizure onset spikes. For both encoding and retrieval, the GLMM was refitted with the addition of these two covariates as well as an interaction term for clinical SOZ lateralization.
Regional analysis
All 67 subjects were included in the regional analysis. Each electrode was grouped into regions corresponding to level 5 of the Talairach atlas, which includes Brodmann areas and the hippocampus (Lancaster et al., 2000). Due to variability in electrode positioning across patients, independent GLMMs were fit on regions containing electrodes from 20 or more patients. For each region, a null model containing serial word position, verbal IQ, and age was fit, and an alternative model with the addition of regional spikes was fit. Significance was determined by the likelihood ratio test and adjusted for familywise error with the Holm-Bonferroni method (Holm, 1979).
Code availability
MATLAB was used for spike detection and extraction. R was used for statistical analysis and generation of figures. Scripts for the statistical analysis and generation of figures are available at http://github.com/hoameng/cognitive-spike-2016.
Results
A total of 244/6144 channels were identified as artefact and removed from the analysis. Over 900 detected spikes were randomly selected from all patients and validated by a board certified epileptologist (K.D.). Positive predictive rate was 72.2%. False positive rate was 15.5%. Of all detected spikes, 12.3% were deemed indeterminate. An example of a detected spike is shown in Supplementary Fig. 1.
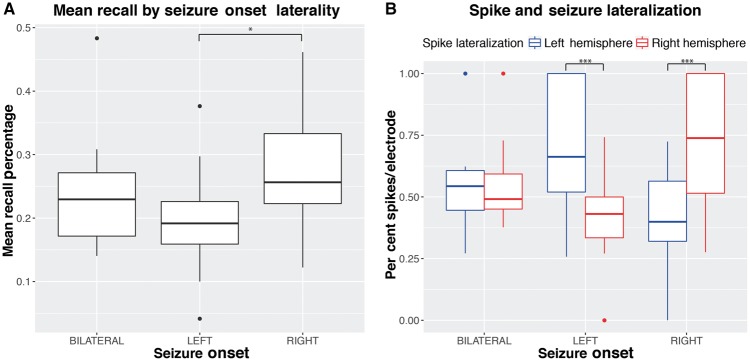
Subjects on average recalled at a rate of 24% (range: 4–48%, SD = 9%). Older age was significantly associated with a decrease in mean recall rates [t(54) = −2.9, P = 0.005, = 0.1], but not average spike rates (Supplementary Fig. 2A). There was no significant difference in mean recall rates or mean spike rates by sex (Supplementary Fig. 2B). Initial words were recalled with greater accuracy (Supplementary Fig. 2C). Greater verbal IQ was associated with greater recall accuracy [t(40) = 3.133, P = 0.003, = 0.177] (Supplementary Fig. 3). Averaged across patients, 23.7% (SD = 11.7%) of words that failed to be recalled occurred with no spikes. Patients with left lateralized onset regions (n = 23) had lower mean recall rates than patients with right lateralized onsets (n = 26) [t(47) = 3.1, P = 0.003, d = 0.9] (Fig. 2). In addition, a greater percentage of spikes per electrode occurred ipsilateral to the seizure onset region [left onset: t(36.3) = 4.2, P < 0.001, d = 1.33; right onset: t(38.1) = 4.5, P < 0.001, d = 1.34] (Fig. 2).
Figure 2.
Mean recall (A) and spike lateralization (B) grouped by seizure onset lateralization. Boxplots indicate median, 25th, and 75th percentiles. Significance at *P < 0.01, ***P < 0.001.
Encoding
During the encoding phase, an increased number of spikes across all electrodes were associated with decreased recall [ = 8.64, P = 0.003]. In patients with left lateralized SOZ, spikes outside of the SOZ had a significant effect on recall, whereas spikes within did not. In patients with right lateralized onset regions, spikes were not significantly associated with recall performance, whether within or outside the SOZ (Table 1). In the majority of patients, spikes were more frequent outside the SOZ [P < 0.001, t(66) = 7.78, Supplementary Fig. 5].
Table 1.
Estimated odds ratios of effect of spikes relative to the seizure onset zone during encoding
| OR (95% CI) | Z | P | |
|---|---|---|---|
| Right lateralized SOZ (n = 26) | |||
| Spikes within SOZ | 1.005 (0.972–1.039) | −1.189 | 0.235 |
| Spikes outside SOZ | 0.975 (0.935–1.016) | −0.318 | 0.750# |
| Left lateralized SOZ (n = 23) | |||
| Spikes within SOZ | 0.979 (0.925–1.036) | −0.719 | 0.472 |
| Spikes outside SOZ | 0.925 (0.886–0.964) | −3.647 | 0.000265*,# |
A logistic GLMM model was fit to a binary response variable indicating recall success (1) or failure (0). Covariates included spikes within the SOZ, outside the SOZ, and interaction of each with clinical SOZ lateralization. ORs are adjusted for age, serial word position, and verbal IQ. Patients with bilateral onsets were not included. OR = odds ratio, CI = confidence interval, significance determined by Wald statistics.
*P < 0.05; #significant difference between left and right lateralized SOZ patients [increased odds of recall for spikes outside the SOZ if right lateralized: odds = 1.087 (1.013–1.112), z = 3.077, P = 0.002].
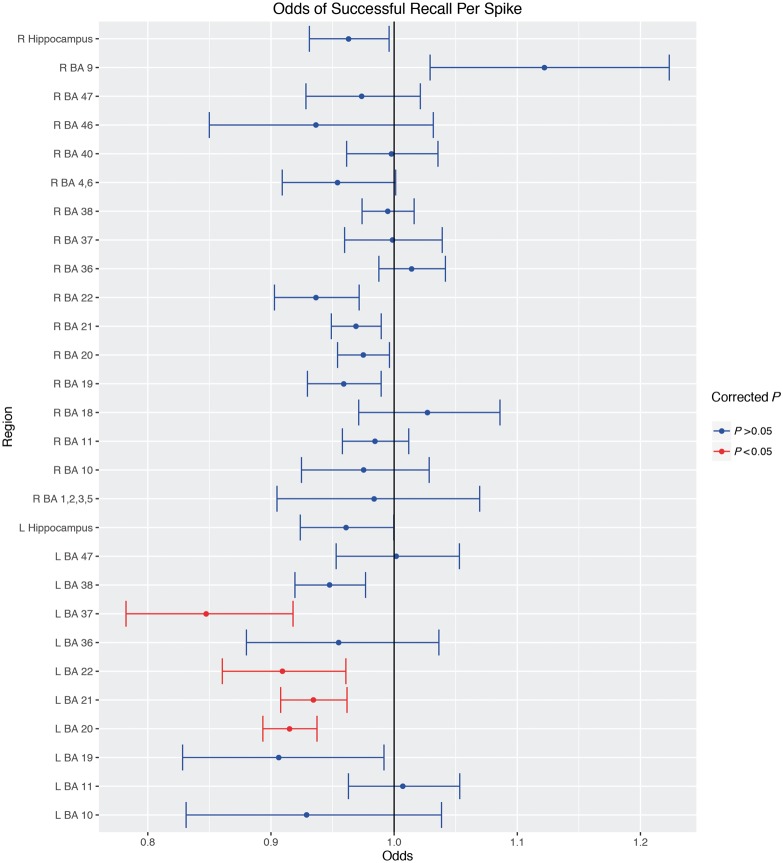
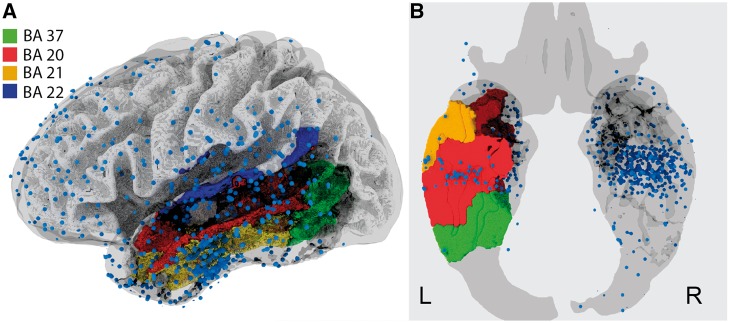
The top 10 regions from the regional analysis are given in Table 2. The odds ratios for all regions are shown in Fig. 3. Left temporal lobe structures such as the fusiform gyrus (BA 37) and inferior temporal gyrus (BA 20) were most significant after multiple comparisons correction for family-wise error with the Holm-Bonferroni method (Holm, 1979). The middle and superior temporal gyri (BA 21/22) were also significant after correction. A plot of the per cent change in epileptiform discharges during failed recall are shown in Fig. 4, separated by SOZ lateralization and the significant regions from the above regional analysis. These three regions are shown along with electrode locations across all subjects in Fig. 5.
Table 2.
Regional effect of spikes during encoding
| n | L/R | Region | Brodmann area | Odds (95% CI) | P (adjusted) | |
|---|---|---|---|---|---|---|
| 22 | L | Fusiform gyrus | BA 37 | 0.847 (0.782–0.918) | 44.0 | <0.001*** |
| 45 | L | Inferior temporal gyrus | BA 20 | 0.915 (0.893–0.937) | 30.2 | <0.001*** |
| 43 | L | Middle temporal gyrus | BA 21 | 0.934 (0.908–0.962) | 29.8 | <0.001*** |
| 22 | L | Superior temporal gyrus | BA 22 | 0.909 (0.861–0.961) | 27.1 | 0.0304* |
| 24 | L | Peristriate cortex | BA 19 | 0.906 (0.828–0.992) | 20.1 | 0.0650 |
| 21 | L | Fusiform gyrus | BA 36 | 0.955 (0.880 1.036) | 15.4 | 0.444 |
| 28 | R | Motor cortex | BA 4, 6 | 0.954 (0.909 1.001) | 12.7 | 1.000 |
| 28 | R | Superior temporal gyrus | BA 22 | 0.936 (0.902–0.972) | 7.3 | 1.000 |
| 35 | L | Sup/mid temporal gyrus | BA 38 | 0.948 (0.919–0.977) | 6.4 | 1.000 |
| 25 | R | Frontal cortex | BA 11 | 0.985 (0.958–1.012) | 5.8 | 1.000 |
Number of patients with electrodes in corresponding regions is shown, along with laterality. Each region is derived from the Talairach atlas, level 5, shown with associated gyri/lobes. The effect of each spike on the odds of recall is also given. Effect size and adjusted P-values from the likelihood ratio test are given after controlling for family-wise error with the Holm-Bonferroni method. significant at *P = 0.05, ***P = 0.001
Figure 3.
Estimated odds of successful recall for each region. Mean odds of successful recall per spike during the encoding period are shown along with 95% CIs. Odds <1 indicate a decrease in the odds of successful recall per spike. Red indicates significant after multiple comparisons correction. L/R = left/right hemisphere.
Figure 4.
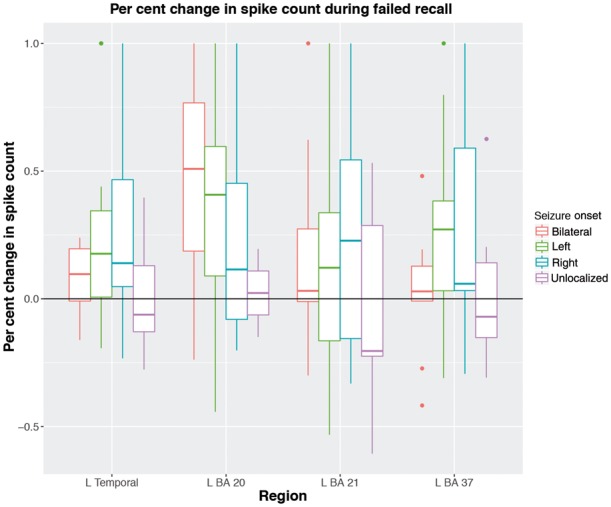
Per cent change in spike count during failed encoding for each patient. Vertical axis represents the per cent change in spike count. The horizontal axis represents the left temporal lobe as well as the significant left temporal regions from our regional analysis. Boxplots represent the percent change of spikes across all patients with the given SOZ lateralization. Only the recall of words in serial position >5 were included to account for the primacy effect.
Figure 5.
Electrode coverage and significant regions. Electrodes (n = 6144; blue dots) across all patients are shown in a 3D view of the left hemisphere (A) and an axial slice (B) on a template brain in Montreal Neurological Institute (MNI) space.
Retrieval
During the retrieval period, increased spiking correlated with decreased retrieval. In other words, a greater number of spikes occurred during unsuccessful retrieval than 1 s prior to a successful recall (Table 3). For left lateralized patients, both spikes within and outside of the SOZ decreased retrieval. For right lateralized patients, only spikes outside the SOZ significantly reduced retrieval. In either case, spikes outside the SOZ had a larger effect on retrieval versus spikes within the SOZ. Regional analysis was not performed due to insufficient time-matched periods across patients and Brodmann areas.
Table 3.
Estimated odds ratios of effect of spikes relative to the seizure onset zone during retrieval
| OR (95% CI) | Z | P | |
|---|---|---|---|
| Right lateralized SOZ (n = 26) | |||
| Spikes within SOZ | 0.995 (0.952–1.040) | −0.213 | 0.831 |
| Spikes outside SOZ | 0.847 (0.810–0.886) | −7.198 | <0.001* |
| Left lateralized SOZ (n = 22) | |||
| Spikes within SOZ | 0.796 (0.738–0.860) | −5.829 | <0.001*,# |
| Spikes outside SOZ | 0.750 (0.705–0.798) | −9.178 | <0.001*,# |
A logistic GLMM model was fit to a binary response variable indicating recall success (1) or failure (0). Covariates included spikes within the SOZ, outside the SOZ, and interaction of each with clinical SOZ lateralization. Odds ratios (OR) are adjusted for age and serial word position. Patients with bilateral onsets were not included. CI = confidence interval, significance determined by Wald statistics. Significance at *P < 0.05; #significant difference between left and right lateralized SOZ patients [increased odds of retrieval for spikes if right lateralized; outside SOZ: odds = 1.130 (1.055–1.210), z = 3.49, P < 0.001, within SOZ: odds = 1.250 (1.198–1.304), z = 5.248, P < 0.001].
Discussion
We showed that in patients with epilepsy, interictal epileptiform spikes that occur after word presentation lead to greater likelihood of failed recall, which suggests that spikes disrupt short term memory encoding. Spikes during the retrieval period also led to reduced recall. These effects were strongest with left lateralized spikes. During the encoding period, spikes outside the SOZ in left lateralized patients significantly reduced recall, a finding not present in right lateralized patients. Similarly, spikes regardless of SOZ significantly affected retrieval, though again the effect was greater with spikes outside the SOZ. Finally, we observed that spikes in specific temporal lobe structures functionally implicated in verbal and auditory word processing and verbal memory were most impactful. Our regional observations support the importance of the temporal neocortex in memory encoding and extend it by elucidating the spatial distribution of spikes relative to the seizure onset zone, the lateralization of spikes, and the quantification of the effect of spikes in the verbal word recall task.
As our findings rest on the accuracy of our spike detections, we vetted a random subset of our spikes equally represented across all patients. In this group, our detections performed with a positive predictive value of 72%. Furthermore, we observed an expected association between spike and seizure onset lateralization (Fig. 2).
During encoding, the observation that spikes outside the SOZ affect recall greater than spikes within the SOZ suggests that epileptic regions may have some degree of dysfunction at baseline that, in a verbal word memory task, manifests most prominently in left lateralized patients (Table 1). These observations related to the SOZ are supported neuropathologically by studies showing neuronal loss in more than 90% of temporal lobe epilepsies as detailed in a review by Sutula et al. (2003). In addition, electrophysiological network studies have also shown dynamic uncoupling of the seizure onset region (Warren et al., 2010; Burns et al., 2014). This finding has potential implications in the clinical realm. Clinical tests are routinely used in an attempt to identify regions of eloquent cortex in order to weigh the functional consequences versus the potential benefits of surgical resection. As new focal surgical techniques, such as laser ablation, have led to improved cognitive outcomes by limiting damage to collateral structures (Drane et al., 2015), limiting the extent of resection may lead to improved cognitive outcome. This is supported by the concept of a ‘nociferous cortex’, where the epileptogenic zone may impair the function of other regions, in this case through spikes. Spikes have previously been shown to provide adjunctive information in the clinical mapping of the seizure onset region in certain populations (Marsh et al., 2010), and our results suggest that identifying spike populations, namely those that do not impact cognition, may improve SOZ localization, surgical planning, and cognitive outcomes from surgical resection.
The regional localization of spikes may also elucidate the function of underlying tissue. Our regional analysis suggests that spikes in primarily left-sided structures lead to poor recall, which agree with previous studies on cognitive impairment that lateralize functional disruption based on cortical interictal epileptiform discharges. Specifically, that the left-sided and right-sided spikes produce verbal and spatial task impairments, respectively (Aarts et al., 1984).
Spikes in the left inferior temporal gyrus (BA 20) and nearby fusiform gyrus (BA 37) most significantly disrupted memory encoding. These regions form the ventral spatial pathway of visual memory and are involved in visual processing of words. Numerous studies have associated inferior temporal lobe function with working memory tasks in both humans and primates (Nobre et al., 1994; Wagner et al., 1998; Kondo et al., 2005). Wagner et al. (1998) demonstrated through functional MRI that the ability to remember a verbal experience is predicted by the activation of regions in the left prefrontal and temporal cortices. Hamame et al. (2012) recently observed increased gamma activity in the inferior temporal gyrus corresponding to increased visuospatial working memory load, suggesting that this region acts as a ‘visual sketchpad’ during memory maintenance. This, however, was a trial of only one patient, and we showed here in 67 patients that spikes in this region likely interfere with memory encoding by interrupting mental imagery of presented words.
During word presentation, subjects were asked to read each word aloud as it was presented, which likely activates both visual and auditory structures involved in word processing in addition to memory. The fusiform gyrus (BA 37) specifically has been functionally implicated in word recognition and is referred to as the visual word form area in a thorough review by McCandliss et al. (2003). Literature in cognition implicates that a critical process in visual word recognition groups the letters of a word together to an integrated perceptual unit (a ‘visual word form’), a function the fusiform gyrus participates in. The visual word form area within the left inferior temporal cortex has been shown to have increased activity through functional MRI during visual word recognition as well as an event related potential ∼250 ms after word presentation (Posner and McCandliss, 1992; Cohen et al., 2002). Wagner et al. (1998) and Hamame et al. (2012) similarly found correlated activity in the fusiform gyrus during verbal memory tasks. Our findings support this body of literature and extend it by suggesting that spikes in the fusiform gyrus may impede successful visual recognition of a word-form and maintenance of verbal memory. We showed in Fig. 4 that in words that were not recalled, there was an increased in spike rate in BA 37 during the corresponding encoding period. Though we were not able to determine a causal relationship between spikes and word recall, these differences begin to appear as more words were shown in a given word list, indicating that the effect of spikes may manifest as a patient finds greater difficulty in memorizing words.
There is extensive work covering the middle and superior temporal lobe’s involvement in memory processing, specifically regarding the medial structures such as the hippocampus and parahippocampal gyrus (Squire and Zola-Morgan, 1991; Squire et al., 2004; Eichenbaum et al., 2007; Baxter, 2009; Raslau et al., 2015). Surprisingly, we did not find these structures to be significantly affected by spikes, with the exception of BA 21 and 22. The relationship of these more lateral regions to memory is unclear. Several imaging studies showed that both BA 21 and 22 are involved with processing of auditory word-form as well as sentence generation (Grasby et al., 1993; Zahn et al., 2000; Ahmad et al., 2003; Monti et al., 2009). In this case, it may play a role similar to the fusiform gyrus for auditory stimuli as subjects were asked to read each word aloud, but additional work is necessary to further parse out these regions’ function in relation to memory.
Of note, a recent study of 10 patients with temporal lobe epilepsy showed that right-sided hippocampal discharges significantly reduced performance in a memory retrieval task in both humans and rats, but not encoding (Kleen et al., 2010, 2013). Though we were unable to directly test retrieval on a regional basis, our findings agree in that there was no effect of hippocampal spikes on encoding. This is to a certain extent surprising, as the hippocampus is believed to play a role in the encoding and retrieval of unconsolidated memory (Raslau et al., 2014). However, it is possible that existing hippocampal pathology prevents the effect of spikes from manifesting, similar to the relationship we found with the SOZ. Relatively healthy tissue, such as those surrounding the SOZ, may exhibit greater spike sensitivity, allowing us to observe functional consequences in the present study.
Furthermore, we found that spikes did not impede encoding in patients with right lateralized SOZs (Table 1). Taken into context with our previous findings showing that left-sided spikes are involved in memory encoding, this can be explained by the lack of spikes in the left hemisphere in right lateralized patients (Fig. 2). However, even in right lateralized patients there is increased spiking during the encoding of words that were not recalled (Fig. 4), which provides further evidence that the effect of spikes is regionally dependent. In an attempt to further identify the regional effects, we conducted a separate analysis only on right lateralized patients, but no regions (on either hemisphere) reached significance during encoding (Supplementary Table 3).
Inferior temporal lobe involvement in memory encoding is also supported in a recent finding by the Restoring Active Memory collaborative research group (Horak et al., 2016). Interestingly, they reported an impact of spikes in parietal lobe structures and in the SOZ, which we did not find. In fact, our analysis suggests that spikes impair recall only if outside the SOZ, and in these cases, only in left lateralized patients. This may be due to variability in the patient population, where for the majority of patients, spikes did not occur more often in the SOZ (Supplementary Fig. 5). Furthermore, our analysis investigated whole-brain sublobar regions with greater specificity at the level of Brodmann areas versus a priori selection of six brain regions. For example, our strongest effect was found in the left fusiform gyrus, part of the inferior temporal lobe, which has associations with verbal word formation instead of with regions traditionally thought to be involved in memory. While some of the differences between the two studies are difficult to reconcile, we believe the main complementary findings are an important step to understanding the impact of spikes on cognition.
During retrieval, spikes reduced recall in both left and right lateralized patients (Table 3). We also observed a greater effect of spikes as well as a diminished difference between effects relative to the SOZ. The differences between retrieval and encoding are difficult to explain, but are likely related to the involvement of other functional regions or circuits during active retrieval, which include regions involved in contextual retrieval (Friedman and Johnson, 2000). This observation has been seen previously by Kleen et al. (2013) who demonstrated that hippocampal spikes reduced performance specifically during retrieval and not encoding. Previous imaging studies also suggest involvement of right lateralized structures, such as the precuneus specifically during retrieval, which may further explain our observed effect of right hemispheric spikes outside the SOZ (Fletcher et al., 1995). Interestingly, this is supported by Kleen et al. (2013), who showed that the effect of hippocampal spikes is lateralized to the right hemisphere during retrieval. This may suggest that in patients with left temporal epilepsy the language dominance has shifted to the right temporal lobe, though in our case left hemisphere spikes still affected retrieval in patients with left lateralized SOZ. Further studies using cognitive tasks designed to test retrieval are needed to more precisely determine the regional effects of spikes.
Quantitative modelling
Interpretation of the model estimates show that each spike in BA 37 reduces the odds of recall by 15%, where in BA 20 the odds of recall are reduced by ∼8% per spike. Supplementary Fig. 4 show the corresponding predicted recall percentages per spike estimated from our model. This implies that increased spiking increases the probability of failed recall and is not necessarily an all-or-nothing mechanism. These findings show that spikes focally and additively impact function in a spatially dependent manner within a given region. Finally, it is important to note that spike occurrence across BA 20, 21, 22 and 37 are correlated (r > 0.46), which is expected as these regions may play similar functional roles, though further work is necessary to determine whether this contribution is additive.
Limitations
Although automated spike detection is a difficult challenge to perfect and validate in the field, we believe an automated algorithm that performs with acceptable true positive rate allows us to still make comparisons between groups. Furthermore, an automated algorithm allows objective detection of spikes that does not suffer from differences in inter-rater reliability.
We have shown that epileptiform discharges in various regions of the temporal lobe affect verbal word processing and memory encoding. Our ability to discern influence in other structures is limited by electrode coverage (Fig. 5). Notably, our electrodes are primarily cortical, with the exception of medial temporal subcortical structures interrogated by depth electrodes. It is also important to note that when assessing the effect of spikes on retrieval, we make an assumption that any time-matched period without any vocal utterance is a period of unsuccessful retrieval. Another task, such as the Sternberg task where recall periods are controlled, should be used to verify these findings.
There is rich literature supporting the notion that interictal epileptiform discharges are associated with cognitive impairments, described by Aarts et al. (1984) as ‘transitory cognitive impairment’. Though spikes may influence memory and other cognitive processes, it remains unclear whether spikes should be treated in clinical management of otherwise well controlled epilepsy (Binnie, 2003). In addition, while we have shown that memory encoding is affected by spikes in a spatially distributed manner, the magnitude of the effect is fairly small depending on the region (roughly a 5% decline in probability for spikes outside the seizure onset zone; Supplementary Fig. 4). Coupled with the observation that recall percentage is limited to begin with, the consequence of spikes on overall patient cognition and quality of life needs to be further studied.
Conclusion
In this study we have shown that interictal epileptiform spikes impede memory encoding when present outside the SOZ and within the left temporal lobe, specifically in the temporal and fusiform gyri. Word retrieval is also affected by spikes irrespective of the SOZ in left lateralized patients, and only by spikes outside the SOZ in right lateralized patients. These results suggest that spikes are not benign and can disrupt visual word recognition as well as verbal memory processes. In addition, our findings support the use of surgical interventions that spare cortex outside of the SOZ. Specifically, the observation that spikes affect a particular region of the brain suggests that this region may be functional in the absence of spikes. Further study is warranted to determine the magnitude of this effect relative to known cognitive deficits in epilepsy patients.
Supplementary Material
Acknowledgments
We thank the Penn Computational Memory Lab for providing data and supporting information.
Glossary
Abbreviations
- BA
Brodmann area
- GLMM
generalized linear mixed model
- SOZ
seizure-onset zone
Funding
This study was funded by the National Institutes of Health (NIH), the Thornton Foundation, and the Mirowski Foundation grants through the University of Pennsylvania: NIH P20NS080181, U01NS073557, K23NS073801, R25GM071745, R01NS099348, and T32NS091006. The International Epilepsy Electrophysiology Portal is funded by the NIH (U24NS063930).
Supplementary material
Supplementary material is available at Brain online.
References
- Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain 1984; 107 (Pt 1): 293–308. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology 2003; 60: 1598–605. [DOI] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron 2009; 61: 667–77. [DOI] [PubMed] [Google Scholar]
- Binnie CD. Cognitive impairment during epileptic form discharges: is it ever justifiable to treat the EEG? Lancet Neurol 2003; 2: 725–30. [DOI] [PubMed] [Google Scholar]
- Blake RV, Wroe SJ, Breen EK, McCarthy RA. Accelerated forgetting in patients with epilepsy: evidence for an impairment in memory consolidation. Brain 2000; 123: 472–83. [DOI] [PubMed] [Google Scholar]
- Brinkmann BH, Bower MR, Stengel KA, Worrell GA, Stead M. Multiscale electrophysiology format: an open-source electrophysiology format using data compression, encryption, and cyclic redundancy check. In: Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, Minneapolis, MN, EMBC, Sept 3–6 2009 p. 7083–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Sharan AD, Sperling MR, Ramayya AG, Evans JJ, Healey MK, et al. Theta and high-frequency activity mark spontaneous recall of episodic memories. J Neurosci 2014; 34: 11355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD, et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J Neurosci 2013; 33: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Santaniello S, Yaffe RB, Jouny CC, Crone NE. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci USA 2014; 111: E5321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 2002; 125: 1054–69. [DOI] [PubMed] [Google Scholar]
- Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 2015; 56: 101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007; 30: 123–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller R, Echauz J, Tcheng T, Litt B, Pless B. Line length: an efficient feature for seizure onset detection. Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, Oct 25–28, 2001 Vol. 2, p. 1707–10. [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RSJ, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory an in vivo study in humans. Brain 1995; 118: 401–16. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc Res Tech 2000; 51: 6–28. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Alkawadri R, Farooque P, Goncharova II, Zaveri HP. Automatic detection of prominent interictal spikes in intracranial EEG: validation of an algorithm and relationsip to the seizure onset zone. Clin Neurophysiol 2014; 125: 1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Khodagholy D, Thesen T, Devinsky O, Buzsáki G. Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat Med 2016; 22: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, Spencer SS, Duckrow RB, Hirsch LJ, Spencer DD, Zaveri HP. Intracranially recorded interictal spikes: relation to seizure onset area and effect of medication and time of day. Clin Neurophysiol 2013; 124: 2119–28. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ. Functional mapping of brain areas implicated in auditory—verbal memory function. Brain 1993; 116: 1–20. [DOI] [PubMed] [Google Scholar]
- Hamame CM, Vidal JR, Ossandon T, Jerbi K, Dalal SS, Minotti L, et al. Reading the mind’s eye: online detection of visuo-spatial working memory and visual imagery in the inferior temporal lobe. Neuroimage 2012; 59: 872–9. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 2006; 8: 504–15. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Elger CE, Helmstaedter C. Long-term memory impairment in patients with focal epilepsy. Epilepsia 2007; 48 (Suppl 9): 26–9. [DOI] [PubMed] [Google Scholar]
- Horak P, Meisenhelter S, Song Y, Testorf M, Kahana M, Viles W, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia 2016; 58; 373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn Sci 2010; 14: 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janca R, Jezdik P, Cmejla R, Tomasek M, Worrell GA, Stead M, et al. Detection of interictal epileptiform discharges using signal envelope distribution modelling: application to epileptic and non-epileptic intracranial recordings. Brain Topogr 2014; 28: 172–83. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol 2010; 67: 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013; 81: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Bénar CG, Aghakhani Y, Andermann F, Dubeau F, et al. Temporal and extratemporal BOLD responses to temporal lobe interictal spikes. Epilepsia 2006; 47: 343–54. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, et al. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage 2005; 24: 1154–63. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol 2012; 98: 279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh ED, Peltzer B, Brown MW, Wusthoff C, Storm PB, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia 2010; 51: 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci 2003; 7: 293–9. [DOI] [PubMed] [Google Scholar]
- Monti MM, Parsons LM, Osherson DN. The boundaries of language and thought in deductive inference. Proc Natl Acad Sci USA 2009; 106: 12554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BB. The serial position effect of free recall. J Exp Psychol 1962; 64: 482–8. [Google Scholar]
- Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010; 51: 883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature 1994; 372: 260–3. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci 2002; 6: 93–102. [DOI] [PubMed] [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. JAMA 1954; 155: 86. [Google Scholar]
- Posner MI, McCandliss BD. Brain circuitry during reading. In: Converging methods for understanding reading and dyslexia. 1992. The MIT Press, Cambridge, MA, p. 305–37. [Google Scholar]
- Raslau FD, Klein AP, Ulmer JL, Mathews V, Mark LP. Memory part 1: overview. AJNR Am J Neuroradiol 2014; 35: 2058–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslau FD, Mark IT, Klein AP, Ulmer JL, Mathews V, Mark LP. Memory part 2: the role of the medial temporal lobe. AJNR Am J Neuroradiol 2015; 36: 846–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R, Lieb JP, Crandall PH. Neuropsychologic correlates of depth spike activity in epileptic patients. Arch Neurol 1978; 35: 699–705. [DOI] [PubMed] [Google Scholar]
- Schwab RS. A method of measuring consciousness in attacks of petit mall epilepsy. Arch Neurol Psychiatry 1939; 41: 215–17. [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 2003; 23: 10809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 2007; 17: 1190–6. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia 2008; 49: 1881–92. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci 2004; 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 1991; 253: 1380–6. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol 2003; 16: 189–95. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998; 281: 1188–91. [DOI] [PubMed] [Google Scholar]
- Wang W-H, Liou H-H, Chen C-C, Chiu M-J, Chen T-F, Cheng T-W, et al. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: a retrospective cross-sectional study. Epilepsy Behav 2011; 22: 728–34. [DOI] [PubMed] [Google Scholar]
- Warren CP, Hu S, Stead M, Brinkmann BH, Bower MR, Worrell GA. Synchrony in normal and focal epileptic brain: the seizure onset zone is functionally disconnected. J Neurophysiol 2010; 104: 3530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SB, Emerson R. Spike detection: a review and comparison of algorithms. Clin Neurophysiol 2002; 113: 1873–81. [DOI] [PubMed] [Google Scholar]
- Zahn R, Huber W, Drews E, Erberich S, Krings T, Willmes K, et al. Hemispheric lateralization at different levels of human auditory word processing: a functional magnetic resonance imaging study. Neurosci Lett 2000; 287: 195–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.